Abstract
Background
Tumor microenvironment, and particularly tumor-associated macrophages (TAMs), represent a key contributing factor in pancreatic ductal adenocarcinoma (PDAC) pathogenesis. Here we report that heparanase (predominant enzyme degrading heparan sulfate, the main polysaccharide found at the cell surface and extracellular matrix) directs tumor-promoting behavior of TAM in PDAC.
Methods
A mouse model of heparanase-overexpressing pancreatic carcinoma (n = 5 mice/group), tumor-associated macrophages ex vivo, primary wild-type and heparanase-null macrophages, and histological specimens from PDAC patients (n = 16), were analyzed, applying immunostaining, enzyme-linked immunosorbent assay, real-time reverse transcription–polymerase chain reaction, cell proliferation, and heparanase activity assays. All statistical tests are two-sided.
Results
We found that overexpression of heparanase is associated with increased TAM infiltration in both experimental (P = .002) and human (P = .01) PDAC. Moreover, macrophages derived from heparanase-rich tumors (which grew faster in mouse hosts), display pronounced procancerous phenotype, evidenced by overexpression of MSR-2, IL-10, CCL2, VEGF, and increased production of IL-6, an important player in PDAC pathogenesis. Furthermore, in vitro heparanase enzyme–rendered macrophages (stimulated by necrotic cells which are often present in PDAC tissue) procancerous, as exemplified by their enhanced production of key cytokines implicated in PDAC (including IL-6), as well as by their ability to induce STAT3 signaling and to augment pancreatic carcinoma cell proliferation. In agreement, we observed activation of STAT3 in experimental and clinical specimens of heparanase-overexpressing PDAC.
Conclusions
Our findings underscore a novel function of heparanase in molecular decision-making that guides cancer-promoting action of TAM and imply that heparanase expression status may become highly relevant in defining a target patient subgroup that is likely to benefit the most from treatment modalities targeting TAM/IL-6/STAT3.
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive and fatal tumor type (1). Currently existing cytotoxic/targeted therapies rarely provide substantial response in PDAC, rendering the disease one of the five most common causes of cancer mortality in developed countries (1). Pancreatic tumor microenvironment, and in particular infiltrating inflammatory cells (largely macrophages), represent an important contributing factor to pancreatic carcinoma aggressiveness and resistance to treatment (2–7). Tumor-associated macrophages (TAMs) are known to supply bioactive molecules (ie, cytokines, growth factors, antiapoptotic proteins) and activate tumor-stimulating signaling pathways (eg, STAT3), thus promoting tumorigenesis in several anatomic sites (including pancreas) (2,6,8–13). It was proposed that once the tumor is initiated and progresses toward malignancy, the macrophage phenotype changes from the “classically” activated (M1) to the alternatively activated type, “M2” (12). More recent data suggest that in contrast to this binary definition, TAMs often share features of both classically and alternatively activated populations, generally oriented toward promoting tumor growth (13).
Considerable progress has been made in deciphering the role of the local cytokine profile in TAM polarization toward tumor-supporting phenotype (8,12,13). Less appreciated is the role of extracellular matrix (ECM)–degrading enzymes (ie, heparanase) in this phenomenon.
Heparanase is a single mammalian endoglycosidase that cleaves heparan sulfate (HS) glycosaminoglycans, ubiquitously found at the cell surface and in the ECM (14,15). HS binds to and assembles ECM proteins, thus playing important roles in ECM integrity, barrier function, and cell interactions with ECM (14,15), cytokines, and growth factors (14,16). Degradation of HS by heparanase therefore affects numerous pathophysiological processes, including tumorigenesis (17) and inflammation (18).
Heparanase is tightly linked to pathogenesis of pancreatic carcinoma (19–22), as well as additional inflammation-associated tumor types (ie, Barrett’s oesophagus adenocarcinoma, hepatocellular carcinoma and colorectal cancer) (23–27). Remarkably, the enzyme is upregulated in essentially all inflammatory conditions linked to these cancer types (ie, chronic pancreatitis [19], Barrett’s oesophagus [23], hepatitis C infection [24] and inflammatory bowel disease [28,29]), suggesting that in tissues in which cancer-related inflammation typically occurs, heparanase may be mechanistically involved in coupling inflammation and tumorigenesis.
Recently the functional importance of the enzyme in modulating macrophage activation by microbial products was demonstrated in the model of ulcerative colitis (28). However, the effect of heparanase on tumor-infiltrating macrophages in the setting of noninfectious “aseptic” inflammation (occurring in the majority of cancer types [30]) has not been elucidated. Indeed, unlike colon, in majority of inflammation-associated tumor sites, including pancreas, nonmicrobial endogenous signals (ie, substances released by damaged/necrotic cells) may contribute to macrophage stimulation through pattern recognition receptors (ie, toll-like receptors [TLR]) (31–33), although these signals alone are often not sufficient to elicit tumor-promoting activity of macrophages (34).
In the present study, we highlight the novel action of the heparanase enzyme in coupling inflammation and cancer during PDAC progression (as a prototype of aseptic inflammation-associated tumor). We found in vitro that heparanase polarizes macrophages, stimulated by necrotic cells (which are abundantly present in both clinical and experimental PDAC [35–37]), toward tumor-promoting phenotype, characterized by augmented expression of several key cytokines driving pancreatic tumorigenesis and by increased ability to promote PDAC cell growth. Utilizing a mouse model of heparanase-overexpressing pancreatic carcinoma, we demonstrated that the enzyme directs protumorigenic behavior of TAM. Overexpression of heparanase resulted in increased TAM infiltration. Moreover, well-recognized markers of protumorigenic macrophage population (ie, MSR-2, CCL-2, IL-10, VEGF) and cytokines (ie, IL-6) tightly implicated in the pathogenesis of PDAC (6,9,13,38) were statistically significantly upregulated in TAM isolated from heparanase-overexpressing mouse tumors. Furthermore, we observed increased activation of STAT3 (a pivotal signaling molecule in inflammation-related cancer, that acts downstream of IL-6 (6,9,10,38) in heparanase-overexpressing pancreatic tumors. Moreover, we found that heparanase overexpession in human PDAC is associated with increased TAM infiltration and augmented STAT3 signaling, validating the clinical relevance of our observations. Taken together, our findings describe what we believe to be a previously unknown role of heparanase in powering cancer-related aseptic inflammation, highly relevant to pathogenesis of PDAC and additional tumor types.
Methods
Pancreatic Carcinoma Model In Vivo
Six week-old male SCID mice per group were anesthetized, a small left abdominal flank incision was made, and the spleen exteriorized. The Panc1-Vo or Panc1-Hpa cells (1.5 × 106) were resuspended in 0.03mL Matrigel and injected subcapsularly to the pancreas. A successful subcapsular intrapancreatic injection of tumor cells was verified by the appearance of a fluid bleb without ip leakage. In some experiments, the cells were injected subcutaneously (five mice per group). Mouse experiments were approved by Institutional Animal Care Committee.
Peritoneal Macrophages Isolation
Peritoneal exudate cells were harvested from male 11- to 12-week-old C57Bl/6 wild-type (wt) and heparanase-null (Hpse-KO [39]) mice. Three days prior to isolation, the mice received intraperitoneal injections of 1.5mL 3% (wt/vol) thioglycollate medium (Difco, Detroit, MI). A total of 3 × 106 cells were seeded in 60-mm dishes (Nunc, Denmark) in a final volume of 3mL incomplete medium and, after one hour of incubation in 5% CO2, 37°C, nonadherent cells were vigorously washed off with jets of medium. Monolayers contained 95% or greater macrophages, as assessed by immunostaining with an antimouse F4/80 monoclonal antibody (Serotec). All cultures were maintained in serum free RPMI-1640 medium. After adherence, macrophages were incubated for 2 hours in standard culture conditions and then used in the subsequent experiments.
Treatment With Necrotic Cells
Macrophages were incubated for indicated times with necrotic Panc02 cells (generated by three freezing/thawing cycles), in the absence or presence of active heparanase, medium was collected, and cells processed for RNA extraction.
TAM Isolation
TAMs were isolated applying isolation procedure described in (40,41) and further detailed in Supplementary Methods (available online). In some experiments, TAMs were isolated applying magnetic CD11b immunobeading, as described (42). Only the preparations containing 95% or more of F4/80-positive macrophages were used in further analysis.
Immunohistochemistry
Formalin-fixed PDAC tissues from 16 nonselected patients (eight males and eight females, average age at diagnosis: males 64 years, females 66 years; without epidemiological differences between the patients) were available from the Deptartment of Pathology, Hadassah Medical Center, Jerusalem. The use of these specimens in research was approved by the Human Subjects Research Ethics Committee of the Hadassah Medical Center as exempt from institutional review board review because the study does not meet Common Rule Section 101(b) criteria for “research involving human subjects.” Tissue samples were deidentified and impossible for us to trace back to the patient’s identity. Paraffin-embedded slides were deparaffinized and incubated in 3% H2O2. Antigen unmasking was carried out by heating (20 minutes) in a microwave oven in 10mM Tris buffer containing 1mM EDTA. Slides were incubated with primary antibody antiheparanase monoclonal antibody # 01385-126 (kindly provided by Dr. P. Kussie; ImClone Systems Inc., New York, NY) (43), diluted (1:200) in CAS-Block (Invitrogen) or with CAS-Block alone, as a control. Appropriate secondary antibodies (Nichirei) were then added and slides incubated at room temperature for 30 minutes. Mousestain kit (Nichirei) was used when primary mouse antibody was applied to stain mouse tissues. Color was developed using the DAB substrate kit (Thermo Scientific), followed by counterstaining with Mayer’s hematoxylin. Controls without addition of primary antibody showed low or no background staining in all cases.
Statistical Analysis
The results are presented as the mean plus or minus standard deviation. Differences between groups were assessed by the unpaired Student’s t test or the Mann–Whitney test; Pearson’s correlation coefficient was calculated to measure the strength of the association between the two variables, unless otherwise stated; P values of .05 or less were considered statistically significant. All statistical tests were two-sided.
Results
Effect of Heparanase Overexpression on Pancreatic Carcinoma Growth In Vivo vs In Vitro
Heparanase was previously linked to increased aggressiveness and decreased survival in PDAC patients (19–22), however, little is known about the exact mode of heparanase action in pancreatic tumor growth. In order to investigate how heparanase influences pancreatic tumor progression, we utilized Panc1 PDAC cell line, which expresses relatively low levels of endogenous heparanase (Supplementary Figure 1, available online). Panc1 cells were stably transfected with vector encoding for human heparanase under constitutive CMV promoter (Panc1-Hpa) or empty vector (Panc1-Vo), and overexpression of heparanase in Panc1-Hpa cells was confirmed by real-time reverse transcription–polymerase chain reaction (qRT-PCR) and enzymatic activity assay (Figure 1A). As shown in Figure 1B, tumors produced in SCID mice by Panc1-Hpa cells grew faster, and differences in tumor progression reached statistical significance on day 28 (two-sided Mann–Whitney test P = .05). Intriguingly, comparison of the growth rate in vitro did not reveal any increase in proliferation rate of Panc1-Hpa vs Panc1-Vo cells (Figure 1C), suggesting contribution of microenvironment in accelerated progression of Panc1-Hpa tumors growing in the mouse host. Of note, no difference in angiogenisis was detected between Panc1-Hpa and Panc1-Vo tumors (in line with the lack of previous reports linking heparanase to PDAC angiogenesis [19–22] and perhaps reflecting the unusual nature of PDAC vascularity [4]).
Figure 1.
Effect of heparanase overexpression on Panc1 carcinoma. A) Heparanase overexpression in Panc1-Hpa cells was confirmed by enzymatic activity assay. Inset: heparanase mRNA expression in Panc1-Vo and Panc1-Hpa cells, measured by real-time reverse transcription–polymerase chain reaction and normalized to actin mRNA. Data shown are representative of three independent experiments. B) Changes in primary tumor growth in SCID mice injected with Panc1-Vo cells (gray line) and Panc1-Hpa cells (black line), n = 5, *two-sided Mann–Whitney test P = .05. C) In vitro proliferation rate of Panc1-Vo cells (gray line) and Panc1-Hpa cells (black line) assessed by MTS assay. Each data point shows the mean of pentaplicate cultures. Error bars represent standard deviation.
Macrophage Accumulation in Panc1-Hpa Tumors
The key contribution of TAM to pancreatic cancer–promoting microenvironment (3,5,7,9), along with the emerging ability of heparanase to modulate macrophage responses to microbial agents (28), led us to hypothesize that the enzyme may confer procancerous phenotype to TAM under “sterile” conditions occurring in PDAC development, accounting (at least in part) for the enhanced progression of Panc1-Hpa tumors. To test this hypothesis, we first compared the degree of macrophage infiltration in Panc1-Hpa vs Panc1-Vo tumors, applying immunostaining with antibodies directed against specific mouse macrophage markers (44) Mac2 (Figure 2A) and F4/80 (Figure 4A, middle panels). Quantitation of macrophage number per 0.03mm2 microscopic field revealed statistically significantly increased macrophage infiltration in Panc1-Hpa tumors vs Panc1-Vo tumors (Student’s t test P = .002) (Figure 2B).
Figure 2.

Macrophage accumulation in Panc1-Hpa tumors. Tissue specimens from Panc1-Hpa and Panc1-Vo tumors were stained with anti-Mac2 antibody. A) Mac2-positive macrophages (green) infiltrating Panc1-Hpa (bottom) and Panc1-Vo (top) tumors. Scale bars: 50 μm. B) Mac2-positive macrophages in tissue specimens derived from Panc1-Hpa (black bar) and Panc1-Vo (gray bar) tumors were quantified per 0.03mm2 microscopic field (all the fields chosen for analysis were located ≥1mm from the tumor border), based on six sections from three mice per group (*two-sided Student’s t test P = .002). Error bars represent standard deviation.
Figure 4.
Levels of IL-6–expressing macrophages in Panc1-Hpa and Panc1-Vo tumors. A) Panc1-Hpa and Panc1-Vo tumors were harvested on experimental day 28 and processed for double immunofluorescent analysis using anti-IL-6 (red) and anti-F4/80 (yellow) antibodies. Orange arrows indicate IL-6–expressing macrophages. Cell nuclei were counterstained with DRAQ5 (blue). Scale bars: 50 μm. Insets: enlarged images of the area delineated by dashed lines (original magnification X630). Scale bars: 25 μm. B) IL-6–expressing macrophages were quantified per 0.06mm2 microscopic field, based on six sections from three mice per group; error bars represent standard deviation (*two-sided Student’s t test P = .02).
Effect of Heparanase on Tumor-Promoting Phenotype of Macrophages
We next tested whether heparanase affects TAM polarization toward cancer-promoting phenotype by analyzing macrophages isolated from Panc1-Hpa and Panc1-Vo tumors. Applying qRT-PCR (Figure 3A) and enzyme-linked immunosorbent assay (ELISA) (Figure 3B), we revealed that Panc1-Hpa tumor-derived macrophages exhibited statistically significantly increased levels of known markers of procancerous TAM population (ie, macrophage scavenger receptor 2 [MSR-2]; chemokine [C-C motif] ligand 2 [CCL-2, ie, monocyte chemotactic protein-1; potent chemoattractant responsible for macrophages recruitment]; VEGF, IL-10, and IL-6) (8,13). Additionally, a four-fold increase in TNFα secretion was detected by ELISA in Panc1-Hpa vs -Vo tumor-derived macrophages (24 pg/mL vs 6 pg/mL, Student’s t test P = .03). Importantly, primary macrophages, isolated from peritoneal lavage of Panc1-Hpa and Panc1-Vo tumor-bearing mice, expressed extremely low levels of these markers/cytokines as compared with TAMs from the same animals (Figure 3A). Moreover, no difference in expression of MSR-2, CCL-2, IL-10, VEGF, or IL-6 was detected between primary macrophages isolated from mice bearing Panc1-Hpa vs Panc1-Vo tumors (Figure 3A).
Figure 3.
Heparanase levels and tumor-associated macrophage (TAM) procancerous phenotype in Panc1 tumors. A) On day 28 posttumor inoculation, primary macrophages (PMɸ) were isolated from peritoneal lavage of Panc1-Vo and Panc1-Hpa tumor–bearing mice (three or more mice per group), and TAM were isolated from Panc1-Vo (Vo) and Panc1-Hpa (Hpa) tumors. Inset: Immunostaining confirmed that 95% or more of the cells used in further experiments were F4/80 positive. Scale bar: 25 μm. Expression of the markers of procancerous macrophage population (MSR-2, CCL-2; IL-10, VEGF, and IL-6) was assessed by real-time reverse transcription–polymerase chain reaction (in triplicates). PMɸ isolated from Panc1-Vo tumors were used as reference cell population for fold increase calculation, two-sided Student’s t test *P = .04; **P = .03; ***P = .01; ****P = .005. B) IL-6 secretion by tumor-associated macrophage isolated from Panc1-Vo (Vo) and Panc1-Hpa (Hpa) tumors was evaluated (in triplicates) by ELISA, **two-sided Student’s t test P = .03. Data shown are representative of three independent experiments. Error bars represent SD.
One of the cytokines overexpressed by Panc1-Hpa tumor-derived TAM, namely IL-6 (Figure 3), is particularly important in PDAC pathogenesis (6,9,38). We therefore further validated IL-6 induction and examined its cellular source in heparanase-rich pancreatic carcinoma, applying combined immunofluorescent staining with anti–IL-6 and anti-F4/80 antibodies. We detected markedly increased levels of IL-6 protein in Panc1-Hpa vs -Vo tumors (Figure 4A) and confirmed that TAMs represent an important (although not the sole) source of IL-6 in Panc1-Hpa tumors, as evidenced by a statistically significant increase in the fraction of IL-6 positive macrophages, visualized by colocalized IL-6 and F4/80 fluorescent signals (Student’s t test P = .02) (Figure 4, A and B).
Of note, in vitro treatment of macrophages with purified recombinant heparanase alone had no effect on expression of IL-6 and additional cytokines (Figure 5A–C). This notion, taken together with the emerging role of necrosis-related inflammation in cancer (including, but not limited to, PDAC) (35,36,45) and invariable occurrence of necrotic regions in Panc1 tumors growing in vivo (Supplementary Figure 2, available online), prompted us to test whether the enzyme rather acts by polarizing macrophages stimulated by necrotic cells toward tumor-promoting phenotype. Indeed, signals originating in necrotic cells were shown to affect macrophage behavior (31–33), although these signals alone are often not sufficient to elicit tumor-promoting activity of macrophages (34). We first compared production of tumor-promoting cytokines by primary peritoneal macrophages (isolated from C57/BL6 mice) in response to stimulation by necrotic Panc02 cells (syngeneic C57/BL6 mouse pancreatic carcinoma cell line [46]), in the absence or presence of recombinant active heparanase, to recapitulate conditions occurring in vivo. As shown in Figure 5, A-C, a marked increase in IL-6, IL-10, and CCL-2 expression was detected in macrophages stimulated by necrotic cells (NC) in the presence of heparanase. Notably, this effect was dependent on heparanase enzymatic activity, since heat-inactivated heparanase did not affect macrophage activation by NC.
Figure 5.
Heparanase effect on tumor-promoting phenotype of macrophages stimulated by necrotic cells. A-C) Production of cytokines implicated in tumor-promoting action of tumor-associated macrophage in the presence of heparanase. Mouse peritoneal macrophages, isolated as described in Methods, were either untreated (Mф) or coincubated (6 hours, 37°C) with necrotic Panc02 cells (NC), in the absence (gray bars) or presence (black bars) of active recombinant heparanase (Hpa, 0.8 µg/ml). Expression of IL-6 (A), IL-10 (B), and CCL-2 (C) was assessed by real-time reverse transcription–polymerase chain reaction, *two-sided Student’s t test P < .05. In some experiments, live Panc02 cells (LC) were used as a control (A). D-F) Effect of conditioned medium of macrophages stimulated by NC in the presence of heparanase on STAT3 signaling and proliferation in Panc02 carcinoma cells. Panc02 cells were seeded in quadruplicates and incubated for 30 minutes (D, E) or for 24 hours (F) with medium that has been conditioned (6 hours, 37°C) by untreated or NC-stimulated Mф in the absence (gray bars) or presence (black bars) of active heparanase (Hpa, 0.8 µg/ml). D) Panc02 cells were then stained with anti p-STAT3 antibody (red). Cell nuclei were counterstained with DRAQ5 (blue). Overlay (pink) represents cells positive for nuclear-localized p-STAT3. Scale bars: 50 μm. E) Quantification of the degree of association between p-STAT3 and Draq5 staining within Panc02 cells was performed using colocalization tools of Zen software. Bar graph shows the thresholded Pearson’s correlation coefficient values for p-STAT3/Draq5 colocalization (**P < .001). F) Bar graph demonstrates the fold change in Panc02 cell proliferation, analyzed by MTS assay. ***Two-sided Student’s t test P = .04. Error bars represent standard deviation.
As mentioned above, IL-6 is the major cancer-promoting cytokine, which induces several pathways leading to tumor growth, survival, angiogenesis, and resistance to therapies. One important mechanism of IL-6 procancerous action is activation of STAT3, a critical component of tumor-stimulating signaling in pancreas and other organs (6,9,10,38) responsible for the upregulation of genes promoting proliferation and aggressiveness of cancer cells (6). Importantly, we found that medium conditioned by NC-stimulated macrophages pretreated with heparanase markedly enhances STAT3 signaling (Figure 5, D and E) and proliferation (Figure 5F) of Panc02 cell in vitro, as compared with the medium conditioned by NC-stimulated macrophages without heparanase pretreatment (Figure 5D). In further agreement with the proposed role of heparanase, macrophages isolated from heparanase-deficient (Hpse-KO) mice (39) expressed statistically significantly lower levels of IL-6 in response to stimulation by NC, as compared to wt macrophages (Supplementary Figure 3A, available online), and exogenously added heparanase restored responsiveness of Hpse-KO macrophages, as evident by a three-fold increase in IL-6 expression levels following stimulation by NC in the presence of recombinant enzyme (Student’s t test P < .001). Accordingly, the medium conditioned by NC-stimulated Hpse-KO macrophages had no effect on Panc02 cell growth in vitro, while the medium conditioned by NC-activated wt macrophages induced a 1.3-fold increase in Panc02 proliferation (Supplementary Figure 3B, available online). These observations further validate heparanase role in conferring tumor-promoting activity to macrophages.
Activation of STAT3 in Heparanase-Overexpressing Experimental and Human PDAC
We then compared the activation status of STAT3 in Panc1- Hpa vs Vo tumors. As shown in Figure 6, immunofluorescent staining with antiphosphoSTAT3 antibody revealed a marked increase in tumor cells positive for nuclear localized phospho-STAT3 in Panc1-Hpa as compared with Panc1-Vo tumors. Importantly, when the activation status of STAT3 was compared in Panc1-Hpa cells vs Panc1-Vo cells cultured in vitro, no nuclear-localized phospho-STAT3 was detected in either sublines (Supplementary Figure 4, available online), emphasizing the contribution of stromal components (ie, macrophages) to this phenomenon.
Figure 6.
Levels of nuclear phospho-STAT3 in Panc1-Hpa and Panc1-Vo tumors. Panc1-Hpa and Panc1-Vo tumors were harvested on experimental day 28 and processed for immunofluorescent analysis using anti- phospho-STAT3 (p-STAT3) antibody (red). Cell nuclei were counterstained with DRAQ5 (blue). Overlay (pink) represents cells positive for nuclear-localized p-STAT3. Five mice per group were analyzed, and representative photographs are shown. Scale bars: 50 μm.
Next, to validate the relevance of our findings in a clinical setting, we analyzed tissue specimens derived from 16 nonselected PDAC patients, applying antibodies directed against heparanase and CD68 (human macrophage–specific marker). Immunostaining revealed that tumors with high heparanase levels also displayed increased TAM infiltration (Table 1 and Figure 7), localized mainly in the areas of heparanase expression (Figure 7). Statistical analysis confirmed that overexpression of heparanase in the PDAC tumors is statistically significantly associated with increased macrophage infiltration (two-sided Fisher’s exact test, P = .01). Moreover, we revealed augmented STAT3 signaling (evident by the presence of nuclear phospho-STAT3) in human PDAC specimens with high heparanase/high CD68 levels by immunostaining of serial sections (Supplementary Figure 5, top, available online), as compared with tumors displaying low heparanase/low CD68 levels (Supplementary Figure 5, bottom, available online).
Table 1.
Extent of heparanase expression in human pancreatic ductal adenocarcinoma specimens with low vs high degree of tumor-associated macrophage infiltration
| Degree of TAM infiltration | Low (CD68-negative) | High (CD68-positive) | ||||
| Heparanase staining score | 0 | 1 | 2 | 0 | 1 | 2 |
| % per condition | 100 | 0 | 0 | 0 | 41.7 | 58.3 |
Figure 7.
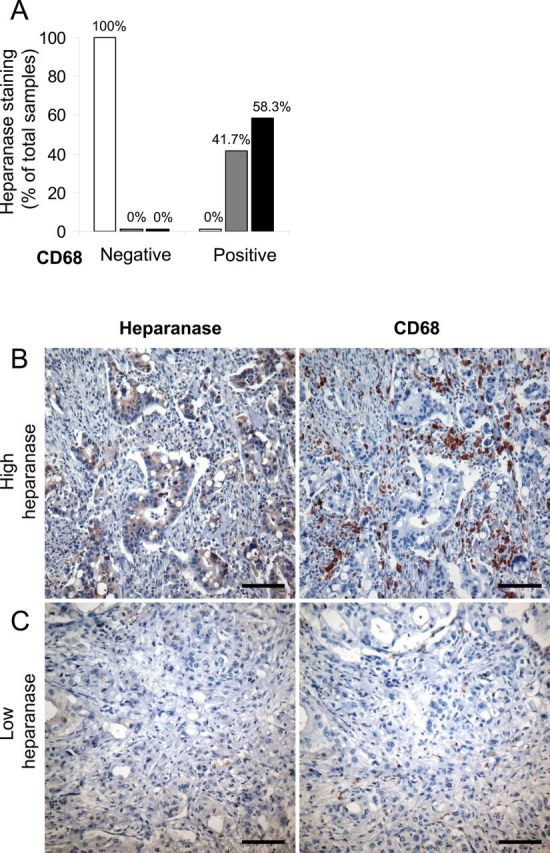
Heparanase expression in human pancreatic ductal adenocarcinoma specimens with low vs high degree of tumor-associated macrophage infiltration. Serial sections of human pancreatic carcinoma tissue samples (n = 16) were processed for immunohistochemistry with antiheparanase (left panels) and anti-CD68 (human macrophage specific marker, right panels) antibodies. A) Sections were then scored according to heparanase staining intensity (white bars: low/no staining = 0; gray bars: medium staining = 1; black bars: high staining = 2). Two-sided Fisher’s exact test was applied to verify that overexpression of heparanase in the tumors (ie, staining grades 1and 2) is significantly associated with high macrophage infiltration (P = .01). Representative images for samples with high level of heparanase/ high CD68 (B) or low heparanase/ low CD68 (C) are shown (scale bars: 100 μm, original magnification ×200).
Discussion
Chronic inflammation stimulates PDAC progression through complex interaction between extracellular microenvironment, malignant epithelial cells, and tumor-infiltrating immunocytes (largely macrophages) (6,47). A key role of TAM in numerous inflammation-related cancer progression scenarios, along with reports suggesting that under certain conditions macrophages may oppose malignancy (13,48), highlights the occurrence of the dynamic changes in the TAM phenotype during tumor development. The mechanisms underlying the switch between the pro- and anticancerous action of TAM are under intense investigation: While involvement of tumor cytokine milieu in shaping cancer-supportive phenotype of TAM is well characterized (13), effect of the ECM components and their enzymatic degradation remains underappreciated. Our present observations reveal a previously unknown function of the ECM-degrading enzyme heparanase in directing tumor-promoting behavior of TAM in PDAC.
Based on the pancreatic neuroendocrine tumor model, macrophages were recently suggested to serve (along with the tumor cells) as a source of heparanase (49), but the role of TAM as a cellular target of the enzyme action has not been reported. Here we demonstrate that overexpression of heparanase by PDAC cells is associated with increased TAM infiltration in both experimental and human PDAC, and report that macrophages residing in heparanase-rich tumors (which grow faster in the mouse host), are polarized toward protumor phenotype, characterized by overexpression of MSR-2, IL-10, CCL2, VEGF, and increased production of IL-6, the key player in PDAC pathogenesis (6,9,38).
Since heparanase protein per se did not affect the cytokine production by macrophages, we hypothesized that the enzyme facilitates tumor-promoting action of TAM activated by endogenous ligands originating in PDAC microenvironment. As mentioned above, several elements in tumor microenvironment (eg, ECM components, necrotic cell-derived substances) may affect macrophage behavior through TLR activation (32). Although it is not clear whether these agents alone are able to direct TAM toward tumor-promoting phenotype, endogenous ligand-triggered TLR signaling has been linked to cancer progression (13,31,33,50,51). In particular, it is increasingly apparent that necrotic cancer cells may lead to more aggressive tumors because of the stimulatory role of necrosis-induced inflammation (45). A hallmark of TAMs is their tendency to accumulate into necrotic regions of tumors (8). Of note, necrosis is a common histological feature of both pancreatic inflammation (37) and PDAC (35), and necrotic areas are present in more than 60% of PDAC patients (35), as well as in experimental PDAC (36). Moreover, necrosis is an independent predictor of poor outcome in PDAC (35). In agreement, we noted that necrotic regions were invariably present in Panc1 tumors growing in vivo (although no differences in the extent of necrosis were detected between control and heparanase-overexpressing tumors). Importantly, treatment of NC-stimulated macrophages with active heparanase resulted in induction of key cytokines implicated in PDAC (including IL-6) and facilitated their ability to activate STAT3 (a cornerstone of pancreatic carcinogenesis acting downstream IL-6 [6]) and to augment proliferation of pancreatic carcinoma cells in vitro. In further agreement with the in vitro studies, activation of STAT3 was observed in experimental and clinical specimens of PDAC with increased heparanase levels.
Although our findings validate the hypothesized role of the enzyme in shaping tumor-promoting action of macrophages, a limitation of the present study is that the exact mechanism of heparanase-mediated change in macrophage phenotype is not fully elucidated. One intriguing possibility is that the intact extracellular HS, enzymatic substrate of heparanase, inhibits TLR4 responses and macrophage activation, while its removal relieves this inhibition (52), most likely by increasing accessibility of the TLR to its ligands. This assumption is consistent with the recently reported ability of heparanase to increase accessibility of the adhesion molecules presented on endothelial surface, by means of HS degradation (Supplementary Figure 6, available online) (53). On the other hand, soluble HS fragments themselves were found to stimulate TLR (in particular TLR4) signaling in vitro (52,54,55) and in pancreatic tissue in vivo (56). Importantly, it was previously demonstrated that heparanase is capable of releasing bioactive HS degradation fragments from the ECM/cell surface (16,57). While further investigation is warranted, our present study reveals a novel role of heparanase in the molecular decision-making process that guides changes in the TAM phenotype during tumor development. Moreover, it implies that heparanase expression status may become highly relevant in defining target patient subgroup that is likely to benefit the most from treatment modalities based on TAM/IL-6 signaling targeting, which are currently under intensive preclinical/clinical validation in PDAC and other tumor types (58).
Funding
This work was supported by grants (to ME) from the Israel Cancer Research Fund (ICRF), Israel Science Foundation (grant 806/14), and the National Cancer Institute, National Institutes of Health (grant RO1-CA106456-10).
Supplementary Material
The study sponsors had no role in the design of the study, the collection, analysis, or interpretation of the data, the writing of the manuscript, nor the decision to submit the manuscript for publication.
We are grateful to Dr. Israel Vlodavsky (Rappoport Faculty of Medicine, Technion, Israel) for his continuous help and active collaboration.
The authors have no conflicts of interest to declare.
References
- 1. Raimondi S, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an overview. Nat Rev Gastroenterol Hepatol. 2009;6(12):699–708. [DOI] [PubMed] [Google Scholar]
- 2. Farrow B, Evers BM. Inflammation and the development of pancreatic cancer. Surg Oncol. 2002;10(4):153–169. [DOI] [PubMed] [Google Scholar]
- 3. Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67(19):9518–9527. [DOI] [PubMed] [Google Scholar]
- 4. Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res 2012;18(16):4266–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mitchem JB, Brennan DJ, Knolhoff BL, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73(3):1128–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baumgart S, Ellenrieder V, Fernandez-Zapico ME. Oncogenic transcription factors: cornerstones of inflammation-linked pancreatic carcinogenesis. Gut. 2013;62(2):310–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ino Y, Yamazaki-Itoh R, Shimada K, et al. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer. 2013;108(4):914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86(5):1065–1073. [DOI] [PubMed] [Google Scholar]
- 9. Lesina M, Kurkowski MU, Ludes K, et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19(4):456–469. [DOI] [PubMed] [Google Scholar]
- 10. Fukuda A, Wang SC, Morris JP, 4th, et al. Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer Cell. 2011;19(4):441–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. [DOI] [PubMed] [Google Scholar]
- 12. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. [DOI] [PubMed] [Google Scholar]
- 13. Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iozzo RV, Sanderson RD. Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. J Cell Mol Med. 2011;15(5):1013–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Theocharis AD, Skandalis SS, Tzanakakis GN, Karamanos NK. Proteoglycans in health and disease: novel roles for proteoglycans in malignancy and their pharmacological targeting. FEBS J. 2010;277(19):3904–3923. [DOI] [PubMed] [Google Scholar]
- 16. Kato M, Wang H, Kainulainen V, et al. Physiological degradation converts the soluble syndecan-1 ectodomain from an inhibitor to a potent activator of FGF-2. Nat Med. 1998;4(6):691–697. [DOI] [PubMed] [Google Scholar]
- 17. Ramani VC, Purushothaman A, Stewart MD, et al. The heparanase/syndecan-1 axis in cancer: mechanisms and therapies. Febs J. 2013;280(10):2294–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goldberg R, Meirovitz A, Hirshoren N, et al. Versatile role of heparanase in inflammation. Matrix Biol. 2013;32(5):234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koliopanos A, Friess H, Kleeff J, et al. Heparanase expression in primary and metastatic pancreatic cancer. Cancer Res. 2001;61(12):4655–4659. [PubMed] [Google Scholar]
- 20. Hoffmann AC, Mori R, Vallbohmer D, et al. High expression of heparanase is significantly associated with dedifferentiation and lymph node metastasis in patients with pancreatic ductal adenocarcinomas and correlated to PDGFA and via HIF1a to HB-EGF and bFGF. J Gastrointest Surg. 2008;12(10):1674–1681; discussion 1681–2. [DOI] [PubMed] [Google Scholar]
- 21. Rohloff J, Zinke J, Schoppmeyer K, et al. Heparanase expression is a prognostic indicator for postoperative survival in pancreatic adenocarcinoma. Br J Cancer. 2002;86(8):1270–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Quiros RM, Rao G, Plate J, et al. Elevated serum heparanase-1 levels in patients with pancreatic carcinoma are associated with poor survival. Cancer. 2006;106(3):532–540. [DOI] [PubMed] [Google Scholar]
- 23. Brun R, Naroditsky I, Waterman M, et al. Heparanase expression by Barrett’s epithelium and during esophageal carcinoma progression. Mod Pathol. 2009;22(12):1548–1554. [DOI] [PubMed] [Google Scholar]
- 24. El-Assal ON, Yamanoi A, Ono T, Kohno H, Nagasue N. The clinicopathological significance of heparanase and basic fibroblast growth factor expressions in hepatocellular carcinoma. Clin Cancer Res. 2001;7(5):1299–1305. [PubMed] [Google Scholar]
- 25. Zhang Y, Li L, Wang Y, et al. Downregulating the expression of heparanase inhibits the invasion, angiogenesis and metastasis of human hepatocellular carcinoma. Biochem Biophys Res Commun. 2007;358(1):124–129. [DOI] [PubMed] [Google Scholar]
- 26. Xiong Z, Lu MH, Fan YH, et al. Downregulation of heparanase by RNA interference inhibits invasion and tumorigenesis of hepatocellular cancer cells in vitro and in vivo. Int J Oncol. 2012;40(5):1601–1609. [DOI] [PubMed] [Google Scholar]
- 27. Nobuhisa T, Naomoto Y, Ohkawa T, et al. Heparanase expression correlates with malignant potential in human colon cancer. J Cancer Res Clin Oncol. 2005;131(4):229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lerner I, Hermano E, Zcharia E, et al. Heparanase powers a chronic inflammatory circuit that promotes colitis-associated tumorigenesis in mice. J Clin Invest. 2011;121(5):1709–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Waterman M, Ben-Izhak O, Eliakim R, Groisman G, Vlodavsky I, Ilan N. Heparanase upregulation by colonic epithelium in inflammatory bowel disease. Mod Pathol. 2007;20(1):8–14. [DOI] [PubMed] [Google Scholar]
- 30. Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339(6117):286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Park HD, Lee Y, Oh YK, et al. Pancreatic adenocarcinoma upregulated factor promotes metastasis by regulating TLR/CXCR4 activation. Oncogene. 2011;30(2):201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yu L, Wang L, Chen S. Exogenous or endogenous Toll-like receptor ligands: which is the MVP in tumorigenesis? Cell Mol Life Sci. 2012;69(6):935–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim S, Takahashi H, Lin WW, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457(7225):102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cocco RE, Ucker DS. Distinct modes of macrophage recognition for apoptotic and necrotic cells are not specified exclusively by phosphatidylserine exposure. Mol Biol Cell. 2001;12(4):919–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hiraoka N, Ino Y, Sekine S, et al. Tumour necrosis is a postoperative prognostic marker for pancreatic cancer patients with a high interobserver reproducibility in histological evaluation. Br J Cancer. 2010;103(7):1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ijichi H, Chytil A, Gorska AE, et al. Inhibiting Cxcr2 disrupts tumor-stromal interactions and improves survival in a mouse model of pancreatic ductal adenocarcinoma. J Clin Invest. 2011;121(10):4106–4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kumar V, Abbas A, Fausto N, eds. Pathologic Basis of Disease. 7th ed: Elsevier Saunders; 2005. [Google Scholar]
- 38. Jones SA, Scheller J, Rose-John S. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J Clin Invest. 2011;121(9):3375–3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zcharia E, Jia J, Zhang X, et al. Newly generated heparanase knock-out mice unravel co-regulation of heparanase and matrix metalloproteinases. PLos One. 2009;4(4):e5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Duff MD, Mestre J, Maddali S, Yan ZP, Stapleton P, Daly JM. Analysis of gene expression in the tumor-associated macrophage. J Surg Res. 2007;142(1):119–128. [DOI] [PubMed] [Google Scholar]
- 41. Wang R, Lu M, Zhang J, et al. Increased IL-10 mRNA expression in tumor-associated macrophage correlated with late stage of lung cancer. J Exp Clin Cancer Res. 2011;30:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fujiwara T, Fukushi J, Yamamoto S, et al. Macrophage infiltration predicts a poor prognosis for human ewing sarcoma. Am J Pathol. 2011;179(3):1157–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kelly T, Miao HQ, Yang Y, et al. High heparanase activity in multiple myeloma is associated with elevated microvessel density. Cancer Res. 2003;63(24):8749–8756. [PubMed] [Google Scholar]
- 44. Leenen PJ, de Bruijn MF, Voerman JS, Campbell PA, van Ewijk W. Markers of mouse macrophage development detected by monoclonal antibodies. J Immunol Methods. 1994;174(1–2): 5–19. [DOI] [PubMed] [Google Scholar]
- 45. Proskuryakov SY, Gabai VL. Mechanisms of tumor cell necrosis. Curr Pharm Des. 2010;16(1):56–68. [DOI] [PubMed] [Google Scholar]
- 46. Bauer C, Bauernfeind F, Sterzik A, et al. Dendritic cell-based vaccination combined with gemcitabine increases survival in a murine pancreatic carcinoma model. Gut. 2007;56(9):1275–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lunardi S, Muschel RJ, Brunner TB. The stromal compartments in pancreatic cancer: Are there any therapeutic targets? Cancer Lett. 2014;343(2):147–155. [DOI] [PubMed] [Google Scholar]
- 48. Kim DW, Min HS, Lee KH, et al. High tumour islet macrophage infiltration correlates with improved patient survival but not with EGFR mutations, gene copy number or protein expression in resected non-small cell lung cancer. Br J Cancer. 2008;98(6):1118–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hunter KE, Palermo C, Kester JC, et al. Heparanase promotes lymphangiogenesis and tumor invasion in pancreatic neuroendocrine tumors. Oncogene. 2014;33(14):1799–1808. [DOI] [PubMed] [Google Scholar]
- 50. Ochi A, Nguyen AH, Bedrosian AS, et al. MyD88 inhibition amplifies dendritic cell capacity to promote pancreatic carcinogenesis via Th2 cells. J Exp Med. 2012;209(9):1671–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wu Y, Lu J, Antony S, et al. Activation of TLR4 Is Required for the Synergistic Induction of Dual Oxidase 2 and Dual Oxidase A2 by IFN-gamma and Lipopolysaccharide in Human Pancreatic Cancer Cell Lines. J Immunol. 2013;190(4):1859–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Brunn GJ, Bungum MK, Johnson GB, Platt JL. Conditional signaling by Toll-like receptor 4. Faseb J. 2005;19(7):872–874. [DOI] [PubMed] [Google Scholar]
- 53. Schmidt EP, Yang Y, Janssen WJ, et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med. 2012;18(8):1217–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Johnson GB, Brunn GJ, Kodaira Y, Platt JL. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J Immunol. 2002;168(10):5233–5239. [DOI] [PubMed] [Google Scholar]
- 55. Yu L, Wang L, Chen S. Endogenous toll-like receptor ligands and their biological significance. J Cell Mol Med. 2010;14(11):2592–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Akbarshahi H, Axelsson JB, Said K, Malmstrom A, Fischer H, Andersson R. TLR4 dependent heparan sulphate-induced pancreatic inflammatory response is IRF3-mediated. J Transl Med. 2011;9:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Elkin M, Ilan N, Ishai-Michaeli R, et al. Heparanase as mediator of angiogenesis: mode of action. Faseb J. 2001;15(9):1661–1663. [DOI] [PubMed] [Google Scholar]
- 58. Fang H, Declerck YA. Targeting the tumor microenvironment: from understanding pathways to effective clinical trials. Cancer Res. 2013;73(16):4965–4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







