Abstract
Purpose
The purpose of this study was to compare illness-related anxiety among participants in the Rituximab Extended Schedule or Retreatment Trial (RESORT) randomly assigned to maintenance rituximab (MR) versus rituximab re-treatment (RR). A secondary objective was to examine whether the superiority of MR versus RR on anxiety depended on illness-related coping style.
Patients and Methods
Patients (N = 253) completed patient-reported outcome (PRO) measures at random assignment to MR or RR (baseline); at 3, 6, 12, 24, 36, and 48 months after random assignment; and at rituximab failure. PRO measures assessed illness-related anxiety and coping style, and secondary end points including general anxiety, worry and interference with emotional well-being, depression, and health-related quality of life (HRQoL). Patients were classified as using an active or avoidant illness-related coping style. Independent sample t tests and linear mixed-effects models were used to identify treatment arm differences on PRO end points and differences based on coping style.
Results
Illness-related anxiety was comparable between treatment arms at all time points (P > .05), regardless of coping style (active or avoidant). Illness-related anxiety and general anxiety significantly decreased over time on both arms. HRQoL scores were relatively stable and did not change significantly from baseline for both arms. An avoidant coping style was associated with significantly higher anxiety (18% and 13% exceeded clinical cutoff points at baseline and 6 months, respectively) and poorer HRQoL compared with an active coping style (P < .001), regardless of treatment arm assignment.
Conclusion
Surveillance until RR at progression was not associated with increased anxiety compared with MR, regardless of coping style. Avoidant coping was associated with higher anxiety and poorer HRQoL.
INTRODUCTION
Non-Hodgkin lymphoma (NHL) is the sixth most common cancer in the United States1 and often managed as a chronic disease as a result of high long-term survival rates. Survivors of NHL live with their disease and treatment effects for years; therefore, health-related quality of life (HRQoL) is a central concern for clinical management. For patients with low tumor burden and intact HRQoL, potential benefits of treatment must be weighed against treatment toxicities that compromise HRQoL2–8 and symptom burden associated with recurrence or active disease.8–10 Elevated anxiety and fear of progression have been documented in survivors of NHL.11–14 Survivors of NHL with indolent, incurable disease face unique challenges. Patients offered a watch-and-wait strategy may experience anxiety as a result of lack of active treatment, compounded by anxiety about inevitable disease progression. Alternatively, patients undergoing immune therapy or chemotherapy face HRQoL decrements associated with treatment.
Rituximab as a potential first-line treatment for NHL offers promise. Rituximab is well tolerated, with no measurable detriment to patient-reported HRQoL.15 Patients with NHL randomly assigned to rituximab induction followed by maintenance rituximab (MR) every 8 weeks reported comparable physical well-being to patients randomly assigned to observation.6 Rituximab may offer psychological benefit by providing a viable alternative to the watch-and-wait strategy, reducing anxiety and bolstering HRQoL. Patients with NHL receiving MR reported feeling more in control of their disease, less worry, and less illness-related anxiety compared with patients randomly assigned to observation.16 This presents a clinical challenge regarding whether MR is better than the watch-and-wait strategy given the adverse emotional effects of what patients may perceive as a passive approach to managing their disease with the watch-and-wait approach.
The Eastern Cooperative Oncology Group (ECOG) Rituximab Extended Schedule or Retreatment Trial (RESORT; E4402)17 provided a unique opportunity to prospectively assess anxiety among a large sample of patients with indolent NHL, randomly assigned to MR every 3 months or rituximab re-treatment (RR) at progression. Primary trial results indicate no significant differences between MR and RR on time to treatment failure and disease-related outcomes.17 The purpose of this study was to compare illness-related anxiety among trial participants randomly assigned to MR versus RR. A secondary objective was to examine superiority of MR versus RR with regard to illness-related anxiety given participant coping style for managing illness (active v avoidant). We hypothesized that participants endorsing active coping would report less anxiety on MR compared with RR, because MR is the more active option. We further reasoned that participants reporting avoidant illness-related coping would report less anxiety on RR compared with MR because with RR they could more easily avoid worry about recurrence.
PATIENTS AND METHODS
Study Population
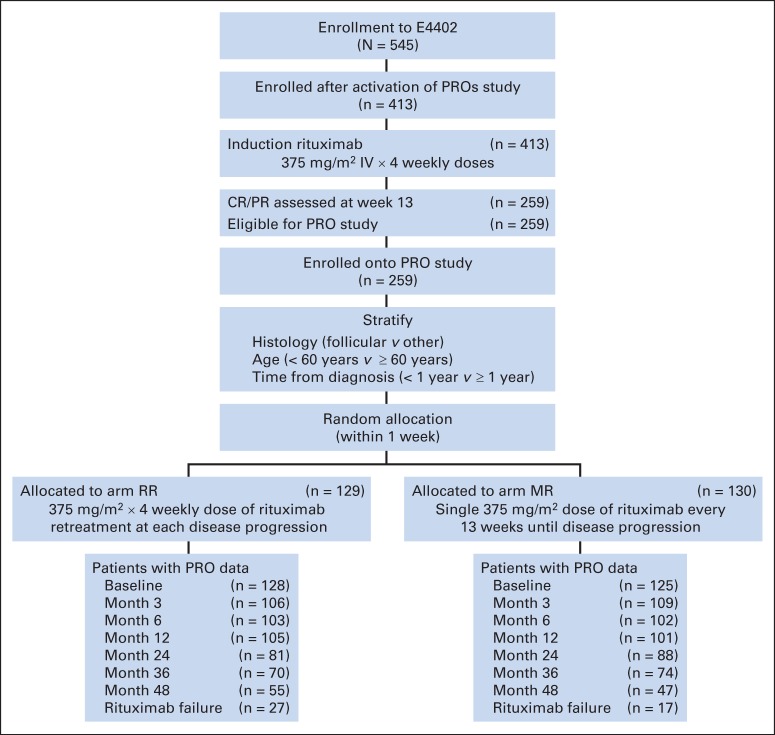
A subgroup of participants (N = 253) enrolled onto RESORT who achieved complete or partial response after 4-week rituximab induction therapy completed patient-reported outcome (PRO) measures. PRO end points were added 18 months after trial activation (November 2003). The study was approved by local human investigations committees at all participating institutions, all participants provided written informed consent, and research was conducted in accordance with the Declaration of Helsinki. All participants enrolled onto RESORT after May 2005 completed PROs. Figure 1 displays the study CONSORT diagram.18 RESORT eligibility criteria included age ≥ 18 years, ECOG performance status of 0 to 1, stage III or IV (modified Ann Arbor staging) biopsy-proven NHL with no evidence of transformation to large-cell histology, and low tumor burden. Primary trial outcomes have been analyzed separately for participants with follicular17 and nonfollicular19 NHL. No significant differences were observed on PRO measures between participants with follicular and nonfollicular NHL; therefore, results presented are based on the combined sample.
Fig 1.
CONSORT diagram. CR, complete response; MR, maintenance rituximab; PR, partial response; PROs, patient-reported outcomes; RR, rituximab re-treatment.
Measures: PROs
PRO measures assessed illness-related anxiety and coping style (active v avoidant) and secondary end points including general anxiety, illness-related worry and interference with emotional well-being, depression, and overall HRQoL. We collaboratively designed this assessment with investigators from the Rituximab Versus Watch-and-Wait trial16 to allow comparison of results. Reasons for not completing PROs were collected using a standardized form.
Illness-related anxiety.
The 22-item Impact of Event Scale–Revised (IES-R)20 is revised from the original 15-item Impact of Event Scale21 and assesses subjective distress caused by traumatic events, such as illness. Per IES-R administration procedures, revised instructions directed participants to answer with respect to having lymphoma. IES-R subscales measure intrusive thoughts, avoidance of reminders of illness, and symptoms of hyperarousal. Items are rated on a 5-point Likert scale from 0 (not at all) to 4 (extremely), yielding a total score of 0 to 88. Higher scores represent greater illness-related anxiety. Analyses of subscale scores yielded similar findings to the IES-R total score, so the latter is reported.
Illness-related coping style.
Four items were written for this protocol to evaluate illness-related coping style (active v avoidant) specific to managing lymphoma, as recommended by coping experts.22 Two items assessed whether medical care reduced anxiety as a result of increased feelings of control (active illness-related coping style). Two questions assessed the degree to which receiving medical care increased anxiety as a reminder of illness (avoidant illness-related coping style). Participants endorsed items using a 5-point Likert scale from 0 (not at all) to 4 (very much), using standard response options from the Functional Assessment of Cancer Therapy (FACT).23
General anxiety and depression.
The Hospital Anxiety and Depression Scale (HADS)24,25 was administered to measure general anxiety (seven items) and depression (seven items). Subscales are scored separately. Total scores for each subscale range from 0 to 21, and higher scores represent greater anxiety or depression.
Illness-related worry and interference with emotional well-being.
Nine items were administered to assess worry about the future as a result of illness and illness-related interference with emotional well-being (illness-related worry). Given targeted study aims, two FACT-Lymphoma26 items and five items from a preliminary version of the National Institutes of Health Patient-Reported Outcomes Measurement Information System Illness Impact item bank27 were selected. Two additional items were written for this study to assess fears about lymphoma progression. Internal consistency of these nine items was good across assessment time points (Cronbach's α = .86 to .89), so items were summed for analysis. The total score ranges from 0 to 36; higher scores represent greater worry about the future and distress.
Loss of control.
Three items from the Mental Adjustment to Cancer Scale28,29 were administered to assess the extent to which patients reported feeling a loss of control over their cancer. The total score ranges from 0 to 9; higher scores represent feeling less control.
HRQoL.
The FACT–General (FACT-G)23,30 is a 27-item assessment used to measure HRQoL, specifically, physical, functional, social, and emotional well-being. The FACT-G has previously been used to investigate HRQoL among patients with indolent lymphoma.31,32 The total score ranges from 0 to 108, with higher scores indicating better HRQoL.
Assessment Schedule
Participants completed PRO measures at the time of random assignment to MR versus RR. This occurred 3 months after registration on RESORT and after 4 weeks of induction rituximab. Herein, this is referred to as the baseline assessment for ease of interpretation, with the clarification that this assessment was administered after rituximab induction and before treatment random assignment. PRO measures were administered at 3, 6, 12, 24, 36, and 48 months after baseline. PRO measures were also completed at the time of rituximab failure, which was defined as any one of the following events: no response to rituximab, time to progression ≤ 26 weeks, initiation of alternative therapy, or inability to complete protocol therapy (as a result of adverse events, patient preference, or any other reason including death).
Statistical Analysis
The primary end point was the difference between participants randomly assigned to MR versus RR on IES-R score change (illness-related anxiety) from baseline to 6 months after random assignment. Six-month assessment corresponded with the third scheduled dose of rituximab for participants randomly assigned to MR. A sample size of 180 patients (90 per arm) would yield 80% power to detect a 4.2-point difference (effect size, 0.42) between groups on IES-R change scores from baseline to 6 months, using two independent sample t test with a two-sided type I error of 0.05, assuming a common standard deviation of 10 for the score changes.
A hierarchical cluster analysis (Ward's linkage) of the four-item illness-related coping style measure was used to categorize participants as having an active illness-related coping style (active) or an avoidant illness-related coping style (avoidant). An IES-R score ≥ 33 was defined as clinically significant illness-related anxiety.20 Scoring ≥ 11 on the HADS-Anxiety and HADS-Depression measures was defined as moderate-severe.24 Patient characteristics were compared between treatment arms and coping style (active v avoidant) using the χ2 test and Fisher's exact test. The proportion of clinically significant anxiety and depression was compared between coping styles using Fisher's exact test. PRO scores and change scores were compared between treatment arms and coping style using two independent sample t tests. Multivariable linear mixed-effects models with random intercept (repeated measures within single patients with unstructured covariance matrices) were fit using the maximum likelihood method to examine the time trend for PRO measures (time included as a continuous variable) and to estimate the average difference in PRO measures between treatment arms and illness-related coping style, after adjusting for other covariates, assuming that any missing data were missing at random. Separate multivariable linear mixed models tested whether the time trend for PRO measures was different between treatment arms (via treatment-by-time interaction term) and whether treatment arm differences depended on coping style (via treatment-by-coping style interaction term). Log-normal survival models were conducted as a sensitivity analysis to analyze longitudinal data that incorporated the potential nonignorable censoring mechanism. The same covariates were adjusted in all models, including age, sex, ECOG performance status, serum lactate dehydrogenase level, Follicular Lymphoma International Prognostic Index,33 and modified Ann Arbor stage. No multicollinearity was found among these covariates using the variance inflation factor method.
No adjustment was made for multiple comparisons. All P values were two-sided, and P = .05 was considered statistically significant. STATA version 11.2 (StataCorp, College Station, TX) was used for all analyses.
RESULTS
Study Population
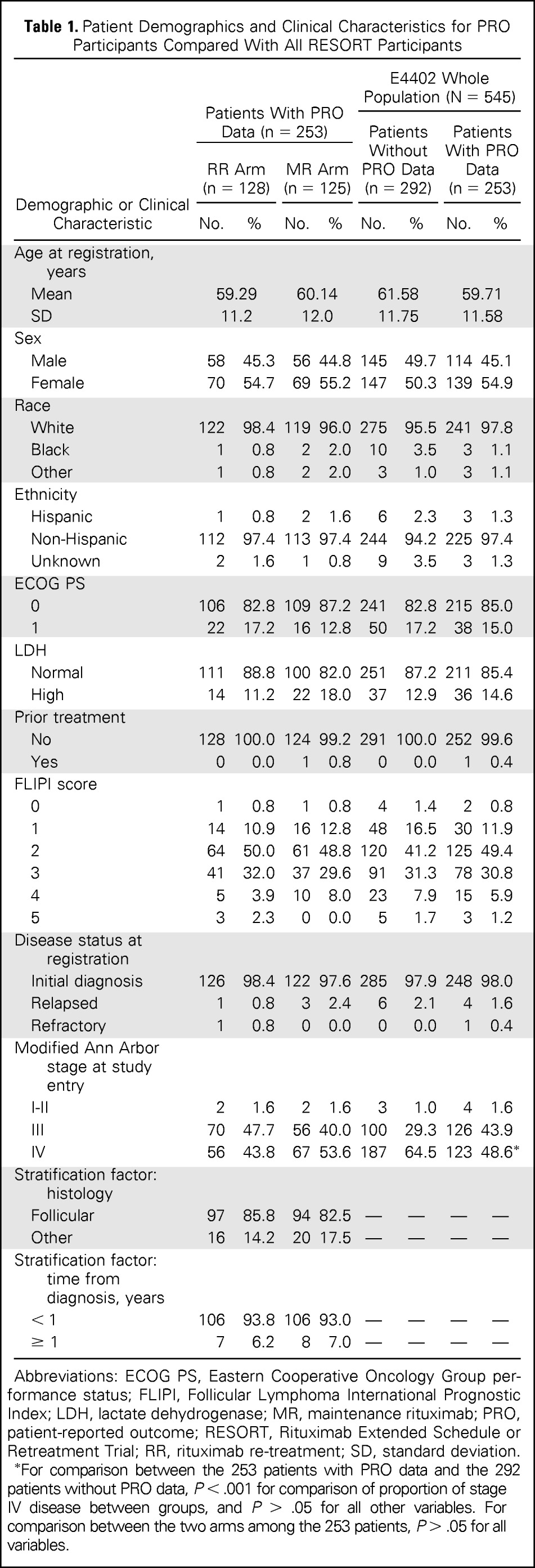
RESORT participants who completed PROs (N = 253; Table 1) were a mean of 59.7 years old (SD, 11.6 years), were predominantly white (n = 241, 97.6%), and mostly had an ECOG performance status of 0 (n = 215, 85.0%). The majority of patients had follicular lymphoma (n = 210, 83.0%). Participants were balanced between treatment arms with regard to demographic and medical characteristics. Participants who completed PROs were similar to those on RESORT who did not complete PRO assessment (n = 292; Table 1) with regard to demographic and medical characteristics, except fewer patients had stage IV disease at study entry in the 253 patients with PRO data (48.6% v 64.5%, respectively).
Table 1.
Patient Demographics and Clinical Characteristics for PRO Participants Compared With All RESORT Participants
| Demographic or Clinical Characteristic | Patients With PRO Data (n = 253) |
E4402 Whole Population (N = 545) |
||||||
|---|---|---|---|---|---|---|---|---|
| RR Arm (n = 128) |
MR Arm (n = 125) |
Patients Without PRO Data (n = 292) |
Patients With PRO Data (n = 253) |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Age at registration, years | ||||||||
| Mean | 59.29 | 60.14 | 61.58 | 59.71 | ||||
| SD | 11.2 | 12.0 | 11.75 | 11.58 | ||||
| Sex | ||||||||
| Male | 58 | 45.3 | 56 | 44.8 | 145 | 49.7 | 114 | 45.1 |
| Female | 70 | 54.7 | 69 | 55.2 | 147 | 50.3 | 139 | 54.9 |
| Race | ||||||||
| White | 122 | 98.4 | 119 | 96.0 | 275 | 95.5 | 241 | 97.8 |
| Black | 1 | 0.8 | 2 | 2.0 | 10 | 3.5 | 3 | 1.1 |
| Other | 1 | 0.8 | 2 | 2.0 | 3 | 1.0 | 3 | 1.1 |
| Ethnicity | ||||||||
| Hispanic | 1 | 0.8 | 2 | 1.6 | 6 | 2.3 | 3 | 1.3 |
| Non-Hispanic | 112 | 97.4 | 113 | 97.4 | 244 | 94.2 | 225 | 97.4 |
| Unknown | 2 | 1.6 | 1 | 0.8 | 9 | 3.5 | 3 | 1.3 |
| ECOG PS | ||||||||
| 0 | 106 | 82.8 | 109 | 87.2 | 241 | 82.8 | 215 | 85.0 |
| 1 | 22 | 17.2 | 16 | 12.8 | 50 | 17.2 | 38 | 15.0 |
| LDH | ||||||||
| Normal | 111 | 88.8 | 100 | 82.0 | 251 | 87.2 | 211 | 85.4 |
| High | 14 | 11.2 | 22 | 18.0 | 37 | 12.9 | 36 | 14.6 |
| Prior treatment | ||||||||
| No | 128 | 100.0 | 124 | 99.2 | 291 | 100.0 | 252 | 99.6 |
| Yes | 0 | 0.0 | 1 | 0.8 | 0 | 0.0 | 1 | 0.4 |
| FLIPI score | ||||||||
| 0 | 1 | 0.8 | 1 | 0.8 | 4 | 1.4 | 2 | 0.8 |
| 1 | 14 | 10.9 | 16 | 12.8 | 48 | 16.5 | 30 | 11.9 |
| 2 | 64 | 50.0 | 61 | 48.8 | 120 | 41.2 | 125 | 49.4 |
| 3 | 41 | 32.0 | 37 | 29.6 | 91 | 31.3 | 78 | 30.8 |
| 4 | 5 | 3.9 | 10 | 8.0 | 23 | 7.9 | 15 | 5.9 |
| 5 | 3 | 2.3 | 0 | 0.0 | 5 | 1.7 | 3 | 1.2 |
| Disease status at registration | ||||||||
| Initial diagnosis | 126 | 98.4 | 122 | 97.6 | 285 | 97.9 | 248 | 98.0 |
| Relapsed | 1 | 0.8 | 3 | 2.4 | 6 | 2.1 | 4 | 1.6 |
| Refractory | 1 | 0.8 | 0 | 0.0 | 0 | 0.0 | 1 | 0.4 |
| Modified Ann Arbor stage at study entry | ||||||||
| I-II | 2 | 1.6 | 2 | 1.6 | 3 | 1.0 | 4 | 1.6 |
| III | 70 | 47.7 | 56 | 40.0 | 100 | 29.3 | 126 | 43.9 |
| IV | 56 | 43.8 | 67 | 53.6 | 187 | 64.5 | 123 | 48.6* |
| Stratification factor: histology | ||||||||
| Follicular | 97 | 85.8 | 94 | 82.5 | — | — | — | — |
| Other | 16 | 14.2 | 20 | 17.5 | — | — | — | — |
| Stratification factor: time from diagnosis, years | ||||||||
| < 1 | 106 | 93.8 | 106 | 93.0 | — | — | — | — |
| ≥ 1 | 7 | 6.2 | 8 | 7.0 | — | — | — | — |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; FLIPI, Follicular Lymphoma International Prognostic Index; LDH, lactate dehydrogenase; MR, maintenance rituximab; PRO, patient-reported outcome; RESORT, Rituximab Extended Schedule or Retreatment Trial; RR, rituximab re-treatment; SD, standard deviation.
For comparison between the 253 patients with PRO data and the 292 patients without PRO data, P < .001 for comparison of proportion of stage IV disease between groups, and P > .05 for all other variables. For comparison between the two arms among the 253 patients, P > .05 for all variables.
Rates of PRO Data Collection
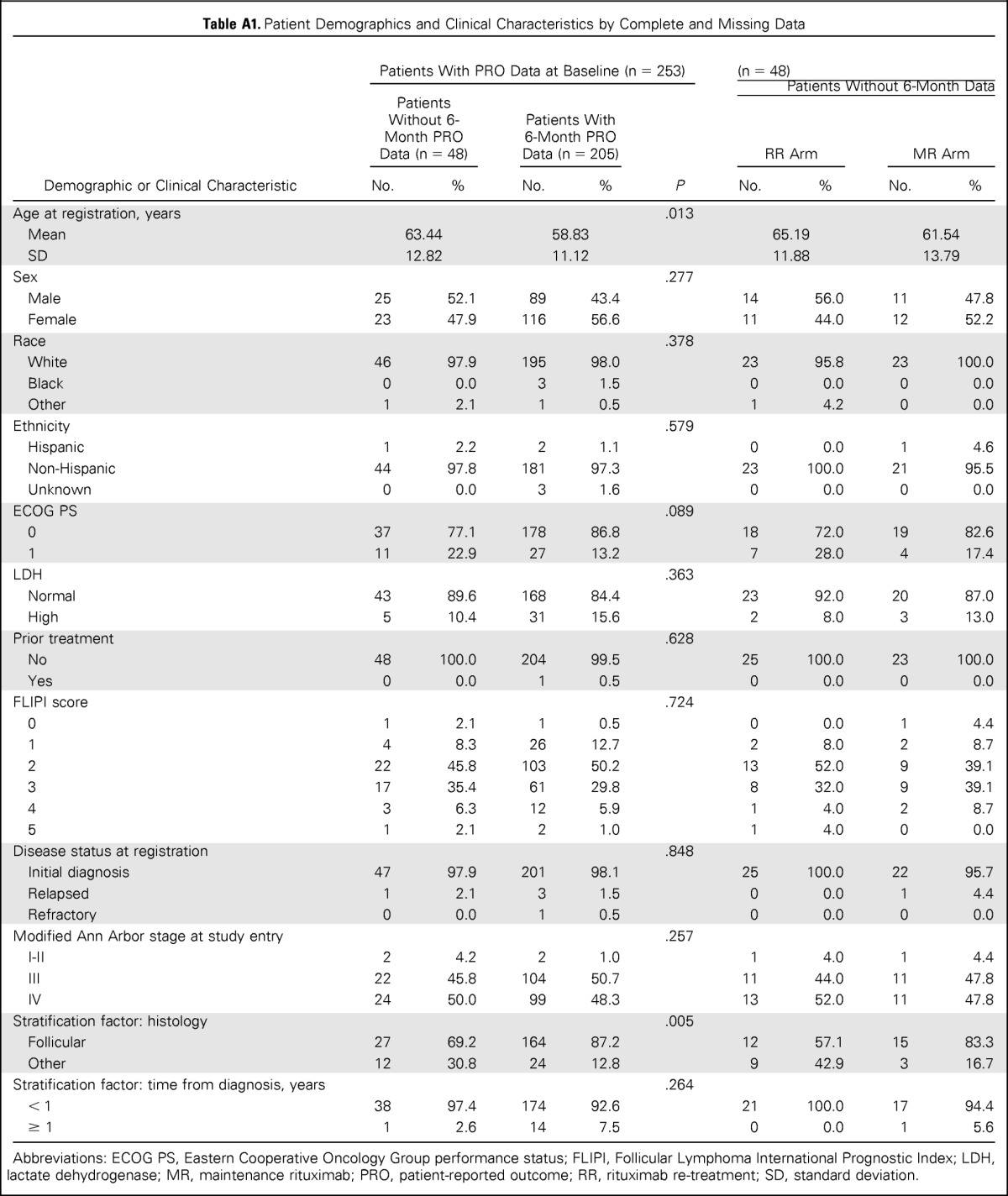
PRO questionnaires were available from 253 patients at baseline (92% of RESORT participants; Fig 1), 215 patients (78%) at month 3, 205 patients (74%) at month 6, and 206 patients (75%) at month 12. Rates of missing PRO data were similar between treatment arms for all assessments. Patients who provided PROs at 6 months were comparable to patients with missing data at 6 months, with the exception of age and histology (Appendix Table A1, online only). All assessments combined, the main reasons for missing PROs were staff error (77%) and patient refusal or missed appointment (18%). Few patients missed assessments as a result of being too ill or death (3%).
Differences Between Treatment Arms on Anxiety and HRQoL
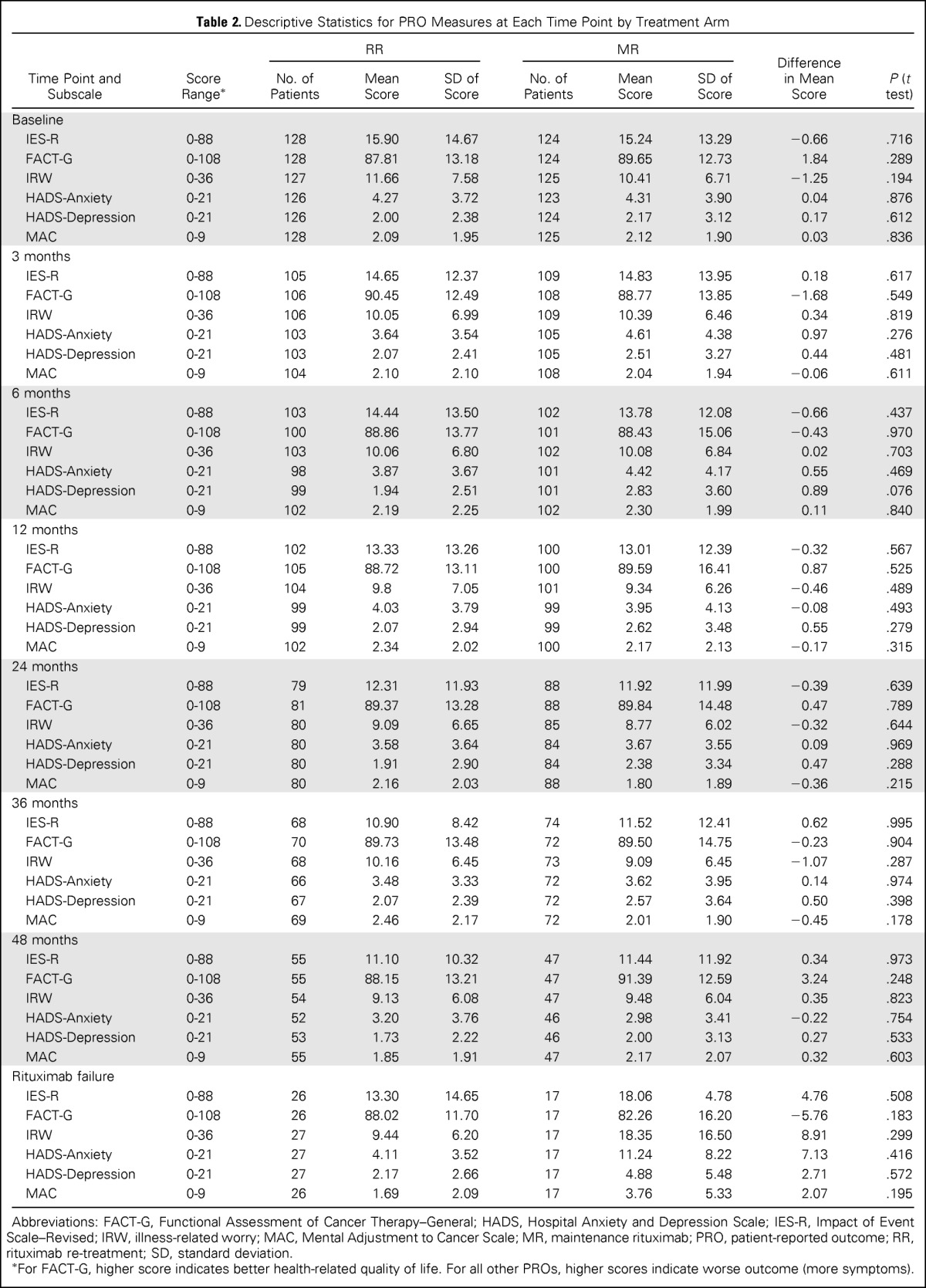
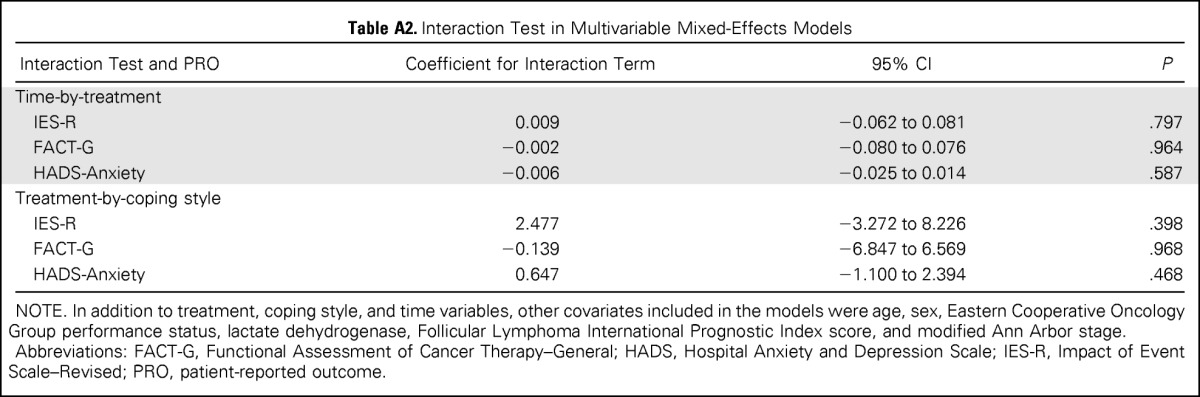
Descriptive statistics for PRO measures at each time point are listed in Table 2. Illness-related anxiety (IES-R) was comparable between treatment arms at all time points (P < .05), and the difference between treatment arms did not exceed the clinically meaningful difference of 4 to 7 points estimated using a distribution-based method.34 Results were similar for secondary end points, including general anxiety, illness-related worry and interference with emotional well-being, loss of control, depression, and overall HRQoL. Longitudinal trajectories of illness-related anxiety, general anxiety, and HRQoL scores were similar for patients receiving MR and RR (Fig 2). For both treatment arms, illness-related anxiety and general anxiety decreased over time. HRQoL scores were relatively stable for both treatment arms and did not change significantly from baseline, indicating that treatment was well tolerated (Fig 2C). Multivariable linear mixed-effects models showed similar results after adjusting for other covariates. The treatment-by-time interaction term was not significant in models for illness-related anxiety, general anxiety, and HRQoL (P > .5 for all; Appendix Table A2, online only), indicating a similar time trend in PRO scores in both arms after adjusting for other covariates. In the mixed-effects models without any interaction term, anxiety declined significantly with time (P < .001 for illness-related anxiety, P = .007 for general anxiety), whereas HRQoL was relatively stable (P = .297; Appendix Table A3, online only). The log-normal survival models produced similar results as the previously mentioned analyses after considering the possible informative censoring mechanism of the missing data (data not shown).
Table 2.
Descriptive Statistics for PRO Measures at Each Time Point by Treatment Arm
| Time Point and Subscale | Score Range* | RR |
MR |
Difference in Mean Score | P (t test) | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Patients | Mean Score | SD of Score | No. of Patients | Mean Score | SD of Score | ||||
| Baseline | |||||||||
| IES-R | 0-88 | 128 | 15.90 | 14.67 | 124 | 15.24 | 13.29 | −0.66 | .716 |
| FACT-G | 0-108 | 128 | 87.81 | 13.18 | 124 | 89.65 | 12.73 | 1.84 | .289 |
| IRW | 0-36 | 127 | 11.66 | 7.58 | 125 | 10.41 | 6.71 | −1.25 | .194 |
| HADS-Anxiety | 0-21 | 126 | 4.27 | 3.72 | 123 | 4.31 | 3.90 | 0.04 | .876 |
| HADS-Depression | 0-21 | 126 | 2.00 | 2.38 | 124 | 2.17 | 3.12 | 0.17 | .612 |
| MAC | 0-9 | 128 | 2.09 | 1.95 | 125 | 2.12 | 1.90 | 0.03 | .836 |
| 3 months | |||||||||
| IES-R | 0-88 | 105 | 14.65 | 12.37 | 109 | 14.83 | 13.95 | 0.18 | .617 |
| FACT-G | 0-108 | 106 | 90.45 | 12.49 | 108 | 88.77 | 13.85 | −1.68 | .549 |
| IRW | 0-36 | 106 | 10.05 | 6.99 | 109 | 10.39 | 6.46 | 0.34 | .819 |
| HADS-Anxiety | 0-21 | 103 | 3.64 | 3.54 | 105 | 4.61 | 4.38 | 0.97 | .276 |
| HADS-Depression | 0-21 | 103 | 2.07 | 2.41 | 105 | 2.51 | 3.27 | 0.44 | .481 |
| MAC | 0-9 | 104 | 2.10 | 2.10 | 108 | 2.04 | 1.94 | −0.06 | .611 |
| 6 months | |||||||||
| IES-R | 0-88 | 103 | 14.44 | 13.50 | 102 | 13.78 | 12.08 | −0.66 | .437 |
| FACT-G | 0-108 | 100 | 88.86 | 13.77 | 101 | 88.43 | 15.06 | −0.43 | .970 |
| IRW | 0-36 | 103 | 10.06 | 6.80 | 102 | 10.08 | 6.84 | 0.02 | .703 |
| HADS-Anxiety | 0-21 | 98 | 3.87 | 3.67 | 101 | 4.42 | 4.17 | 0.55 | .469 |
| HADS-Depression | 0-21 | 99 | 1.94 | 2.51 | 101 | 2.83 | 3.60 | 0.89 | .076 |
| MAC | 0-9 | 102 | 2.19 | 2.25 | 102 | 2.30 | 1.99 | 0.11 | .840 |
| 12 months | |||||||||
| IES-R | 0-88 | 102 | 13.33 | 13.26 | 100 | 13.01 | 12.39 | −0.32 | .567 |
| FACT-G | 0-108 | 105 | 88.72 | 13.11 | 100 | 89.59 | 16.41 | 0.87 | .525 |
| IRW | 0-36 | 104 | 9.8 | 7.05 | 101 | 9.34 | 6.26 | −0.46 | .489 |
| HADS-Anxiety | 0-21 | 99 | 4.03 | 3.79 | 99 | 3.95 | 4.13 | −0.08 | .493 |
| HADS-Depression | 0-21 | 99 | 2.07 | 2.94 | 99 | 2.62 | 3.48 | 0.55 | .279 |
| MAC | 0-9 | 102 | 2.34 | 2.02 | 100 | 2.17 | 2.13 | −0.17 | .315 |
| 24 months | |||||||||
| IES-R | 0-88 | 79 | 12.31 | 11.93 | 88 | 11.92 | 11.99 | −0.39 | .639 |
| FACT-G | 0-108 | 81 | 89.37 | 13.28 | 88 | 89.84 | 14.48 | 0.47 | .789 |
| IRW | 0-36 | 80 | 9.09 | 6.65 | 85 | 8.77 | 6.02 | −0.32 | .644 |
| HADS-Anxiety | 0-21 | 80 | 3.58 | 3.64 | 84 | 3.67 | 3.55 | 0.09 | .969 |
| HADS-Depression | 0-21 | 80 | 1.91 | 2.90 | 84 | 2.38 | 3.34 | 0.47 | .288 |
| MAC | 0-9 | 80 | 2.16 | 2.03 | 88 | 1.80 | 1.89 | −0.36 | .215 |
| 36 months | |||||||||
| IES-R | 0-88 | 68 | 10.90 | 8.42 | 74 | 11.52 | 12.41 | 0.62 | .995 |
| FACT-G | 0-108 | 70 | 89.73 | 13.48 | 72 | 89.50 | 14.75 | −0.23 | .904 |
| IRW | 0-36 | 68 | 10.16 | 6.45 | 73 | 9.09 | 6.45 | −1.07 | .287 |
| HADS-Anxiety | 0-21 | 66 | 3.48 | 3.33 | 72 | 3.62 | 3.95 | 0.14 | .974 |
| HADS-Depression | 0-21 | 67 | 2.07 | 2.39 | 72 | 2.57 | 3.64 | 0.50 | .398 |
| MAC | 0-9 | 69 | 2.46 | 2.17 | 72 | 2.01 | 1.90 | −0.45 | .178 |
| 48 months | |||||||||
| IES-R | 0-88 | 55 | 11.10 | 10.32 | 47 | 11.44 | 11.92 | 0.34 | .973 |
| FACT-G | 0-108 | 55 | 88.15 | 13.21 | 47 | 91.39 | 12.59 | 3.24 | .248 |
| IRW | 0-36 | 54 | 9.13 | 6.08 | 47 | 9.48 | 6.04 | 0.35 | .823 |
| HADS-Anxiety | 0-21 | 52 | 3.20 | 3.76 | 46 | 2.98 | 3.41 | −0.22 | .754 |
| HADS-Depression | 0-21 | 53 | 1.73 | 2.22 | 46 | 2.00 | 3.13 | 0.27 | .533 |
| MAC | 0-9 | 55 | 1.85 | 1.91 | 47 | 2.17 | 2.07 | 0.32 | .603 |
| Rituximab failure | |||||||||
| IES-R | 0-88 | 26 | 13.30 | 14.65 | 17 | 18.06 | 4.78 | 4.76 | .508 |
| FACT-G | 0-108 | 26 | 88.02 | 11.70 | 17 | 82.26 | 16.20 | −5.76 | .183 |
| IRW | 0-36 | 27 | 9.44 | 6.20 | 17 | 18.35 | 16.50 | 8.91 | .299 |
| HADS-Anxiety | 0-21 | 27 | 4.11 | 3.52 | 17 | 11.24 | 8.22 | 7.13 | .416 |
| HADS-Depression | 0-21 | 27 | 2.17 | 2.66 | 17 | 4.88 | 5.48 | 2.71 | .572 |
| MAC | 0-9 | 26 | 1.69 | 2.09 | 17 | 3.76 | 5.33 | 2.07 | .195 |
Abbreviations: FACT-G, Functional Assessment of Cancer Therapy–General; HADS, Hospital Anxiety and Depression Scale; IES-R, Impact of Event Scale–Revised; IRW, illness-related worry; MAC, Mental Adjustment to Cancer Scale; MR, maintenance rituximab; PRO, patient-reported outcome; RR, rituximab re-treatment; SD, standard deviation.
For FACT-G, higher score indicates better health-related quality of life. For all other PROs, higher scores indicate worse outcome (more symptoms).
Fig 2.
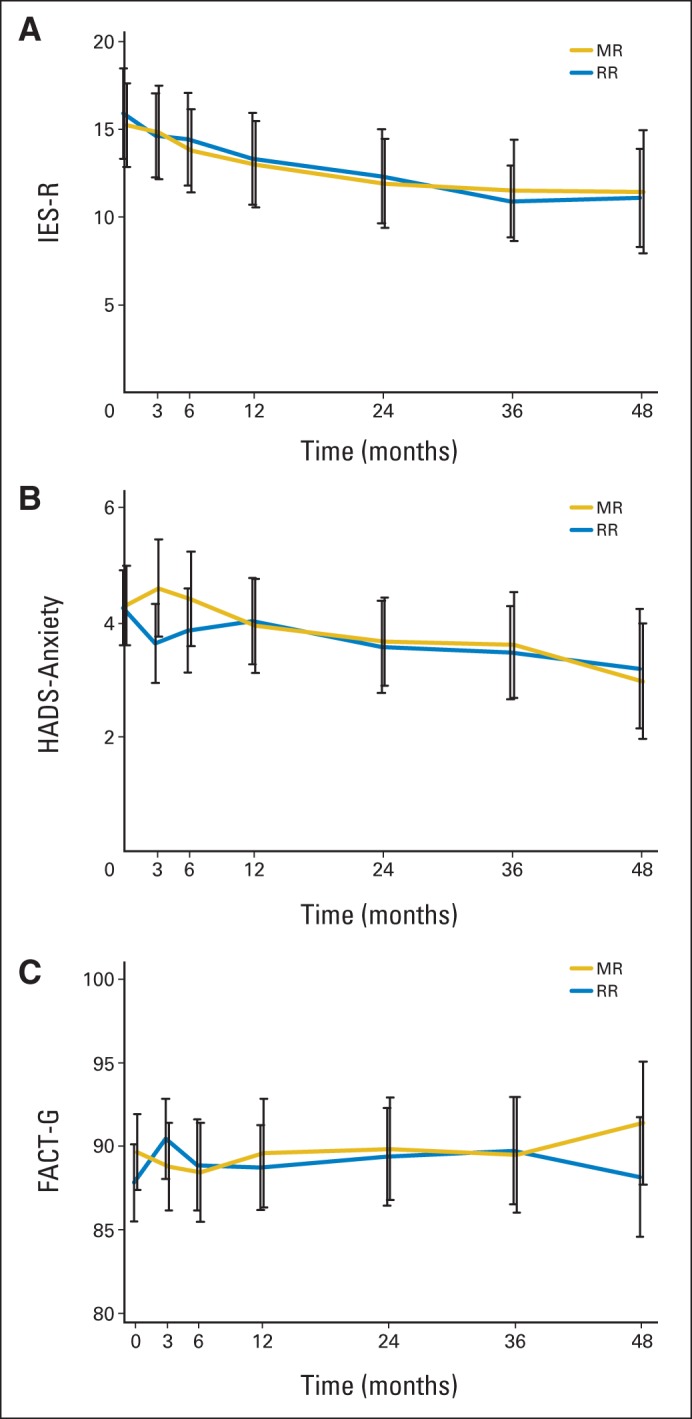
Trajectory of illness-related anxiety and secondary outcomes by treatment arm: (A) Impact of Event Scale–Revised (IES-R), (B) Hospital Anxiety and Depression Scale (HADS) –Anxiety, and (C) Functional Assessment of Cancer Therapy–General (FACT-G) scores. For IES-R, the total score ranges from 0 to 88, and higher scores indicate a higher level of illness-related anxiety. For HADS-Anxiety, the total score ranges from 0 to 28, and higher scores indicate a higher level of general anxiety. The black error bars indicate 95% CIs for the mean scores. For FACT-G, the total score ranges from 0 to 108, and higher scores indicate better quality of life. MR, maintenance rituximab; RR, rituximab re-treatment.
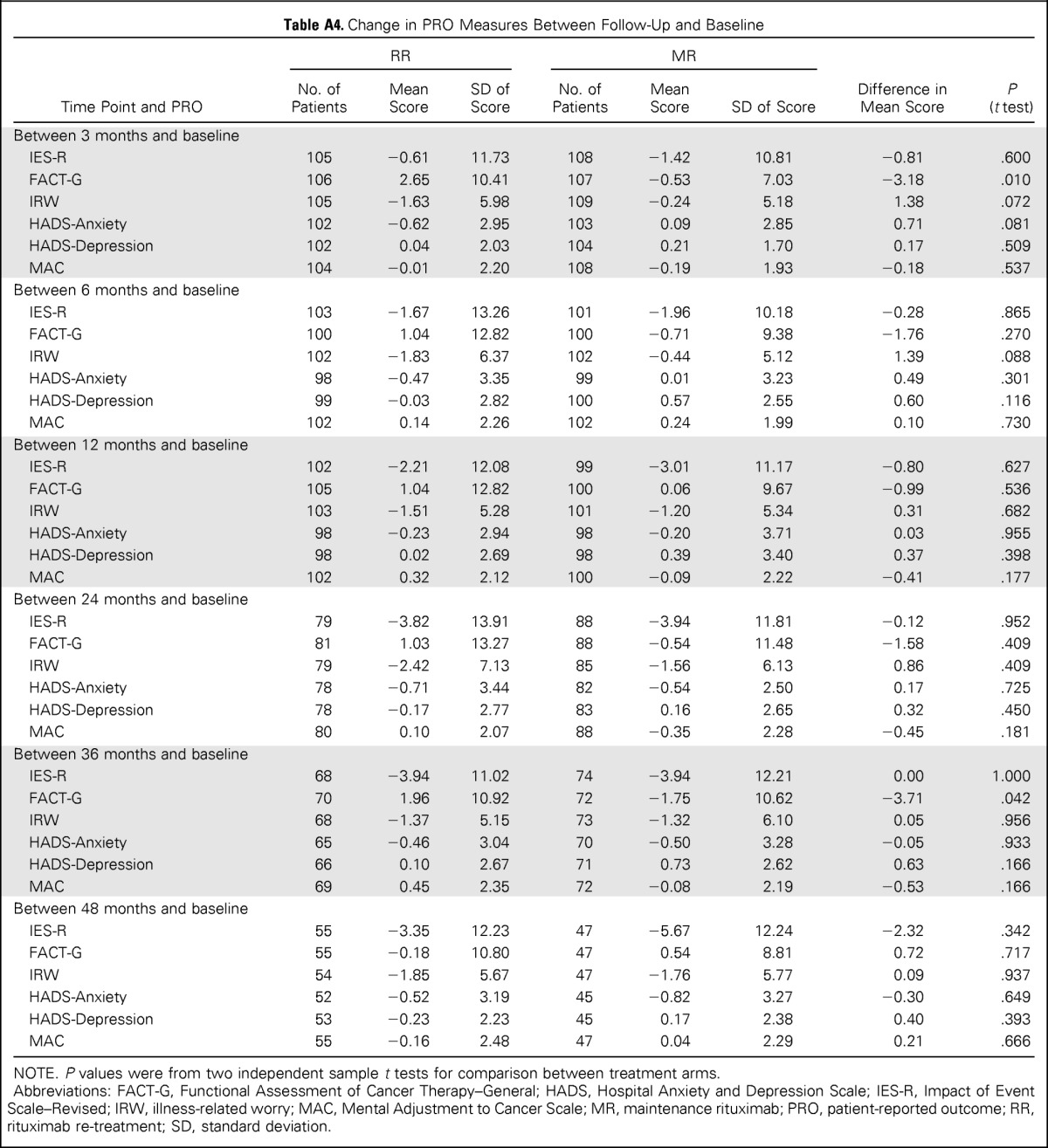
The magnitude of change on PROs from baseline to 6 months was similar for patients on MR and RR (Appendix Table A4, online only). The mean difference in FACT-G change scores (1.76 points) between treatment arms from baseline to 6 months was not statistically significant and below the estimated minimally important difference of 4.2 points.34
The majority of participants (n = 177, 70.0%) were classified as using avoidant illness-related coping. A higher proportion of participants classified as using active illness-related coping versus avoidant coping were randomly assigned to the MR arm (36.0% v 24.2%, respectively; P = .041). Participant groups (active v avoidant) were balanced with regard to demographic and medical characteristics (data not shown). Figure 3 illustrates the trajectory of illness-related anxiety, general anxiety, and HRQoL scores by treatment arm, separated by illness-related coping style (active v avoidant). Overall, the difference between treatment arms did not vary by coping style, and the treatment-by-coping style interaction terms were not statistically significant in the multivariable linear mixed-effects models (Appendix Table A2).
Fig 3.
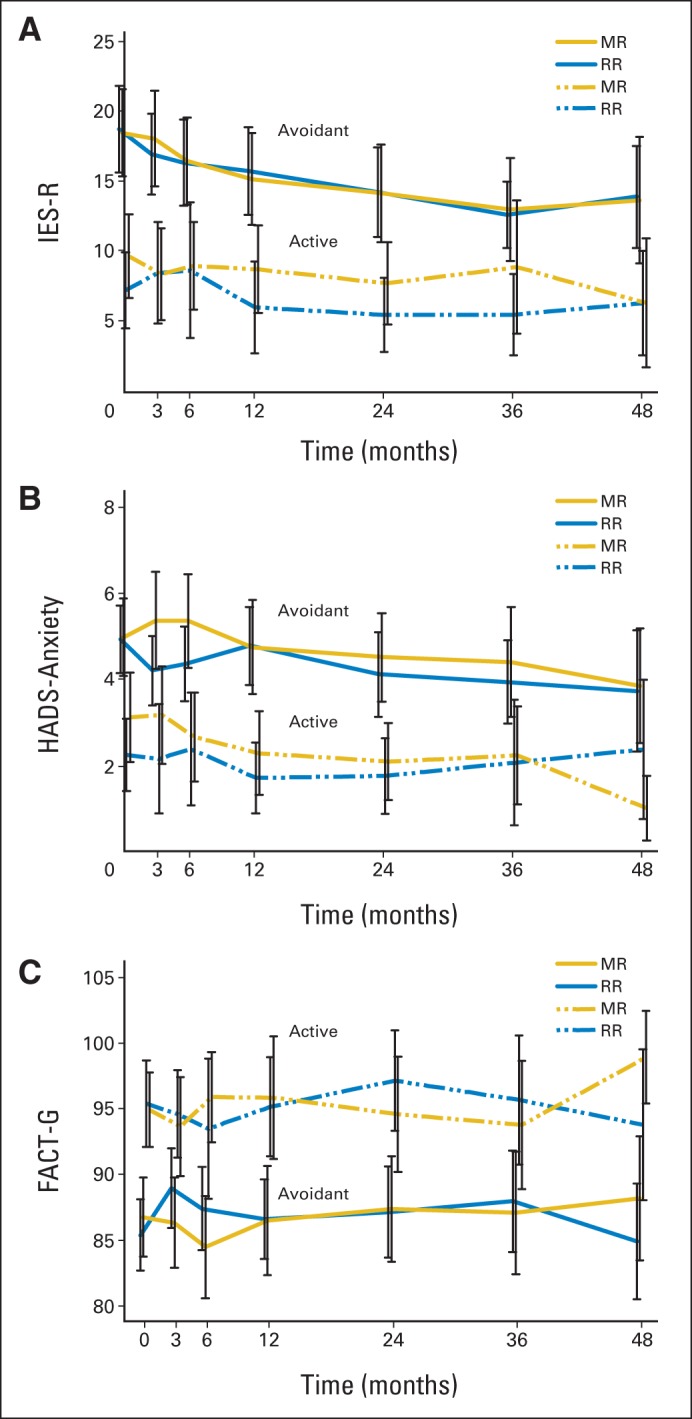
Trajectory of illness-related anxiety and secondary outcomes by treatment arm and coping style: (A) Impact of Event Scale–Revised (IES-R), (B) Hospital Anxiety and Depression Scale (HADS) –Anxiety, and (C) Functional Assessment of Cancer Therapy–General (FACT-G) score. For IES-R, the total score ranges from 0 to 88, and higher scores indicate a higher level of illness-related anxiety. For HADS-Anxiety, the total score ranges from 0 to 21, and higher scores indicate a higher level of general anxiety. The black error bars indicate 95% CIs for the mean scores. For FACT-G, total score ranges from 0 to 108, and higher scores indicate better quality of life. MR, maintenance rituximab; RR, rituximab re-treatment arm.
On both treatment arms, participants classified as avoidant reported significantly higher illness-related anxiety (Fig 3A) and general anxiety (Fig 3B) and poorer HRQoL (Fig 3C) than those classified as using active coping. Avoidant coping was also associated with greater worry about progression and loss of control over disease and higher depression (data not shown). Multivariable liner mixed effects models showed similar results for the comparison between coping styles (coefficient for coping style, 8.25 for IES-R; −2.26 for HADS-Anxiety; and 8.86 for HRQoL; P < .001 for all; Appendix Table A3).
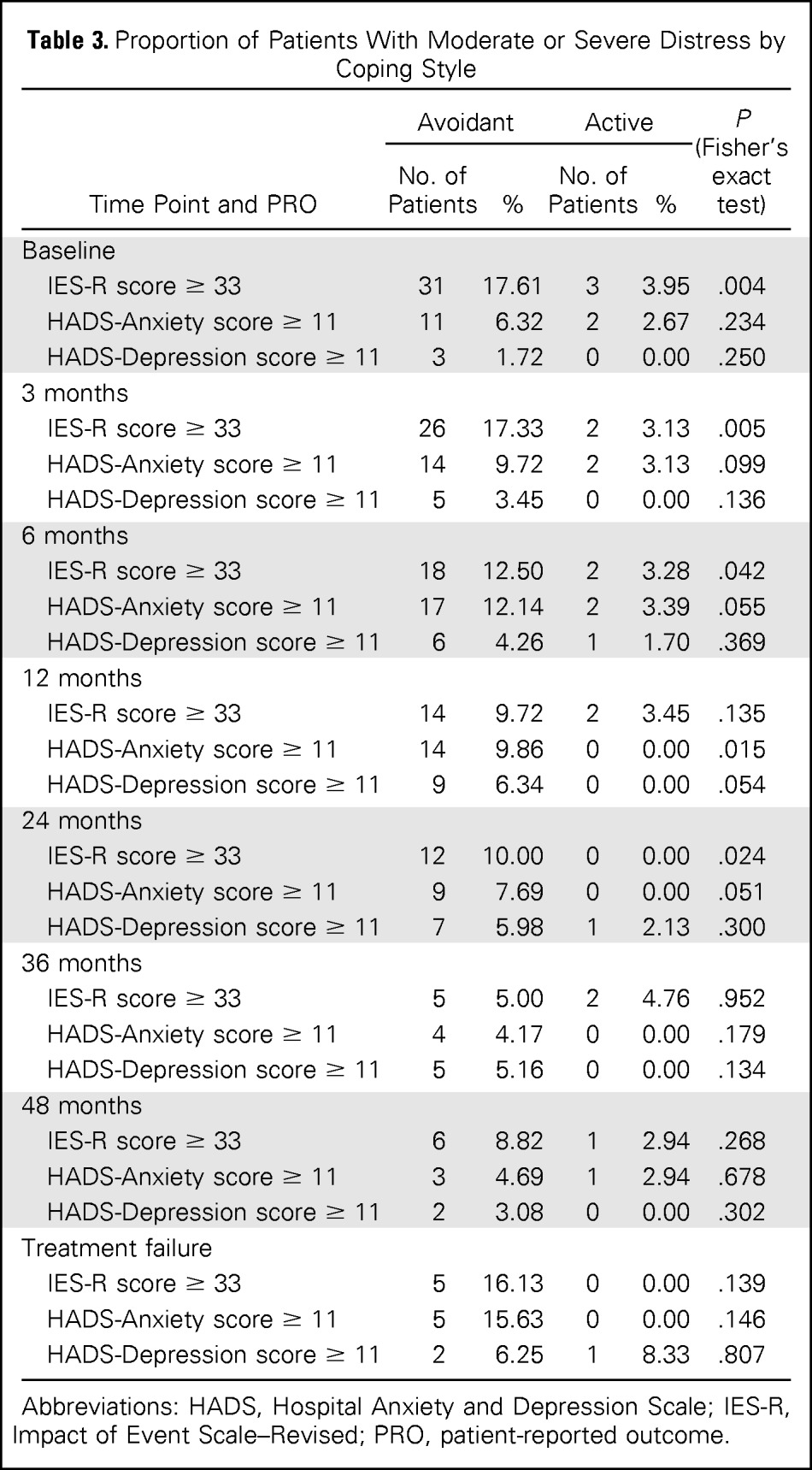
Proportion of Patients With Clinically Significant Anxiety and Depression
A significantly higher proportion of patients classified as using avoidant illness-related coping, compared with patients using active coping, exceeded the established clinical cutoff20 on illness-related anxiety at baseline (17.6% v 4.0%, respectively; P < .01), 3 months (17.3% v 3.1%, respectively; P < .01), 6 months (12.5% v 3.3%, respectively; P < .05), and 24 months (10% v 0%, respectively; P < .05; Table 3). At 12 months, 10% of avoidant copers demonstrated evidence of moderate to severe24 general anxiety (active, 0%; P < .05). At 36 and 48 months, differences between participants based on coping style were less pronounced. There was no significant difference between treatment arms for rates of clinically significant depression.
Table 3.
Proportion of Patients With Moderate or Severe Distress by Coping Style
| Time Point and PRO | Avoidant |
Active |
P (Fisher's exact test) | ||
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||
| Baseline | |||||
| IES-R score ≥ 33 | 31 | 17.61 | 3 | 3.95 | .004 |
| HADS-Anxiety score ≥ 11 | 11 | 6.32 | 2 | 2.67 | .234 |
| HADS-Depression score ≥ 11 | 3 | 1.72 | 0 | 0.00 | .250 |
| 3 months | |||||
| IES-R score ≥ 33 | 26 | 17.33 | 2 | 3.13 | .005 |
| HADS-Anxiety score ≥ 11 | 14 | 9.72 | 2 | 3.13 | .099 |
| HADS-Depression score ≥ 11 | 5 | 3.45 | 0 | 0.00 | .136 |
| 6 months | |||||
| IES-R score ≥ 33 | 18 | 12.50 | 2 | 3.28 | .042 |
| HADS-Anxiety score ≥ 11 | 17 | 12.14 | 2 | 3.39 | .055 |
| HADS-Depression score ≥ 11 | 6 | 4.26 | 1 | 1.70 | .369 |
| 12 months | |||||
| IES-R score ≥ 33 | 14 | 9.72 | 2 | 3.45 | .135 |
| HADS-Anxiety score ≥ 11 | 14 | 9.86 | 0 | 0.00 | .015 |
| HADS-Depression score ≥ 11 | 9 | 6.34 | 0 | 0.00 | .054 |
| 24 months | |||||
| IES-R score ≥ 33 | 12 | 10.00 | 0 | 0.00 | .024 |
| HADS-Anxiety score ≥ 11 | 9 | 7.69 | 0 | 0.00 | .051 |
| HADS-Depression score ≥ 11 | 7 | 5.98 | 1 | 2.13 | .300 |
| 36 months | |||||
| IES-R score ≥ 33 | 5 | 5.00 | 2 | 4.76 | .952 |
| HADS-Anxiety score ≥ 11 | 4 | 4.17 | 0 | 0.00 | .179 |
| HADS-Depression score ≥ 11 | 5 | 5.16 | 0 | 0.00 | .134 |
| 48 months | |||||
| IES-R score ≥ 33 | 6 | 8.82 | 1 | 2.94 | .268 |
| HADS-Anxiety score ≥ 11 | 3 | 4.69 | 1 | 2.94 | .678 |
| HADS-Depression score ≥ 11 | 2 | 3.08 | 0 | 0.00 | .302 |
| Treatment failure | |||||
| IES-R score ≥ 33 | 5 | 16.13 | 0 | 0.00 | .139 |
| HADS-Anxiety score ≥ 11 | 5 | 15.63 | 0 | 0.00 | .146 |
| HADS-Depression score ≥ 11 | 2 | 6.25 | 1 | 8.33 | .807 |
Abbreviations: HADS, Hospital Anxiety and Depression Scale; IES-R, Impact of Event Scale–Revised; PRO, patient-reported outcome.
DISCUSSION
For most malignancies, progression events are associated with cancer-related symptoms and/or shortened survival. As a result, strategies that delay disease recurrence (ie, maintenance) are generally considered beneficial for patients. Indolent NHL is unique, however. Progression events are typically not associated with disease-related symptoms or shortened survival. Why then, have maintenance strategies become so common in these diseases? It is probably because the agent used (rituximab) is extremely well tolerated and has been shown in several trials to improve progression-free survival.6,33,35 Because it is commonly assumed that relapse will generate anxiety, it is reasonable to conclude that strategies that delay relapse (eg, maintenance) should diminish anxiety and improve HRQoL. Consistent with these assumptions are the results from an international trial comparing rituximab maintenance with a watch-and-wait approach for patients with asymptomatic low–tumor burden follicular lymphoma.16 Patients receiving rituximab felt more in control of their disease, had less negative associations with hospital visits, and were less likely to avoid thinking about their disease.
The results from RESORT suggest that an alternative treatment strategy can produce a similar patient-centered outcome. In our study, patients randomly assigned to rituximab at progression reported a similar level of illness-related anxiety, general anxiety, and HRQoL compared with patients receiving MR every 3 months. This result holds even when taking illness-related coping style (active v avoidant) into account. Surveillance until RR at progression was not associated with increased anxiety compared with MR, even among participants who reported emotional benefit from receiving medical care.
We find these results instructive. Patients assigned to a watch-and-wait strategy in the international trial faced the prospect of observation until their disease reached a high–tumor burden state and were then likely managed with immunochemotherapy. Patients assigned to observation and RR in the RESORT trial were to be immediately re-treated with single-agent rituximab at progression. Our data suggest that relapse is not automatically associated with anxiety, if the recurrence will be rapidly addressed with a well-tolerated therapy. The comparison of the two trials is appropriate, because the patient populations were similar and, by design, both trials used the same PRO measures.
We also found that patients who endorsed avoidant coping are at increased risk of illness-related anxiety, general anxiety, and lower HRQoL regardless of rituximab treatment schedule. These individuals may benefit from learning active strategies for managing anxiety and worry about progression. Psychosocial interventions, specifically cognitive-behavioral interventions, are highly successful in reducing anxiety and illness-related distress among survivors of cancer.36–40 Minimizing the influence of patient anxiety on medical decision making will likely enhance patient-provider communication and optimize outcomes by reducing the risk of oversurveillance and overtreatment, a growing concern in oncology care.41,42
There are some limitations to our data. PRO measures were added 18 months after RESORT initiation, meaning not all patients participated. However, patients with PRO data had similar demographic and medical characteristics as the entire RESORT sample. Once added to RESORT, PRO measures were required from all participants to minimize selection bias. We recognize that missing PRO data at follow-up imposes a bias. We collected explanations for missing data to facilitate interpretation of results. Overall, the proportion of missing data was comparable to similar trials.
The RESORT results have significant implications for clinical care. For patients, not treating a malignant condition is counterintuitive.43 However, for patients with follicular NHL, the RESORT trial has demonstrated that MR provides no clinical benefit over RR but requires approximately four times as much rituximab.17 Taken in tandem with findings from this study, clinicians can choose RR over MR and expect similar patient-centered outcomes while saving resources and achieving similar clinical outcomes.
Acknowledgment
Presented at the 54th Annual Meeting of the American Society of Hematology, Atlanta, GA, December 8-11, 2012. This study was conducted by the Eastern Cooperative Oncology Group–American College of Radiology Imaging Network (ECOG-ACRIN) Cancer Research Group (Robert L. Comis, MD, and Mitchell D. Schnall, MD, PhD, group co-chairs).
Glossary Terms
- health-related quality of life (HRQoL):
a broad multidimensional concept that usually includes self-reported measures of physical and mental health.
- patient-reported outcomes:
questionnaires used in a clinical setting to systemically collect information directly from the patient.
Appendix
Table A1.
Patient Demographics and Clinical Characteristics by Complete and Missing Data
| Demographic or Clinical Characteristic | Patients With PRO Data at Baseline (n = 253) |
Patients Without 6-Month Data (n = 48) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Patients Without 6-Month PRO Data (n = 48) |
Patients With 6-Month PRO Data (n = 205) |
P | RR Arm |
MR Arm |
|||||
| No. | % | No. | % | No. | % | No. | % | ||
| Age at registration, years | .013 | ||||||||
| Mean | 63.44 | 58.83 | 65.19 | 61.54 | |||||
| SD | 12.82 | 11.12 | 11.88 | 13.79 | |||||
| Sex | .277 | ||||||||
| Male | 25 | 52.1 | 89 | 43.4 | 14 | 56.0 | 11 | 47.8 | |
| Female | 23 | 47.9 | 116 | 56.6 | 11 | 44.0 | 12 | 52.2 | |
| Race | .378 | ||||||||
| White | 46 | 97.9 | 195 | 98.0 | 23 | 95.8 | 23 | 100.0 | |
| Black | 0 | 0.0 | 3 | 1.5 | 0 | 0.0 | 0 | 0.0 | |
| Other | 1 | 2.1 | 1 | 0.5 | 1 | 4.2 | 0 | 0.0 | |
| Ethnicity | .579 | ||||||||
| Hispanic | 1 | 2.2 | 2 | 1.1 | 0 | 0.0 | 1 | 4.6 | |
| Non-Hispanic | 44 | 97.8 | 181 | 97.3 | 23 | 100.0 | 21 | 95.5 | |
| Unknown | 0 | 0.0 | 3 | 1.6 | 0 | 0.0 | 0 | 0.0 | |
| ECOG PS | .089 | ||||||||
| 0 | 37 | 77.1 | 178 | 86.8 | 18 | 72.0 | 19 | 82.6 | |
| 1 | 11 | 22.9 | 27 | 13.2 | 7 | 28.0 | 4 | 17.4 | |
| LDH | .363 | ||||||||
| Normal | 43 | 89.6 | 168 | 84.4 | 23 | 92.0 | 20 | 87.0 | |
| High | 5 | 10.4 | 31 | 15.6 | 2 | 8.0 | 3 | 13.0 | |
| Prior treatment | .628 | ||||||||
| No | 48 | 100.0 | 204 | 99.5 | 25 | 100.0 | 23 | 100.0 | |
| Yes | 0 | 0.0 | 1 | 0.5 | 0 | 0.0 | 0 | 0.0 | |
| FLIPI score | .724 | ||||||||
| 0 | 1 | 2.1 | 1 | 0.5 | 0 | 0.0 | 1 | 4.4 | |
| 1 | 4 | 8.3 | 26 | 12.7 | 2 | 8.0 | 2 | 8.7 | |
| 2 | 22 | 45.8 | 103 | 50.2 | 13 | 52.0 | 9 | 39.1 | |
| 3 | 17 | 35.4 | 61 | 29.8 | 8 | 32.0 | 9 | 39.1 | |
| 4 | 3 | 6.3 | 12 | 5.9 | 1 | 4.0 | 2 | 8.7 | |
| 5 | 1 | 2.1 | 2 | 1.0 | 1 | 4.0 | 0 | 0.0 | |
| Disease status at registration | .848 | ||||||||
| Initial diagnosis | 47 | 97.9 | 201 | 98.1 | 25 | 100.0 | 22 | 95.7 | |
| Relapsed | 1 | 2.1 | 3 | 1.5 | 0 | 0.0 | 1 | 4.4 | |
| Refractory | 0 | 0.0 | 1 | 0.5 | 0 | 0.0 | 0 | 0.0 | |
| Modified Ann Arbor stage at study entry | .257 | ||||||||
| I-II | 2 | 4.2 | 2 | 1.0 | 1 | 4.0 | 1 | 4.4 | |
| III | 22 | 45.8 | 104 | 50.7 | 11 | 44.0 | 11 | 47.8 | |
| IV | 24 | 50.0 | 99 | 48.3 | 13 | 52.0 | 11 | 47.8 | |
| Stratification factor: histology | .005 | ||||||||
| Follicular | 27 | 69.2 | 164 | 87.2 | 12 | 57.1 | 15 | 83.3 | |
| Other | 12 | 30.8 | 24 | 12.8 | 9 | 42.9 | 3 | 16.7 | |
| Stratification factor: time from diagnosis, years | .264 | ||||||||
| < 1 | 38 | 97.4 | 174 | 92.6 | 21 | 100.0 | 17 | 94.4 | |
| ≥ 1 | 1 | 2.6 | 14 | 7.5 | 0 | 0.0 | 1 | 5.6 | |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; FLIPI, Follicular Lymphoma International Prognostic Index; LDH, lactate dehydrogenase; MR, maintenance rituximab; PRO, patient-reported outcome; RR, rituximab re-treatment; SD, standard deviation.
Table A2.
Interaction Test in Multivariable Mixed-Effects Models
| Interaction Test and PRO | Coefficient for Interaction Term | 95% CI | P |
|---|---|---|---|
| Time-by-treatment | |||
| IES-R | 0.009 | −0.062 to 0.081 | .797 |
| FACT-G | −0.002 | −0.080 to 0.076 | .964 |
| HADS-Anxiety | −0.006 | −0.025 to 0.014 | .587 |
| Treatment-by-coping style | |||
| IES-R | 2.477 | −3.272 to 8.226 | .398 |
| FACT-G | −0.139 | −6.847 to 6.569 | .968 |
| HADS-Anxiety | 0.647 | −1.100 to 2.394 | .468 |
NOTE. In addition to treatment, coping style, and time variables, other covariates included in the models were age, sex, Eastern Cooperative Oncology Group performance status, lactate dehydrogenase, Follicular Lymphoma International Prognostic Index score, and modified Ann Arbor stage.
Abbreviations: FACT-G, Functional Assessment of Cancer Therapy–General; HADS, Hospital Anxiety and Depression Scale; IES-R, Impact of Event Scale–Revised; PRO, patient-reported outcome.
Table A3.
Multivariable Mixed-Effects Models for PROs
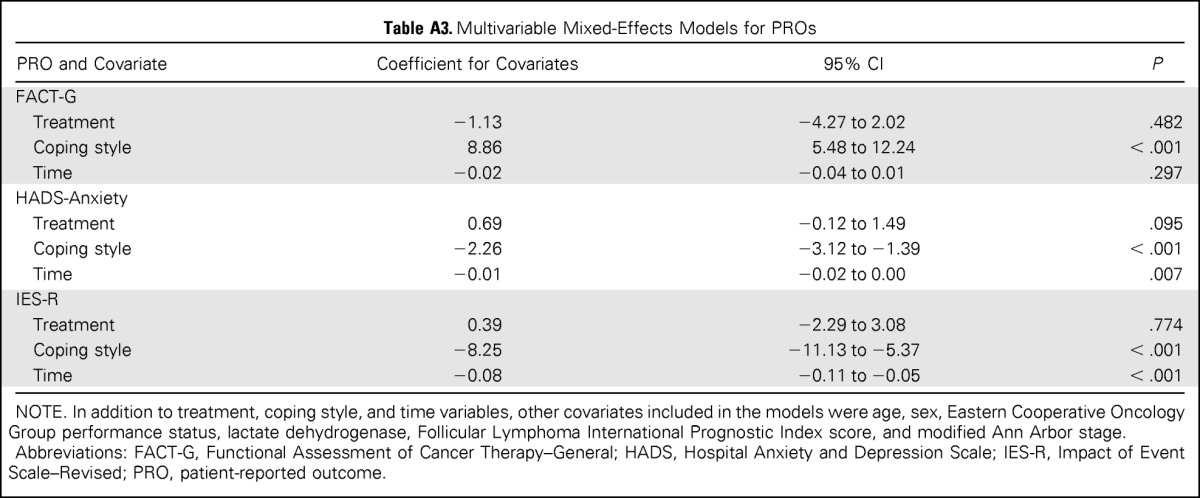
| PRO and Covariate | Coefficient for Covariates | 95% CI | P |
|---|---|---|---|
| FACT-G | |||
| Treatment | −1.13 | −4.27 to 2.02 | .482 |
| Coping style | 8.86 | 5.48 to 12.24 | < .001 |
| Time | −0.02 | −0.04 to 0.01 | .297 |
| HADS-Anxiety | |||
| Treatment | 0.69 | −0.12 to 1.49 | .095 |
| Coping style | −2.26 | −3.12 to −1.39 | < .001 |
| Time | −0.01 | −0.02 to 0.00 | .007 |
| IES-R | |||
| Treatment | 0.39 | −2.29 to 3.08 | .774 |
| Coping style | −8.25 | −11.13 to −5.37 | < .001 |
| Time | −0.08 | −0.11 to −0.05 | < .001 |
NOTE. In addition to treatment, coping style, and time variables, other covariates included in the models were age, sex, Eastern Cooperative Oncology Group performance status, lactate dehydrogenase, Follicular Lymphoma International Prognostic Index score, and modified Ann Arbor stage.
Abbreviations: FACT-G, Functional Assessment of Cancer Therapy–General; HADS, Hospital Anxiety and Depression Scale; IES-R, Impact of Event Scale–Revised; PRO, patient-reported outcome.
Table A4.
Change in PRO Measures Between Follow-Up and Baseline
| Time Point and PRO | RR |
MR |
Difference in Mean Score | P (t test) | ||||
|---|---|---|---|---|---|---|---|---|
| No. of Patients | Mean Score | SD of Score | No. of Patients | Mean Score | SD of Score | |||
| Between 3 months and baseline | ||||||||
| IES-R | 105 | −0.61 | 11.73 | 108 | −1.42 | 10.81 | −0.81 | .600 |
| FACT-G | 106 | 2.65 | 10.41 | 107 | −0.53 | 7.03 | −3.18 | .010 |
| IRW | 105 | −1.63 | 5.98 | 109 | −0.24 | 5.18 | 1.38 | .072 |
| HADS-Anxiety | 102 | −0.62 | 2.95 | 103 | 0.09 | 2.85 | 0.71 | .081 |
| HADS-Depression | 102 | 0.04 | 2.03 | 104 | 0.21 | 1.70 | 0.17 | .509 |
| MAC | 104 | −0.01 | 2.20 | 108 | −0.19 | 1.93 | −0.18 | .537 |
| Between 6 months and baseline | ||||||||
| IES-R | 103 | −1.67 | 13.26 | 101 | −1.96 | 10.18 | −0.28 | .865 |
| FACT-G | 100 | 1.04 | 12.82 | 100 | −0.71 | 9.38 | −1.76 | .270 |
| IRW | 102 | −1.83 | 6.37 | 102 | −0.44 | 5.12 | 1.39 | .088 |
| HADS-Anxiety | 98 | −0.47 | 3.35 | 99 | 0.01 | 3.23 | 0.49 | .301 |
| HADS-Depression | 99 | −0.03 | 2.82 | 100 | 0.57 | 2.55 | 0.60 | .116 |
| MAC | 102 | 0.14 | 2.26 | 102 | 0.24 | 1.99 | 0.10 | .730 |
| Between 12 months and baseline | ||||||||
| IES-R | 102 | −2.21 | 12.08 | 99 | −3.01 | 11.17 | −0.80 | .627 |
| FACT-G | 105 | 1.04 | 12.82 | 100 | 0.06 | 9.67 | −0.99 | .536 |
| IRW | 103 | −1.51 | 5.28 | 101 | −1.20 | 5.34 | 0.31 | .682 |
| HADS-Anxiety | 98 | −0.23 | 2.94 | 98 | −0.20 | 3.71 | 0.03 | .955 |
| HADS-Depression | 98 | 0.02 | 2.69 | 98 | 0.39 | 3.40 | 0.37 | .398 |
| MAC | 102 | 0.32 | 2.12 | 100 | −0.09 | 2.22 | −0.41 | .177 |
| Between 24 months and baseline | ||||||||
| IES-R | 79 | −3.82 | 13.91 | 88 | −3.94 | 11.81 | −0.12 | .952 |
| FACT-G | 81 | 1.03 | 13.27 | 88 | −0.54 | 11.48 | −1.58 | .409 |
| IRW | 79 | −2.42 | 7.13 | 85 | −1.56 | 6.13 | 0.86 | .409 |
| HADS-Anxiety | 78 | −0.71 | 3.44 | 82 | −0.54 | 2.50 | 0.17 | .725 |
| HADS-Depression | 78 | −0.17 | 2.77 | 83 | 0.16 | 2.65 | 0.32 | .450 |
| MAC | 80 | 0.10 | 2.07 | 88 | −0.35 | 2.28 | −0.45 | .181 |
| Between 36 months and baseline | ||||||||
| IES-R | 68 | −3.94 | 11.02 | 74 | −3.94 | 12.21 | 0.00 | 1.000 |
| FACT-G | 70 | 1.96 | 10.92 | 72 | −1.75 | 10.62 | −3.71 | .042 |
| IRW | 68 | −1.37 | 5.15 | 73 | −1.32 | 6.10 | 0.05 | .956 |
| HADS-Anxiety | 65 | −0.46 | 3.04 | 70 | −0.50 | 3.28 | −0.05 | .933 |
| HADS-Depression | 66 | 0.10 | 2.67 | 71 | 0.73 | 2.62 | 0.63 | .166 |
| MAC | 69 | 0.45 | 2.35 | 72 | −0.08 | 2.19 | −0.53 | .166 |
| Between 48 months and baseline | ||||||||
| IES-R | 55 | −3.35 | 12.23 | 47 | −5.67 | 12.24 | −2.32 | .342 |
| FACT-G | 55 | −0.18 | 10.80 | 47 | 0.54 | 8.81 | 0.72 | .717 |
| IRW | 54 | −1.85 | 5.67 | 47 | −1.76 | 5.77 | 0.09 | .937 |
| HADS-Anxiety | 52 | −0.52 | 3.19 | 45 | −0.82 | 3.27 | −0.30 | .649 |
| HADS-Depression | 53 | −0.23 | 2.23 | 45 | 0.17 | 2.38 | 0.40 | .393 |
| MAC | 55 | −0.16 | 2.48 | 47 | 0.04 | 2.29 | 0.21 | .666 |
NOTE. P values were from two independent sample t tests for comparison between treatment arms.
Abbreviations: FACT-G, Functional Assessment of Cancer Therapy–General; HADS, Hospital Anxiety and Depression Scale; IES-R, Impact of Event Scale–Revised; IRW, illness-related worry; MAC, Mental Adjustment to Cancer Scale; MR, maintenance rituximab; PRO, patient-reported outcome; RR, rituximab re-treatment; SD, standard deviation.
Support information appears at the end of this article.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Support
Supported in part by Public Health Service Grants No. CA21115, CA23318, CA66636, CA49957, CA21076, C47145, C43650, CA035267, C46116, CA21076, C45488, and C43650 from the National Cancer Institute, National Institutes of Health, and the Department of Health and Human Services.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Lynne I. Wagner, Michael E. Williams, John C. Krauss, David Cella, Sandra J. Horning, Brad S. Kahl
Provision of study materials or patients: Lynne I. Wagner, Michael E. Williams, Ranjana H. Advani, Ronald S. Go, Thomas M. Habermann, Joseph W. Leach, Brian O'Connor, Stephen J. Schuster, Sandra J. Horning, Brad S. Kahl
Collection and assembly of data: Lynne I. Wagner, Michael E. Williams, Ranjana H. Advani, Ronald S. Go, Thomas M. Habermann, Joseph W. Leach, Brian O'Connor, Stephen J. Schuster, Sandra J. Horning, Brad S. Kahl
Data analysis and interpretation: Lynne I. Wagner, Fengmin Zhao, Fangxin Hong, Michael E. Williams, Randy D. Gascoyne, David Cella, Brad S. Kahl
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Anxiety and Health-Related Quality of Life Among Patients With Low–Tumor Burden Non-Hodgkin Lymphoma Randomly Assigned to Two Different Rituximab Dosing Regimens: Results From ECOG Trial E4402 (RESORT)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Lynne I. Wagner
Consulting or Advisory Role: Gilead Sciences
Fengmin Zhao
No relationship to disclose
Fangxin Hong
No relationship to disclose
Michael E. Williams
Consulting or Advisory Role: Genentech/Roche
Research Funding: Genentech/Roche (Inst)
Randy D. Gascoyne
Consulting or Advisory Role: Genentech/Roche
Speakers' Bureau: Seattle Genetics
John C. Krauss
No relationship to disclose
Ranjana H. Advani
Honoraria: Millennium Takeda, General Electric/Clarient
Research Funding: Millennium Takeda, Seattle Genetics, Genentech/Roche, Allos Therapeutics, Pharmacyclics, Janssen Pharmaceuticals, Celgene
Travel, Accommodations, Expenses: Genentech, Seattle Genetics
Ronald S. Go
No relationship to disclose
Thomas M. Habermann
No relationship to disclose
Joseph W. Leach
Consulting or Advisory Role: Biodesix
Brian O'Connor
No relationship to disclose
Stephen J. Schuster
No relationship to disclose
David Cella
No relationship to disclose
Sandra J. Horning
Employment: Genentech/Roche
Leadership: Genentech/Roche
Stock or Other Ownership: Roche/Genentech
Brad S. Kahl
Consulting or Advisory Role: Genentech/Roche
Research Funding: Genentech/Roche
REFERENCES
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. Cancer. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Voliotis D, Diehl V. Challenges in treating hematologic malignancies. Semin Oncol. 2002;29:30–39. doi: 10.1053/sonc.2002.33531. [DOI] [PubMed] [Google Scholar]
- 3.Foster T, Miller JD, Boye ME, et al. Economic burden of follicular non-Hodgkin's lymphoma. Pharmacoeconomics. 2009;27:657–679. doi: 10.2165/11314820-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Smith SK, Zimmerman S, Williams CS, et al. Health status and quality of life among non-Hodgkin lymphoma survivors. Cancer. 2009;115:3312–3323. doi: 10.1002/cncr.24391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith SK, Mayer DK, Zimmerman S, et al. Quality of life among long-term survivors of non-Hodgkin lymphoma: A follow-up study. J Clin Oncol. 2013;31:272–279. doi: 10.1200/JCO.2011.40.6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hochster H, Weller E, Gascoyne RD, et al. Maintenance rituximab after cyclophosphamide, vincristine, and prednisone prolongs progression-free survival in advanced indolent lymphoma: Results of the randomized phase III ECOG1496 Study. J Clin Oncol. 2009;27:1607–1614. doi: 10.1200/JCO.2008.17.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oerlemans S, Mols F, Issa DE, et al. A high level of fatigue among long-term survivors of non-Hodgkin's lymphoma: Results from the longitudinal population-based PROFILES registry in the south of the Netherlands. Haematologica. 2013;98:479–486. doi: 10.3324/haematol.2012.064907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen RE, Arora NK, Bellizzi KM, et al. Health-related quality of life among survivors of aggressive non-Hodgkin lymphoma. Cancer. 2013;119:672–680. doi: 10.1002/cncr.27781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith SK, Crespi CM, Petersen L, et al. The impact of cancer and quality of life for post-treatment non-Hodgkin lymphoma survivors. Psychooncology. 2010;19:1259–1267. doi: 10.1002/pon.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pettengell R, Donatti C, Hoskin P, et al. The impact of follicular lymphoma on health-related quality of life. Ann Oncol. 2008;19:570–576. doi: 10.1093/annonc/mdm543. [DOI] [PubMed] [Google Scholar]
- 11.Allart P, Soubeyran P, Cousson-Gélie F. Are psychosocial factors associated with quality of life in patients with haematological cancer? A critical review of the literature. Psychooncology. 2013;22:241–249. doi: 10.1002/pon.3026. [DOI] [PubMed] [Google Scholar]
- 12.Smith SK, Zimmerman S, Williams CS, et al. Post-traumatic stress outcomes in non-Hodgkin's lymphoma survivors. J Clin Oncol. 2008;26:934–941. doi: 10.1200/JCO.2007.12.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montgomery C, Pocock M, Titley K, et al. Individual quality of life in patients with leukaemia and lymphoma. Psychooncology. 2002;11:239–243. doi: 10.1002/pon.557. [DOI] [PubMed] [Google Scholar]
- 14.Montgomery C, Pocock M, Titley K, et al. Predicting psychological distress in patients with leukaemia and lymphoma. J Psychosom Res. 2003;54:289–292. doi: 10.1016/s0022-3999(02)00396-3. [DOI] [PubMed] [Google Scholar]
- 15.Witzens-Harig M, Reiz M, Heiss C, et al. Quality of life during maintenance therapy with the anti-CD20 antibody rituximab in patients with B cell non-Hodgkin's lymphoma: Results of a prospective randomized controlled trial. Ann Hematol. 2009;88:51–57. doi: 10.1007/s00277-008-0560-2. [DOI] [PubMed] [Google Scholar]
- 16.Ardeshna KM, Qian W, Smith P, et al. Rituximab versus a watch-and-wait approach in patients with advanced-stage, asymptomatic, non-bulky follicular lymphoma: An open-label randomised phase 3 trial. Lancet Oncol. 2014;15:424–435. doi: 10.1016/S1470-2045(14)70027-0. [DOI] [PubMed] [Google Scholar]
- 17.Kahl BS, Hong F, Williams ME, et al. Rituximab Extended Schedule or Retreatment Trial (RESORT) for low tumor burden follicular lymphoma: Eastern Cooperative Oncology Group Protocol E4402. J Clin Oncol. 2014;32:3096–3102. doi: 10.1200/JCO.2014.56.5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calvert M, Blazeby J, Altman DG, et al. Reporting of patient-reported outcomes in randomized trials: The CONSORT PRO extension. JAMA. 2013;309:814–822. doi: 10.1001/jama.2013.879. [DOI] [PubMed] [Google Scholar]
- 19.Williams ME, Hong F, Kahl BS, et al. A subgroup analysis of small lymphocytic and marginal zone lymphomas in the Eastern Cooperative Oncology Group protocol E4402 (RESORT): A randomized phase III study comparing two different rituximab dosing strategies for low tumor burden indolent non-Hodgkin lymphoma. J Clin Oncol. 2012;(suppl):30. abstr 8007. [Google Scholar]
- 20.Weiss D, Marmar C. The Impact of Events Scale-Revised. In: Wilson J, Keane T, editors. Assessing Psychological Trauma and PTSD (ed 2) New York, NY: Guilford Press; 2004. pp. 168–189. [Google Scholar]
- 21.Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: A measure of subjective stress. Psychosom Med. 1979;41:209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Folkman S, Lazarus RS. The relationship between coping and emotion: Implications for theory and research. Soc Sci Med. 1988;26:309–317. doi: 10.1016/0277-9536(88)90395-4. [DOI] [PubMed] [Google Scholar]
- 23.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 24.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 25.Moorey S, Greer S, Watson M, et al. The factor structure and factor stability of the Hospital Anxiety and Depression Scale in patients with cancer. Br J Psychiatry. 1991;158:255–259. doi: 10.1192/bjp.158.2.255. [DOI] [PubMed] [Google Scholar]
- 26.Hlubocky FJ, Webster K, Cashy J, et al. The development and validation of a measure of health-related quality of life for non-Hodgkin's lymphoma: The Functional Assessment of Cancer Therapy-Lymphoma (FACT-Lym) Lymphoma. 2013;2013:1–9. [Google Scholar]
- 27.Lai JS, Garcia SF, Salsman JM, et al. The psychosocial impact of cancer: Evidence in support of independent general positive and negative components. Qual Life Res. 2012;21:195–207. doi: 10.1007/s11136-011-9935-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watson M, Law M, dos Santos M, et al. The Mini-MAC: Further development of the mental adjustment to cancer scale. J Psychosoc Oncol. 1994;12:33–46. [Google Scholar]
- 29.Osborne RH, Elsworth GR, Kissane DW, et al. The Mental Adjustment to Cancer (MAC) scale: Replication and refinement in 632 breast cancer patients. Psychol Med. 1999;29:1335–1345. doi: 10.1017/s0033291799001142. [DOI] [PubMed] [Google Scholar]
- 30.Cella D. Elmhurst, IL: FACIT.org; 1997. Manual of the Functional Assessment of Chronic Illness Therapy (FACIT Scales) Version 4. [Google Scholar]
- 31.Webster K, Cashy J, Cella D, et al. Measuring quality of life (QOL) among patients with non-Hodgkin's lymphoma (NHL): The Functional Assessment of Cancer Therapy-Lymphoma (FACT-LYM) Qual Life Res. 2005;14:2103. [Google Scholar]
- 32.Yost KJ, Thompson CA, Eton DT, et al. The Functional Assessment of Cancer Therapy-General (FACT-G) is valid for monitoring quality of life in patients with non-Hodgkin lymphoma. Leuk Lymphoma. 2013;54:290–297. doi: 10.3109/10428194.2012.711830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghielmini M, Schmitz SF, Cogliatti SB, et al. Prolonged treatment with rituximab in patients with follicular lymphoma significantly increases event-free survival and response duration compared with the standard weekly x 4 schedule. Blood. 2004;103:4416–4423. doi: 10.1182/blood-2003-10-3411. [DOI] [PubMed] [Google Scholar]
- 34.Yost KJ, Eton DT. Combining distribution- and anchor-based approaches to determine minimally important differences: The FACIT experience. Eval Health Prof. 2005;28:172–191. doi: 10.1177/0163278705275340. [DOI] [PubMed] [Google Scholar]
- 35.Salles G, Seymour JF, Offner F, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): A phase 3, randomised controlled trial. Lancet. 2011;377:42–51. doi: 10.1016/S0140-6736(10)62175-7. [DOI] [PubMed] [Google Scholar]
- 36.Stanton AL. Psychosocial concerns and interventions for cancer survivors. J Clin Oncol. 2006;24:5132–5137. doi: 10.1200/JCO.2006.06.8775. [DOI] [PubMed] [Google Scholar]
- 37.Jacobsen PB, Jim HS. Psychosocial interventions for anxiety and depression in adult cancer patients: Achievements and challenges. CA Cancer J Clin. 2008;58:214–230. doi: 10.3322/CA.2008.0003. [DOI] [PubMed] [Google Scholar]
- 38.Antoni MH, Wimberly SR, Lechner SC, et al. Reduction of cancer-specific thought intrusions and anxiety symptoms with a stress management intervention among women undergoing treatment for breast cancer. Am J Psychiatry. 2006;163:1791–1797. doi: 10.1176/ajp.2006.163.10.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Traeger L, Greer JA, Fernandez-Robles C, et al. Evidence-based treatment of anxiety in patients with cancer. J Clin Oncol. 2012;30:1197–1205. doi: 10.1200/JCO.2011.39.5632. [DOI] [PubMed] [Google Scholar]
- 40.Leykin Y, Thekdi SM, Shumay DM, et al. Internet interventions for improving psychological well-being in psycho-oncology: Review and recommendations. Psychooncology. 2012;21:1016–1025. doi: 10.1002/pon.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schnipper LE, Smith TJ, Raghavan D, et al. American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: The top five list for oncology. J Clin Oncol. 2012;30:1715–1724. doi: 10.1200/JCO.2012.42.8375. [DOI] [PubMed] [Google Scholar]
- 42.Schnipper LE, Lyman GH, Blayney DW, et al. American Society of Clinical Oncology 2013 top five list in oncology. J Clin Oncol. 2013;31:4362–4370. doi: 10.1200/JCO.2013.53.3943. [DOI] [PubMed] [Google Scholar]
- 43.Kahl B. Is there a role for “watch and wait” in follicular lymphoma in the rituximab era? ASH Education Program Book. 2012;2012:433–438. doi: 10.1182/asheducation-2012.1.433. [DOI] [PubMed] [Google Scholar]