Abstract
Purpose
The Radiation Therapy Oncology Group 9804 study identified good-risk patients with ductal carcinoma in situ (DCIS), a breast cancer diagnosis found frequently in mammographically detected cancers, to test the benefit of radiotherapy (RT) after breast-conserving surgery compared with observation.
Patients and Methods
This prospective randomized trial (1998 to 2006) in women with mammographically detected low- or intermediate-grade DCIS, measuring less than 2.5 cm with margins ≥ 3 mm, compared RT with observation after surgery. The study was designed for 1,790 patients but was closed early because of lower than projected accrual. Six hundred thirty-six patients from the United States and Canada were entered; tamoxifen use (62%) was optional. Ipsilateral local failure (LF) was the primary end point; LF and contralateral failure were estimated using cumulative incidence, and overall and disease-free survival were estimated using the Kaplan-Meier method.
Results
Median follow-up time was 7.17 years (range, 0.01 to 11.33 years). Two LFs occurred in the RT arm, and 19 occurred in the observation arm. At 7 years, the LF rate was 0.9% (95% CI, 0.0% to 2.2%) in the RT arm versus 6.7% (95% CI, 3.2% to 9.6%) in the observation arm (hazard ratio, 0.11; 95% CI, 0.03 to 0.47; P < .001). Grade 1 to 2 acute toxicities occurred in 30% and 76% of patients in the observation and RT arms, respectively; grade 3 or 4 toxicities occurred in 4.0% and 4.2% of patients, respectively. Late RT toxicity was grade 1 in 30%, grade 2 in 4.6%, and grade 3 in 0.7% of patients.
Conclusion
In this good-risk subset of patients with DCIS, with a median follow-up of 7 years, the LF rate was low with observation but was decreased significantly with the addition of RT. Longer follow-up is planned because the timeline for LF in this setting seems protracted.
INTRODUCTION
Ductal carcinoma in situ (DCIS) of the breast is stage 0 cancer, considered non–life threatening, and the fourth most common cancer diagnosed in women, after invasive breast, lung, and colon cancers. In screening programs in the United States, DCIS accounts for approximately 25% of all new breast cancers.1 After the demonstration that breast-conserving surgery (BCS) and radiation (RT) produced results equivalent to mastectomy by both the National Surgical Adjuvant Breast and Bowel Project (NSABP) and the Milan Cancer Institute trials for early invasive breast cancers,2,3 four large prospective trials were designed to address the effectiveness of BCS and RT for women with DCIS compared with BCS alone.4–7 The trials produced strikingly similar results; the addition of RT resulted in a reduction in the risk of local failure (LF) in the breast of ≥ 50%. Of the LFs, approximately half were DCIS and half were invasive breast cancer. However, survival is excellent and seems to be independent of local treatment.
More recently, an understanding of DCIS as not just one disease but a group of related subtypes of cancers has emerged, with a spectrum of LF risk. Is there a low-risk DCIS for which the benefit of RT would not be seen? Several groups addressed this intriguing issue by assessing clinical and pathologic factors in their DCIS patient databases and modeling for recurrence; examples include the Van Nuys Recurrence Score8 and the Memorial Sloan-Kettering Nomogram.9 The Radiation Therapy Oncology Group (RTOG) 9804 phase III trial was conceived to address the question of RT benefit in a good-risk DCIS subset. The definition for good risk was derived from the best available clinical information at the time and was aimed at addressing the smaller size and lower grade lesions detected by screening mammography.
PATIENTS AND METHODS
This trial was conducted by the RTOG and reviewed and approved by the American College of Radiology Institutional Review Board (IRB) and local IRBs from participating sites. All patients gave written informed consent in accordance with each center's IRB guidelines.
The primary objective was to assess the role of RT versus observation after BCS in decreasing or delaying the appearance of invasive cancer or DCIS LF and preventing the need for mastectomy. Secondary end points included disease-free survival (DFS).
Another objective was to demonstrate a working pathology classification system for DCIS. The study opened shortly after the Consensus Conference on the Classification of Ductal Carcinoma In Situ in 1997.10 Definitions for DCIS subtypes developed at that meeting were adopted for this study and were used in developing College of American Pathology guidelines.11
The study developed a pathology teaching Web site to assist pathologists in identifying low-risk patients, because funding for central pathology review was not available. Another objective included a planned review by a breast pathologist of 10% of all patients, recently reported separately.12
Eligibility
Women with DCIS detected by mammogram or incidentally found in tissue of an otherwise benign biopsy were eligible. The DCIS was unicentric, low or intermediate nuclear grade, and less than 2.5 cm, on pathology or imaging, based on the work of Lagios et al.13 Minimal margin width was 3 mm to ink. All patients had a negative postexcision mammogram, and women in the RT arm began treatment within 12 weeks of final surgery.
Patients were not eligible if they were less than age 26 years, had active connective tissue disorders, or were pregnant or lactating. The usual exclusions of women with prior cancers were applied, except for those with basal or squamous skin cancers or in situ carcinoma of the cervix.
Women taking hormone therapy at the time of study entry were excluded. Tamoxifen was allowed but only if started within 4 weeks from the diagnosis of DCIS.
Treatment
Tamoxifen was required in both arms when the study opened, based on information from the NSABP B24 study comparing RT with or without tamoxifen.14 RTOG 9804 was amended in 2001 to make tamoxifen optional, based on the conflicting data regarding tamoxifen in DCIS from the United Kingdom, Australia, and New Zealand trial results.7 Tamoxifen dose was 20 mg daily for 5 years and was added as a stratification factor based on intent to use in 2001.
For women randomly assigned to whole-breast RT, the dose was 50 Gy in 25 fractions or 50.4 Gy in 28 fractions. The study was amended in 2001 to include 42.5 Gy in 16 fractions; no boost was allowed in the study. Standard whole-breast tangent fields were used, planned with either conventional or computed tomography simulation. Dose was prescribed to the lung–chest wall interface at the isocenter, and maximum dose to the planning target volume allowed was 15%. Compensators, wedges, and dynamic therapy were techniques used to achieve this constraint. All patients were observed every 3 months for the first year, then every 6 months for 2 years, and then yearly. Mammograms were required yearly.
Design
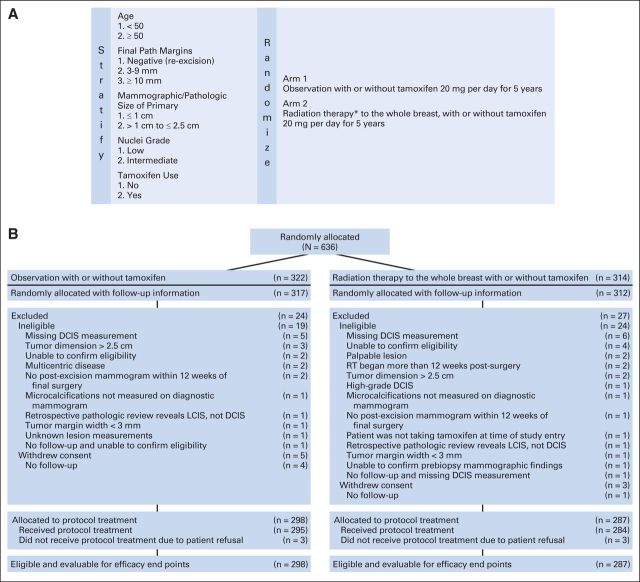
The primary end point was LF, which was defined as biopsy-proven invasive or DCIS recurrence measured from date of random assignment to date of failure or last follow-up for censored patients. Patients were stratified by age (< v ≥ 50 years), DCIS size (≤ v > 1 cm), and pathology margins (negative re-excision v 3 to 9 mm v ≥ 10 mm). When tamoxifen was made optional, tamoxifen use (yes v no) and nuclear grade (low v intermediate) were added as stratification factors. Patients were randomly assigned 1:1 to RT versus observation as described by Zelen15 (Fig 1A) using a permuted block random assignment scheme. The revised design, after the 2001 amendment, was based on the assumption that two thirds of the patients would receive tamoxifen and that low- and intermediate-grade distribution would be equally frequent. In addition, the LF rates for the control arm based on tamoxifen use and nuclear grade were assumed to be 3%, 5%, 7%, and 10% for yes/low, no/low, yes/intermediate, and no/intermediate, respectively, with an overall assumed LF rate of 6% for the observation arm. The primary hypothesis was that the addition of RT would reduce the LF rate from 6% to 3.5% (hazard ratio [HR], 0.58).
Fig 1.
(A) Schema and (B) CONSORT patient flow diagram of the Radiation Therapy Oncology Group (RTOG) 9804 study. (*) Radiation therapy dose consisted of one of the following: 1.8 Gy per fraction for 28 fractions, for a total dose of 50.4 Gy; 2.0 Gy per fraction for 25 fractions, for a total dose of 50.0 Gy; or 2.656 Gy per fraction for 16 fractions, for a total dose of 42.5 Gy. DCIS, ductal carcinoma in situ; LCIS, lobular carcinoma in situ.
On the basis of these assumptions and the sequential design approach of Kim and Tsiatis16 with three treatment comparisons (two interim analyses and one final analysis) using two-sided log-rank test statistics, a maximum of 129 local recurrence events was required to detect the hypothesized reduction in the LF cumulative incidence with a statistical power of 80% and significance level of P = .05. The target sample size was 1,790 patients.
Statistical Methods
All analyses were performed using SAS version 9.2 statistical software (SAS Institute, Cary, NC). Secondary end points included overall survival (OS), defined as death from any cause; contralateral breast failure (CBF); distant failure (DF), defined as progression of disease beyond the treated breast and regional nodes; and salvage mastectomy failure (MF), defined as removal of the study breast. Death, LF, CBF, DF, and MF were considered failures for DFS. Secondary end points were measured from the date of random assignment to date of failure or last follow-up for censored patients.
Cumulative incidence methods17 were used to estimate the rates of LF, CBF, DF, and MF, with death as the competing risk. OS and DFS rates were estimated using the Kaplan-Meier method.18 Tests of the statistical significance of the effect of RT on all end points were carried out using the log-rank test.19 For LF, CBF, DF, and MF, Gray's test comparing treatment arms is also shown.20 Univariable Cox proportional hazards models21 were used to test for treatment differences (observation v RT) and are coded such that HR less than 1 indicates a decreased risk of failure for the RT arm. All eligible patients are included in the intent-to-treat analysis, based on the treatment arm to which they were randomly assigned. The data were also analyzed with the inclusion of patients found to be ineligible, because the most common reasons for ineligibility included missing DCIS size, inability to confirm all eligibility criteria, or tumor reportedly larger than 2.5 cm, and patients who withdrew consent but had follow-up information before withdrawal (Fig 1B).
Acute toxicities (≤ 90 days from treatment start) were scored using the National Cancer Institute Common Toxicity Criteria version 2.0. Late RT toxicities (> 90 days from treatment start) were scored using the RTOG/European Organisation for Research and Treatment of Cancer Late Radiation Morbidity Scheme.
RESULTS
Administrative Data
This study opened in December 1999, and as a result of not meeting targeted accrual, it closed in July 2006, with a total of 636 patients of the 1,790 planned, entered from almost 200 institutions. At the time the trial closed, the required number of events for the first protocol-specified interim analysis of LF had not been reached, so no interim analyses were performed. The Data Monitoring Committee recommended that all patients be observed for a minimum of 5 years and then the study results be reported. No protocol-specified primary end point interim analyses occurred before this reporting. All analyses are based on data through March 2012. Forty-three women were ineligible on review, of whom 41 had follow-up information, and eight women withdrew consent, of whom three had follow-up information. Reasons for ineligibility are shown in the CONSORT diagram (Fig 1B).
Median age was 58 years. Table 1 lists the patient and tumor characteristics in the two randomized arms. Median follow-up for all patients is 7.17 years (range, 0.01 to 11.33 years; 25th percentile, 5.92 years; 75th percentile, 8.87 years), and median follow-up for alive patients is 7.35 years (range, 0.03 to 11.33 years; 25th percentile, 5.99 years; 75th percentile, 8.93 years).
Table 1.
Patient Demographics and Clinical Characteristics

| Demographic or Clinical Characteristic | Observation ± Tamoxifen (n = 298) |
Radiotherapy ± Tamoxifen (n = 287) |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Age, years | ||||
| Median | 58 | 58 | ||
| Range | 35-84 | 34-85 | ||
| Q1-Q3 | 51-68 | 51-67 | ||
| Largest dimension of DCIS lesion on pathology slide, cm | ||||
| Median | 0.5 | 0.5 | ||
| Range | 0.1-2.5 | 0.1-2.5 | ||
| Q1-Q3 | 0.3-0.8 | 0.3-0.8 | ||
| Race | ||||
| White | 246 | 82.6 | 227 | 79.1 |
| African American | 21 | 7.0 | 22 | 7.7 |
| Hispanic | 12 | 4.0 | 17 | 5.9 |
| American Indian | 5 | 1.7 | 2 | 0.7 |
| Asian | 13 | 4.4 | 14 | 4.9 |
| Native Hawaiian or other Pacific Islander | 1 | 0.3 | 4 | 1.4 |
| Other | 0 | 0.0 | 1 | 0.3 |
| Age, years | ||||
| < 50 | 61 | 20.5 | 54 | 18.8 |
| ≥ 50 | 237 | 79.5 | 233 | 81.2 |
| Final microscopic margins, mm | ||||
| ≥ 3-9 | 106 | 35.6 | 104 | 36.2 |
| ≥ 10 | 48 | 16.1 | 45 | 15.7 |
| Negative by negative re-excision | 144 | 48.3 | 138 | 48.1 |
| Mammographic size of primary tumor, cm | ||||
| ≤ 1 | 217 | 72.8 | 207 | 72.1 |
| > 1 | 81 | 27.2 | 80 | 27.9 |
| Nuclear grade | ||||
| 1 | 131 | 44.0 | 121 | 42.2 |
| 2 | 167 | 56.0 | 166 | 57.8 |
| Tumor location | ||||
| Left breast | 148 | 49.7 | 142 | 49.5 |
| Right breast | 150 | 50.3 | 145 | 50.5 |
| Intention to use tamoxifen | ||||
| No | 91 | 30.5 | 90 | 31.4 |
| Yes | 207 | 69.5 | 197 | 68.6 |
Abbreviations: DCIS, ductal carcinoma in situ; Q1, first quartile; Q3, third quartile.
Study End Points
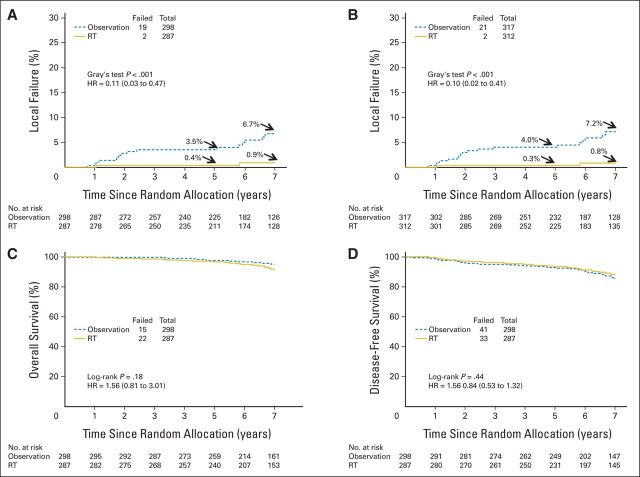
Cumulative rates of LF in the ipsilateral breast at 5 and 7 years were 0.4% and 0.9% in the RT arm versus 3.5% and 6.7% in the observation arm, respectively (Fig 2A; Table 2; log-rank and Gray's test, P < .001; HR, 0.11; 95% CI, 0.03 to 0.47). The histology of the LFs was invasive in 42.1% and noninvasive in 57.9% of patients in the observation arm; in the RT arm, one patient each experienced invasive and noninvasive LF. Results were essentially the same when including the ineligible patients with follow-up data (n = 41) or who withdrew consent (n = 3); the LF rates at 5 and 7 years were 0.3% and 0.8% in the RT arm versus 4.0% and 7.2% in the observation arm, respectively (Table 2; log-rank and Gray's test, P < .001; HR, 0.10; 95% CI, 0.02 to 0.41; Fig 2B). LF rates by tamoxifen use and nuclear grade were 1.2%, 5.3%, 2%, and 8.4% for yes/low, no/low, yes/intermediate, and no/intermediate, respectively. The risk of CBF at 7 years was 3.9% in the RT arm and 4.8% in the observation arm (Table 2; log-rank and Gray's test, P = .86 and P = .88, respectively; HR, 1.07; 95% CI, 0.48 to 2.39).
Fig 2.
(A) Local failure in ipsilateral breast for all eligible patients (n = 585). (B) Local failure in ipsilateral breast for all accrued patients with follow-up (n = 629). (C) Disease-free survival (n = 585). (D) Overall survival (n = 585). HR, hazard ratio; RT, radiation therapy.
Table 2.
Efficacy End Points
| End Point and Arm | No. of Patients | No. of Failures | LF at 5 Years (%) |
LF at 7 Years (%) |
Log-Rank/Gray's Test P | Hazard Ratio | 95% CI | ||
|---|---|---|---|---|---|---|---|---|---|
| Estimated Rate | 95% CI | Estimated Rate | 95% CI | ||||||
| Local failure in treated breast | |||||||||
| All eligible patients | 585 | ||||||||
| Observation ± tamoxifen | 298 | 19 | 3.5 | 1.4 to 5.7 | 6.7 | 3.4 to 10.0 | < .001 | 0.11 | 0.03 to 0.47 |
| Radiotherapy ± tamoxifen | 287 | 2 | 0.4 | 0.0 to 1.1 | 0.9 | 0.0 to 2.2 | |||
| All accrued patients with follow-up* | 629 | ||||||||
| Observation ± tamoxifen | 317 | 21 | 4.0 | 1.8 to 6.3 | 7.2 | 3.9 to 10.5 | < .001 | 0.10 | 0.02 to 0.41 |
| Radiotherapy ± tamoxifen | 312 | 2 | 0.3 | 0.0 to 1.0 | 0.8 | 0.0 to 2.0 | |||
| Contralateral breast failure | 585 | ||||||||
| Observation ± tamoxifen | 298 | 12 | 2.2 | 0.4 to 3.9 | 4.8 | 2.0 to 7.7 | .86/.88 | 1.07 | 0.48 to 2.39 |
| Radiotherapy ± tamoxifen | 287 | 12 | 3.4 | 1.2 to 5.6 | 3.9 | 1.5 to 6.4 | |||
| Overall survival | 585 | ||||||||
| Observation ± tamoxifen | 298 | 15 | 97.5 | 94.8 to 98.8 | 95.1 | 91.4 to 97.2 | .18 | 1.56 | 0.81 to 3.01 |
| Radiotherapy ± tamoxifen | 287 | 22 | 96.7 | 93.7 to 98.3 | 91.7 | 87.2 to 94.7 | |||
| Disease-free survival | 585 | ||||||||
| Observation ± tamoxifen | 298 | 41 | 92.7 | 89.1 to 95.2 | 85.6 | 80.4 to 89.4 | .44 | 0.84 | 0.53 to 1.32 |
| Radiotherapy ± tamoxifen | 287 | 33 | 93.4 | 89.7 to 95.8 | 88.0 | 83.1 to 91.6 | |||
Ineligible patients with follow-up and patients who withdrew consent but had follow-up information before withdrawal are included.
Mastectomy rates were low; in the observation arm, eight women underwent subsequent mastectomy, including four unilateral mastectomies for an ipsilateral LF, three bilateral mastectomies for ipsilateral LF (n = 2) and bilateral LF (n = 1), and one elective bilateral mastectomy. In the RT arm, four women had mastectomies, all bilateral. Indications included ipsilateral failure (n = 1), contralateral failure (n = 1), bilateral failure (n = 1), and one elective surgery. The 7-year cumulative incidence of mastectomy was 2.8% (95% CI, 0.7% to 4.9%) in the observation arm and 1.5% (95% CI, 0% to 3.0%) in the RT arm. As expected, the OS (Fig 2C) and DFS (Fig 2D) rates were excellent and not different between the two arms (Table 2).
Patients assigned to the RT arm had a higher rate of grade 1 and 2 acute toxicities than patients in the observation arm (76% v 30%, respectively; P < .001). The rate of toxicities ≥ grade 3 was 4% in both arms. Late RT toxicity was grade 1 in 30.0%, grade 2 in 4.6%, and grade 3 in 0.7% of patients. There was no late grade 4 or 5 RT toxicity.
DISCUSSION
To our knowledge, this is the first prospective randomized trial comparing RT with observation in a subset of women with defined, mammographically detected good-risk DCIS. Other groups have also reported lower rates of in-breast recurrence after excision alone when selecting for good-risk DCIS. One other multi-institutional prospective study has reported similar results to RTOG 9804 in this specific population of DCIS. Hughes et al22 recently reported a 7-year LF rate of 10.5% in a low-risk DCIS cohort, with observation only, in women after lumpectomy on the Eastern Cooperative Oncology Group (ECOG) 5194 DCIS single-arm prospective trial. The low- or intermediate-grade patients in this trial matched the eligibility for RTOG 9804.
Another prospective study by the Dana-Farber group of observation for low-risk DCIS was recently updated. That study was planned for 200 women, but it closed early because of LF events exceeding protocol stopping rules. Good-risk criteria for this study included mammographically detected DCIS, measuring ≤ 2.5 cm, with a predominant nuclear grade of 1 or 2, and a margin of ≥ 1 cm or a negative re-excision if re-excised. With a median follow-up time of 11 years in 143 women, the cumulative incidence of LF at 10 years was 15.5%. Of note, unlike our study and the ECOG study, the presence of high nuclear grade did not exclude patients, as long as this was not the dominant grade. In the updated analysis, the presence of any high nuclear grade was associated with a relative risk ratio of 14.0 in their model.23
Our results, together with ECOG 5194, imply that clinical pathologic criteria can be used to define a cohort of patients with DCIS who can be expected to have a much lower rate of in-breast recurrence without RT in the first 7 years after lumpectomy than previously reported in past randomized trials. For comparison, the LF rates at 5 years in NSABP B17,4 in patients not selected for good risk, were 20.9% with no RT and 9.6% with RT. For many women, these new data may support their decision for omission of adjuvant RT after BCS, particularly because the rate of mastectomy in RTOG 9804 in both treatment arms was low. However, RTOG 9804 confirms that RT provides significant benefit in further reducing in-breast recurrence for women who opt to receive it.
Despite the similarity of the patient populations in RTOG 9804 and the ECOG 5194 low-risk cohort, there was some difference in the LF rates reported at a similar median follow-up of 7 years (6.6% v 10.5%, respectively). This may reflect different utilization rates of tamoxifen, which was taken by 31.3% of women accrued to ECOG 5194 compared with 62% of women in the RTOG trial. The NSABP B24 study of women with DCIS treated with BCS and then randomly assigned to RT versus RT and tamoxifen demonstrated the interaction between RT and tamoxifen in further lowering the risk of LF.14 Although the NSABP B24 study did not have a treatment arm with tamoxifen and no RT, a phase III randomized controlled trial from the United Kingdom, Australia, and New Zealand in DCIS did contain such an arm. In a recent follow-up report, women assigned to tamoxifen only had a significant reduction in new breast events, and tamoxifen specifically was noted to reduce the risk of DCIS LF in the ipsilateral breast, compared with the no tamoxifen/no RT arm.24 In RTOG 9804, there are so few events at this time that the data cannot support or refute the role of tamoxifen in the treatment of low-risk DCIS. Data on tamoxifen use in study follow-up are being collected, and with more events expected in the future, a meaningful analysis may be able to be performed at a later date.
The rates of LF in RTOG 9804 at 5 and 7 years, even in the observation arm, are low. But what is likely to happen with longer follow-up time? The Early Breast Cancer Trialists' Collaborative Group overview demonstrated that although the relative benefit of RT remained stable, the absolute benefit increased with time; at 5 years after random assignment, the absolute reduction in risk was 10.5% (SE, 1.2%; LF rate: 7.6% with RT v 18.1% with observation), whereas at 10 years, it was 15.2% (SE, 1.6%; LF rate: 12.9% with RT v 28.1% with observation).25 Solin et al26 retrospectively looked at 15-year results for 1,003 women with DCIS, detected mammographically and treated with BCS and RT, in a collaborative study of 10 institutions. At 5 years, the LF rate for the high-grade lesions with comedonecrosis was 12%, and for those without these features, the LF rate was 3%. However, at 10 years, the lower grade DCIS group essentially caught up with the higher risk group, with an LF rate of 15% in the low-grade group compared with 18% in the high-grade group. These data suggest that lower risk DCIS may have a low rate of LF in the first 5 years that continues to increase over time. The RTOG 9804 data will need continued follow-up to fully realize the rates of LF in this cohort of low-risk patients.
It is recognized that this study closed early, with 636 patients accrued of the original planned 1,790 women and approximately 20% of the planned events; therefore, this study has significantly reduced statistical power for the original hypothesis. Given that, although these results are not definitive, in the original hypothesis, it was estimated that the addition of RT would reduce the risk of LF from 6% to 3.5% (HR, 0.58). To date, the LF rate in the RT arm is less than 1%, yielding a statistically significant difference between the two arms as a result of the much larger than anticipated hazard reduction (HR, 0.11). Because, to our knowledge, this is the first prospective randomized DCIS study for good-risk patients, these data suggest the original estimate based on prior studies that included high-risk DCIS was too high. The magnitude of proportional reduction in local recurrence in this study from RT (HR, 0.10) is much larger than that seen in the four prior trials comparing RT with observation (HR, ∼0.46) after lumpectomy for DCIS.25 At this time in follow-up, RTOG 9804 has few events and wide CIs. Follow-up is continuing, and future analyses with longer follow-up will determine the reliability of this proportional reduction.
In conclusion, the RTOG 9804 trial in DCIS successfully identified a subset of women with good-risk DCIS based on standard pathology features including nuclear grade, size, and margin width. Although the addition of RT significantly decreased the LF rate for the patients accrued to this study, the full clinical implications of these results will require further follow-up, given the historic patterns of LF over 10 to 15 years from diagnosis of good-risk DCIS.
Supplementary Material
Footnotes
See accompanying article on page 686
Written on behalf of the Radiation Therapy Oncology Group.
Supported by Radiation Therapy Oncology Group Grant No. U10 CA 21661 and Community Clinical Oncology Program Grant No. U10 CA 37422 from the National Cancer Institute.
Presented in part at the 48th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 1-5, 2012, and the 54th Annual Meeting of the American Society of Radiation Oncology, Boston, MA, October 28-31, 2012.
This article's contents are the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT00003857.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Beryl McCormick, Kathryn Winter, Clifford Hudis, Henry Mark Kuerer, Barbara L. Smith, Nour Sneige, Eric A. Strom, Julia White
Provision of study materials or patients: Eileen Rakovitch, Eric A. Strom, Julia White
Collection and assembly of data: Kathryn Winter, Eileen Rakovitch, Amit Shah, Isabelle Germain, Alan C. Hartford, Afshin Rashtian, Eleanor M. Walker, Albert Yuen, Jeannette L. Wilcox, Laura A. Vallow, William Small Jr, Anthony T. Pu, Kevin Kerlin
Data analysis and interpretation: Beryl McCormick, Kathryn Winter, Jennifer Moughan, William Small Jr
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
RTOG 9804: A Prospective Randomized Trial for Good-Risk Ductal Carcinoma In Situ Comparing Radiotherapy With Observation
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Beryl McCormick
No relationship to disclose
Kathryn Winter
Consulting or Advisory Role: Roche
Clifford Hudis
Other Relationship: Breast Cancer Research Foundation
Henry Mark Kuerer
Consulting or Advisory Role: Gerson Lehrman Group, Lightpoint Medical
Research Funding: Genomic Health (Inst)
Patents, Royalties, Other Intellectual Property: McGraw-Hill Publishing
Eileen Rakovitch
Research Funding: Genomic Health
Barbara L. Smith
No relationship to disclose
Nour Sneige
No relationship to disclose
Jennifer Moughan
No relationship to disclose
Amit Shah
No relationship to disclose
Isabelle Germain
No relationship to disclose
Alan C. Hartford
No relationship to disclose
Afshin Rashtian
No relationship to disclose
Eleanor M. Walker
No relationship to disclose
Albert Yuen
No relationship to disclose
Eric A. Strom
No relationship to disclose
Jeannette L. Wilcox
No relationship to disclose
Laura A. Vallow
No relationship to disclose
William Small Jr
Honoraria: Zeiss
Speakers' Bureau: Zeiss
Travel, Accommodations, Expenses: Zeiss
Anthony T. Pu
Leadership: Radiological Associates of Sacramento
Stock or Other Ownership: Radiological Associates of Sacramento
Kevin Kerlin
Employment: 21st Century Oncology
Julia White
Honoraria: Genomic Health, Bayer Healthcare, Qfix, Varian
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 3.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 4.Fisher B, Dignam J, Wolmark N, et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: Findings from the National Surgical Adjuvant Breast and Bowel Project B-17. J Clin Oncol. 1998;16:441–452. doi: 10.1200/JCO.1998.16.2.441. [DOI] [PubMed] [Google Scholar]
- 5.Julien JP, Bijker N, Fentimen IS, et al. Radiotherapy in breast conserving treatment for ductal carcinoma in situ: First results of the EORTC randomized phase III trial 10853—EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. Lancet. 2000;355:528–533. doi: 10.1016/s0140-6736(99)06341-2. [DOI] [PubMed] [Google Scholar]
- 6.Emdin SO, Granstrand B, Ringberg A, et al. SweDCIS: Radiotherapy after sector resection for ductal carcinoma in situ of the breast—Results of a randomized trial in a population offered mammography screening. Acta Oncol. 2006;45:536–543. doi: 10.1080/02841860600681569. [DOI] [PubMed] [Google Scholar]
- 7.Houghton J, George WD, Cuzick J, et al. Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia and New Zealand: Randomized controlled trial. Lancet. 2003;362:95–102. doi: 10.1016/s0140-6736(03)13859-7. [DOI] [PubMed] [Google Scholar]
- 8.Silverstein MJ, Lagios MD, Groshen S, et al. The influence of margin width on local control of ductal carcinoma in situ of the breast. N Engl J Med. 1999;340:1455–1461. doi: 10.1056/NEJM199905133401902. [DOI] [PubMed] [Google Scholar]
- 9.Rudloff U, Jacks LM, Goldberg JI, et al. Nomogram for predicting the risk of local recurrence after breast-conserving surgery for ductal carcinoma in situ. J Clin Oncol. 2010;28:3762–3769. doi: 10.1200/JCO.2009.26.8847. [DOI] [PubMed] [Google Scholar]
- 10.The Consensus Conference Committee. Consensus Conference on the classification of ductal carcinoma in situ. Cancer. 1997;80:1798–1802. doi: 10.1002/(sici)1097-0142(19971101)80:9<1798::aid-cncr15>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 11.Lester SC, Connolly JL, Amin MB. College of American Pathologists protocol for the reporting of ductal carcinoma in situ. Arch Pathol Lab Med. 2009;133:13–14. doi: 10.5858/133.1.13. [DOI] [PubMed] [Google Scholar]
- 12.Woodward W, Sneige N, Winter K, et al. Web based pathology assessment in RTOG 98-04. J Clin Pathol. 2014;67:777–780. doi: 10.1136/jclinpath-2014-202370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lagios MD, Margolin FR, Westdahl PR, et al. Mammographically detected duct carcinoma in situ: Frequency of local recurrence following tylectomy and prognostic effect of nuclear grade on local recurrence. Cancer. 1989;63:618–624. doi: 10.1002/1097-0142(19890215)63:4<618::aid-cncr2820630403>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 14.Fisher B, Dignam J, Wolmark N, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999;353:1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 15.Zelen M. The randomization and stratification of patients to clinical trials. J Chronic Dis. 1974;27:365–375. doi: 10.1016/0021-9681(74)90015-0. [DOI] [PubMed] [Google Scholar]
- 16.Kim K, Tsiatis AA. Study duration for clinical trials with survival response and early stopping rule. Biometrics. 1990;46:81–92. [PubMed] [Google Scholar]
- 17.Kalbfleisch J, Prentice R. The Statistical Analysis of Failure Time Data. New York, NY: John Wiley & Sons; 1980. pp. 167–169. [Google Scholar]
- 18.Kaplan EL, Meier P. Nonparameteric estimation from incomplete observations. J Am Statist Assoc. 1958;53:457–481. [Google Scholar]
- 19.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 20.Gray R. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 21.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 22.Hughes LL, Wang M, Page DL, et al. Local excision alone without irradiation for ductal carcinoma in situ of the breast: A trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2009;27:5319–5324. doi: 10.1200/JCO.2009.21.8560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong JS, Chen YH, Gadd MA, et al. Eight-year update of a prospective study of wide excision alone for small low- or intermediate-grade ductal carcinoma in situ (DCIS) Breast Cancer Res Treat. 2014;143:343–350. doi: 10.1007/s10549-013-2813-6. [DOI] [PubMed] [Google Scholar]
- 24.Cuzick J, Sestak I, Pinder SE, et al. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: Long-term results from the UK/ANZ DCIS trial. Lancet Oncol. 2011;12:21–29. doi: 10.1016/S1470-2045(10)70266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Early Breast Cancer Trialists' Collaborative Group. Correa C, McGale P, et al. Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr. 2010;2010:162–177. doi: 10.1093/jncimonographs/lgq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solin LJ, Kurtz J, Fourquet A, et al. Fifteen-year results of breast-conserving surgery and definitive breast irradiation for the treatment of ductal carcinoma in situ of the breast. J Clin Oncol. 1996;14:754–763. doi: 10.1200/JCO.1996.14.3.754. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.