Abstract
Purpose
We examined whether the survival advantage of androgen-deprivation therapy with radiotherapy (ADT plus RT) relative to ADT alone for men with locally advanced prostate cancer reported in two randomized trials holds in real-world clinical practice and extended the evidence to patients poorly represented in the trials.
Methods
We conducted nonrandomized effectiveness studies of ADT plus RT versus ADT in three groups of patients diagnosed between 1995 and 2007 and observed through 2009 in the SEER-Medicare data set: (1) the randomized clinical trial (RCT) cohort, which included men age 65 to 75 years and was most consistent with participants in the randomized trials; (2) the elderly cohort, which included men age > 75 years with locally advanced prostate cancer; and (3) the screen-detected cohort, which included men age ≥ 65 years with screen-detected high-risk prostate cancer. We evaluated cause-specific and all-cause mortality using propensity score, instrumental variable (IV), and sensitivity analyses.
Results
In the RCT cohort, ADT plus RT was associated with reduced cause-specific and all-cause mortality relative to ADT alone (cause-specific propensity score–adjusted hazard ratio [HR], 0.43; 95% CI, 0.37 to 0.49; all-cause propensity score–adjusted HR, 0.63; 95% CI, 0.59 to 0.67). Effectiveness estimates for the RCT cohort were not significantly different from those from randomized trials (P > .1). In the elderly and screen-detected cohorts, ADT plus RT was also associated with reduced cause-specific and all-cause mortality. IV analyses produced estimates similar to those from propensity score–adjusted methods.
Conclusion
Older men with locally advanced or screen-detected high-risk prostate cancer who receive ADT alone risk decrements in cause-specific and overall survival.
INTRODUCTION
Although clinically localized prostate cancers often present as indolent malignancies in the prostate-specific antigen (PSA) era, locally advanced prostate cancers are more aggressive tumors. Locally advanced disease involves extension of tumor beyond the confines of the prostate gland (clinical stage T3). Ten-year cause-specific mortality approaches 25%.1,2
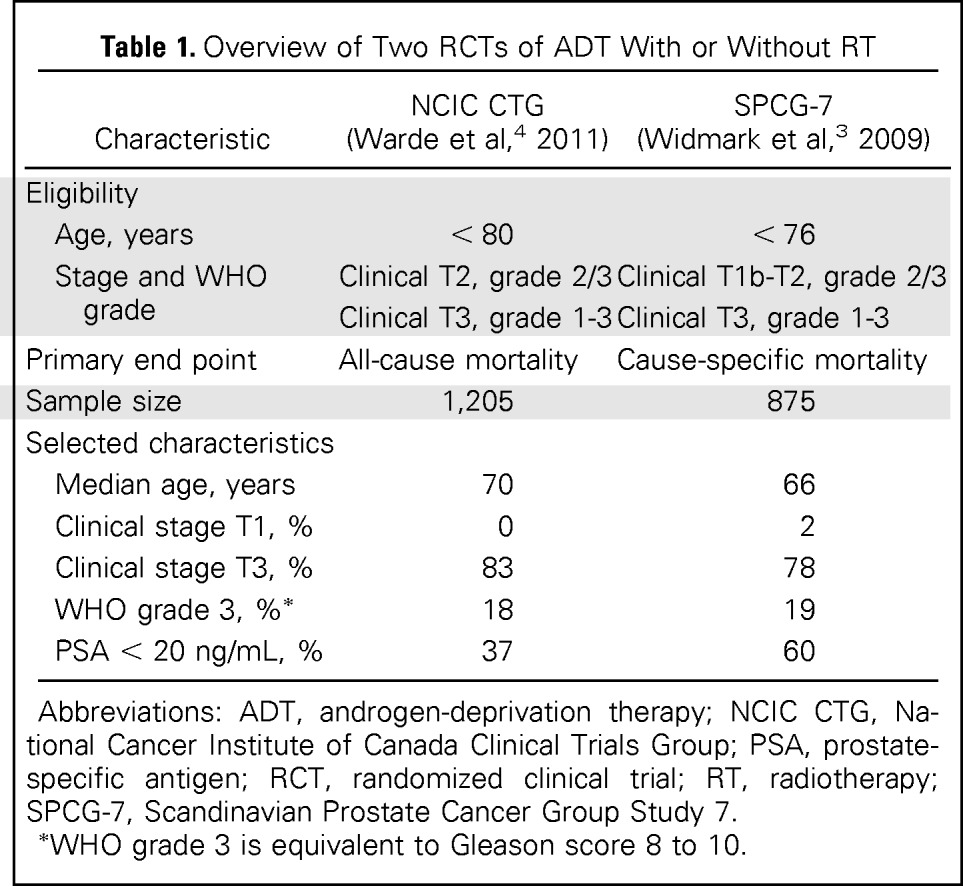
For decades, clinicians were uncertain as to whether the addition of prostate radiotherapy (RT) to systemic androgen-deprivation therapy (ADT) improved survival for patients with locally advanced cancers. Two recent landmark randomized clinical trials (RCTs) demonstrated that ADT plus RT leads to a large and significant reduction in overall and cause-specific mortality compared with ADT alone (Table 1).3,4 However, elderly men and patients with screen-detected high-risk cancers were poorly represented in or excluded from the RCTs. Screen-detected high-risk prostate cancer involves poorly differentiated or undifferentiated tumors detected by PSA screening (clinical stage T1c; Gleason score 8 to 10) and, like locally advanced cancers, are associated with substantial cause-specific mortality.1,2
Table 1.
Overview of Two RCTs of ADT With or Without RT
| Characteristic | NCIC CTG (Warde et al,4 2011) | SPCG-7 (Widmark et al,3 2009) |
|---|---|---|
| Eligibility | ||
| Age, years | < 80 | < 76 |
| Stage and WHO grade | Clinical T2, grade 2/3 | Clinical T1b-T2, grade 2/3 |
| Clinical T3, grade 1-3 | Clinical T3, grade 1-3 | |
| Primary end point | All-cause mortality | Cause-specific mortality |
| Sample size | 1,205 | 875 |
| Selected characteristics | ||
| Median age, years | 70 | 66 |
| Clinical stage T1, % | 0 | 2 |
| Clinical stage T3, % | 83 | 78 |
| WHO grade 3, %* | 18 | 19 |
| PSA < 20 ng/mL, % | 37 | 60 |
Abbreviations: ADT, androgen-deprivation therapy; NCIC CTG, National Cancer Institute of Canada Clinical Trials Group; PSA, prostate-specific antigen; RCT, randomized clinical trial; RT, radiotherapy; SPCG-7, Scandinavian Prostate Cancer Group Study 7.
WHO grade 3 is equivalent to Gleason score 8 to 10.
Despite these efficacy findings, ADT alone is a common treatment for prostate cancer in the United States, particularly among elderly patients in their 70s and 80s. Among men age > 75 years diagnosed with locally advanced or high-risk screen-detected cancers, 40% receive ADT alone, even though it is not curative, and patients risk debilitating adverse effects.5–7 Moreover, prostate cancer incidence and aggressiveness increase with advancing age, and US demographic trends favor a shift in age distribution toward older men.8
The lack of evidence to guide prostate cancer treatment decisions among older men and those with screen-detected high-risk tumors stands as a special priority among the many evidence gaps in the treatment of prostate cancer. Therefore, we conducted nonrandomized observational studies to examine whether the strong survival advantage of ADT plus RT relative to ADT alone reported in the two efficacy trials holds in real-world clinical practice and to extend the evidence to two prevalent subgroups of patients poorly represented in the trials: (1) men age > 75 years with locally advanced prostate cancer; and (2) men age ≥ 65 years with screen-detected high-risk prostate cancer.
METHODS
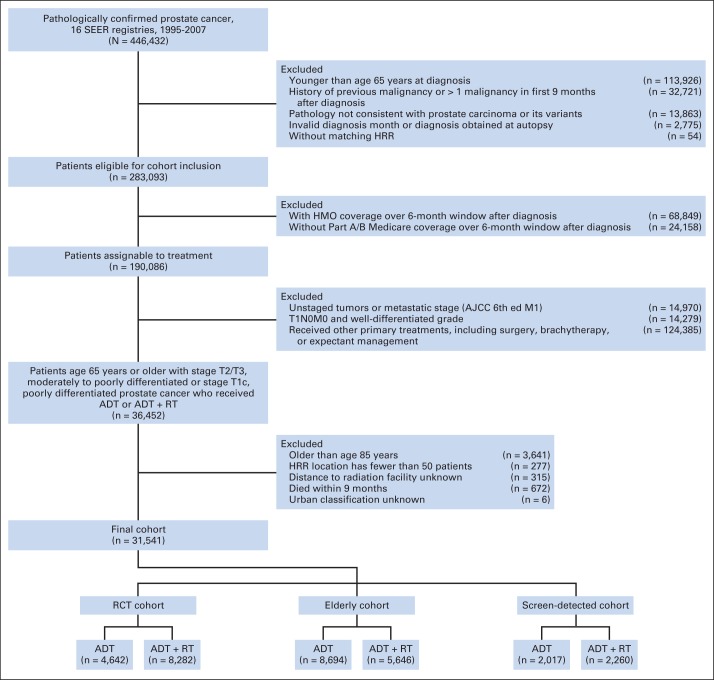
We conducted retrospective cohort studies using the SEER-Medicare database (approved by institutional review board).9 We designed the primary cohort (ie, RCT cohort) to be closest in composition to the eligibility criteria for the two efficacy trials (Table 1). The RCT cohort included men age 65 to 75 years with clinical stage T2 and moderately or poorly differentiated prostate cancer (WHO grade 2 or 3, respectively) or clinical stage T3 and WHO grade 1, 2, or 3 prostate cancer. WHO grades 2 and 3 correspond to Gleason scores 5 to 7 and 8 to 10, respectively. We then identified two other cohorts: (1) men age 76 to 85 years with clinical stage T2 and WHO grade 2 or 3 prostate cancer or clinical stage T3 and WHO grade 1, 2, or 3 prostate cancer (ie, elderly cohort); and (2) men age 65 to 85 years with screen-detected (clinical T1c) high-risk (WHO grade 3) prostate cancer (ie, screen-detected cohort). After exclusions (Appendix Fig A1, online only), the final cohorts included 31,451 patients (RCT cohort: ADT, n = 4,642; ADT plus RT, n = 8,282; elderly cohort: ADT, n = 8,694; ADT plus RT, n = 5,646; screen-detected cohort: ADT, n = 2,017; ADT plus RT, n = 2,260).
Definition of Variables
ADT and ADT plus RT were assigned based on identification from Medicare files.10,11 ADT was defined as orchiectomy or ≥ one dose of a gonadotropin-releasing hormone agonist within the first 9 months of diagnosis.12
Patient characteristics included age, race, ethnicity, and marital status. Clinical characteristics included American Joint Committee on Cancer T stage, N stage, and WHO grade. Comorbidities were identified by classifying inpatient and outpatient claims for the 12-month interval preceding prostate cancer diagnosis into 17 categories.13 Demographic variables included diagnosis year, SEER registry location, county of residence population, and median household income in census tract of residence (US$).
The primary outcomes were time to death resulting from any cause and time to death resulting from prostate cancer (all-cause and cause-specific mortality). Cause of death was determined from SEER. The observation time for follow-up was the time from diagnosis until the Medicare date of death or end of follow-up (December 31, 2009).
Statistical Analysis
Confounding by indication is an important concern for all observational studies, but particularly for older men with prostate cancer treated in routine clinical practice. Therefore, we used several complementary analytic approaches to account for measured and unmeasured confounding in the cohorts under study.
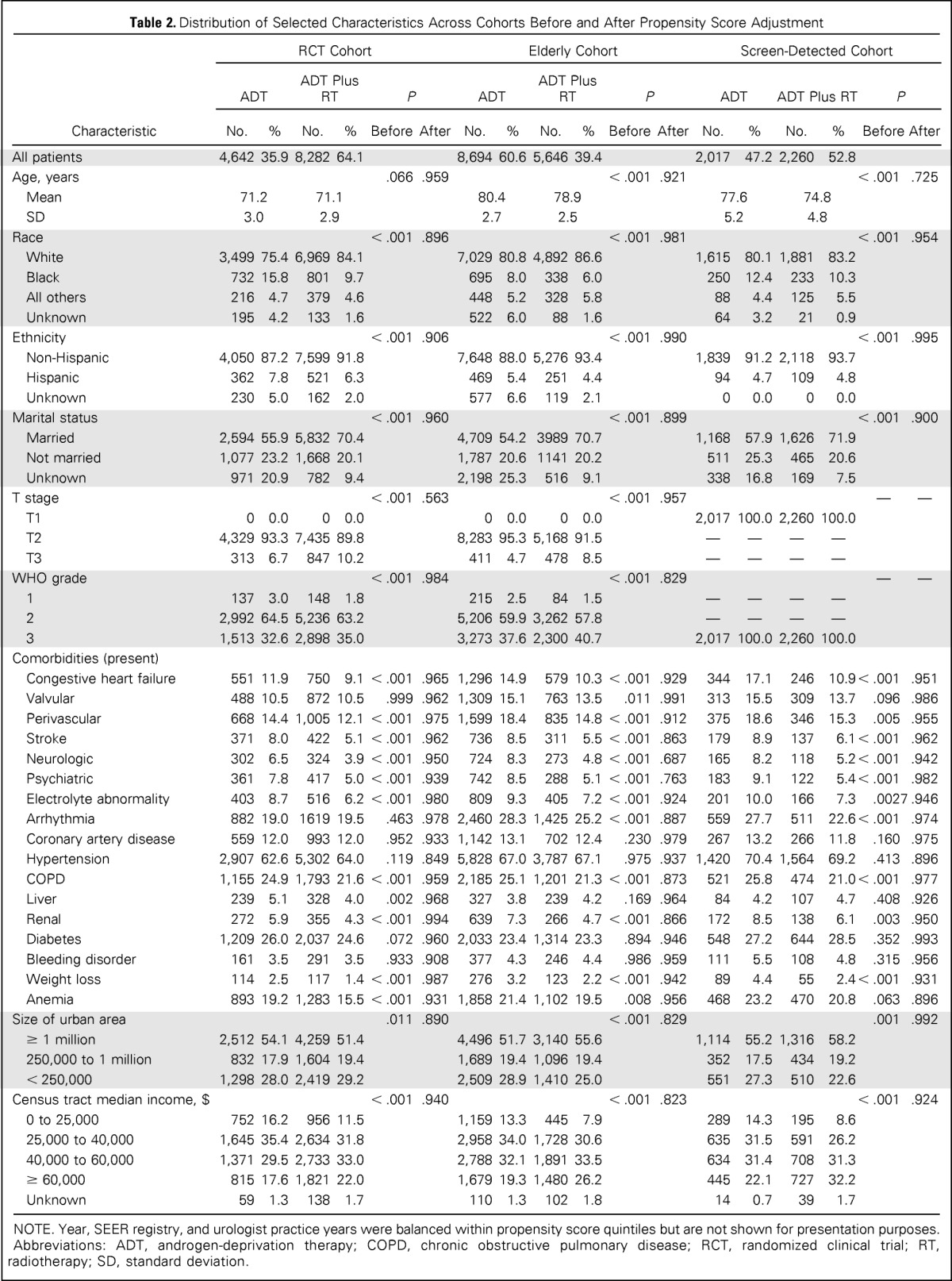
In our propensity score approach, we estimated propensity scores separately within the three analytic cohorts using multivariable logistic regression, with receipt of ADT plus RT as the outcome of interest, adjusting for all variables and, in addition, interactions among race, 17 comorbid disease groupings, and age. Missing values were entered into models as a separate category. This approach to missing data treats missing data as a covariate, which can systematically differ in its distribution between treatment groups.14,15 We used Cochran-Mantel-Haenszel tests to determine whether covariates were balanced within propensity score quintiles and found that all covariates were balanced (Table 2). In our final Cox adjusted proportional hazards models, we included the propensity score as a continuous variable.16,17
Table 2.
Distribution of Selected Characteristics Across Cohorts Before and After Propensity Score Adjustment
| Characteristic | RCT Cohort |
Elderly Cohort |
Screen-Detected Cohort |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ADT |
ADT Plus RT |
P |
ADT |
ADT Plus RT |
P |
ADT |
ADT Plus RT |
P |
||||||||||
| No. | % | No. | % | Before | After | No. | % | No. | % | Before | After | No. | % | No. | % | Before | After | |
| All patients | 4,642 | 35.9 | 8,282 | 64.1 | 8,694 | 60.6 | 5,646 | 39.4 | 2,017 | 47.2 | 2,260 | 52.8 | ||||||
| Age, years | .066 | .959 | < .001 | .921 | < .001 | .725 | ||||||||||||
| Mean | 71.2 | 71.1 | 80.4 | 78.9 | 77.6 | 74.8 | ||||||||||||
| SD | 3.0 | 2.9 | 2.7 | 2.5 | 5.2 | 4.8 | ||||||||||||
| Race | < .001 | .896 | < .001 | .981 | < .001 | .954 | ||||||||||||
| White | 3,499 | 75.4 | 6,969 | 84.1 | 7,029 | 80.8 | 4,892 | 86.6 | 1,615 | 80.1 | 1,881 | 83.2 | ||||||
| Black | 732 | 15.8 | 801 | 9.7 | 695 | 8.0 | 338 | 6.0 | 250 | 12.4 | 233 | 10.3 | ||||||
| All others | 216 | 4.7 | 379 | 4.6 | 448 | 5.2 | 328 | 5.8 | 88 | 4.4 | 125 | 5.5 | ||||||
| Unknown | 195 | 4.2 | 133 | 1.6 | 522 | 6.0 | 88 | 1.6 | 64 | 3.2 | 21 | 0.9 | ||||||
| Ethnicity | < .001 | .906 | < .001 | .990 | < .001 | .995 | ||||||||||||
| Non-Hispanic | 4,050 | 87.2 | 7,599 | 91.8 | 7,648 | 88.0 | 5,276 | 93.4 | 1,839 | 91.2 | 2,118 | 93.7 | ||||||
| Hispanic | 362 | 7.8 | 521 | 6.3 | 469 | 5.4 | 251 | 4.4 | 94 | 4.7 | 109 | 4.8 | ||||||
| Unknown | 230 | 5.0 | 162 | 2.0 | 577 | 6.6 | 119 | 2.1 | 0 | 0.0 | 0 | 0.0 | ||||||
| Marital status | < .001 | .960 | < .001 | .899 | < .001 | .900 | ||||||||||||
| Married | 2,594 | 55.9 | 5,832 | 70.4 | 4,709 | 54.2 | 3989 | 70.7 | 1,168 | 57.9 | 1,626 | 71.9 | ||||||
| Not married | 1,077 | 23.2 | 1,668 | 20.1 | 1,787 | 20.6 | 1141 | 20.2 | 511 | 25.3 | 465 | 20.6 | ||||||
| Unknown | 971 | 20.9 | 782 | 9.4 | 2,198 | 25.3 | 516 | 9.1 | 338 | 16.8 | 169 | 7.5 | ||||||
| T stage | < .001 | .563 | < .001 | .957 | — | — | ||||||||||||
| T1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 2,017 | 100.0 | 2,260 | 100.0 | ||||||
| T2 | 4,329 | 93.3 | 7,435 | 89.8 | 8,283 | 95.3 | 5,168 | 91.5 | — | — | — | — | ||||||
| T3 | 313 | 6.7 | 847 | 10.2 | 411 | 4.7 | 478 | 8.5 | — | — | — | — | ||||||
| WHO grade | < .001 | .984 | < .001 | .829 | — | — | ||||||||||||
| 1 | 137 | 3.0 | 148 | 1.8 | 215 | 2.5 | 84 | 1.5 | — | — | — | — | ||||||
| 2 | 2,992 | 64.5 | 5,236 | 63.2 | 5,206 | 59.9 | 3,262 | 57.8 | — | — | — | — | ||||||
| 3 | 1,513 | 32.6 | 2,898 | 35.0 | 3,273 | 37.6 | 2,300 | 40.7 | 2,017 | 100.0 | 2,260 | 100.0 | ||||||
| Comorbidities (present) | ||||||||||||||||||
| Congestive heart failure | 551 | 11.9 | 750 | 9.1 | < .001 | .965 | 1,296 | 14.9 | 579 | 10.3 | < .001 | .929 | 344 | 17.1 | 246 | 10.9 | < .001 | .951 |
| Valvular | 488 | 10.5 | 872 | 10.5 | .999 | .962 | 1,309 | 15.1 | 763 | 13.5 | .011 | .991 | 313 | 15.5 | 309 | 13.7 | .096 | .986 |
| Perivascular | 668 | 14.4 | 1,005 | 12.1 | < .001 | .975 | 1,599 | 18.4 | 835 | 14.8 | < .001 | .912 | 375 | 18.6 | 346 | 15.3 | .005 | .955 |
| Stroke | 371 | 8.0 | 422 | 5.1 | < .001 | .962 | 736 | 8.5 | 311 | 5.5 | < .001 | .863 | 179 | 8.9 | 137 | 6.1 | < .001 | .962 |
| Neurologic | 302 | 6.5 | 324 | 3.9 | < .001 | .950 | 724 | 8.3 | 273 | 4.8 | < .001 | .687 | 165 | 8.2 | 118 | 5.2 | < .001 | .942 |
| Psychiatric | 361 | 7.8 | 417 | 5.0 | < .001 | .939 | 742 | 8.5 | 288 | 5.1 | < .001 | .763 | 183 | 9.1 | 122 | 5.4 | < .001 | .982 |
| Electrolyte abnormality | 403 | 8.7 | 516 | 6.2 | < .001 | .980 | 809 | 9.3 | 405 | 7.2 | < .001 | .924 | 201 | 10.0 | 166 | 7.3 | .0027 | .946 |
| Arrhythmia | 882 | 19.0 | 1619 | 19.5 | .463 | .978 | 2,460 | 28.3 | 1,425 | 25.2 | < .001 | .887 | 559 | 27.7 | 511 | 22.6 | < .001 | .974 |
| Coronary artery disease | 559 | 12.0 | 993 | 12.0 | .952 | .933 | 1,142 | 13.1 | 702 | 12.4 | .230 | .979 | 267 | 13.2 | 266 | 11.8 | .160 | .975 |
| Hypertension | 2,907 | 62.6 | 5,302 | 64.0 | .119 | .849 | 5,828 | 67.0 | 3,787 | 67.1 | .975 | .937 | 1,420 | 70.4 | 1,564 | 69.2 | .413 | .896 |
| COPD | 1,155 | 24.9 | 1,793 | 21.6 | < .001 | .959 | 2,185 | 25.1 | 1,201 | 21.3 | < .001 | .873 | 521 | 25.8 | 474 | 21.0 | < .001 | .977 |
| Liver | 239 | 5.1 | 328 | 4.0 | .002 | .968 | 327 | 3.8 | 239 | 4.2 | .169 | .964 | 84 | 4.2 | 107 | 4.7 | .408 | .926 |
| Renal | 272 | 5.9 | 355 | 4.3 | < .001 | .994 | 639 | 7.3 | 266 | 4.7 | < .001 | .866 | 172 | 8.5 | 138 | 6.1 | .003 | .950 |
| Diabetes | 1,209 | 26.0 | 2,037 | 24.6 | .072 | .960 | 2,033 | 23.4 | 1,314 | 23.3 | .894 | .946 | 548 | 27.2 | 644 | 28.5 | .352 | .993 |
| Bleeding disorder | 161 | 3.5 | 291 | 3.5 | .933 | .908 | 377 | 4.3 | 246 | 4.4 | .986 | .959 | 111 | 5.5 | 108 | 4.8 | .315 | .956 |
| Weight loss | 114 | 2.5 | 117 | 1.4 | < .001 | .987 | 276 | 3.2 | 123 | 2.2 | < .001 | .942 | 89 | 4.4 | 55 | 2.4 | < .001 | .931 |
| Anemia | 893 | 19.2 | 1,283 | 15.5 | < .001 | .931 | 1,858 | 21.4 | 1,102 | 19.5 | .008 | .956 | 468 | 23.2 | 470 | 20.8 | .063 | .896 |
| Size of urban area | .011 | .890 | < .001 | .829 | .001 | .992 | ||||||||||||
| ≥ 1 million | 2,512 | 54.1 | 4,259 | 51.4 | 4,496 | 51.7 | 3,140 | 55.6 | 1,114 | 55.2 | 1,316 | 58.2 | ||||||
| 250,000 to 1 million | 832 | 17.9 | 1,604 | 19.4 | 1,689 | 19.4 | 1,096 | 19.4 | 352 | 17.5 | 434 | 19.2 | ||||||
| < 250,000 | 1,298 | 28.0 | 2,419 | 29.2 | 2,509 | 28.9 | 1,410 | 25.0 | 551 | 27.3 | 510 | 22.6 | ||||||
| Census tract median income, $ | < .001 | .940 | < .001 | .823 | < .001 | .924 | ||||||||||||
| 0 to 25,000 | 752 | 16.2 | 956 | 11.5 | 1,159 | 13.3 | 445 | 7.9 | 289 | 14.3 | 195 | 8.6 | ||||||
| 25,000 to 40,000 | 1,645 | 35.4 | 2,634 | 31.8 | 2,958 | 34.0 | 1,728 | 30.6 | 635 | 31.5 | 591 | 26.2 | ||||||
| 40,000 to 60,000 | 1,371 | 29.5 | 2,733 | 33.0 | 2,788 | 32.1 | 1,891 | 33.5 | 634 | 31.4 | 708 | 31.3 | ||||||
| ≥ 60,000 | 815 | 17.6 | 1,821 | 22.0 | 1,679 | 19.3 | 1,480 | 26.2 | 445 | 22.1 | 727 | 32.2 | ||||||
| Unknown | 59 | 1.3 | 138 | 1.7 | 110 | 1.3 | 102 | 1.8 | 14 | 0.7 | 39 | 1.7 | ||||||
NOTE. Year, SEER registry, and urologist practice years were balanced within propensity score quintiles but are not shown for presentation purposes.
Abbreviations: ADT, androgen-deprivation therapy; COPD, chronic obstructive pulmonary disease; RCT, randomized clinical trial; RT, radiotherapy; SD, standard deviation.
We constructed multivariable Cox proportional hazards models to compare cause-specific and all-cause mortality between ADT and ADT plus RT. We used the Schoenfeld residuals test to evaluate for violations of the proportional hazards assumption. Although the assumption was violated in some models, the addition of an interaction between treatment and propensity score substantially decreased deviation from the proportional hazards assumption; the results of this interaction model were nearly identical to those of our primary model. To account for the presence of competing risks of death in our analysis of cause-specific mortality, we used a competing causes of death approach (tabulating separately numbers of men alive, dead as result of prostate cancer, and dead as result of other cause) via Fine and Gray semiparametric modeling.18,19
For presentation purposes, we present adjusted cumulative incidence curves for the three cohorts using the Breslow estimator for the cumulative hazard.20 We adjusted these curves for measured confounders by setting the propensity score to 0.5. That is, the cumulative incidence curves graphically represent the cumulative probability of death for patients equally likely to receive ADT or ADT plus RT.
Instrumental Variable Analysis
We performed secondary analyses to compare all-cause mortality using quasiexperimental instrumental variable (IV) methods, which serve as a tool to simulate a randomized experiment in assigning patients to treatment groups.21 We did not examine cause-specific mortality, because IV methods for competing risks analyses have not been fully developed.
We formulated the IV as the local area treatment rate. The IV was created by assigning patients with nonmetastatic prostate cancer in the SEER-Medicare data set to hospital referral regions as defined by the Dartmouth Atlas of Health Care on the basis of their zip code at diagnosis and then dividing the number of patients who received aggressive treatment (either surgery or RT) by the total number of patients with prostate cancer in the hospital referral region.22 The IV, as a measure of local area treatment aggressiveness, captures regionally distinct structural care variation driven by factors beyond patient characteristics.
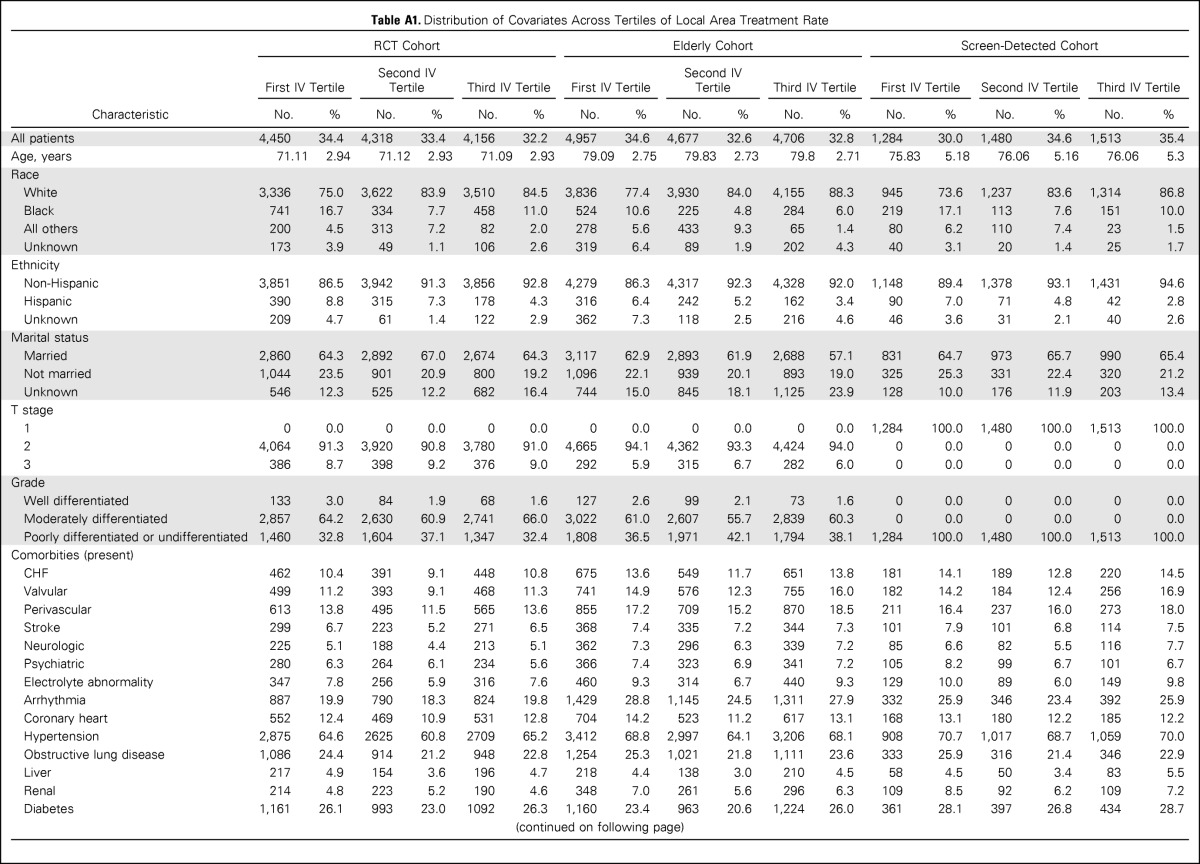
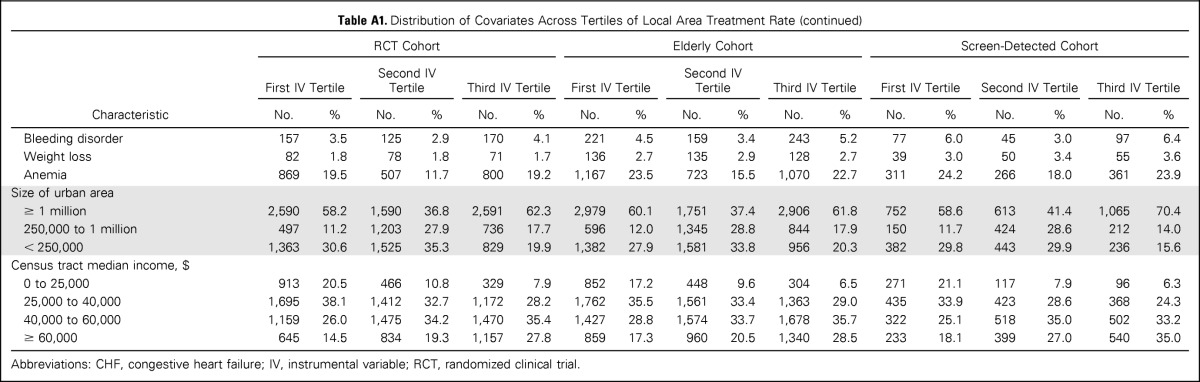
The instrument was strongly associated with treatment assignment (F statistic for RCT, elderly, and screen-detected cohorts, 23.3, 55.2, and 17.7, respectively). When the analytic cohorts were divided across IV tertiles, prognostically important covariates like age, tumor stage, grade, and comorbidities were balanced. Patient race, ethnicity, and area-level median income remained unbalanced (Appendix Table A1, online only). We used the two-stage residual inclusion method for IV estimation (Appendix, online only).23
Our final effect estimates included unadjusted, propensity score–adjusted, and IV-adjusted hazard ratio (HR) estimates of the effectiveness of ADT plus RT relative to ADT. In the primary RCT cohort, we compared our effectiveness results with the efficacy results of the two randomized trials using Wald's test (which compares estimators that are stochastically independent).24 We also conducted secondary analyses of the screen-detected cohort to evaluate effect estimates among patients age 76 to 85 years with T1 high-risk prostate cancer.
Sensitivity Analysis for Unmeasured Confounding
In addition to the IV analyses, we conducted sensitivity analyses to assess how strong and imbalanced a binary unmeasured confounding variable would need to be to change the significance of the estimated propensity score–adjusted HRs. As an example, we hypothesized a distribution for performance status, a variable that is not collected in the SEER-Medicare program and is a plausible unmeasured confounder. We assumed that poor performance status among the elderly would be associated with a hazard of death ranging from 1.25 to 3.5 (or higher) and prevalence estimates between 10% and 50%.25 We assumed a higher prevalence of poor performance status in the ADT group than ADT-plus-RT group. We varied imbalances in prevalence between the treatment groups and the strength of the hazard associated with the example unmeasured confounder to assess its influence on estimated HRs and their statistical significance (ie, whether upper bound of 95% CI crossed 1.0). These results would be applicable to other unmeasured binary confounders.
Statistical modeling was performed using R software (version 2.13.0; Vienna, Austria). Statistical significance was set at .05, and all tests were two tailed.
RESULTS
Table 2 lists selected baseline characteristics of the ADT and ADT-plus-RT groups in the RCT, elderly, and screen-detected cohorts. Across cohorts, patients in the ADT-plus-RT groups were more likely to have greater disease severity (higher-stage and -grade tumors) and fewer comorbid conditions; some differences between treatment groups were statistically significant but unlikely to be clinically meaningful. In the elderly and screen-detected cohorts, patients in the ADT-plus-RT groups were younger. In comparison with the published RCTs (Table 1), the RCT cohort was of similar age but had higher-grade and lower-stage cancer.
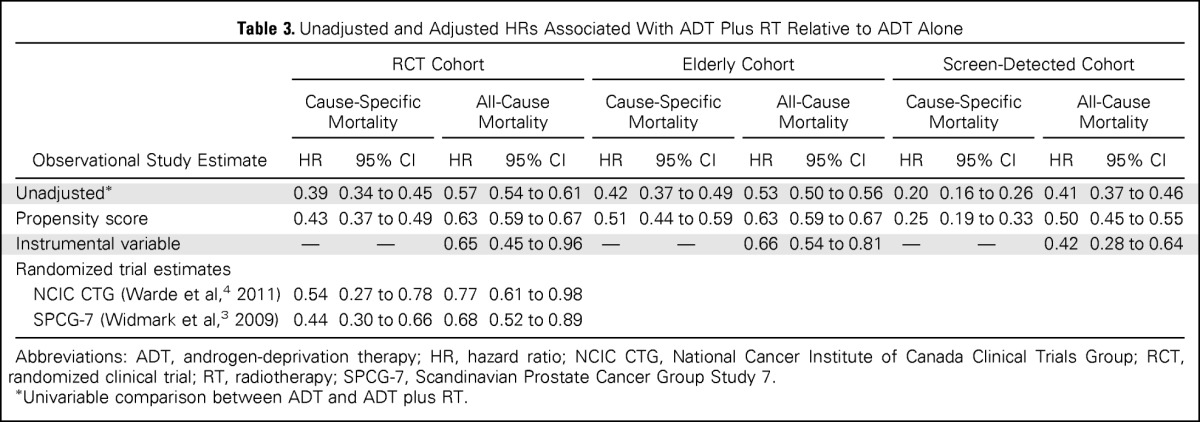
Table 3 lists HRs associated with ADT plus RT relative to ADT from unadjusted, propensity score–adjusted, and IV analyses. In the RCT cohort, in unadjusted Cox models, ADT plus RT was associated with reduced cause-specific mortality (HR, 0.39; 95% CI, 0.34 to 0.45) and all-cause mortality (HR, 0.57; 95% CI, 0.54 to 0.61). After adjusting for measured confounders using propensity score methods, the HRs were attenuated but remained significant (cause-specific propensity score–adjusted HR, 0.43; 95% CI, 0.37 to 0.49; all-cause propensity score–adjusted HR, 0.63; 95% CI, 0.59 to 0.67). IV analysis produced similar survival estimates of ADT plus RT versus ADT (all-cause HR, 0.65; 95% CI, 0.45 to 0.96). Effectiveness point estimates of cause-specific and all-cause mortality for the RCT cohort were not significantly different from the efficacy estimates from the published RCTs (Wald's P > .1 for all comparisons).
Table 3.
Unadjusted and Adjusted HRs Associated With ADT Plus RT Relative to ADT Alone
| Observational Study Estimate | RCT Cohort |
Elderly Cohort |
Screen-Detected Cohort |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cause-Specific Mortality |
All-Cause Mortality |
Cause-Specific Mortality |
All-Cause Mortality |
Cause-Specific Mortality |
All-Cause Mortality |
|||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Unadjusted* | 0.39 | 0.34 to 0.45 | 0.57 | 0.54 to 0.61 | 0.42 | 0.37 to 0.49 | 0.53 | 0.50 to 0.56 | 0.20 | 0.16 to 0.26 | 0.41 | 0.37 to 0.46 |
| Propensity score | 0.43 | 0.37 to 0.49 | 0.63 | 0.59 to 0.67 | 0.51 | 0.44 to 0.59 | 0.63 | 0.59 to 0.67 | 0.25 | 0.19 to 0.33 | 0.50 | 0.45 to 0.55 |
| Instrumental variable | — | — | 0.65 | 0.45 to 0.96 | — | — | 0.66 | 0.54 to 0.81 | — | — | 0.42 | 0.28 to 0.64 |
| Randomized trial estimates | ||||||||||||
| NCIC CTG (Warde et al,4 2011) | 0.54 | 0.27 to 0.78 | 0.77 | 0.61 to 0.98 | ||||||||
| SPCG-7 (Widmark et al,3 2009) | 0.44 | 0.30 to 0.66 | 0.68 | 0.52 to 0.89 | ||||||||
Abbreviations: ADT, androgen-deprivation therapy; HR, hazard ratio; NCIC CTG, National Cancer Institute of Canada Clinical Trials Group; RCT, randomized clinical trial; RT, radiotherapy; SPCG-7, Scandinavian Prostate Cancer Group Study 7.
Univariable comparison between ADT and ADT plus RT.
In the elderly cohort, ADT plus RT was associated with reduced cause-specific mortality (propensity score–adjusted HR, 0.51; 95% CI, 0.44 to 0.59) and all-cause mortality (propensity score–adjusted HR, 0.63; 95% CI, 0.59 to 0.67). In the screen-detected cohort, ADT plus RT was associated with reduced cause-specific mortality (propensity score–adjusted HR 0.25; 95% CI, 0.19 to 0.33) and all-cause mortality (propensity score–adjusted HR, 0.50; 95% CI, 0.45 to 0.55). In secondary analyses of the screen-detected cohort restricted to elderly men age 76 to 85 years, results were similar to the main findings. For the elderly and screen-detected cohorts, IV analyses produced HRs similar to those from propensity score–adjusted methods.
Table 4 lists adjusted cumulative incidence of cause-specific and all-cause mortality for patients with equal likelihood of receiving ADT or ADT plus RT (propensity score, 0.5) in the three cohorts. In the RCT cohort, with a mean follow-up of 6.0 years in the ADT group and 6.5 years in the ADT-plus-RT group, the cumulative incidence at 7 years of cause-specific mortality was 9.8% (95% CI, 8.9% to 10.7%) in the ADT group and 4.4% (95% CI, 3.9% to 4.9%) in the ADT-plus-RT group; for all-cause mortality, it was 39.2% (95% CI, 37.6% to 54.8%) in the ADT group and 24.6% (95% CI, 23.6% to 25.7%) in the ADT-plus-RT group. The cumulative incidence of cause-specific and all-cause mortality at 7 years for patients in the RCT cohort was similar to findings in the published randomized trials. Figure 1 graphically displays the adjusted cumulative incidence of cause-specific and other-cause mortality for patients with equal likelihood of receiving ADT or ADT plus RT in the three cohorts (Appendix Fig A2, online only, shows all-cause mortality).
Table 4.
Adjusted Cumulative Incidence of Cause-Specific and All-Cause Mortality
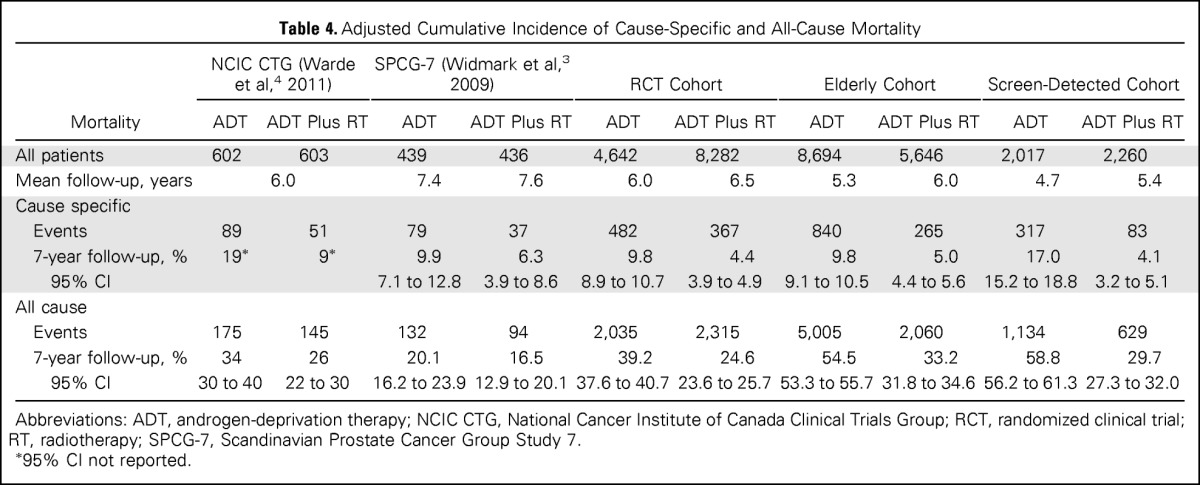
| Mortality | NCIC CTG (Warde et al,4 2011) |
SPCG-7 (Widmark et al,3 2009) |
RCT Cohort |
Elderly Cohort |
Screen-Detected Cohort |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| ADT | ADT Plus RT | ADT | ADT Plus RT | ADT | ADT Plus RT | ADT | ADT Plus RT | ADT | ADT Plus RT | |
| All patients | 602 | 603 | 439 | 436 | 4,642 | 8,282 | 8,694 | 5,646 | 2,017 | 2,260 |
| Mean follow-up, years | 6.0 | 7.4 | 7.6 | 6.0 | 6.5 | 5.3 | 6.0 | 4.7 | 5.4 | |
| Cause specific | ||||||||||
| Events | 89 | 51 | 79 | 37 | 482 | 367 | 840 | 265 | 317 | 83 |
| 7-year follow-up, % | 19* | 9* | 9.9 | 6.3 | 9.8 | 4.4 | 9.8 | 5.0 | 17.0 | 4.1 |
| 95% CI | 7.1 to 12.8 | 3.9 to 8.6 | 8.9 to 10.7 | 3.9 to 4.9 | 9.1 to 10.5 | 4.4 to 5.6 | 15.2 to 18.8 | 3.2 to 5.1 | ||
| All cause | ||||||||||
| Events | 175 | 145 | 132 | 94 | 2,035 | 2,315 | 5,005 | 2,060 | 1,134 | 629 |
| 7-year follow-up, % | 34 | 26 | 20.1 | 16.5 | 39.2 | 24.6 | 54.5 | 33.2 | 58.8 | 29.7 |
| 95% CI | 30 to 40 | 22 to 30 | 16.2 to 23.9 | 12.9 to 20.1 | 37.6 to 40.7 | 23.6 to 25.7 | 53.3 to 55.7 | 31.8 to 34.6 | 56.2 to 61.3 | 27.3 to 32.0 |
Abbreviations: ADT, androgen-deprivation therapy; NCIC CTG, National Cancer Institute of Canada Clinical Trials Group; RCT, randomized clinical trial; RT, radiotherapy; SPCG-7, Scandinavian Prostate Cancer Group Study 7.
95% CI not reported.
Fig 1.
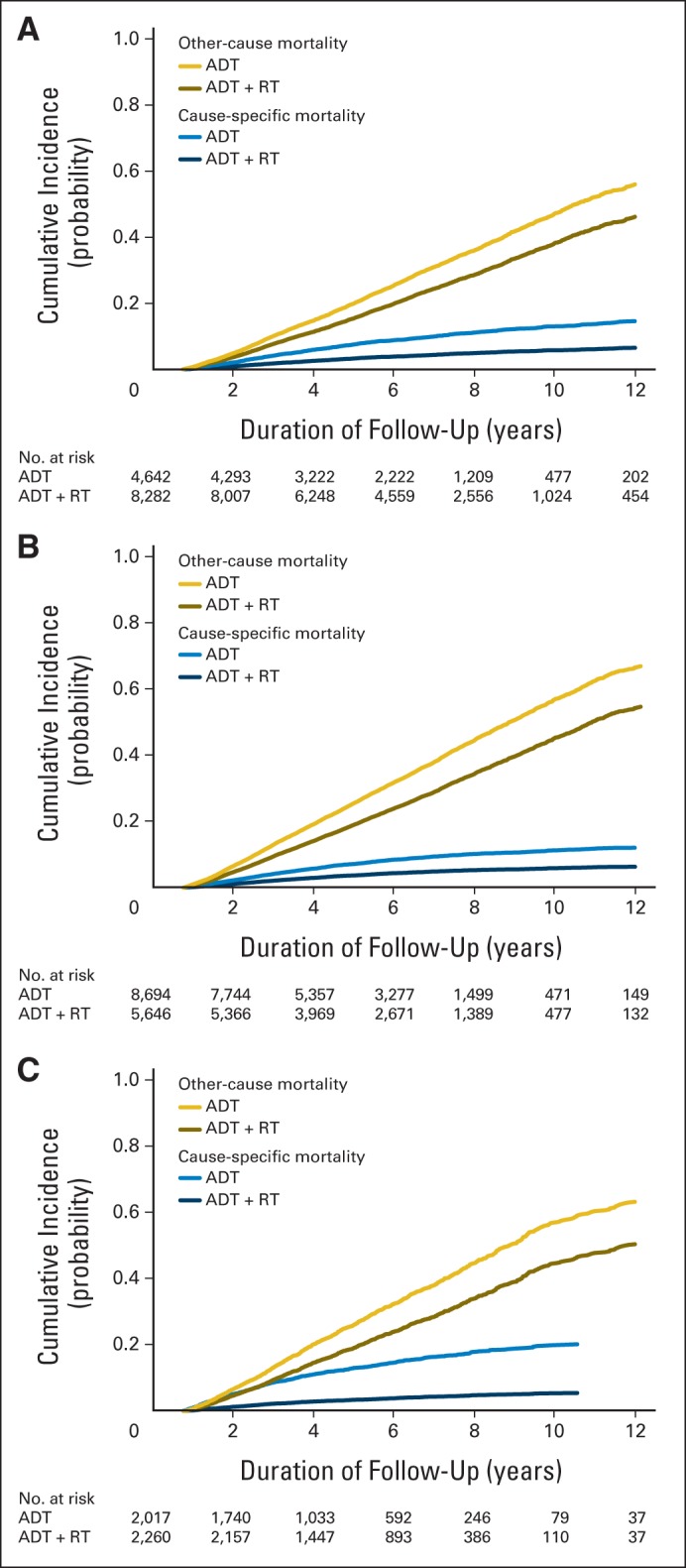
Adjusted cumulative incidence of cause-specific and other-cause mortality for (A) randomized clinical trial (RCT) cohort, (B) elderly cohort, and (C) screen-detected cohort. ADT, androgen-deprivation therapy; RT, radiotherapy.
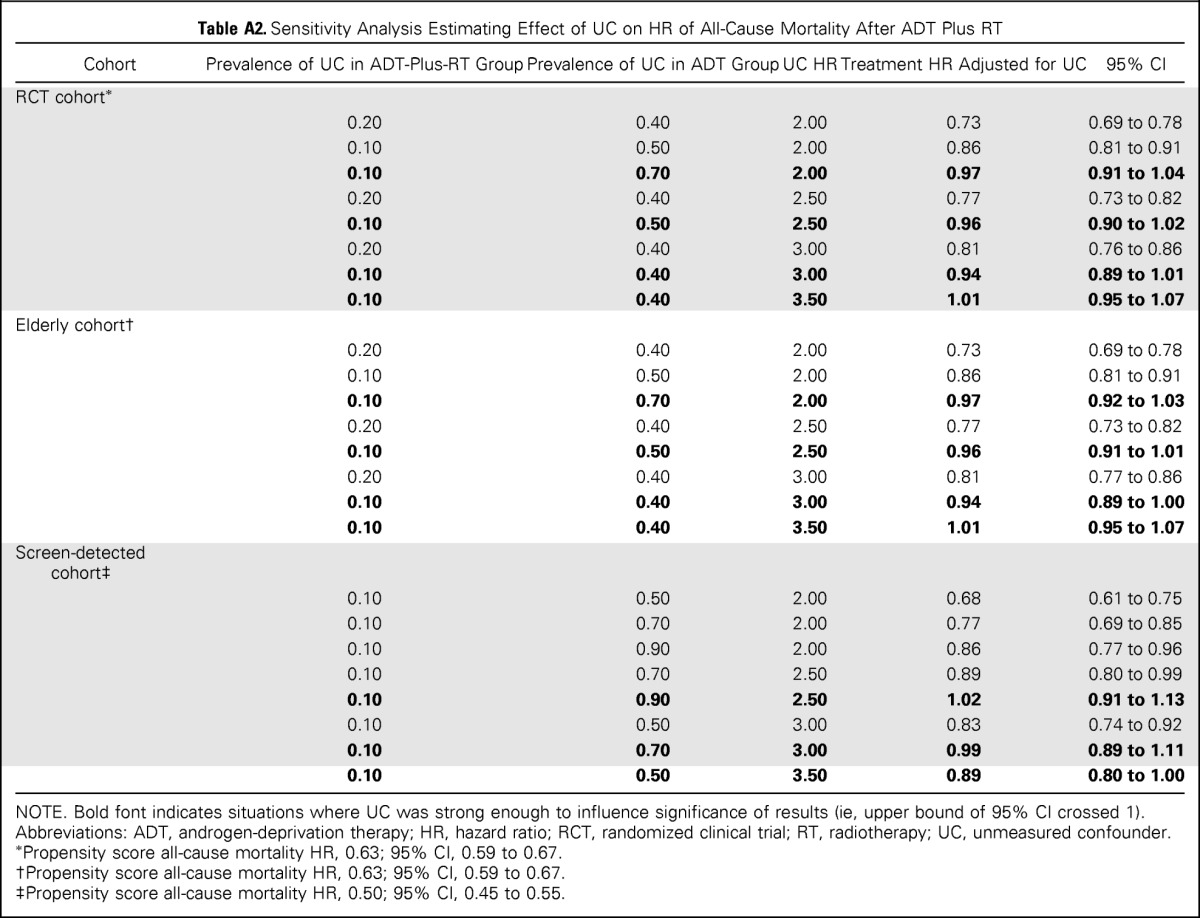
The sensitivity analysis for all-cause mortality (Appendix Table A2, online only) shows, for example, that an extreme unmeasured confounder (HR, 2.5) would eliminate the significant benefit of ADT plus RT in the RCT and elderly cohorts if its prevalence in the ADT and ADT-plus-RT groups were five-fold different. The unmeasured confounder would have to be even more strongly associated with death or have greater imbalance between treatment groups to affect the significance of results in the screen-detected cohort. The sensitivity analysis for cause-specific mortality was even less sensitive to unmeasured confounding than all-cause mortality (data not shown).
DISCUSSION
We conducted this study to assess whether the survival advantage of ADT plus RT versus ADT alone for locally advanced prostate cancer reported in two randomized trials holds in real-world practice and to extend the evidence base to prevalent patient subgroups poorly represented in the trials. Among a cohort of patients in the SEER-Medicare database whose patient characteristics were closest to the eligibility criteria for the randomized trials, we found that ADT plus RT was associated with a reduction in cause-specific and all-cause mortality, commensurate with efficacy estimates observed in the randomized trials. Furthermore, we found that ADT plus RT was associated with survival benefits of similar magnitude in elderly men age 76 to 85 years with locally advanced prostate cancer and older men age 65 to 85 years with screen-detected high-risk prostate cancer.
Our results are consistent with and extend the findings of prior studies that have examined the role ADT and RT in the treatment of older men with locally advanced or high-risk prostate cancer. In the efficacy literature (Table 1), the Scandinavian Prostate Cancer Group Study 7 (SPCG-7) randomly assigned 875 men with a mean age of 66 years and demonstrated a large and significant reduction in cause-specific and all-cause mortality with ADT plus RT.3 The National Cancer Institute of Canada Clinical Trials Group (NCIC CTG) randomly assigned 1,205 men with a median age of 70 years and also confirmed a substantial reduction in mortality with ADT plus RT.4 Both trials were powered for survival outcomes.
The baseline characteristics of the population-based RCT cohort reflect the heterogeneity of routine clinical practice. The age distribution of the RCT cohort was similar to that in the NCIC CTG trial, which enrolled older patients than the SPCG-7. The clinical characteristics of the RCT cohort were more similar to those of SPCG-7 participants (who had more favorable disease severity than those in NCIC CTG trial). In subgroup analyses reported by the SPCG-7, the benefits of RT held among patients age 67 to 75 years and those with clinical stage T1b/T2 as well as T3 tumors.3 The weight of the evidence from these efficacy trials and our effectiveness RCT cohort suggests that ADT plus RT is a highly effective treatment for locally advanced and high-risk prostate cancers.
In the observational literature, studies have consistently demonstrated that elderly men with locally advanced or high-risk prostate cancer benefit from treatment.1,2,5,6,26–28 A long-term analysis of men in the Prostate Cancer Outcomes Study showed that elderly men with high-risk prostate cancer had meaningful cause-specific mortality after adjusting for treatment (nearly 20% at 10 years among men age > 70 years) despite also having high other-cause mortality. Not surprisingly, we also observed high other-cause mortality and comorbid disease in the elderly and screen-detected cohorts.
In the screen-detected cohort, cause-specific mortality was higher among patients treated with ADT alone than in the other cohorts, which seems improbable and may have partially contributed to the more marked effectiveness estimate of ADT plus RT among men with screen-detected high-risk cancer. These findings held among elderly men with screen-detected cancer as well. Despite our attempts to control for hidden bias in this cohort, it is likely that selection effects or clinical understaging are more pronounced among older men with screen-detected cancer who receive ADT alone. Thus, we echo previous calls for enhancements to the richness of available data sets to conduct observational cancer comparative-effectiveness research, because elderly patients will continue to be under-represented in randomized trials.
Lacking randomization, our study is unable to confirm a causal effect of ADT plus RT on reduced mortality within the three cohorts. The significant reduction in other-cause mortality in all three cohorts may be a marker of residual confounding by indication. However, the main elements of the Hill29 model of causal inference offer some reassurance in the validity of our findings. The survival benefit associated with ADT plus RT was strong across the three cohorts and robust to unmeasured confounding; consistent across cohorts using both propensity score and IV methods; coherent with findings from the randomized trials for trial-eligible patients; and biologically plausible, in that locally advanced and high-risk prostate cancers are aggressive tumors regardless of age at presentation, and treatment of the prostate with RT is hypothesized to slow the progression of micrometastasic disease.30
Our study has other limitations. Cause of death may be misclassified in tumor registries, although such misclassification is likely to be nondifferential between treatment groups; thus, we would not expect it to have a meaningful impact on effect estimates for cause-specific mortality.31,32 We were unable to ascertain important clinical variables from SEER registry data, such as baseline PSA or radiation dose or field. Although the IV results confirmed findings of the propensity-score models, we caution that the IV balanced many but not all observable variables. We adjusted for observable variables in IV models; however, we cannot verify that unmeasured confounders were balanced using IV methods.33 Moreover, the IV itself, as a measure of area-level health care aggressiveness, could be confounded by geographic variation in PSA screening rates.
In conclusion, our findings suggest ADT plus RT reduces both overall and cause-specific mortality in patients with locally advanced prostate cancer treated in routine practice who meet the eligibility criteria for two pivotal RCTs as well as two prevalent patient subgroups not represented in the RCTs (ie, elderly patients and those with screen-detected high-risk prostate cancer). The absence of randomized evidence comparing ADT plus RT with ADT alone in these subgroups remains an evidence gap. However, our findings raise a provocative hypothesis that in the United States, men age > 75 years with locally advanced prostate cancer or men age > 65 years with high-risk screen-detected prostate cancer who receive ADT alone risk decrements in cause-specific and overall survival.
Acknowledgment
We used the linked SEER-Medicare database and acknowledge the efforts of the Applied Research Program; National Cancer Institute; Office of Research, Development and Information; Centers for Medicare and Medicaid Services; Information Management Services; and SEER program tumor registries in the creation of the SEER-Medicare database. We thank Robert Sunderland, MS, for programming assistance.
Glossary Terms
- androgen-deprivation therapy (ADT):
treatment that suppresses or blocks the production or action of male hormones.
- comparative-effectiveness research:
the generation and synthesis of evidence that compares the benefits and harms of alternative methods to prevent, diagnose, treat, and monitor a clinical condition or to improve the delivery of care. The purpose of comparative effectiveness research is to assist consumers, clinicians, purchasers, and policy makers to make informed decisions that will improve health care at the individual and population levels.
Appendix
Detailed Review of Instrumental Variable Analyses
We conducted instrumental variable (IV) analyses using the two-stage residual inclusion method that involves fitting two regression models. The first model examines the association between exposure (ie, treatment assignment [androgen-deprivation therapy (ADT) v ADT plus radiotherapy]) as the dependent variable and the IV (ie, local area treatment rate) as the independent variable, controlling for all baseline covariates. The second model examines the outcome as the dependent variable and uses, as the independent variables, exposure, residuals (differences between predicted and observed responses) from the first model, and covariates that do not appear balanced by the IV. In our approach, we included the propensity score in the second-stage model. This procedure theoretically yields asymptotically unbiased estimates of the effect of exposure on outcome, whether confounders are measured or not, provided three assumptions are met (Zohoori N et al: Ann Epidemiol 7:251-257, 1997; Brookhart MA et al: Pharmacoepidemiol Drug Saf 19:537-554, 2010; Rassen JA et al: Am J Epidemiol 169:273-284, 2009).21,33
The first assumption is that the IV is correlated with exposure, which is testable with standard methods of correlation association. If instruments are poor predictors of treatment, they are considered weak instruments, at risk of contributing to bias in observational studies (Staiger D et al: Econometrica 65:557-586, 1997; Small D et al: J Am Stat Assoc 103:924-933, 2008; Hennessy S et al: J Clin Epidemiol 61:1285-1288, 2008). We assessed the strength of each candidate IV on the basis of the F statistic. F statistic values < 10 indicate weak instruments. The second assumption is that the instruments should not be directly associated with outcomes except through treatment assignment. The third assumption is that the association between instruments, treatment assignment, and outcomes should not be confounded by unmeasured variables that may themselves be associated with outcome.
To assess the second and third conditions, termed the independence and exclusion assumptions, we examined means and frequencies of observed covariates (eg, age, comorbid disease, grade, race) across levels of the IV (Appendix Table A2). The purpose was to gain an understanding of covariate balance and the potential for confounding of the IV itself through empiric evaluation of the relationships among instruments, measured confounders, treatment assignment, and outcomes (Hernán MA et al: Epidemiology 17:360-372, 2006). It is important to note that the last two assumptions are fundamentally unverifiable, because it is impossible to determine with any statistical testing whether candidate IVs are themselves unconfounded (Rassen JA et al: J Clin Epidemiol 62:1226-1232, 2009). These two assumptions can be defended based on the theoretic argument that the IV (treatment aggressiveness of broad geographic region where patient lives) is independent of the patient's unmeasured risk factors that may affect outcome independent of treatment.
IV approaches have often been used in the context of a linear regression model, which yields risk differences rather than risk ratios, but have been extended to multiplicative models such as logistic regression and Cox proportional hazards models (Zohoori N et al: Ann Epidemiol 7:251-257, 1997). Although such extensions are approximations, they have been shown to produce IV-adjusted odds ratios or hazard ratios with low bias and good precision (Rassen JA et al: Am J Epidemiol 169:273-284, 2009; Kahn J et al: Health Serv Res 44:862-879, 2009).23
The IV analysis compares the effect on outcome for a given patient resulting from changes in treatment induced by the IV. Thus, the treatment effect produced by IV analysis applies to what has been called the marginal or complying patient population, defined as patients whose treatment status depends strongly on the instrument. In the case of a local area treatment effect IV, the marginal population is defined as patients who would receive ADT plus radiotherapy in geographic regions with higher but not lower treatment rates.
Table A1.
Distribution of Covariates Across Tertiles of Local Area Treatment Rate
| Characteristic | RCT Cohort |
Elderly Cohort |
Screen-Detected Cohort |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First IV Tertile |
Second IV Tertile |
Third IV Tertile |
First IV Tertile |
Second IV Tertile |
Third IV Tertile |
First IV Tertile |
Second IV Tertile |
Third IV Tertile |
||||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| All patients | 4,450 | 34.4 | 4,318 | 33.4 | 4,156 | 32.2 | 4,957 | 34.6 | 4,677 | 32.6 | 4,706 | 32.8 | 1,284 | 30.0 | 1,480 | 34.6 | 1,513 | 35.4 |
| Age, years | 71.11 | 2.94 | 71.12 | 2.93 | 71.09 | 2.93 | 79.09 | 2.75 | 79.83 | 2.73 | 79.8 | 2.71 | 75.83 | 5.18 | 76.06 | 5.16 | 76.06 | 5.3 |
| Race | ||||||||||||||||||
| White | 3,336 | 75.0 | 3,622 | 83.9 | 3,510 | 84.5 | 3,836 | 77.4 | 3,930 | 84.0 | 4,155 | 88.3 | 945 | 73.6 | 1,237 | 83.6 | 1,314 | 86.8 |
| Black | 741 | 16.7 | 334 | 7.7 | 458 | 11.0 | 524 | 10.6 | 225 | 4.8 | 284 | 6.0 | 219 | 17.1 | 113 | 7.6 | 151 | 10.0 |
| All others | 200 | 4.5 | 313 | 7.2 | 82 | 2.0 | 278 | 5.6 | 433 | 9.3 | 65 | 1.4 | 80 | 6.2 | 110 | 7.4 | 23 | 1.5 |
| Unknown | 173 | 3.9 | 49 | 1.1 | 106 | 2.6 | 319 | 6.4 | 89 | 1.9 | 202 | 4.3 | 40 | 3.1 | 20 | 1.4 | 25 | 1.7 |
| Ethnicity | ||||||||||||||||||
| Non-Hispanic | 3,851 | 86.5 | 3,942 | 91.3 | 3,856 | 92.8 | 4,279 | 86.3 | 4,317 | 92.3 | 4,328 | 92.0 | 1,148 | 89.4 | 1,378 | 93.1 | 1,431 | 94.6 |
| Hispanic | 390 | 8.8 | 315 | 7.3 | 178 | 4.3 | 316 | 6.4 | 242 | 5.2 | 162 | 3.4 | 90 | 7.0 | 71 | 4.8 | 42 | 2.8 |
| Unknown | 209 | 4.7 | 61 | 1.4 | 122 | 2.9 | 362 | 7.3 | 118 | 2.5 | 216 | 4.6 | 46 | 3.6 | 31 | 2.1 | 40 | 2.6 |
| Marital status | ||||||||||||||||||
| Married | 2,860 | 64.3 | 2,892 | 67.0 | 2,674 | 64.3 | 3,117 | 62.9 | 2,893 | 61.9 | 2,688 | 57.1 | 831 | 64.7 | 973 | 65.7 | 990 | 65.4 |
| Not married | 1,044 | 23.5 | 901 | 20.9 | 800 | 19.2 | 1,096 | 22.1 | 939 | 20.1 | 893 | 19.0 | 325 | 25.3 | 331 | 22.4 | 320 | 21.2 |
| Unknown | 546 | 12.3 | 525 | 12.2 | 682 | 16.4 | 744 | 15.0 | 845 | 18.1 | 1,125 | 23.9 | 128 | 10.0 | 176 | 11.9 | 203 | 13.4 |
| T stage | ||||||||||||||||||
| 1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1,284 | 100.0 | 1,480 | 100.0 | 1,513 | 100.0 |
| 2 | 4,064 | 91.3 | 3,920 | 90.8 | 3,780 | 91.0 | 4,665 | 94.1 | 4,362 | 93.3 | 4,424 | 94.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| 3 | 386 | 8.7 | 398 | 9.2 | 376 | 9.0 | 292 | 5.9 | 315 | 6.7 | 282 | 6.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Grade | ||||||||||||||||||
| Well differentiated | 133 | 3.0 | 84 | 1.9 | 68 | 1.6 | 127 | 2.6 | 99 | 2.1 | 73 | 1.6 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Moderately differentiated | 2,857 | 64.2 | 2,630 | 60.9 | 2,741 | 66.0 | 3,022 | 61.0 | 2,607 | 55.7 | 2,839 | 60.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Poorly differentiated or undifferentiated | 1,460 | 32.8 | 1,604 | 37.1 | 1,347 | 32.4 | 1,808 | 36.5 | 1,971 | 42.1 | 1,794 | 38.1 | 1,284 | 100.0 | 1,480 | 100.0 | 1,513 | 100.0 |
| Comorbities (present) | ||||||||||||||||||
| CHF | 462 | 10.4 | 391 | 9.1 | 448 | 10.8 | 675 | 13.6 | 549 | 11.7 | 651 | 13.8 | 181 | 14.1 | 189 | 12.8 | 220 | 14.5 |
| Valvular | 499 | 11.2 | 393 | 9.1 | 468 | 11.3 | 741 | 14.9 | 576 | 12.3 | 755 | 16.0 | 182 | 14.2 | 184 | 12.4 | 256 | 16.9 |
| Perivascular | 613 | 13.8 | 495 | 11.5 | 565 | 13.6 | 855 | 17.2 | 709 | 15.2 | 870 | 18.5 | 211 | 16.4 | 237 | 16.0 | 273 | 18.0 |
| Stroke | 299 | 6.7 | 223 | 5.2 | 271 | 6.5 | 368 | 7.4 | 335 | 7.2 | 344 | 7.3 | 101 | 7.9 | 101 | 6.8 | 114 | 7.5 |
| Neurologic | 225 | 5.1 | 188 | 4.4 | 213 | 5.1 | 362 | 7.3 | 296 | 6.3 | 339 | 7.2 | 85 | 6.6 | 82 | 5.5 | 116 | 7.7 |
| Psychiatric | 280 | 6.3 | 264 | 6.1 | 234 | 5.6 | 366 | 7.4 | 323 | 6.9 | 341 | 7.2 | 105 | 8.2 | 99 | 6.7 | 101 | 6.7 |
| Electrolyte abnormality | 347 | 7.8 | 256 | 5.9 | 316 | 7.6 | 460 | 9.3 | 314 | 6.7 | 440 | 9.3 | 129 | 10.0 | 89 | 6.0 | 149 | 9.8 |
| Arrhythmia | 887 | 19.9 | 790 | 18.3 | 824 | 19.8 | 1,429 | 28.8 | 1,145 | 24.5 | 1,311 | 27.9 | 332 | 25.9 | 346 | 23.4 | 392 | 25.9 |
| Coronary heart | 552 | 12.4 | 469 | 10.9 | 531 | 12.8 | 704 | 14.2 | 523 | 11.2 | 617 | 13.1 | 168 | 13.1 | 180 | 12.2 | 185 | 12.2 |
| Hypertension | 2,875 | 64.6 | 2625 | 60.8 | 2709 | 65.2 | 3,412 | 68.8 | 2,997 | 64.1 | 3,206 | 68.1 | 908 | 70.7 | 1,017 | 68.7 | 1,059 | 70.0 |
| Obstructive lung disease | 1,086 | 24.4 | 914 | 21.2 | 948 | 22.8 | 1,254 | 25.3 | 1,021 | 21.8 | 1,111 | 23.6 | 333 | 25.9 | 316 | 21.4 | 346 | 22.9 |
| Liver | 217 | 4.9 | 154 | 3.6 | 196 | 4.7 | 218 | 4.4 | 138 | 3.0 | 210 | 4.5 | 58 | 4.5 | 50 | 3.4 | 83 | 5.5 |
| Renal | 214 | 4.8 | 223 | 5.2 | 190 | 4.6 | 348 | 7.0 | 261 | 5.6 | 296 | 6.3 | 109 | 8.5 | 92 | 6.2 | 109 | 7.2 |
| Diabetes | 1,161 | 26.1 | 993 | 23.0 | 1092 | 26.3 | 1,160 | 23.4 | 963 | 20.6 | 1,224 | 26.0 | 361 | 28.1 | 397 | 26.8 | 434 | 28.7 |
| Bleeding disorder | 157 | 3.5 | 125 | 2.9 | 170 | 4.1 | 221 | 4.5 | 159 | 3.4 | 243 | 5.2 | 77 | 6.0 | 45 | 3.0 | 97 | 6.4 |
| Weight loss | 82 | 1.8 | 78 | 1.8 | 71 | 1.7 | 136 | 2.7 | 135 | 2.9 | 128 | 2.7 | 39 | 3.0 | 50 | 3.4 | 55 | 3.6 |
| Anemia | 869 | 19.5 | 507 | 11.7 | 800 | 19.2 | 1,167 | 23.5 | 723 | 15.5 | 1,070 | 22.7 | 311 | 24.2 | 266 | 18.0 | 361 | 23.9 |
| Size of urban area | ||||||||||||||||||
| ≥ 1 million | 2,590 | 58.2 | 1,590 | 36.8 | 2,591 | 62.3 | 2,979 | 60.1 | 1,751 | 37.4 | 2,906 | 61.8 | 752 | 58.6 | 613 | 41.4 | 1,065 | 70.4 |
| 250,000 to 1 million | 497 | 11.2 | 1,203 | 27.9 | 736 | 17.7 | 596 | 12.0 | 1,345 | 28.8 | 844 | 17.9 | 150 | 11.7 | 424 | 28.6 | 212 | 14.0 |
| < 250,000 | 1,363 | 30.6 | 1,525 | 35.3 | 829 | 19.9 | 1,382 | 27.9 | 1,581 | 33.8 | 956 | 20.3 | 382 | 29.8 | 443 | 29.9 | 236 | 15.6 |
| Census tract median income, $ | ||||||||||||||||||
| 0 to 25,000 | 913 | 20.5 | 466 | 10.8 | 329 | 7.9 | 852 | 17.2 | 448 | 9.6 | 304 | 6.5 | 271 | 21.1 | 117 | 7.9 | 96 | 6.3 |
| 25,000 to 40,000 | 1,695 | 38.1 | 1,412 | 32.7 | 1,172 | 28.2 | 1,762 | 35.5 | 1,561 | 33.4 | 1,363 | 29.0 | 435 | 33.9 | 423 | 28.6 | 368 | 24.3 |
| 40,000 to 60,000 | 1,159 | 26.0 | 1,475 | 34.2 | 1,470 | 35.4 | 1,427 | 28.8 | 1,574 | 33.7 | 1,678 | 35.7 | 322 | 25.1 | 518 | 35.0 | 502 | 33.2 |
| ≥ 60,000 | 645 | 14.5 | 834 | 19.3 | 1,157 | 27.8 | 859 | 17.3 | 960 | 20.5 | 1,340 | 28.5 | 233 | 18.1 | 399 | 27.0 | 540 | 35.0 |
Abbreviations: CHF, congestive heart failure; IV, instrumental variable; RCT, randomized clinical trial.
Table A2.
Sensitivity Analysis Estimating Effect of UC on HR of All-Cause Mortality After ADT Plus RT
| Cohort | Prevalence of UC in ADT-Plus-RT Group | Prevalence of UC in ADT Group | UC HR | Treatment HR Adjusted for UC | 95% CI |
|---|---|---|---|---|---|
| RCT cohort* | |||||
| 0.20 | 0.40 | 2.00 | 0.73 | 0.69 to 0.78 | |
| 0.10 | 0.50 | 2.00 | 0.86 | 0.81 to 0.91 | |
| 0.10 | 0.70 | 2.00 | 0.97 | 0.91 to 1.04 | |
| 0.20 | 0.40 | 2.50 | 0.77 | 0.73 to 0.82 | |
| 0.10 | 0.50 | 2.50 | 0.96 | 0.90 to 1.02 | |
| 0.20 | 0.40 | 3.00 | 0.81 | 0.76 to 0.86 | |
| 0.10 | 0.40 | 3.00 | 0.94 | 0.89 to 1.01 | |
| 0.10 | 0.40 | 3.50 | 1.01 | 0.95 to 1.07 | |
| Elderly cohort† | |||||
| 0.20 | 0.40 | 2.00 | 0.73 | 0.69 to 0.78 | |
| 0.10 | 0.50 | 2.00 | 0.86 | 0.81 to 0.91 | |
| 0.10 | 0.70 | 2.00 | 0.97 | 0.92 to 1.03 | |
| 0.20 | 0.40 | 2.50 | 0.77 | 0.73 to 0.82 | |
| 0.10 | 0.50 | 2.50 | 0.96 | 0.91 to 1.01 | |
| 0.20 | 0.40 | 3.00 | 0.81 | 0.77 to 0.86 | |
| 0.10 | 0.40 | 3.00 | 0.94 | 0.89 to 1.00 | |
| 0.10 | 0.40 | 3.50 | 1.01 | 0.95 to 1.07 | |
| Screen-detected cohort‡ | |||||
| 0.10 | 0.50 | 2.00 | 0.68 | 0.61 to 0.75 | |
| 0.10 | 0.70 | 2.00 | 0.77 | 0.69 to 0.85 | |
| 0.10 | 0.90 | 2.00 | 0.86 | 0.77 to 0.96 | |
| 0.10 | 0.70 | 2.50 | 0.89 | 0.80 to 0.99 | |
| 0.10 | 0.90 | 2.50 | 1.02 | 0.91 to 1.13 | |
| 0.10 | 0.50 | 3.00 | 0.83 | 0.74 to 0.92 | |
| 0.10 | 0.70 | 3.00 | 0.99 | 0.89 to 1.11 | |
| 0.10 | 0.50 | 3.50 | 0.89 | 0.80 to 1.00 |
NOTE. Bold font indicates situations where UC was strong enough to influence significance of results (ie, upper bound of 95% CI crossed 1).
Abbreviations: ADT, androgen-deprivation therapy; HR, hazard ratio; RCT, randomized clinical trial; RT, radiotherapy; UC, unmeasured confounder.
Propensity score all-cause mortality HR, 0.63; 95% CI, 0.59 to 0.67.
Propensity score all-cause mortality HR, 0.63; 95% CI, 0.59 to 0.67.
Propensity score all-cause mortality HR, 0.50; 95% CI, 0.45 to 0.55.
Fig A1.
Definition of study cohort. ADT, androgen-deprivation therapy; AJCC, American Joint Committee on Cancer; HMO, health maintenance organization; HRR, hospital referral region; RCT, randomized clinical trial; RT, radiotherapy.
Fig A2.
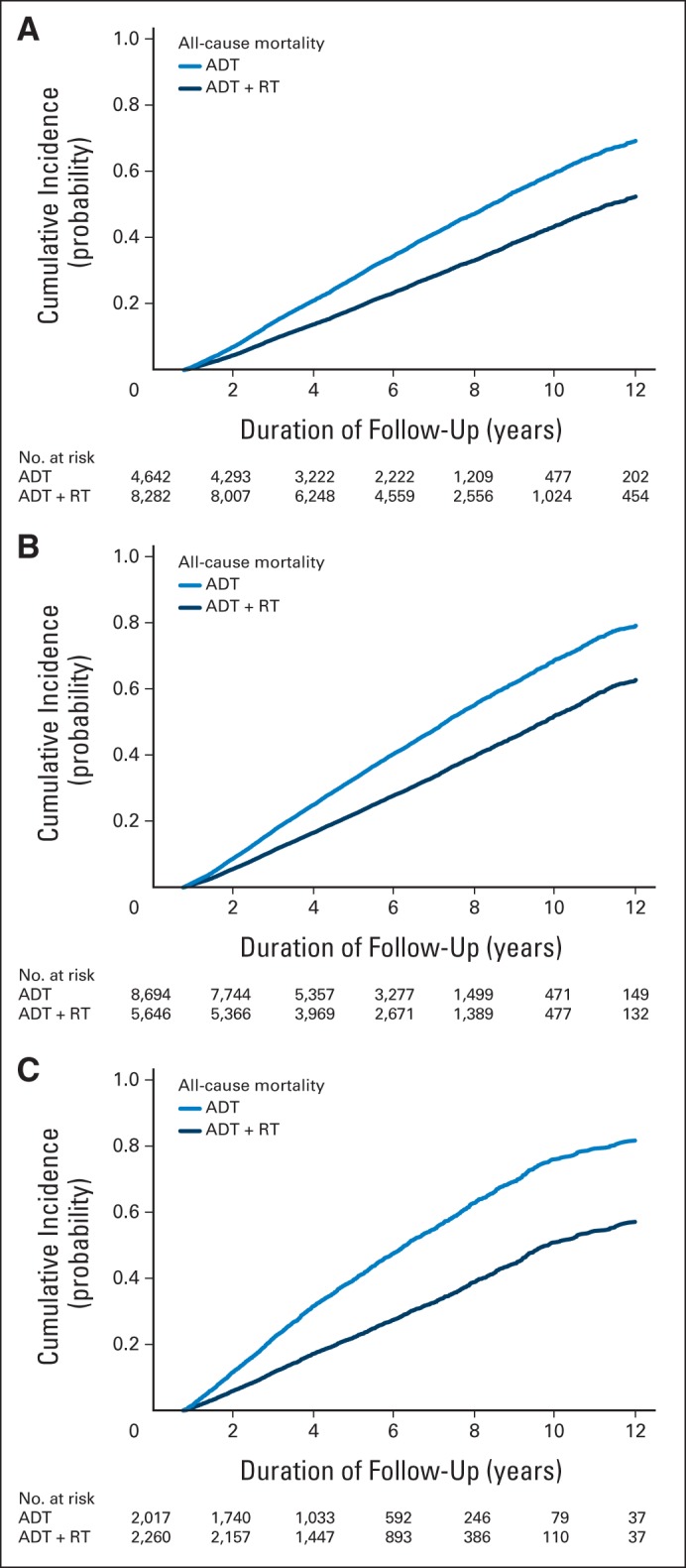
Adjusted cumulative incidence of all-cause mortality for (A) randomized clinical trial (RCT) cohort, (B) elderly cohort, and (C) screen-detected cohort. ADT, androgen-deprivation therapy; RT, radiotherapy.
Footnotes
See accompanying editorial on page 676
Supported by Grants No. RC4-CA155809 from the National Institutes of Health, Nos. RC2-CA148310, K12-CA076931, K07-CA163616, and UC2-CA148310 from the National Cancer Institute, Nos. P30-CA016520 and P30-CA06927 from the US Public Health Service, and No. MRSG-121988 from the American Cancer Society.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
The funding agencies did not participate in the design or conduct of the study; in the collection, analysis, or interpretation of the data; or in the preparation, review, or approval of the manuscript. The interpretation and reporting of these data are the sole responsibility of the authors.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Justin E. Bekelman, Nandita Mitra, Katrina Armstrong
Financial support: Justin E. Bekelman, Daniel Polsky, Katrina Armstrong
Collection and assembly of data: Justin E. Bekelman, Elizabeth A. Handorf
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Effectiveness of Androgen-Deprivation Therapy and Radiotherapy for Older Men With Locally Advanced Prostate Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Justin E. Bekelman
No relationship to disclose
Nandita Mitra
No relationship to disclose
Elizabeth A. Handorf
Research Funding: Pfizer (Inst)
Robert G. Uzzo
Speakers' Bureau: Pfizer, Janssen
Stephen Hahn
No relationship to disclose
Daniel Polsky
No relationship to disclose
Katrina Armstrong
No relationship to disclose
REFERENCES
- 1.Lu-Yao GL, Albertsen PC, Moore DF, et al. Outcomes of localized prostate cancer following conservative management. JAMA. 2009;302:1202–1209. doi: 10.1001/jama.2009.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daskivich TJ, Fan KH, Koyama T, et al. Effect of age, tumor risk, and comorbidity on competing risks for survival in a U.S. population-based cohort of men with prostate cancer. Ann Intern Med. 2013;158:709–717. doi: 10.7326/0003-4819-158-10-201305210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Widmark A, Klepp O, Solberg A, et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): An open randomised phase III trial. Lancet. 2009;373:301–308. doi: 10.1016/S0140-6736(08)61815-2. [DOI] [PubMed] [Google Scholar]
- 4.Warde P, Mason M, Ding K, et al. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: A randomised, phase 3 trial. Lancet. 2011;378:2104–2111. doi: 10.1016/S0140-6736(11)61095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu-Yao GL, Albertsen PC, Moore DF, et al. Survival following primary androgen deprivation therapy among men with localized prostate cancer. JAMA. 2008;300:173–181. doi: 10.1001/jama.300.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts CB, Albertsen PC, Shao YH, et al. Patterns and correlates of prostate cancer treatment in older men. Am J Med. 2011;124:235–243. doi: 10.1016/j.amjmed.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 8.Jacobsen L, Mather M, Lee M, et al. America's Aging Population. http://www.prb.org/pdf11/aging-in-america.pdf.
- 9.Potosky AL, Riley GF, Lubitz JD, et al. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31:732–748. [PubMed] [Google Scholar]
- 10.Shahinian VB, Kuo YF, Gilbert SM. Reimbursement policy and androgen-deprivation therapy for prostate cancer. N Engl J Med. 2010;363:1822–1832. doi: 10.1056/NEJMsa0910784. [DOI] [PubMed] [Google Scholar]
- 11.Bekelman JE, Mitra N, Efstathiou J, et al. Outcomes after intensity-modulated versus conformal radiotherapy in older men with nonmetastatic prostate cancer. Int J Radiat Oncol Biol Phys. 2011;81:e325–e334. doi: 10.1016/j.ijrobp.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shahinian VB, Kuo YF, Freeman JL, et al. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 13.Elixhauser A, Steiner C, Harris D, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Mattei A. Estimating and using propensity score in presence of missing background data: An application to assess the impact of childbearing on wellbeing. Stat Methods Appt. 2009;18:257–273. [Google Scholar]
- 15.Rosenbaum P, Robin D. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. 1984;79:516–524. [Google Scholar]
- 16.Rosenbaum P, Rubin D. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 17.Rubin D. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127:757–763. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 18.Gaynor J, Feuer E, Tan C, et al. On the use of cause-specific failure and conditional failure probabilities: Examples from clinical oncology data. J Am Stat Assoc. 1993;88:400–409. [Google Scholar]
- 19.Fine J, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stats Assoc. 1999;94:496–509. [Google Scholar]
- 20.Klein JP, Moeschberger ML. Survival Analysis: Techniques for Censored and Truncated Data (ed 2) Springer, New York: NY; 2003. [Google Scholar]
- 21.Brookhart MA, Stürmer T, Glynn RJ, et al. Confounding control in healthcare database research: Challenges and potential approaches. Med Care. 2010;48(suppl):S114–S120. doi: 10.1097/MLR.0b013e3181dbebe3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wennberg J, Gittelsohn J. Small area variations in health care delivery. Science. 1973;182:1102–1108. doi: 10.1126/science.182.4117.1102. [DOI] [PubMed] [Google Scholar]
- 23.Terza JV, Basu A, Rathouz PJ. Two-stage residual inclusion estimation: Addressing endogeneity in health econometric modeling. J Health Econ. 2008;27:531–543. doi: 10.1016/j.jhealeco.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clogg CC, Petkova E, Haritou A. Statistical methods for comparing regression coefficients between models. Am J Sociol. 1995;100:1271–1293. [Google Scholar]
- 25.Spillman B. Changes in elderly disability rates and the implications for health care utilization and cost. Milbank Q. 2004;82:157–194. doi: 10.1111/j.0887-378X.2004.00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bechis SK, Carroll PR, Cooperberg MR. Impact of age at diagnosis on prostate cancer treatment and survival. J Clin Oncol. 2011;29:235–241. doi: 10.1200/JCO.2010.30.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong YN, Mitra N, Hudes G, et al. Survival associated with treatment vs observation of localized prostate cancer in elderly men. JAMA. 2006;296:2683–2693. doi: 10.1001/jama.296.22.2683. [DOI] [PubMed] [Google Scholar]
- 28.Potosky AL, Haque R, Cassidy-Bushrow AE, et al. Effectiveness of primary androgen-deprivation therapy for clinically localized prostate cancer. J Clin Oncol. 2014;32:1324–1330. doi: 10.1200/JCO.2013.52.5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill AB. The environment and disease: Association or causation? Proc R Soc Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan A, Parker C. Radiotherapy in locally advanced prostate cancer. Lancet. 2009;373:274–276. doi: 10.1016/S0140-6736(08)61816-4. [DOI] [PubMed] [Google Scholar]
- 31.Albertsen PC, Walters S, Hanley JA. A comparison of cause of death determination in men previously diagnosed with prostate cancer who died in 1985 or 1995. J Urol. 2000;163:519–523. [PubMed] [Google Scholar]
- 32.Penson DF, Albertsen PC, Nelson PS, et al. Determining cause of death in prostate cancer: Are death certificates valid? J Natl Cancer Inst. 2001;93:1822–1823. doi: 10.1093/jnci/93.23.1822. [DOI] [PubMed] [Google Scholar]
- 33.Angrist J, Imbens G, Rubin D. Identification of causal effects using instrumental variables. J Am Stat Assoc. 1996;91:444–455. [Google Scholar]