Abstract
Background. We aimed to characterize seminal hepatitis C virus (HCV) RNA dynamics in human immunodeficiency virus (HIV)–positive men with acute HCV infection given its potential role in sexual transmission of HCV.
Methods. Men with acute HCV infection (duration, ≤12 months) or chronic HCV infection (duration, >12 months) were prospectively recruited. Paired semen and blood samples were assayed for HCV RNA levels. Results were analyzed using χ2, Fisher exact, Mann–Whitney U, and Kruskal–Wallis tests.
Results. Eighteen men (27.3%) had acute HCV and HIV coinfection, 22 (33.3%) had chronic HCV infection and HIV coinfection, and 26 (39.4%) had chronic HCV monoinfection. HCV RNA was detected in semen specimens from 29 of 66 men (43.9%). The median HCV RNA level in blood was 4.0 log IU/mL higher than that in semen. HCV RNA levels were correlated in semen and blood (r2 = 0.142). Neither HIV positivity nor acute HCV infection was associated with an increased frequency of seminal HCV RNA detection. Among men with acute HCV and HIV coinfection, the median HCV RNA level in blood specimens from those with seminal HCV RNA was higher than that in blood specimens from those without seminal HCV RNA (P = .001). Seminal HCV RNA was detected in ≥1 sample for 26 of 35 men (74.3%) attending follow up.
Conclusions. HCV RNA was detected in semen during both acute and chronic HCV infection. This was unaffected by HIV positivity or the phase of HCV infection. Elevated seminal HCV RNA levels could contribute to sexual transmission of HCV, but other factors, including high-risk behaviors, may be the main drivers for HCV transmission in HIV-infected individuals.
Keywords: hepatitis C virus, HIV, sexual transmission of HCV, semen, men who have sex with men
Increasing incidence rates of acute hepatitis C virus (HCV) infection in human immunodeficiency virus (HIV)–positive men who have sex with men (MSM) have been reported in many industrialized nations [1–6]. Transmission occurs predominantly through the permucosal route, with most individuals denying injection drug use (IDU). This implies transmission across an intact or disrupted mucosal barrier, usually following sexual exposure. Among HIV-uninfected heterosexuals, sexual transmission of HCV is rare, with one study estimating a frequency of 1 transmission per 190 000 sexual acts [7]. Sexual transmission of HCV is considered to be less efficient than for HIV, probably owing to a lower level of virus in genital secretions and the absence of suitable target cells in the anogenital mucosal [8].
For HIV-infected MSM, the biological mechanism underlying sexual transmission of HCV remains unclear, although is likely related to multiple factors. Behavioral factors include serosorting with respect to HIV status, involvement in high-risk sexual activities, and use of permucosally administered recreational drugs. Biological factors include HIV coinfection and sexually transmitted infections (STIs), particularly ulcerative mucocutaneous infections, such as syphilis and lymphogranuloma venereum [9–11]. Permucosal transmission may be related to direct transfer of virus in blood present during sex [10], exposure to fomites [12, 13], or exposure to semen containing virus [14].
Although several studies have examined seminal HCV RNA levels, the dynamics of HCV in semen remain poorly characterized. Early studies failed to identify seminal HCV RNA in men with chronic HCV infection [15, 16], although most published since 2000 reported detection rates of 10%–40% [14, 17, 18]. Seminal levels of HCV RNA in HIV-infected men may be elevated [14], although the data are conflicting [19]. A possible relationship between HCV RNA levels in blood and semen has also been identified [14, 17, 20]. Of those studies examining RNA levels longitudinally, most describe an intermittent process [14, 21, 22].
Only 1 study reported seminal HCV RNA levels in acute HCV infection [23], with similarly low levels of detection in acute and chronic infection. However, it could be postulated that acute infection differs from chronic infection, owing to fluctuating viral dynamics in blood among individuals with acute infection [24].
No study has examined the possible relationship between STIs and seminal HCV RNA level, although epidemiological data imply an association between STIs and incident HCV infection in HIV-positive MSM engaging in high-risk sex but denying IDU [1, 2, 9–11, 25]. Furthermore, data from HIV-monoinfected men suggest an association between seminal HIV RNA level and concomitant STIs [26, 27].
We aimed to characterize the dynamics of seminal HCV RNA levels in acute men with acute HCV and HIV coinfection through comparison of these data with those for individuals with chronic HCV, with or without HIV coinfection. We also sought to describe the possible relationship between STIs and seminal HCV RNA levels.
METHODS
This study was conducted according to the Declaration of Helsinki and approved by the research ethics committee of St Vincent's Hospital (Sydney, Australia).
Study Design
This was a longitudinal cohort study of HCV RNA–positive men not currently receiving therapy for HCV who were recruited prospectively between November 2009 and October 2013 from the hepatitis clinic at St Vincent's Hospital.
Participant Selection
Seventy men participated and were assigned to one of 3 groups: acute or recent HCV infection plus HIV-1 infection (AHCV/HIV+), chronic HCV infection plus HIV-1 infection (CHCV/HIV+), and chronic HCV infection plus no HIV infection (CHCV/HIV−). A fourth group (acute HCV infection plus no HIV infection) comprised only 4 individuals and was therefore excluded from subsequent analysis. Subjects with chronic HCV infection were those who tested positive for HCV RNA for >12 months. Subjects with acute or recent HCV infection were those who tested positive for anti-HCV antibody or HCV RNA ≤6 months before enrollment and had either (1) acute clinical infection (defined as symptomatic seroconversion or an alanine aminotransferase level of >10 times the upper limit of normal, excluding other causes of acute hepatitis, within 12 months before enrollment) or (2) asymptomatic HCV infection with seroconversion (defined as a negative result of a test for anti-HCV antibody during the 12 months before the initial positive result of a test for anti-HCV antibody).
The date of HCV acquisition was estimated according to previously described formulae (Supplementary Table 1) [28]. “Duration of infection” was defined as the time from the estimated date of HCV infection to the date of the baseline visit [28]. Individuals were excluded if HCV RNA was undetectable at enrollment. All participants provided written, informed consent for the collection of samples and subsequent analysis. Participants provided demographic details and sexual and drug-use histories through completion of a questionnaire. Clinical and laboratory data were collected from hospital records. The most likely mode of HCV transmission was determined by the clinician at initial screening. When both sexual and IDU-associated exposures occurred within 6 months of the estimated date of acquisition, the IDU route was assigned.
Samples
Paired blood and semen samples were collected in ethylenediaminetetraacetic acid–coated containers from each individual. Repeat samples were available from 35 men between 12 and 24 weeks following their first visit. The reasons that 31 men did not provide repeat samples were as follows: subsequent initiation of anti-HCV therapy (15 cases), failure to reattend the clinic (14 cases), and illness (2 cases). Semen samples were taken to the laboratory, where liquefaction was allowed to occur, and were centrifuged at 800 ×g for 10 minutes. The seminal plasma supernatant was harvested and recentrifuged at 1350 ×g for 15 minutes, and the resultant supernatant was stored at −80°C until required.
After removal of the seminal plasma, the cell pellet, consisting of spermatozoa and inflammatory cells, was resuspended in up to 13 mL of phosphate-buffered saline and centrifuged at 700 ×g for 10 minutes. The supernatant was discarded, and the pellet was resuspended in an additional 1 mL of phosphate-buffered saline. This was divided into 1-mL aliquots and microcentrifuged at 12 000 ×g for 5 minutes. The supernatant was discarded by tipping, and the pellet was stored at −80°C.
Participants were tested for Chlamydia trachomatis and Neisseria gonorrhoeae with nucleic acid amplification testing (NAAT) of first-void urine samples and, for MSM, pharyngeal and rectal samples. Rectal specimens positive for chlamydial infection by NAAT underwent serotyping for lymphogranuloma venereum. Anogenital ulcers were swabbed for detection of herpes simplex virus type 1 (HSV-1) and HSV-2 DNA. All men had Treponema pallidum serologic tests performed.
Detection of HCV
HCV RNA was extracted and quantified in blood specimens by the Abbott m2000 RealTime automated system, according to the manufacturer's instructions.
Validation was performed for quantification of HCV RNA levels in semen by spiking seminal plasma from HCV-uninfected volunteers with blood from a genotype 3a HCV RNA–positive donor with a known HCV RNA level (Roche Ampliprep/Taqman HCV Monitor assay) to produce a reducing HCV RNA concentration of 6, 5, and 4 log IU/mL. Experiments were performed in triplicate. RNA extraction and quantification was performed by the m2000 system. Three further validations were undertaken with blood specimens from donors infected with HCV genotype 1a or 3a, to produce a reducing HCV RNA concentration from 4.5 to 1.0 log IU/mL in HCV RNA–negative seminal plasma. The lower limit of detection was 1.8 log IU/mL. HCV RNA levels were then quantified in participating men in aliquots of 200 µL of seminal plasma or an estimated 100 µL of seminal cell pellet suspended in lysis buffer.
Statistical Analysis
Results were analyzed using the χ2 and Fisher exact tests for categorical variables as appropriate. Mann–Whitney U and Kruskal–Wallis tests were used for continuous variables. The r2 coefficient of determination was used to test the strength of correlation between 2 continuous variables. The Wilcoxon rank test was used to assess for differences between paired continuous variables. Statistically significant findings were defined as those with a P value of <.05.
RESULTS
Participant Characteristics
Baseline characteristics of participants are displayed in Table 1. For the AHCV/HIV+ group, the median duration of HCV infection was 12.5 weeks (interquartile range [IQR], 5.6–24.2 weeks); 2 (11.1%) had an infection duration of ≤4 weeks, 1 (5.6%) was negative for anti-HCV immunoglobulin G, 6 (33.3%) had jaundice, and 2 (11.1%) later cleared HCV spontaneously. Men in the AHCV/HIV+ group were younger (median age, 45.3 years; IQR, 36.5–49.0 years) than those in the CHCV/HIV+ group (median age, 49.7 years; IQR, 43.9–54.3 years) and those in the CHCV/HIV− group (median age, 52.1 years; IQR, 46.8–56.0 years). The AHCV/HIV+ group was more likely to have a clinician-assigned sexual risk for HCV (10/18 [55.6%]), compared with the CHCV/HIV+ group (3/22 [13.6%]) and the CHCV/HIV− group (3/26 [11.5%]). Five of 18 men (27.8%) in the AHCV/HIV+ group who considered themselves to have acquired HCV sexually were assigned an IDU risk by the clinician. Most HIV-infected individuals were MSM (39/40 [97.5%]) and had achieved a suppressed blood HIV RNA level by means of antiretroviral therapy (32/40 [80.0%]). The median blood HCV RNA level in the AHCV/HIV+ group (5.8 log IU/mL; IQR, 4.4–6.2 log IU/mL) was lower than that in the CHCV/HIV+ group (6.4 log IU/mL; IQR, 5.5–6.7 log IU/mL) and the CHCV/HIV− group (6.1 log IU/mL; IQR, 5.6–6.4 log IU/mL). The median ALT level in the AHCV/HIV+ group (121.53 U/L; IQR, 60.0–374.3 U/L) was greater than that in the CHCV/HIV+ group (75.5 U/L; IQR, 42.5–139.8 IU/L) and the CHCV/HIV− group (86.5 U/L; IQR, 47.8–171.0 U/L). Half of AHCV/HIV+ men receiving an STI screen (8/16) had an STI detected, which was considerably more than the percentage in the CHCV/HIV+ group (2/20 [10.0%]) and the CHCV/HIV− group (1/20 [5.0%]). Detailed sexual and drug-use behaviors for the preceding 6 months were available for 16 AHCV/HIV+ men (88.9%). These demonstrate multiple competing risks. Seven men (43.8%) had a lifetime history of IDU (methamphetamine use in 6 cases), of whom 5 (71.4%) reported injecting methamphetamine on a less-than-weekly basis. The median number of sex partners in the preceding six months was 20 (IQR, 8–42 partners); 12 men (75.0%) reported participation in group sex (Supplementary Table 2).
Table 1.
Baseline Characteristics for 66 Participating Men, According to Human Immunodeficiency Virus (HIV) Status and Phase of Hepatitis C Virus (HCV) Infection
| Characteristic | AHCV/HIV+ | CHCV/HIV+ | CHCV/HIV− | All |
|---|---|---|---|---|
| Participants | 18 (27.3) | 22 (33.3) | 26 (39.4) | 66 (100) |
| Age, y | 45.3 (36.5–49.0) | 49.7 (43.9–54.3) | 52.1 (46.8–56.0) | 49.0 (43.8–53.3) |
| Australian born | 13 (72.2) | 18 (81.8) | 19 (73.1) | 50 (75.8) |
| MSM | 18 (100) | 21 (95.5) | 7 (26.9) | 46 (69.7) |
| Route of HCV acquisitiona | ||||
| Injection drug use | 8 (44.4) | 19 (86.4) | 15 (57.7) | 42 (63.6) |
| Sexual | 10 (55.6) | 3 (13.6) | 3 (11.5) | 16 (24.2) |
| Other | 0 | 0 | 8 (30.8) | 8 (12.1) |
| Blood HCV RNA level, log IU/mL | 5.8 (4.4–6.2) | 6.4 (5.5–6.7) | 6.1 (5.6–6.4) | 6.1 (5.5–6.5) |
| HCV genotype | ||||
| 1a | 10 (55.6) | 16 (72.7) | 13 (50.0) | 39 (59.1) |
| 3a | 5 (27.8) | 3 (13.6) | 9 (34.6) | 17 (25.8) |
| 4a | 1 (5.6) | 1 (4.5) | 1 (3.8) | 3 (4.5) |
| Other | 2 (11.1) | 2 (9.1) | 3 (11.5) | 7 (10.6) |
| Seminal plasma volume, mL | 1.0 (0.5–1.3) | 1.0 (0.5–1.5) | 1.0 (0.4–1.5) | 1.0 (0.5–1.5) |
| HIV positive | 18 (100) | 22 (100) | 0 | 40 (60.6) |
| CD4 T-cell cell count, cells/mm3 | 576.0 (337.0–715.8) | 643.5 (455.5–1105.0) | NA | 598.0 (409.5–787.5) |
| Receiving cART, by HIV RNA level | ||||
| Any | 15 (83.3) | 20 (90.9) | NA | 35 (87.5) |
| <50 IU/mL | 12 (80.0) | 20 (100) | NA | 32 (80.0) |
| ALT level, U/L | 121.5 (60.0–374.3) | 75.5 (42.5–139.8) | 86.5 (47.8–171.0) | 91.0 (47.8–174.0) |
| Full STI screen performed | 16 (88.9) | 20 (90.9) | 20 (76.9) | 56 (84.8) |
| Urethral STI | 1 (5.6)b | 0 | 0 | 1 (1.5) |
Data are no. (%) of participants or median value (interquartile range). See Methods for descriptions of the study groups.
Abbreviations: ALT, alanine aminotransferase; cART, combination antiretroviral therapy; MSM, men who have sex with men; NA, not applicable; STI, sexually transmitted infection.
a As determined by the clinician.
b Due to Chlamydia trachomatis.
HCV RNA in Semen
Twenty-nine men (43.9%) had HCV RNA detected in seminal plasma at baseline. Men with detectable seminal HCV RNA had a higher median blood level of HCV RNA (6.2 log IU/mL; IQR, 5.8–6.7 log IU/mL) than those with undetectable seminal HCV RNA (5.8 log IU/mL; IQR, 4.8–6.3 log IU/mL; P = .002, by the Mann–Whitney U test). The frequency of HCV RNA detection in semen was similar between HIV-infected men (15/40 [37.5%]) and HIV-uninfected men (14/26 [53.8%]; P = .191) and between men with acute HCV infection (7/18 [38.9%]) and those with chronic HCV infection (22/48 [45.8%]; P = .613). Urethral STI was not associated with detection of HCV RNA in semen (P = 1.000). Table 2 outlines factors associated with detection of HCV RNA in seminal plasma.
Table 2.
Factors Associated With Detection of Hepatitis C Virus (HCV) RNA in Seminal Plasma for 66 Participants
| Factor | HCV RNA in Seminal Plasma |
P Valuea | |
|---|---|---|---|
| Present | Absent | ||
| Participants | 29 (43.9) | 37 (56.1) | … |
| Age, y | 49.8 (44.8–53.0) | 48.2 (43.7–55.0) | .736 |
| HIV positive | 15 (51.7) | 25 (67.6) | .191 |
| Acute HCV infection | |||
| Present | 7 (24.1) | 11 (29.7) | .613 |
| Duration, wks | 13.7 (7.7–22.4) | 9.1 (5.4–29.5) | .783 |
| Blood HCV RNA level, log IU/mL | 6.2 (5.8–6.7) | 5.8 (4.8–6.3) | .002 |
| HIV parameter | |||
| CD4 T-cell count, cells/mm3 | 580.0 (324.0–754.0) | 663.0 (451.0–1017.0) | .170 |
| HIV RNA level <50 IU mL | 11 (73.3) | 21 (84.0) | .444 |
| HCV genotype | |||
| 1a | 15 (51.7) | 24 (64.9) | .281 |
| 3a | 8 (27.6) | 9 (24.3) | .764 |
| Seminal plasma volume, mL | 1.1 (0.5–2.3) | 0.90 (0.5–1.4) | .143 |
| STI screen performed | 25 (86.2) | 31 (83.8) | … |
| Urethral STI | 0 | 1 (3.2) | 1.000 |
| Sexual mode of HCV acquisition | 6 (20.7) | 10 (27.0) | .551 |
Data are no. (%) of participants or median value (interquartile range).
Abbreviations: HIV, human immunodeficiency virus; STI, sexually transmitted infection.
a By the χ2, Fisher exact, or Mann–Whitney U tests.
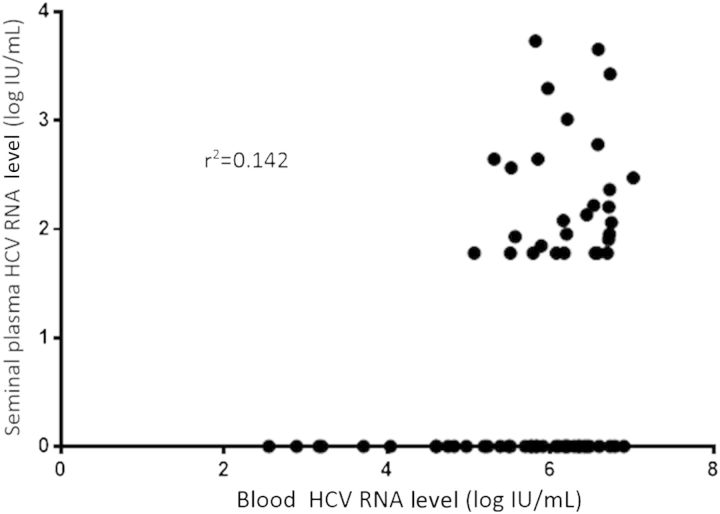
HCV RNA levels were weakly correlated in seminal plasma and blood (r2 = 0.142; P = .002; Figure 1). The blood HCV RNA level had a threshold, 5 log IU/mL, below which seminal HCV RNA was not detected. The seminal HCV RNA level ranged from <1.8 log IU/mL to 3.7 log IU/mL; 5 individuals, all of whom were infected with HIV, had an HCV RNA level of >3 log IU/mL, compared with 0 HIV-uninfected men (5/40 vs 0/26; P = .148). The median HCV RNA level was 4.0 log IU/mL greater in blood than seminal plasma (6.1 log IU/mL [IQR, 5.4–6.5 log IU/mL] vs 2.1 log IU/mL [IQR, 1.8–2.6 log IU/mL]). The median seminal HCV RNA level was similar across the 3 groups, with values of 2.2 log IU/mL (IQR, 1.9–3.3 log IU/mL) in the AHCV/HIV+ group, 2.3 log IU/mL (IQR, 1.8–3.4 log IU/mL) in the CHCV/HIV+ group, and 2.0 log IU/mL (IQR, 1.8–2.4 log IU/mL]) in the CHCV/HIV− group (P = .431, by the Kruskal–Wallis test).
Figure 1.
Correlation of seminal and blood hepatitis C virus (HCV) RNA concentration.
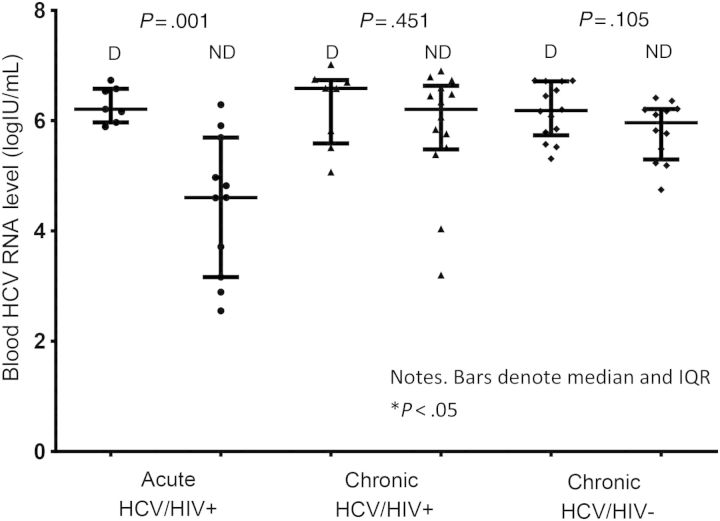
Among men in AHCV/HIV+ group, the median blood HCV RNA level in those with detectable HCV RNA in semen was significantly higher than that in those with undetectable HCV RNA in semen (6.2 log IU/mL [IQR, 6.0–6.6 log IU/mL] vs 4.6 log IU/mL [IQR, 3.2–5.7 log IU/mL]; P = .001), but the difference was not significant in the CHCV/HIV+ group (6.6 log IU/mL [IQR, 5.6–6.7] vs 6.2 log IU/mL [IQR, 5.5–6.6 log IU/mL]; P = .451) or the CHCV/HIV− group (6.2 log IU/mL [IQR, 5.7–6.7 log IU/mL] vs 6.0 log IU/mL [IQR, 5.3–6.2 log IU/mL]; P = .105; Figure 2). A correlation between blood and seminal HCV RNA levels in the AHCV/HIV+ group was observed (r2 = 0.426; P = .003).
Figure 2.
Detection (D) or no detection (ND) of hepatitis C virus (HCV) RNA in seminal plasma according to baseline blood HCV RNA level. See Methods for descriptions of the study groups. Abbreviation: HIV, human immunodeficiency virus.
To determine whether the cellular fraction of semen could also contain HCV that was possibly not detected in the plasma component, the cell pellet was analyzed. Of 11 individuals for whom both seminal plasma and cell pellets were assayed, 4 (36.4%) had detectable HCV RNA in cell pellets, and 6 (54.5%) had detectable HCV RNA in seminal plasma. For 2 men with detectable HCV RNA in seminal plasma, HCV RNA was not detected in the cell pellet. For 4 men, HCV RNA was detectable in both seminal plasma and cell pellets, although at a reduced magnitude in cell pellets (median reduction, 0.6 log IU/mL). For 5 men, HCV RNA was not detected in seminal pellets or plasma. Therefore, reduced detection of HCV RNA in cell pellets versus seminal plasma was observed.
Longitudinal Detection of HCV RNA in Semen
Thirty-five men (53.0%) reattended the clinic at a median duration of 18.0 weeks (IQR, 14.6–25.6 weeks) after enrollment. Median blood HCV RNA levels at baseline (6.0 log IU/mL; IQR, 5.4–6.6 log IU/mL) were similar to those at follow-up (6.0 log IU/mL; IQR, 5.6–6.5 log IU/mL; P = .576, by the Wilcoxon test). Two men (5.7%), both of whom were in the AHCV/HIV+ group, had an STI (rectal chlamydial infection and rectal gonorrhea, respectively). Nine men for whom HCV RNA was undetectable in seminal plasma at baseline had HCV RNA detected during follow-up; of these, 7 were from the CHCV/HIV+ group and 1 each was from the CHCV/HIV− and AHCV/HIV+ groups. Considering both baseline and follow-up samples, 38 of 66 participants (57.6%) had seminal HCV RNA detected at some point.
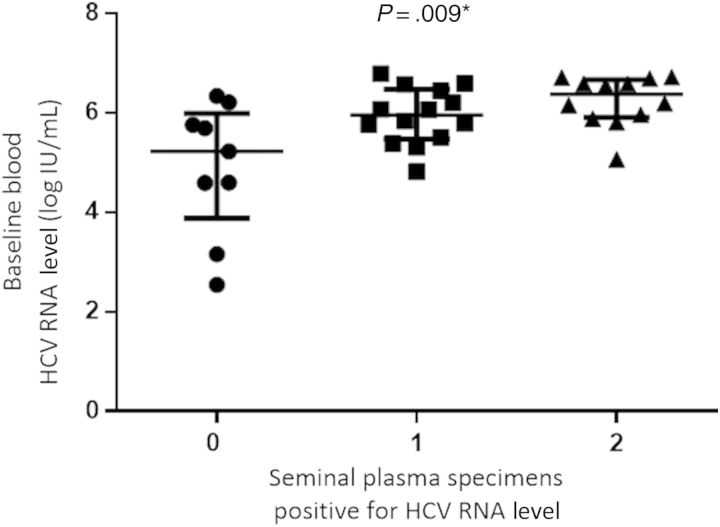
Baseline levels of HCV RNA in blood were associated with longitudinal detection of seminal HCV RNA. Among men with 2, 1, and 0 seminal plasma samples positive for HCV RNA, the level of HCV RNA in blood was 6.4 log IU/mL (IQR, 5.9–6.7 log IU/mL), 6.0 log IU/mL (IQR, 5.5–6.5 log IU/mL), and 5.2 log IU/mL (IQR, 3.9–6.0 log IU/mL), respectively (P = .009, by the Kruskal–Wallis test; Figure 3).
Figure 3.
Longitudinal hepatitis C virus (HCV) RNA detection in seminal plasma according to baseline blood HCV RNA level.
For the AHCV/HIV+ group, a relationship was observed between the baseline HCV RNA level in blood and longitudinal detection of seminal HCV RNA. For men with 2, 1, and 0 semen samples positive for HCV RNA, the level of HCV RNA in blood was 6.0 log IU/mL (IQR, 5.9–6.2 log IU/mL), 6.2 log IU/mL (IQR, 4.8–6.6 log IU/mL), and 4.6 log IU/mL (IQR, 2.9–5.2 log IU/mL), respectively (P = .019). For men with chronic HCV infection, there was no significant difference in baseline HCV RNA level in blood according to the number of seminal specimens in which HCV RNA was detected.
DISCUSSION
Overall, 43.9% of men had seminal HCV RNA detected, with the frequency increasing to 56.7% during longitudinal sampling. A relationship was identified between HCV RNA levels in blood and semen in HIV-infected men with recently acquired HCV (P = .001). HCV RNA levels in this group were correlated, although levels in semen were 4.0 log IU/mL lower than those in blood (r2 = 0.426). Furthermore, longitudinal detection of HCV in semen was associated with HCV RNA levels in blood in the AHCV/HIV+ group (P = .019). These relationships were not identified in men with chronic HCV infection, irrespective of HIV status, or in a previous study of individuals with acute HCV infection and HIV coinfection [23].
As previously reported, a threshold of 5 log IU/mL was identified, below which detection of seminal HCV RNA did not occur [14, 17]. The only factor associated with HCV RNA detection in semen was the HCV RNA level in blood, which suggests that a largely passive process determines seminal dynamics. Seminal HCV RNA levels were generally low (median, 2.1 log IU/mL), although a small but important proportion of men (5/66 [7.6%]) had levels of >3 log IU/mL. Seminal virus may well be infectious, because only 10–20 viral particles are required to establish a productive infection [29, 30]. Nevertheless, the infectiousness of seminal HCV remains to be confirmed, such as by using luciferase reporter assays [31].
This study did not identify HIV coinfection as associated with an increased level of seminal HCV RNA. Although 5 HIV-infected men, compared with 0 HIV-uninfected individuals, had seminal HCV RNA levels of >3 log IU/mL, perhaps implying heightened infectiousness, this difference was not statistically significant (P = .148), and too few HIV-negative individuals with acute HCV infection were identified to examine semen dynamics in this group. Previous reports of the possible role of HIV in determining seminal HCV dynamics are conflicting [14, 19, 32]. Neither CD4 T-cell count nor blood HIV RNA level was associated with HCV RNA detection in this and previous studies, suggesting that any possible interaction is not mediated by immunosuppression. Furthermore, there appears to be no association between the seminal HIV RNA level and the seminal HCV RNA level [21]. Any interaction is likely to be indirect, perhaps mediated by HIV-associated immunoactivation. Alternatively, HIV infection may increase susceptibility to HCV infection, such as through impairment of rectal immune responses, which are permanently depleted following primary HIV infection [33].
The proportion of individuals with detectable seminal HCV RNA rose to 57.6% after inclusion of individuals with undetectable seminal HCV RNA at baseline but detectable HCV RNA at follow-up. This represents a higher level of HCV RNA detection, compared with previous reports [14, 17, 18, 20–22, 32]. For men with 2 clinic visits, 14 of 35 (40.0%) had HCV RNA detected intermittently. Previous studies have also highlighted an intermittent process, which may represent a truly episodic phenomenon or reflect low levels of seminal HCV RNA (median, 2.1 log IU/mL) close to the threshold of detection [14, 21]. Single-time-point assays are therefore likely to underestimate seminal HCV RNA levels. The high level of seminal detection identified here probably also reflects the high sensitivity of the m2000 assay. Future work with the m2000 involving intensive sampling during the very early acute stage of HCV infection could improve characterization of seminal HCV RNA dynamics.
As previously reported, HCV RNA was detected in the seminal pellet at lower levels than for seminal plasma, suggesting that HCV in the male reproductive tract is predominantly extracellular [14, 18, 19]. Briat et al performed limiting-dilution cloning and phylogenetic analysis for 2 individuals with chronic HCV infection and reported clustering of quasispecies in blood and semen [14]. Conventional Sanger sequencing for the HCV core E2 region, followed by phylogenetic analysis, for an individual in the current study also identified clustering between blood-derived and semen-derived sequences (data are available on request). This further supports the notion that HCV is unlikely to replicate in semen. The finding of some individuals with elevated HCV RNA levels in blood but undetectable HCV RNA in semen could be explained by the presence of seminal viremia below the lower limit of detection of the assay (<1.8 log IU/mL), intermittent detection, or preferential transfer of some quasispecies into semen [14].
Urethral STI was not associated with seminal detection of HCV RNA, a finding for which there are several possible explanations. First, although many STIs were observed in the AHCV/HIV+ group, only 1 STI was urethral, so a relationship may have been missed. Second, the epidemiological association of STIs with HCV transmission may represent a marker for high-risk activities. Third, STIs may increase mucosal susceptibility to HCV infection, rather than HCV shedding. Given strong relationships between seminal herpesvirus DNA and seminal HIV-1 RNA, a possible interaction between herpesviruses (eg, HSV-2) and seminal HCV remains to be explored [27, 34].
Several other characteristics of this cohort are noteworthy. First, most in the AHCV/HIV+ group (10/18 [55.6%]) were infected with HCV through the sexual route, implying that permucosal exposure remains the major transmission route in this group and underlining the potential importance of seminal virus. However, a significant minority (8/18 [44.4%]) had an IDU exposure, as in a previous Australian study [35]. IDU-associated exposure to HCV in HIV-positive MSM in other developed countries is probably less common [3, 4, 36]. Interestingly, 5 individuals (27.8%) in the AHCV/HIV+ group considered themselves to have acquired HCV sexually but were assigned an IDU route by the clinician, raising the possibility that estimates of sexual transmission may be conservative in Australia. Men in the CHCV/HIV+ group were more likely to have acquired HCV through IDU than those in the AHCV/HIV+ group, although the difficulty of establishing the mode of transmission many years after HCV acquisition may make a comparison problematic. However, sexual transmission could represent an increasingly important mode of transmission among HIV-positive MSM in recent years [1]. Second, a majority of those in the AHCV/HIV+ group reported participating in group sex, which is known to be associated with HCV transmission [9]. Third, as noted previously, methamphetamine was the preferred injected drug for those reporting IDU [9].
The study has limitations, particularly with regard to uncertainty about true date of infection and the small number of individuals in the very early stages of HCV infection. Only 1 individual with early acute HCV infection was identified (duration, <4 weeks), and only 1 man had a very high level of HCV RNA in blood (>7 log IU/mL). It is not, therefore, possible to ascertain whether, during very early acute HCV infection, extremely high HCV RNA levels in blood may translate into higher HCV levels in semen, thus representing a period of enhanced infectiousness.
In our study, presentation of recently acquired HCV infection was variable, as reflected in the wide IQRs for the baseline HCV RNA level in blood (median, 5.8 log IU/mL; IQR, 4.4–6.2 log IU/mL) and ALT level (median, 121.5 U/L; IQR, 60.0–374.3 U/L), compared with values for men with chronic HCV infection. HCV RNA levels in blood in the AHCV/HIV+ group were lower than in the CHCV/HIV+ group, probably because of low numbers of subjects with early acute infection, which is known to be associated with elevated HCV RNA levels. Additionally, the group contained several individuals with very low HCV RNA levels, consistent with the period of fluctuating viremia frequently observed during the first few months of HCV infection. This is likely to have reduced the overall median HCV RNA level. Nevertheless, the proportion with spontaneous clearance of HCV (2/18 [11.1%]) was at the lower bound of the range reported for other HIV-positive cohorts (0%–40%) [37]. The lack of individuals included with very early infection reflects the difficulties with diagnosis of acute HCV infection, in which many individuals are asymptomatic and identified through (often relatively moderate) increases in the ALT level. Uncertainty around the true date of infection is common, especially for the majority of patients who do not present with jaundice, as was typical in our study. Nevertheless, despite the broader eligibility criteria than in many studies, most patients enrolled had had infection for ≤6 months (ie, acute infection), as calculated through standard methods.
This study has important implications for public health interventions, particularly in view of the urgent need for effective interventions to reduce HCV transmission in HIV-positive MSM. Current sources of information for MSM reflect the uncertainty surrounding the role of seminal virus for HCV transmission. This study highlights that seminal HCV RNA can be detected in individuals with both recently acquired and chronic HCV infection. It is plausible that high HCV RNA levels in semen could translate into an increased risk of sexual transmission. It is important to note, however, that because neither HIV infection nor recent HCV infection was significantly associated with seminal HCV RNA detection, sexual transmission of HCV may not be driven primarily by seminal virus. More likely, transmission occurs through a combination of factors, with seminal HCV RNA playing a role in the context of high-risk behaviors. Nonetheless, given that HCV infections in many individuals with recently acquired HCV are undiagnosed, safer-sex messages should be reinforced, particularly with regard to condom use. Equally, HIV-infected MSM with HCV coinfection, especially those with high levels of HCV RNA in blood, should be counseled regarding their possible heightened sexual infectiousness. These messages should complement existing strategies for reducing transmission risks associated with permucosal blood exposure from traumatic sexual practices and parenteral exposure from IDU.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Rebecca Hickey and the staff and patients of the Immunology and Infectious Diseases B Ambulatory Care Unit, St Vincent's Hospital; the staff of the Viral Hepatitis Clinical Research Programme, Kirby Institute, UNSW Australia (Sydney, Australia); and the HIV Immunovirology Laboratory, St Vincent's Hospital.
Financial support. This work was supported by the Australian National Health and Medical Research Council (grant 568859).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Wandeler G, Gsponer T, Bregenzer A, et al. Hepatitis C Virus Infections in the Swiss HIV Cohort Study: A Rapidly Evolving Epidemic. Clin Infect Dis. 2012;55:1408–16. doi: 10.1093/cid/cis694. [DOI] [PubMed] [Google Scholar]
- 2.Sun H-Y, Chang S-Y, Yang Z-Y, et al. Recent Hepatitis C Virus Infections in HIV-Infected Patients in Taiwan: Incidence and Risk Factors. J Clin Microbiol. 2012;50:781–7. doi: 10.1128/JCM.06014-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishijima T, Shimbo T, Komatsu H, et al. Incidence and risk factors for incident hepatitis C infection among men who have sex with men with HIV-1 infection in a large urban HIV clinic in Tokyo. J Acquir Immune Defic Syndr. 2014;65:213–7. doi: 10.1097/QAI.0000000000000044. [DOI] [PubMed] [Google Scholar]
- 4.van der Helm JJ, Prins M, del Amo J, et al. The hepatitis C epidemic among HIV-positive MSM: incidence estimates from 1990 to 2007. AIDS. 2011;25:1083–91. doi: 10.1097/QAD.0b013e3283471cce. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez C, Plaza Z, Vispo E, et al. Scaling up epidemics of acute hepatitis C and syphilis in HIV-infected men who have sex with men in Spain. Liver Int. 2013;33:1357–62. doi: 10.1111/liv.12212. [DOI] [PubMed] [Google Scholar]
- 6.van de Laar TJ, van der Bij AK, Prins M, et al. Increase in HCV incidence among men who have sex with men in Amsterdam most likely caused by sexual transmission. J Infect Dis. 2007;196:230–8. doi: 10.1086/518796. [DOI] [PubMed] [Google Scholar]
- 7.Terrault NA, Dodge JL, Murphy EL, et al. Sexual transmission of hepatitis C virus among monogamous heterosexual couples: the HCV partners study. Hepatology. 2013;57:881–9. doi: 10.1002/hep.26164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terrault NA. Sexual activity as a risk factor for hepatitis C. Hepatology. 2002;36:S99–105. doi: 10.1053/jhep.2002.36797. [DOI] [PubMed] [Google Scholar]
- 9.Danta M, Brown D, Bhagani S, et al. Recent epidemic of acute hepatitis C virus in HIV-positive men who have sex with men linked to high-risk sexual behaviours. AIDS. 2007;21:983–91. doi: 10.1097/QAD.0b013e3281053a0c. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt AJ, Rockstroh JK, Vogel M, et al. Trouble with bleeding: risk factors for acute hepatitis C among HIV-positive gay men from Germany--a case-control study. PLoS One. 2011;6:e17781. doi: 10.1371/journal.pone.0017781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sexual transmission of hepatitis C virus among HIV-infected men who have sex with men--New York City, 2005–2010. MMWR Morb Mortal Wkly Rep. 2011;60:945–50. [PubMed] [Google Scholar]
- 12.Macias J, Palacios RB, Claro E, et al. High prevalence of hepatitis C virus infection among noninjecting drug users: association with sharing the inhalation implements of crack. Liver Int. 2008;28:781–6. doi: 10.1111/j.1478-3231.2008.01688.x. [DOI] [PubMed] [Google Scholar]
- 13.de Vries HJ, van der Bij AK, Fennema JS, et al. Lymphogranuloma venereum proctitis in men who have sex with men is associated with anal enema use and high-risk behavior. Sex Transm Dis. 2008;35:203–8. doi: 10.1097/OLQ.0b013e31815abb08. [DOI] [PubMed] [Google Scholar]
- 14.Briat A, Dulioust E, Galimand J, et al. Hepatitis C virus in the semen of men coinfected with HIV-1: Prevalence and origin. AIDS. 2005;19:1827–35. doi: 10.1097/01.aids.0000189847.98569.2d. [DOI] [PubMed] [Google Scholar]
- 15.Semprini AE, Persico T, Thiers V, et al. Absence of hepatitis C virus and detection of hepatitis G virus/GB virus C RNA sequences in the semen of infected men. J Infect Dis. 1998;177:848–54. doi: 10.1086/515257. [DOI] [PubMed] [Google Scholar]
- 16.Hsu HH, Wright TL, Luba D, et al. Failure to detect hepatitis C virus genome in human secretions with the polymerase chain reaction. Hepatology. 1991;14:763–7. doi: 10.1002/hep.1840140504. [DOI] [PubMed] [Google Scholar]
- 17.Pekler VA, Robbins WA, Nyamathi A, Yashina TL, Leak B, Robins TA. Use of versant™ TMA and bDNA 3.0 assays to detect and quantify hepatitis C virus in semen. J Clin Lab Anal. 2003;17:264–70. doi: 10.1002/jcla.10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bourlet T, Lornage J, Maertens A, et al. Prospective evaluation of the threat related to the use of seminal fractions from hepatitis C virus-infected men in assisted reproductive techniques. Hum Reprod. 2009;24:530–5. doi: 10.1093/humrep/den414. [DOI] [PubMed] [Google Scholar]
- 19.Halfon P, Giorgetti C, Bourliere M, et al. Medically assisted procreation and transmission of hepatitis C virus: absence of HCV RNA in purified sperm fraction in HIV co-infected patients. AIDS. 2006;20:241–6. doi: 10.1097/01.aids.0000200532.56490.fe. [DOI] [PubMed] [Google Scholar]
- 20.Bourlet T, Levy R, Maertens A, et al. Detection and characterization of hepatitis C virus RNA in seminal plasma and spermatozoon fractions of semen from patients attempting medically assisted conception. J Clin Microbiol. 2002;40:3252–5. doi: 10.1128/JCM.40.9.3252-3255.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasquier C, Bujan L, Daudin M, et al. Intermittent detection of hepatitis C virus (HCV) in semen from men with human immunodeficiency virus type 1 (HIV-1) and HCV. J Med Virol. 2003;69:344–9. doi: 10.1002/jmv.10295. [DOI] [PubMed] [Google Scholar]
- 22.Cassuto NG, Sifer C, Feldmann G, et al. A modified RT-PCR technique to screen for viral RNA in the semen of hepatitis C virus-positive men. Hum Reprod. 2002;17:3153–6. doi: 10.1093/humrep/17.12.3153. [DOI] [PubMed] [Google Scholar]
- 23.Turner J, Aarons E, O'Farrell S, et al. Hepatitis C viral loads in the semen of HIV positive men during acute and chronic hepatitis C infection. Presented at: 2nd Joint Conference BHIVA/BASHH; 20–23 April, Oral No. 5, Manchester, UK.2010. [Google Scholar]
- 24.McGovern BH, Birch CE, Bowen MJ, et al. Improving the diagnosis of acute hepatitis C virus infection with expanded viral load criteria. Clin Infect Dis. 2009;49:1051–60. doi: 10.1086/605561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gotz HM, van Doornum G, Niesters HG, den Hollander JG, Thio HB, de Zwart O. A cluster of acute hepatitis C virus infection among men who have sex with men--results from contact tracing and public health implications. AIDS. 2005;19:969–74. doi: 10.1097/01.aids.0000171412.61360.f8. [DOI] [PubMed] [Google Scholar]
- 26.Politch JA, Mayer KH, Welles SL, et al. Highly active antiretroviral therapy does not completely suppress HIV in semen of sexually active HIV-infected men who have sex with men. AIDS. 2012;26:1535–43. doi: 10.1097/QAD.0b013e328353b11b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zuckerman RA, Lucchetti A, Whittington WL, et al. HSV suppression reduces seminal HIV-1 levels in HIV-1/HSV-2 co-infected men who have sex with men. AIDS. 2009;23:479–83. doi: 10.1097/QAD.0b013e328326ca62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matthews GV, Hellard M, Haber P, et al. Characteristics and treatment outcomes among HIV-infected individuals in the Australian Trial in Acute Hepatitis C. Clin Infect Dis. 2009;48:650–8. doi: 10.1086/596770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shata MT, Tricoche N, Perkus M, et al. Exposure to low infective doses of HCV induces cellular immune responses without consistently detectable viremia or seroconversion in chimpanzees. Virology. 2003;314:601–16. doi: 10.1016/s0042-6822(03)00461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katayama K, Kumagai J, Komiya Y, et al. Titration of hepatitis C virus in chimpanzees for determining the copy number required for transmission. Intervirology. 2004;47:57–64. doi: 10.1159/000076643. [DOI] [PubMed] [Google Scholar]
- 31.Doerrbecker J, Behrendt P, Mateu-Gelabert P, et al. Transmission of hepatitis C virus among people who inject drugs: viral stability and association with drug preparation equipment. J Infect Dis. 2013;207:281–7. doi: 10.1093/infdis/jis677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leruez-Ville M, Kunstmann JM, De Almeida M, et al. Detection of hepatitis C virus in the semen of infected men. Lancet. 2000;356:42–3. doi: 10.1016/S0140-6736(00)02435-1. [DOI] [PubMed] [Google Scholar]
- 33.Rueda CM, Velilla PA, Chougnet CA, et al. HIV-induced T-cell activation/exhaustion in rectal mucosa is controlled only partially by antiretroviral treatment. PLoS One. 2012;7:e30307. doi: 10.1371/journal.pone.0030307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gianella S, Smith DM, Vargas MV, et al. Shedding of HIV and human herpesviruses in the semen of effectively treated HIV-1-infected men who have sex with men. Clin Infect Dis. 2013;57:441–7. doi: 10.1093/cid/cit252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matthews GV, Pham ST, Hellard M, et al. Patterns and characteristics of hepatitis C transmission clusters among HIV-positive and HIV-negative individuals in the Australian trial in acute hepatitis C. Clin Infect Dis. 2011;52:803–11. doi: 10.1093/cid/ciq200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor LE, Holubar M, Wu K, et al. Incident Hepatitis C Virus Infection among US HIV-Infected Men Enrolled in Clinical Trials. Clin Infect Dis. 2011;52:812–8. doi: 10.1093/cid/ciq201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Acute hepatitis C in HIV-infected individuals: recommendations from the European AIDS Treatment Network (NEAT) consensus conference; AIDS; 2011. pp. 399–409. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.