Abstract
In January 2014, the US government temporarily designated 5F-PB-22, along with three other synthetic cannabinoids (AB-FUBINACA, ADB-PINACA and PB-22), into Schedule I. Over the course of a 4-month time period (July–October 2013), our laboratory quantitatively identified 5F-PB-22 in specimens obtained from four postmortem cases. We describe the four cases, to include pertinent autopsy findings and decedent histories, together with quantitative results for 5F-PB-22 determined in postmortem blood and antemortem serum. Samples were prepared via a liquid–liquid extraction at pH 10.2 into hexane : ethyl acetate. Instrumental analysis was achieved with liquid chromatography coupled with electrospray ionization tandem mass spectrometry operating in multiple reaction monitoring mode. Two ion transitions were monitored for the analyte of interest, and one ion transition was monitored for the internal standard. The observed concentration range of 5F-PB-22 is 1.1–1.5 ng/mL for three postmortem blood specimens and one antemortem serum specimen. Three of the decedents experienced abrupt, sudden death; however, one decedent expired after a rapidly deteriorating hospital course.
Introduction
In the USA, synthetic cannabinoids have been detected in products obtained via smoke shops, gas stations and the Internet (1–5). Many of these substances are surmised to be full agonists of cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2). The consumption of synthetic cannabinoids and related products has been linked to adverse effects including agitation, confusion, hypertension, respiration issues, seizures, tachycardia, paranoia, hallucinations, psychoses and acute kidney injury (6–12). They have also been associated with several driving under the influence or impairment cases and have been implicated in human deaths (13–16).
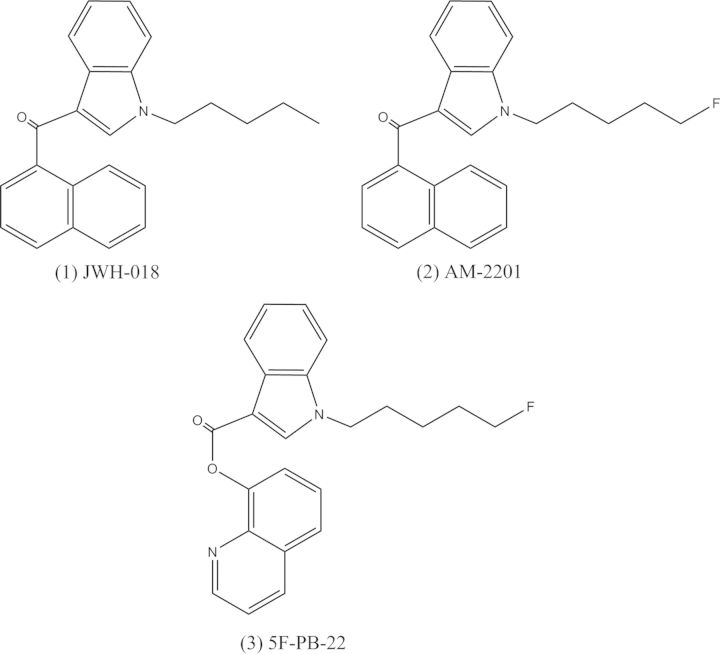
During the summer of 2012, President Obama signed legislation which placed 15 synthetic cannabinoid compounds and 5 overall cannabinoid structural classes into Schedule I of the Controlled Substances Act (CSA). These compounds include, but are not limited to, AM-2201, JWH-018, JWH-019, JWH-073 and JWH-122 (17). The tetramethylcyclopropylindole cannabinoids, UR-144 and XLR-11, became prevalent for much of the remaining portion of 2012 and were ultimately controlled by the Federal government in April 2013 (4, 18). In early 2013, a new wave of compounds that contained a large change in chemical structure emerged and are classified as quinolinyl carboxylate derivatives. 1-(5-Fluoropentyl)-1H-indole-3-carboxylic acid 8-quinolinyl ester, also known as 5F-PB-22, is a quinolinyl carboxylate derivative that differs from the earlier generation naphthoylindole, AM-2201, by replacing the naphthalene group with an 8-hydroxyquinoline moiety (Figure 1). Pharmacological and toxicological data for this compound do not currently exist, but similar to other synthetic cannabinoids, it is expected to be a CB1 and CB2 receptor agonist. Currently, this compound is considered a controlled substance in the states of Minnesota and Florida. On 10 January 2014, the US government temporarily placed four synthetic cannabinoids, including 5F-PB-22, into Schedule I of the CSA via emergency schedule (19).
Figure 1.
Chemical structure comparison of JWH-018, AM-2201 and 5F-PB-22.
There are no published reports of the detection of this compound in postmortem toxicology cases. We describe a liquid chromatography with tandem mass spectrometry (LC–MS-MS) method for the quantitation of 5F-PB-22 in blood and report a series of four fatalities involving these compounds.
Specimen Collection and Testing Protocol
Forensic pathologists collected blood specimens in polypropylene tubes containing sodium fluoride and EDTA during standard autopsy procedures before transporting to the laboratory at ambient temperature for toxicological analyses. Methodologies utilized in the analyses include an enzyme-linked immunosorbent assay (ELISA) screen for classical cannabinoids and opiates/opioids, a liquid chromatography–time-of-flight mass spectrometry screen (LC–ToF) for other abused drugs and therapeutic agents, and a headspace-gas chromatography with flame ionization detection (GC–FID) screen for volatile compounds. Synthetic cannabinoids were analyzed via a directed analysis by LC–MS-MS.
Materials
The 5F-PB-22 reference standard was obtained from Cayman Chemical Company (Ann Arbor, MI, USA). The internal standard, JWH-018-d9, was purchased from Cerilliant Corporation (Round Rock, TX, USA). Acetone (ACS grade), acetonitrile (HPLC grade), ethyl acetate (GC–MS grade), hexane (GC–MS grade), isopropanol (ACS grade), methanol (HPLC grade), sodium bicarbonate (USP grade) and sodium carbonate (ACS grade) were acquired from Fisher Scientific (Pittsburgh, PA, USA). Formic acid (98%) was purchased from Sigma-Aldrich, Inc. (St. Louis, MO, USA).
Extraction
A 50-µL aliquot of JWH-018-d9 internal standard solution in acetonitrile (25 ng/mL) and 500 µL of sodium bicarbonate buffer (pH 10.2) were added to 500 µL of blood. The mixture was vortex-mixed, and 5 mL of hexane : ethyl acetate (98 : 2) was added. The specimens were mixed via a test tube rocker for 3 min and centrifuged for 3 min at 3,000 rpm. The supernatant was transferred to a clean test tube and evaporated to dryness under nitrogen gas flow. The residue was reconstituted in 200 µL of deionized (DI) water : acetonitrile (50 : 50) and transferred to a glass autosampler vial.
Instrumental Analysis
Instrumental analysis was performed via a Waters (Milford, MA, USA) Acquity UltraPerformance® Liquid Chromatograph coupled with a Waters Quattro Premier XE tandem mass spectrometer. Chromatographic separation was performed by injecting 10 µL of vial extract onto a Waters Acquity UPLC® BEH C18 column (2.1 × 100 mm, 1.7 µm particle size), held 60°C, using a gradient elution. Electrospray ionization (ESI) mass spectrometry was performed in positive ionization multiple reaction monitoring mode. Two ion transitions were monitored for the analyte of interest, and one ion transition was monitored for the internal standard. The instrumental methods are summarized in Tables I and II.
Table I.
Elution Gradient
| Total time (min) | Flow rate (mL/min) | %A | %B |
|---|---|---|---|
| Initial | 0.500 | 58.0 | 42.0 |
| 0.30 | 0.500 | 58.0 | 42.0 |
| 5.60 | 0.500 | 34.0 | 66.0 |
| 8.00 | 0.500 | 24.0 | 76.0 |
| 8.50 | 0.500 | 0.0 | 100.0 |
| 8.51 | 0.500 | 58.0 | 42.0 |
| Mobile phases | A (0.1% formic acid in DI water); B (0.1% formic acid in acetonitrile) | ||
| Retention times (min) | 5F-PB-22 (4.75) JWH-018-d9 (5.7) |
||
Table II.
The Mass Spectrometer Method
| Analyte | Ion transition | Type | Dwell time (ms) | Cone voltage (V) | Collision energy (eV) |
|---|---|---|---|---|---|
| 5F-PB-22 | 377.1 > 232.2 | Quantifying | 0.02 | 24 | 24 |
| 5F-PB-22 | 377.1 > 144.1 | Qualifying | 0.02 | 24 | 42 |
| JWH-018-d9 | 351.2 > 154.9 | Internal standard | 0.02 | 45 | 26 |
| Capillary voltage | Extractor voltage | Source temperature | Desolvation temperature | Desolvation gas flow | Collision gas flow |
| 0.60 kV | 3 V | 140°C | 450°C | 850 L/h | 0.30 mL/min |
Method Validation
The analytical method for the determination of synthetic cannabinoids, including 5F-PB-22, in blood specimens was validated as a quantitative assay. Linearity, including limit of detection (LOD), lower limit of quantitation (LLOQ) and upper limit of quantitation (ULOQ), imprecision and accuracy, matrix selectivity, exogenous drug interferences, ion suppression and carryover were assessed. The overall validation methodology used was previously published and is a standard validation procedure for quantitative mass spectrometry-based assays in our laboratory facility (13). Currently, other synthetic cannabinoids monitored in the assay include AB-PINACA, ADB-PINACA, AM-2201, BB-22, Cl-2201, JWH-015, JWH-018, JWH-019, JWH-073, JWH-081, JWH-122, JWH-210, JWH-250, MAM-2201, PB-22, UR-144 and XLR-11.
For 5F-PB-22, the blood assay was linear from 0.5 ng/mL (LLOQ) to 10 ng/mL (ULOQ), with a LOD of 0.1 ng/mL. The intrarun %CV values for the high control specimen (8 ng/mL) were between 2.2 and 4.5% and the interrun %CV values were between 1.6 and 5.6%, while the accuracy values were between 96.5 and 101.2%. The intrarun %CV values for the low control specimen (2 ng/mL) were between 3.4 and 6.6% and the interrun %CV values were between 2.6 and 7.7%, while the accuracy values were between 97.0 and 103.1%. While some matrix effects were observed, the internal standard did compensate for suppression or enhancement of the analyte signal. Of the 100 drugs and metabolites analyzed as possible interferences, none were noted. No carryover was detected in blank extracts injected immediately following a specimen spiked with 100 ng/mL of 5F-PB-22. The average ion transition ratio during analytical method validation for 5F-PB-22 was 1.7 (n = 30).
Case Reports
The four case summaries describing the detection of 5F-PB-22 in postmortem and antemortem specimens received by AIT Laboratories occurred during the time period of July–October 2013 (Table III).
Table III.
Case Results
| Case number | Blood source | 5F-PB-22 (ng/mL) | Other toxicology |
|---|---|---|---|
| 1 | Femoral (postmortem) | 1.1 | Ethanol (0.033 g/dL), amiodarone, caffeine |
| 2 | Serum (antemortem, Day 1, 10 : 20) | Not tested | THCCOOH (246 ng/mL) |
| Serum (antemortem, Day 1, 19 : 54) | Not tested | THCCOOH (176 ng/mL), piperacillin, levofloxacin | |
| Serum (antemortem, Day 2, 04 : 00) | 1.3 | Lorazepam (19.5 ng/mL) | |
| 3 | Iliac (postmortem) | 1.5 | None |
| 4 | Superior vena cava (postmortem) | 1.5 | None |
Case 1
A 17-year-old male was drinking alcohol with a friend over the course of a day and reportedly had been using other drugs including synthetic cannabinoids. The friend stated during the early morning hours of the next day that the decedent appeared ‘to begin gasping for air and fell to the ground’. Resuscitative attempts were immediately initiated by the friend and his parent, while 911 was called. The decedent was pronounced dead at a medical center. An autopsy revealed no significant injuries or natural diseases. The remarkable toxicology findings included a femoral blood concentration for 5F-PB-22 of 1.1 ng/mL, an ethanol concentration of 0.033 g/dL, amiodarone (administered during resuscitative efforts) and caffeine. Based on the known circumstances of death and the autopsy and toxicology findings, the cause of death was attributed to 5F-PB-22 (synthetic cannabinoid) intoxication with the manner of death as accident.
Case 2
A 27-year-old male presented to a local emergency room after being discovered by his girlfriend in the early morning hours appearing quite ill and diaphoretic. He was transferred to a tertiary care facility with a diagnosis of acute liver and kidney injuries. A current or past history of alcohol abuse or dependence was denied by the patient; however, he admitted a history of marijuana use of several times per week. Evaluation in the Medical Intensive Care Unit indicated severe liver injury, severe coagulopathy, acute kidney injury, acute respiratory failure, hypoxemia, severe anion gap metabolic and lactic acidosis. His clinical condition deteriorated markedly over the next 12 h. This was punctuated by a brief episode of cardiac arrest and pulseless electrical activity and poor oxygenation secondary to acute respiratory distress syndrome likely the result of aspiration and pulmonary contusions following chest compressions. He progressed to critically ill status due to circulatory failure, respiratory failure, central nervous system failure, renal failure and severe metabolic derangement. A pair of hospital serum specimens obtained a day before death, but ∼9.5 h apart in time of collection, indicated the presence of THCCOOH at concentrations of 246 and 176 ng/mL. A different serum specimen, collected ∼7 h before death, revealed 5F-PB-22 at a concentration of 1.3 ng/mL. The autopsy revealed the cause of death to be fulminant liver failure in the setting of THC (marijuana) and 5F-PB-22 (synthetic cannabinoid) exposure. The manner of death was certified as undetermined.
Case 3
An 18-year-old male was pronounced dead in the home of friends during the afternoon following a night of partying. The decedent, accompanied by two friends, attended a party at another residence, which included a number of people known only to the decedent. Friends observed that the decedent consumed numerous beers, mixed alcoholic beverages and smoked synthetic marijuana (K2/Spice). The decedent was described to be very intoxicated. At some point in the evening, the friends and decedent departed the party to return to the friends' home. The decedent retired for a nap, having been reported last seen alive and well at 0945 h. He was later discovered unresponsive, not breathing, cool to the touch and pulseless at 1342 h. Bilateral pulmonary vasocongestion and congestion in the abdominal organs (liver, spleen and kidneys) were observed at autopsy. A concentration of 1.5 ng/mL of 5F-PB-22 was determined in iliac blood. The cause of death was attributed to sudden death, in association with synthetic cannabinoid use. The manner of death was classified as accident.
Case 4
A 19-year-old male attended a Saturday night party as evidenced by some photographs showing him ‘unconscious with drinking involved’. He returned to his home around noon the next day and indicated to his mother that he felt lightheaded and retired to his bed. The decedent was discovered deceased by his brother the next morning. The pertinent findings at autopsy included bilateral pulmonary edema, necrotizing granulomatous inflammation with histoplasma microorganisms and congestion of viscera. The toxicology findings observed in superior vena cava blood were notable for the presence of 5F-PB-22 at a concentration of 1.5 ng/mL. The stated cause of death was suspected acute drug intoxication using the synthetic cannabinoid 5F-PB-22. The manner of death was accident.
Discussion
Here, we describe four postmortem case reports that include the detection and quantitative determination of the synthetic cannabinoid, 5F-PB-22, in blood or antemortem serum. The cases were received by our laboratory during a 4-month period (July–October 2013), prior to 5F-PB-22, and three other synthetic cannabinoid compounds, gaining the designation as Schedule I in the CSA. The decedents in all cases were young males; three teenage and one 27 year old. Three cases (1, 3 and 4) presented as relatively sudden or abrupt episodes following a history of ‘partying’ in the company of others. In Case 2, the decedent presented with several acute medical problems which progressively became worse during an intensive care unit hospital stay. The most prominent autopsy findings were associated with Case 2 (fulminant liver failure); Cases 3 and 4 exhibited relatively nonspecific findings (pulmonary edema, visceral congestion and pulmonary granulomatous inflammatory changes). The observed concentration range of 5F-PB-22 is 1.1–1.5 ng/mL for three postmortem blood specimens (femoral, iliac and superior vena cava sites) and one antemortem serum specimen. Other remarkable toxicology findings included ethanol (Case 1) and THCCOOH (Case 2); other findings were consistent with medical intervention (Case 2).
Although synthetic cannabinoids have been associated with sudden death in the USA and abroad, the exact physiological mechanisms for causation, or initiators to contributing factors, remain unclear. In Case 1, a possible anaphylactic etiology was ruled out by the forensic pathologist. Sudden onset cardiac dysrhythmias or seizure suggest plausible mechanisms. In contrast, Case 2 presented as a protracted, rapidly deteriorating clinical event over 24 h culminating in acute hepatic failure. Another possible biochemical etiology to these unfolding sequelae may reside in a yet undetermined or unidentified metabolic intermediate of 5F-PB-22. It is noteworthy that a distinct feature of the quinolinyl carboxylate synthetic cannabinoids is an ester linkage. The earlier waves of compounds, such as JWH-018, AM-2201 and XLR-11, contained a ketone linkage between the indole moiety and the naphthoyl or tetramethylcyclopropyl groups. The ester bond may be susceptible to in vivo hydrolysis reactions catalyzed by carboxylesterase enzymes. This mechanism could cause the accumulation of a metabolite that is perhaps analogous to the toxic mechanism peculiar to paracetamol (acetaminophen) and the accumulation of a quinone metabolite and its reactions with hepatocellular proteins and nucleic acids. Because of the ease of degradation by in vivo hydrolysis reactions, it may be prudent to investigate the possible accumulation of probable metabolites in the blood.
Clearly, further investigation is required with respect to the pharmacokinetics of 5F-PB-22 and other synthetic cannabinoids, their role in human toxidromes and their relevance to detection in postmortem casework. Important point sources for this information will continue to include the US National Network of Poison Information Centers, reporting emergency departments and urgent care centers and medical examiner/coroner systems with their attendant toxicology laboratories.
Conflict of interest
None declared.
References
- 1.Penn H.J., Langman L.J., Unold D., Shields J., Nichols J.H. Detection of synthetic cannabinoids in herbal incense products. Clinical Biochemistry. 2011;44:1163–1165. doi: 10.1016/j.clinbiochem.2011.06.078. [DOI] [PubMed] [Google Scholar]
- 2.Logan B.K., Reinhold L.E., Xu A., Diamond F.X. Identification of synthetic cannabinoids in herbal incense blends in the United States. Journal of Forensic Sciences. 2012;57:1168–1180. doi: 10.1111/j.1556-4029.2012.02207.x. [DOI] [PubMed] [Google Scholar]
- 3.Shanks K.G., Dahn T., Behonick G., Terrell A. Analysis of first and second generation legal highs for synthetic cannabinoids and synthetic stimulants by ultra-performance liquid chromatography and time of flight mass spectrometry. Journal of Analytical Toxicology. 2012;36:360–371. doi: 10.1093/jat/bks047. [DOI] [PubMed] [Google Scholar]
- 4.Shanks K.G., Behonick G.S., Dahn T., Terrell A. Identification of novel third-generation synthetic cannabinoids in products by ultra-performance liquid chromatography and time-of-flight mass spectrometry. Journal of Analytical Toxicology. 2013;37:517–525. doi: 10.1093/jat/bkt062. [DOI] [PubMed] [Google Scholar]
- 5.Presley B.C., Jansen-Varnum S.A., Logan B.K. Analysis of synthetic cannabinoids in botanical material: a review of analytical methods and findings. Forensic Science Review. 2013;25:27–46. [PubMed] [Google Scholar]
- 6.Tofighi B., Lee J.D. Internet highs—seizures after consumption of synthetic cannabinoids purchased online. Journal of Addiction Medicine. 2012;6:240–241. doi: 10.1097/ADM.0b013e3182619004. [DOI] [PubMed] [Google Scholar]
- 7.Young A.C., Schwarz E., Medina G., Obafemi A., Feng S.Y., Kane C., et al. Cardiotoxicity associated with the synthetic cannabinoid, K9, with laboratory confirmation. The American Journal of Emergency Medicine. 2011;30:1320.e5–1320.e7. doi: 10.1016/j.ajem.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Lapoint J., James L.P., Moran C.L., Nelson L.S., Hoffman R.S., Moran J.H. Severe toxicity following synthetic cannabinoid ingestion. Clinical Toxicology. 2011;49:760–764. doi: 10.3109/15563650.2011.609822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thornton S.L., Wood C., Friesen M.W., Gerona R.R. Synthetic cannabinoid use associated with acute kidney injury. Clinical Toxicology. 2013;51:189–190. doi: 10.3109/15563650.2013.770870. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC) Acute kidney injury associated with synthetic cannabinoid use—multiple states, 2012. MMWR Morbidity and Mortality Weekly Report. 2013;62:93–98. [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC) Notes from the field: severe illness associated with synthetic cannabinoid use—Brunswick, Georgia, 2013. MMWR Morbidity and Mortality Weekly Report. 2013;62:939. [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention (CDC) Notes from the field: severe illness associated with reported use of synthetic marijuana – Colorado, August-September 2013. MMWR Morbidity and Mortality Weekly Report. 2013;62:1016–1017. [PMC free article] [PubMed] [Google Scholar]
- 13.Shanks K.G., Dahn T., Terrell A.R. Detection of JWH-018 and JWH-073 by UPLC-MS-MS in postmortem whole blood casework. Journal of Analytical Toxicology. 2012;36:145–152. doi: 10.1093/jat/bks013. [DOI] [PubMed] [Google Scholar]
- 14.Kronstrand R., Roman M., Andersson M., Eklund A. Toxicological findings of synthetic cannabinoids in recreational users. Journal of Analytical Toxicology. 2013;37:534–541. doi: 10.1093/jat/bkt068. [DOI] [PubMed] [Google Scholar]
- 15.Yeakel J.K., Logan B.K. Blood synthetic cannabinoid concentrations in cases of suspected impaired driving. Journal of Analytical Toxicology. 2013;37:547–551. doi: 10.1093/jat/bkt065. [DOI] [PubMed] [Google Scholar]
- 16.Gurney S.M.R., Scott K.S., Kacinko S.L., Presley B.C., Logan B.K. Pharmacology, toxicology, and adverse effects of synthetic cannabinoid drugs. Forensic Science Review. 2014;26:53–78. [PubMed] [Google Scholar]
- 17.Drug Enforcement Administration. Establishment of drug codes for 26 substances. Federal Register. 2013;78(3):664–666. [PubMed] [Google Scholar]
- 18.Drug Enforcement Administration, Department of Justice. Schedules of controlled substances: temporary placement of three synthetic cannabinoids into Schedule I. Final rule. Federal Register. 2013;78:28735–28739. [PubMed] [Google Scholar]
- 19.Drug Enforcement Administration, Department of Justice. Schedules of controlled substances: temporary placement of four synthetic cannabinoids into Schedule I. Final order. Federal Register. 2014;79:7577–7582. [PubMed] [Google Scholar]