Abstract
Methylmercury, polychlorinated biphenyls (PCBs), and perfluorinated compounds (PFCs) are ubiquitous and persistent environmental chemicals with known or suspected toxic effects on the nervous system and the immune system. Animal studies have shown that tissue damage can elicit production of autoantibodies. However, it is not known if autoantibodies similarly will be generated and detectable in humans following toxicant exposures. Therefore, we conducted a pilot study to investigate if autoantibodies specific for neural and non-neural antigens could be detected in children at age 7 years who have been exposed to environmental chemicals. Both prenatal and age-7 exposures to mercury, PCBs, and PFCs were measured in 38 children in the Faroe Islands who were exposed to widely different levels of these chemicals due to their seafood-based diet. Concentrations of IgM and IgG autoantibodies specific to both neural (neurofilaments, cholineacetyltransferase, astrocyte glial fibrillary acidic protein, and myelin basic protein) and non-neural (actin, desmin, and keratin) antigens were measured and the associations of these autoantibody concentrations with chemical exposures were assessed using linear regression. Age-7 blood-mercury concentrations were positively associated with titers of multiple neural- and non-neural-specific antibodies, mostly of the IgM isotype. Additionally, prenatal blood-mercury and -PCBs were negatively associated with anti-keratin IgG and prenatal PFOS was negatively associated with anti-actin IgG. These exploratory findings demonstrate that autoantibodies can be detected in the peripheral blood following exposure to environmental chemicals. The unexpected association of exposures with antibodies specific for non-neural antigens suggests that these chemicals may have toxicities that have not yet been recognized.
Keywords: autoantibodies, mercury, polychlorinated biphenyls, perfluorinated compounds, prenatal, childhood, neurotoxicity, immunotoxicity
Methylmercury, polychlorinated biphenyls (PCBs), and perfluorinated compounds (PFCs) are environmental chemicals that are toxic to both the nervous system and the immune system (Crinnion, 2011; DeWitt et al., 2012; Hong et al., 2012; Post et al., 2012). Methylmercury is primarily known as a neurotoxicant (Hong et al., 2012), but it also is known to accumulate in numerous non-neural tissues (Nielsen et al., 1994; Rodrigues et al., 2010; Usuki et al., 1998). PCBs, which are also known to be neurotoxic (Crinnion, 2011), may affect many different cell types through interaction with their cellular receptors (aryl hydrocarbon receptor and ryanodine receptors), which are broadly expressed (Gasiewicz and Rucci, 1984; Igarashi et al., 1999; Lanner et al., 2010; Mason and Okey, 1982; Quintana et al., 2008) and regulate numerous transcriptional processes (Denison et al., 2011). PFCs are thought to be neurotoxic as well (Gump et al., 2011; Mariussen, 2012; Slotkin et al., 2008), although their main effect may be immunotoxicity (Dong et al., 2013; Grandjean et al., 2012; Granum et al., 2013). The diverse toxicity potentials and potential for broad transcriptional regulatory activity of these chemicals suggest that they may have toxicities beyond what has been identified thus far.
Exposure to such toxicants may leave behind evidence, in the form of long-lived antibodies specific to self-antigens derived from damaged tissues. Autoantibodies have been detected in the blood following damage to tissues in the periphery, such as damage to heart tissue following intraoperative myocardial ischemia (Bledzhyants et al., 2007) and liver damage during hepatitis B infection (McFarlane et al., 1995). Furthermore, autoantibodies have been detected in the peripheral blood after damage to brain tissue including following stroke (Bornstein et al., 2001), head trauma (Marchi et al., 2013), and neurodegenerative diseases (Diamond et al., 2013), as well as following exposure to neurotoxic chemicals (Abou-Donia et al., 2013; El-Fawal et al., 1996; El-Fawal and McCain, 2008; El-Fawal and O'Callaghan, 2008). These findings suggest that autoantibodies may be used as surrogate measures of the occurrence (state), and possibly the severity (stage), of tissue damage from chemical exposure.
Because methylmercury has well-established neurotoxic effects (Hong et al., 2012), exposure to this chemical might be expected to be associated with development of autoantibodies specific for neural antigens. In fact, autoantibodies specific to neurofilaments, astrocyte glial fibrillary acidic protein (GFAP), and myelin basic protein (MBP) were found in the peripheral blood following methylmercury exposure in rats (El-Fawal et al., 1996). However, this has not yet been demonstrated in humans. Additionally, recent evidence suggests a link between PCBs and autoimmune responses including anti-nuclear and anti-thyroid antibodies. However, the primary target, and thus autoantibody profile, may differ depending on the specific mixture of congeners, dose, and sex (Cebecauer et al., 2009; Gallagher et al., 2013; Gu et al., 2009). Therefore, we conducted a pilot study to assess the ability of autoantibodies to serve as biomarkers of past and present exposures to environmental chemicals.
MATERIALS AND METHODS
Subjects
A selection of 38 samples was made from a birth cohort established between 1986 and 1987 as part of the Children's Health and the Environment in the Faroes (CHEF) project at the National Hospital in Tórshavn, Faroe Islands. This cohort has been described previously (Grandjean and Weihe, 1993). The sample selection was constrained by the availability of sufficient serum from children who donated a blood sample at age 7. Additionally, the selection aimed at providing a wide coverage of exposure levels.
Exposure measurements
As markers of methylmercury exposure, the total mercury concentrations were measured in cord blood and maternal hair as well as in the child's blood and hair at age 7 using cold-vapor atomic absorption spectroscopy (Grandjean and Weihe, 1993). Prenatal exposures to the PFCs perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) were assessed from analyses of cord blood; postnatal exposures were assessed from serum samples obtained from the child at approximately age 7. Analysis of coded samples for PFC concentrations was carried out by online solid-phase extraction and analysis using high-pressure liquid chromatography with tandem mass spectrometry (Haug et al., 2009). PCBs were measured in cord blood and from the child's serum at age 7. We used a simplified ΣPCB concentration calculated as the sum of congeners CB-138, CB-153, and CB-180 multiplied by 2 (Grandjean et al., 1995). Because the prenatal and age-7 exposures were measured in different media (e.g., prenatal PCBs in cord blood vs. age-7 PCBs in serum lipid), comparison of exposure levels between these time points requires consideration of conversion factors. However, we chose to use the analytical data without any conversions for the purposes of the present study.
Antibody measurements
Serum levels of autoantibodies to neurotypic and gliotypic proteins were determined as previously described (El-Fawal et al., 1996; El-Fawal and O'Callaghan, 2008). Purified human proteins NF-L, NF-M, and NF-H, GFAP, actin, keratin, and desmin (American Research Products, Belmont, MA), choline acetyltransferase (ChAT) and MBP (Sigma-Aldrich, St. Louis, MO) were prepared in a 10 mM Tris-buffered saline (pH 7.4) at a concentration of 5 μg/ml. MBP was used at a concentration of 25 μg/ml. The choice of antigens was aimed at providing a representation of the cellular heterogeneity of the nervous system, secondary targets (i.e., muscle) and the more ubiquitous actin. Keratin was selected based on published reports of anti-keratin antibodies in hepatopathology and atopic dermatitis. Flat-bottomed Immulon microtiter plates (Fisher Scientific) were coated with 100 μl of the protein solution and incubated overnight at 4°C. The plates were washed three times with 10 mM Tris containing 0.05% Tween-80. Non-specific binding sites were blocked for 30 min at room temperature with 0.5% skim milk solution prepared in the Tris Buffer. Serum dilutions of 1:100 were prepared in the skim milk solution and 100 μl added per well, in triplicate. Plates were incubated for 2 h at room temperature and then washed three times with skim milk solutions containing 0.05% Tween-80. Alkaline phosphate goat anti-human IgG or IgM (Jackson Immunoresearch, West Grover, PA), at a 1:3000 dilution, was added to each well and the plates were incubated for 1 h. Following three washes with skim milk-Tween-80 solution and two washes with 10 mM Tris, the alkaline phosphatase substrate (p-nitrophenyl-phosphate, Bio-Rad, Richmond, CA) was added. The reaction was stopped by the addition of 100 μl 0.4 N NaOH per well. Plates were read at 405 nm in a plate reader (Tecan Spectrafluor). Generation of the standard curve for calculation of ng/ml.equivalence (ng/ml.eq) was performed using commercially available mouse monoclonal antibodies against these neuroantigens (American Research Products). The range of the 8-point standard curve was 0–2000 ng/ml and the limit of detection (LOD) for the assay was 0.002 ng/ml. All values from samples obtained from this cohort fell above the LOD and below the highest value in the standard curve.
Statistical analyses
All analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, NC). Differences between concentrations of IgM and IgG autoantibodies were assessed using a paired t-test. Linear regressions were performed to assess the associations of autoantibody levels with chemical exposures. Autoantibody values were natural log (ln)-transformed in order to obtain normally distributed residuals. Chemical exposure values were ln-transformed to allow the expression of the estimates as the percent change in autoantibody titer per two-fold increase in exposure. Residuals of the linear regression models indicated that the relationship between ln-transformed chemical values and ln-transformed antibody values were approximately linear and therefore that linear regression was an appropriate model for these data. For graphs in which autoantibody titers are displayed within tertiles of chemical exposure, cut points for each exposure were chosen so that each tertile contained approximately the same number of observations.
The mother's hair-mercury and child's hair-mercury were highly correlated with cord blood-mercury and age-7 blood-mercury, respectively, and therefore did not provide additional information to the model. The PFCs measured included perfluorohexane sulfonate and perfluorononanoic acid, but the values, especially in cord blood, were very low and were therefore disregarded. Child's sex was also assessed as a covariate in all models; however, adjustment for this did not change the association between the exposures and the autoantibodies and therefore was not included in the final models.
RESULTS
Among the 38 children whose samples were used in the present analyses, there were 16 males and 22 females with a mean age of 6.6 years at the time of sampling for autoantibody measurements and childhood exposures. The distributions of each of the prenatal and age-7 exposures to mercury, PCBs, and PFCs are shown in Table 1. A few of these exposures were moderately positively correlated with each other (Supplementary table 1).
TABLE 1. Distributions of Exposure Levels.
| Exposure | Age | Unit | N | Geometric mean (IQRa) |
|---|---|---|---|---|
| Mercury | Prenatal | μg/l cord blood | 38 | 31 (11–64) |
| Mercury | Age 7 | μg/l whole blood | 37 | 10 (5.5–23) |
| PCB | Prenatal | μg/l cord blood | 37 | 2.0 (1.3–2.9) |
| PCB | Age 7 | μg/g serum lipid | 33 | 1.6 (0.97–2.9) |
| PFOA | Prenatal | μg/l cord blood | 37 | 0.68 (0.41–0.98) |
| PFOA | Age 7 | μg/l serum | 34 | 4.3 (3.4–5.8) |
| PFOS | Prenatal | μg/l cord blood | 37 | 3.1 (2.5–3.9) |
| PFOS | Age 7 | μg/l serum | 34 | 27 (22–35) |
aIQR, interquartile range.
The distributions of the titers of each of the autoantibodies are shown in Table 2. The concentrations of IgG were higher than those of IgM specific for the same antigen for all neural antigens. However, IgG was lower than IgM for all of the non-neural antigens (actin, desmin, and keratin). Many of the autoantibodies were moderately to highly positively correlated with several other autoantibodies (Supplementary table 2).
TABLE 2. Distributions of Autoantibody Concentrations (μg/l) (N = 38).
| Antigen | Isotype | Geometric mean (IQRa) |
|---|---|---|
| NF-L** | IgM | 90 (29–245) |
| IgG | 3171 (1930–4731) | |
| NF-M** | IgM | 42 (30–57) |
| IgG | 91 (43–143) | |
| NF-H* | IgM | 151 (69–254) |
| IgG | 349 (111–1063) | |
| GFAP** | IgM | 103 (48–213) |
| IgG | 713 (605–837) | |
| MBP** | IgM | 93 (35–234) |
| IgG | 908 (552–1705) | |
| ChAT** | IgM | 2.2 (0.7–5.8) |
| IgG | 84 (57–124) | |
| Actin** | IgM | 926 (655–1300) |
| IgG | 581 (413–708) | |
| Desmin | IgM | 1.2 (0.46–2.4) |
| IgG | 1.1 (0.27–1.6) | |
| Keratin** | IgM | 1338 (824–3265) |
| IgG | 88 (7.6–947) |
aIQR, interquartile range.
Difference between IgM and IgG nominal *p < 0.001, **p < 0.0001.
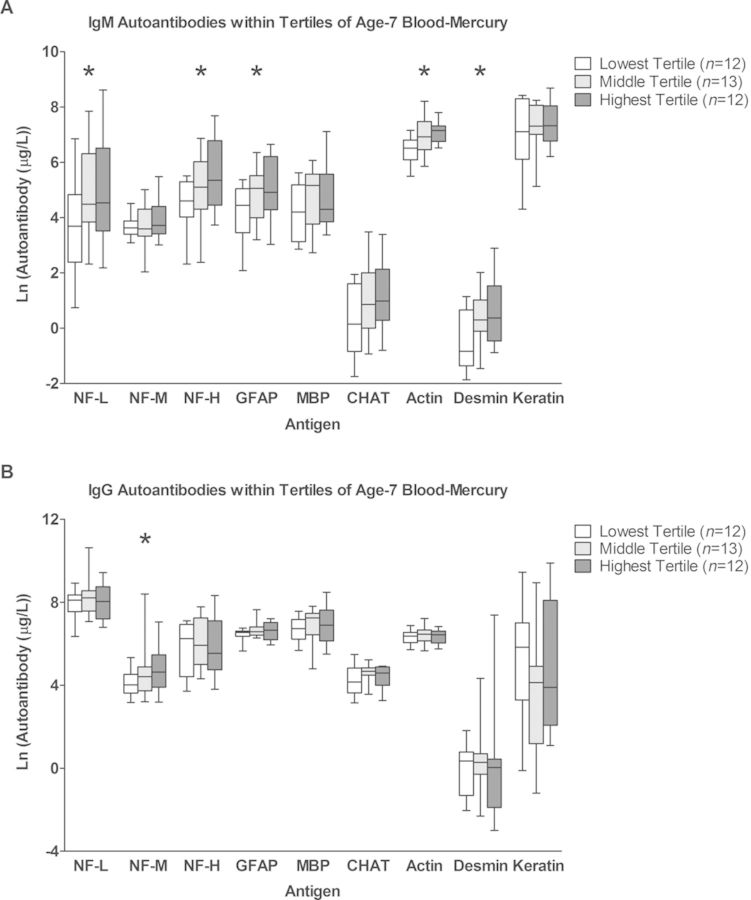
The association of each chemical exposure was individually assessed with each autoantibody titer using linear regression and the estimates for these associations (expressed as the percent change in antibody concentration per two-fold increase in exposure) are shown in Supplementary table 3. Age-7 mercury showed significant positive associations with multiple autoantibodies. These associations were almost exclusively with IgM and included the neural antigens NF-L (53.1% increase per two-fold increase in age-7 mercury), NF-H (40.0% increase), GFAP (30.4% increase), and the non-neural antigens actin (18.7% increase) and desmin (40.1% increase). NF-M-specific IgG was also significantly positively associated with age-7 mercury, showing an estimated 30.0% increase per two-fold increase in age-7 mercury. The antibody concentrations within tertiles of age-7 mercury are shown in Figure 1 and illustrate the dose-dependent increase in these autoantibody concentrations with increasing age-7 mercury concentrations.
FIG. 1.
Autoantibody titers within tertiles of age-7 blood-mercury. Age-7 blood-mercury values were separated into three groups of low, middle, and high values. For each antigen, distributions of autoantibody titers within each tertile are shown for IgM (A) and IgG (B) isotypes. Box plots show medians, interquartile ranges, and 95% confidence intervals. * indicate that the association between age-7 blood-mercury exposure and autoantibody titer for that antigen was significant based on linear regression analysis (p-value < 0.05).
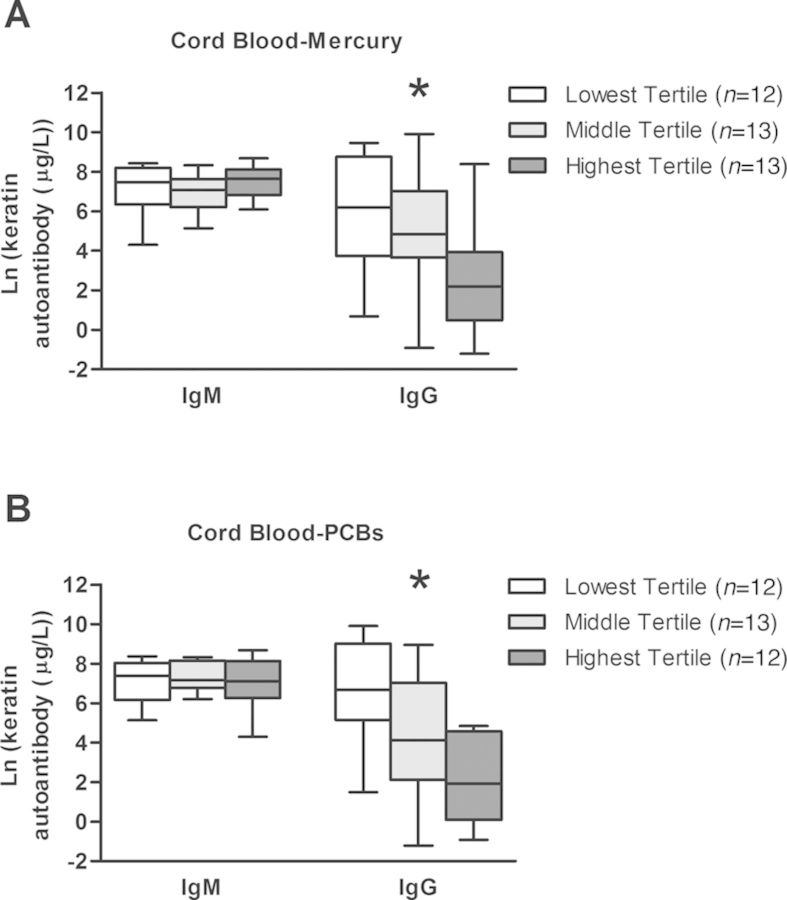
Prenatal mercury and prenatal PCB exposures were found to be strongly negatively associated with the IgG isotype specific solely for the keratin antigen (Fig. 2 and Supplementary table 3). Estimates indicate that anti-keratin IgG antibodies decreased by 59.9% and 77.3% with a two-fold increase in prenatal-mercury and prenatal-PCBs, respectively. When prenatal-mercury and prenatal-PCBs were included in the same model, the estimates for the strength of their association with anti-keratin IgG were modestly attenuated, although they remained significant, resulting in an estimated 46.8% and 67.0% decrease in autoantibodies with two-fold increases in prenatal-mercury and prenatal-PCBs, respectively (results not shown).
FIG. 2.
Keratin-specific autoantibody titers within tertiles of cord blood-mercury and cord blood-PCBs. Cord blood-mercury and -PCB values were separated into three groups of low, middle, and high values. Distributions of the IgM and IgG keratin-specific autoantibody titers within each tertile of cord blood-mercury (A) and cord blood-PCBs (B) are shown. Box plots show medians, interquartile ranges, and 95% confidence intervals. * indicate that the association between age-7 blood-mercury exposure and autoantibody titer for that antigen was significant based on linear regression analysis (p-value < 0.05).
Age-7 PCBs were not found to be associated with any of the autoantibodies in the univariate models (Supplementary table 3). Additionally, PFOA was not found to be associated with any of the autoantibodies, at either the prenatal or the age-7 time point. However, although age-7 PFOS was not associated with any of the autoantibodies, prenatal PFOS was negatively associated with one autoantibody, actin-specific IgG, with an estimated 22% decrease in this autoantibody with a two-fold increase in prenatal PFOS exposure.
As PCBs and PFCs have known suppressive effects on antibody levels, suppression of the concentrations of autoantibodies due to concomitant exposure to PFCs or PCBs was considered. Therefore, PCBs and PFCs were evaluated as covariates in the models of the association of age-7 blood-mercury with autoantibodies. However, adjustment for neither PFOA nor PFOS, measured either prenatally or at age 7, nor PCBs measured at age 7, changed the estimates of the effects of age-7 mercury on autoantibody titers (results not shown).
Finally, evaluation of the effect of including prenatal mercury and age-7 mercury in the same model was conducted. In this model, the strength of the estimates of the association of age-7-mercury with autoantibodies modestly increased and the p-values decreased, resulting in the associations with ChAT-specific IgM and MBP-specific IgG to become significant (results not shown). However, this did not change the interpretation of the associations.
DISCUSSION
Autoantibodies have been suggested to be potentially useful for detection of tissue damage following chemical exposure (El-Fawal et al., 1999; Evans, 1995). Therefore, the present study investigated the relationship between prenatal and childhood exposures to pervasive environmental chemicals and the development of antibodies specific for self-antigens. Despite our small sample size, we were able to detect substantial associations between prenatal mercury, prenatal PCBs, and childhood mercury exposure with the autoantibody titers. This has not been previously demonstrated in humans. Additionally, the associations with non-neural antigen-specific autoantibodies suggest that these chemicals may have toxicities on tissues not previously recognized. These findings demonstrate that autoantibodies can be detected following exposures to environmental chemicals and suggest that such antibodies may be useful biomarkers of tissue damage.
Interestingly, most of the autoantibodies that showed an association with age-7 blood-mercury were of the IgM isotype rather than IgG. This suggests that the tissue damage and the release of self-antigens may have occurred relatively recently or is on-going. Indeed, following exposure of rats to the neurotoxic chemical trimethyltin, IgM isotype autoantibodies were detected at the highest concentrations at week 1 following exposure compared with week 2 and 3, whereas the IgG isotype autoantibodies started to increase at week 2 and were the highest at week 3 (El-Fawal and O'Callaghan, 2008). It should also be pointed out that this paradigm is based on experimental models and evidence from vaccination studies. In chronic exposures, such as in the present study, IgG titers may indicate the initial or historical insult, whereas IgM and its association with age-7 mercury suggests an ongoing insult. For example, although both IgG and IgM isotypes are detected in multiple sclerosis patients, the latter isotype, against gangliosides, correlated with neurological disability (Vyshkina and Kalman, 2008).
The mechanism(s) through which mercury acts to produce the observed combination of associations with both neural and non-neural antigen-specific antibodies is not clear. All of the autoantibodies associated with age-7 mercury exposure may be related to each other through methylmercury's known neurotoxic effects. Methylmercury travels from the blood through the blood brain barrier and into to the brain (Aschner and Clarkson, 1988) where it can cause apoptotic cell death of neurons (Nagashima et al., 1996), releasing neural antigens. Apoptotic cells are thought to be capable of eliciting autoantibodies (Navratil et al., 2006) and this phenomenon may explain the association of neural antigen-specific antibodies with mercury in the present study. Additionally, although actin is not a protein specific to neural tissue, it is ubiquitously expressed, including in neural tissue (Matus et al., 1982). Therefore, apoptosis of nerve cells could release actin antigens and stimulate actin-specific autoantibody production in the same manner. Desmin, on the other hand, is not expressed in neural tissue—it is specific to muscle cells (Paulin and Li, 2004). It is conceivable that muscle atrophy secondary to peripheral nerve damage may release desmin antigens and result in generation of desmin-specific antibodies. Alternatively, accumulation of mercury in muscle tissues (Nielsen et al., 1994; Usuki et al., 1998) may directly induce apoptosis of muscle cells and release the desmin antigen that may then induce desmin-specific antibody production.
The present study did not find an association of neural antigen-specific autoantibodies with prenatal mercury exposure despite the fact that prenatal methylmercury exposure is known to cause substantial and diffuse neurotoxic effects. It is unclear why this was not reflected by an association of autoantibodies with prenatal mercury exposure as it was for childhood exposure. A possible explanation relates to the incomplete development of the adaptive immune system early in life. Although fetuses and newborns have the capacity to produce antibodies, the magnitude and durability of those antibodies are substantially limited by the immaturity of the immune system during these developmental periods (Siegrist and Aspinall, 2009). Therefore, it is possible that prenatal mercury exposure caused tissue damage that resulted in development of autoantibodies, but that those antibodies did not persist through age 7, resulting in the lack of association of prenatal mercury with autoantibodies at age 7. Alternatively, it is plausible that IgG titers, which were higher than IgM, indicate successful development of immunological memory to earlier antigenic challenges occurring perinatally and in early childhood but that the dose response between exposure and autoantibody was lost over time, whereas IgM reflects current status at the time of sampling at age 7. Finally, the nature of the toxicity of mercury on the nervous system may differ by stage of development such that antibodies are elicited by childhood exposure but not by prenatal exposure.
Associations of prenatal mercury and prenatal PCBs with IgG antibodies specific for the non-neural antigen keratin were also found. Given the multiple comparisons that were performed in the present analysis, these associations may be a result of random variation. However, the fact that these prenatal exposures were associated with autoantibodies of the IgG isotype is consistent with the insult occurring in the past, rather than recently. The inverse associations between these exposures and the keratin autoantibody titers were unexpected. This not only suggests that these chemicals may have an immunosuppressive effect, but also that this effect may occur only when exposed during early development. However, why this immunosuppressive effect was specific to keratin autoantibodies is unclear. It is interesting that we have previously reported that PCB exposure was negatively associated with the presence of atopic dermatitis (Grandjean et al., 2010), a disease of the skin that has been associated with keratin-specific autoantibodies (Natter et al., 1998; Samoylikov et al., 2013). Assaying for autoantibodies specific to a larger panel of autoantigens, as well as other immunoglobulin isotypes, would help determine if this is reflective of a suppressive effect due to toxicity specifically targeted to a keratin-expressing cell type or of more global immunological dysregulation.
Finally, we found a general lack of associations of autoantibodies with PFC exposure. Although, we did find that prenatal PFOS was negatively associated with one autoantibody, anti-actin IgG. However, because actin is a ubiquitous protein, the specific tissue damage that may be associated with prenatal PFOS exposure is unknown. It is also possible that the association with actin-specific antibodies was found by chance, especially because most of the other estimates of the associations with prenatal PFOS are in the opposite direction. A larger study should be conducted to determine if the association with anti-actin IgG can be replicated and also to confirm all other associations that were found is this study.
Certain PFCs are carcinogenic (Lau et al., 2007), immunotoxic (Dong et al., 2013; Grandjean et al., 2012; Granum et al., 2013), and may also be neurotoxic (Gump et al., 2011; Mariussen, 2012; Slotkin et al., 2008). Although the carcinogenicity associated with some PFCs has been attributed to the ability to act as peroxisome proliferator-activated receptor alpha (PPARα) agonists (Lindstrom et al., 2011; Vanden Heuvel et al., 2006), it is unclear if other toxicities are also PPARα-dependent (Harada and Koizumi, 2009). PPARα activation decreases expression of the transcription factor T-bet (Gocke et al., 2009), and T-bet promotes antibody isotype switching in B cells and possibly autoantibody production (Peng et al., 2002). Although suppression of T-bet expression by PPARα has been demonstrated only in T cells so far, similar effects in B cells could conceivably lead to protection from autoantibody production. However, the associations observed between PFCs and autoantibodies do not support this notion, which is in agreement with a recent study in mice that failed to identify associations of neural and non-neural autoantibody concentrations with PFOA exposure (Hu et al., 2012).
The blood concentrations of the PFCs in these Faroese children were similar to those found in other countries at comparable time periods, including in the United States (Kato et al., 2011). However, the concentrations of both mercury (CDC, 2004) and PCBs (Nichols et al., 2007) were substantially higher in these Faroese children compared with many other countries, except for populations that also have high exposure to these chemicals due to their seafood-based diets, such as the Inuit (Llop et al., 2012). This highlights the importance of determining if alterations in autoantibody titers also are seen in populations that experience exposures lower than these Faroese children.
The finding that all children had detectable levels of all autoantibodies evaluated is not surprising. It has been shown both in healthy newborns and in healthy adults that the human antibody repertoire contains thousands of antibodies specific to self antigens (Merbl et al., 2007; Nagele et al., 2013). However, many of the studies that have measured these natural autoantibodies have neither identified the specific antigens that the antibodies were binding nor were able to determine the concentrations of these autoantibodies. Therefore, normal ranges of concentrations are not known for most autoantibodies. Consequently, we were unable to identify children in the present study with autoantibody concentrations outside expected ranges. Such information would be crucial to facilitate any future implementation of autoantibodies as biomarkers of tissue damage associated with environmental chemical exposure.
The present study found that environmental chemical exposures were associated with concentrations of antibodies specific to both neural and non-neural antigens. Whether these autoantibodies are side events or perhaps pathogenic so that they are involved in subsequent development of autoimmune disease would require further investigation. Nevertheless, the detection of neural antigen-specific autoantibodies may be of particular interest because these antibodies may be used as accessible biomarkers of chemically induced damage that may lead to neurological delays, deficits, or diseases. Follow-up studies should be conducted on the larger cohort of children to assess if the presence and concentrations of these autoantibodies can predict the development, and possibly severity, of neurological and/or autoimmune outcomes. Finally, the associations with non-neural antigen-specific autoantibodies were unexpected and suggest that these chemicals may cause toxicities that have yet to be recognized. Future studies should confirm these associations with non-neural antigen-specific autoantibodies and evaluate the nature of the tissue damage that is causing them.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institute of Environmental Health Sciences at the National Institutes of Health (ES012199, ES011687, and ES009797).
Supplementary Material
Disclaimer: The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS or NIH.
REFERENCES
- Abou-Donia M. B., Abou-Donia M. M., ElMasry E. M., Monro J. A., Mulder M. F. Autoantibodies to nervous system-specific proteins are elevated in sera of flight crew members: Biomarkers for nervous system injury. J. Toxicol. Environ. Health. Part A. 2013;76:363–380. doi: 10.1080/15287394.2013.765369. [DOI] [PubMed] [Google Scholar]
- Aschner M., Clarkson T. W. Uptake of methylmercury in the rat brain: Effects of amino acids. Brain Res. 1988;462:31–39. doi: 10.1016/0006-8993(88)90581-1. [DOI] [PubMed] [Google Scholar]
- Bledzhyants D. A., Muratov R. M., Movsesyan R. R., Podlubnaya Z. A. Autoantibodies to myosin light chains in the blood as early marker of myocardial injury after aortocoronary bypass surgery. Bull. Experiment. Biol. Med. 2007;144:241–245. doi: 10.1007/s10517-007-0300-y. [DOI] [PubMed] [Google Scholar]
- Bornstein N. M., Aronovich B., Korczyn A. D., Shavit S., Michaelson D. M., Chapman J. Antibodies to brain antigens following stroke. Neurology. 2001;56:529–530. doi: 10.1212/wnl.56.4.529. [DOI] [PubMed] [Google Scholar]
- CDC. Blood mercury levels in young children and childbearing-aged women—United States, 1999–2002. MMWR. 2004;53:1018–1020. [PubMed] [Google Scholar]
- Cebecauer L., Radikova Z., Rovensky J., Koska J., Imrich R., Ksinantova L., Susienkova K., Vigas M., Klimes I., Langer P. Increased prevalence and coincidence of antinuclear and antithyroid antibodies in the population exposed to high levels of polychlorinated pollutants cocktail. Endocrine Regulations. 2009;43:75–81. [PubMed] [Google Scholar]
- Crinnion W. J. Polychlorinated biphenyls: Persistent pollutants with immunological, neurological, and endocrinological consequences. Altern. Med. Rev. 2011;16:5–13. [PubMed] [Google Scholar]
- Denison M. S., Soshilov A. A., He G., DeGroot D. E., Zhao B. Exactly the same but different: Promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicol. Sci. 2011;124:1–22. doi: 10.1093/toxsci/kfr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt J. C., Peden-Adams M. M., Keller J. M., Germolec D. R. Immunotoxicity of perfluorinated compounds: Recent developments. Toxicol. Pathol. 2012;40:300–311. doi: 10.1177/0192623311428473. [DOI] [PubMed] [Google Scholar]
- Diamond B., Honig G., Mader S., Brimberg L., Volpe B. T. Brain-reactive antibodies and disease. Annu. Rev. Immunol. 2013;31:345–385. doi: 10.1146/annurev-immunol-020711-075041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong G. H., Tung K. Y., Tsai C. H., Liu M. M., Wang D., Liu W., Jin Y. H., Hsieh W. S., Lee Y. L., Chen P. C. Serum polyfluoroalkyl concentrations, asthma outcomes, and immunological markers in a case-control study of Taiwanese children. Environ. Health Perspect. 2013;121:507–513. doi: 10.1289/ehp.1205351. 513e501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Fawal H. A., Gong Z., Little A. R., Evans H. L. Exposure to methylmercury results in serum autoantibodies to neurotypic and gliotypic proteins. Neurotoxicology. 1996;17:531–539. [PubMed] [Google Scholar]
- El-Fawal H. A., McCain W. C. Antibodies to neural proteins in organophosphorus-induced delayed neuropathy (OPIDN) and its amelioration. Neurotoxicol. Teratol. 2008;30:161–166. doi: 10.1016/j.ntt.2008.01.005. [DOI] [PubMed] [Google Scholar]
- El-Fawal H. A., O'Callaghan J. P. Autoantibodies to neurotypic and gliotypic proteins as biomarkers of neurotoxicity: Assessment of trimethyltin (TMT) Neurotoxicology. 2008;29:109–115. doi: 10.1016/j.neuro.2007.09.009. [DOI] [PubMed] [Google Scholar]
- El-Fawal H. A., Waterman S. J., De Feo A., Shamy M. Y. Neuroimmunotoxicology: Humoral assessment of neurotoxicity and autoimmune mechanisms. Environ. Health Perspect. 1999;107:767–775. doi: 10.1289/ehp.99107s5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans H. L. Markers of neurotoxicity: From behavior to autoantibodies against brain proteins. Clin. Chem. 1995;41:1874–1881. [PubMed] [Google Scholar]
- Gallagher C. M., McElroy A. E., Smith D. M., Golightly M. G., Meliker J. R. Polychlorinated biphenyls, mercury, and antinuclear antibody positivity, NHANES 2003–2004. Int. J. Hyg. Environ. Health. 2013;216:721–727. doi: 10.1016/j.ijheh.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Gasiewicz T. A., Rucci G. Cytosolic receptor for 2,3,7,8-tetrachlorodibenzo-p-dioxin. Evidence for a homologous nature among various mammalian species. Mol. Pharmacol. 1984;26:90–98. [PubMed] [Google Scholar]
- Gocke A. R., Hussain R. Z., Yang Y., Peng H., Weiner J., Ben L. H., Drew P. D., Stuve O., Lovett-Racke A. E., Racke M. K. Transcriptional modulation of the immune response by peroxisome proliferator-activated receptor-{alpha} agonists in autoimmune disease. J. Immunol. 2009;182:4479–4487. doi: 10.4049/jimmunol.0713927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P., Andersen E. W., Budtz-Jorgensen E., Nielsen F., Molbak K., Weihe P., Heilmann C. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA. 2012;307:391–397. doi: 10.1001/jama.2011.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P., Poulsen L. K., Heilmann C., Steuerwald U., Weihe P. Allergy and sensitization during childhood associated with prenatal and lactational exposure to marine pollutants. Environ. Health Perspect. 2010;118:1429–1433. doi: 10.1289/ehp.1002289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P., Weihe P. Neurobehavioral effects of intrauterine mercury exposure: Potential sources of bias. Environ. Res. 1993;61:176–183. doi: 10.1006/enrs.1993.1062. [DOI] [PubMed] [Google Scholar]
- Grandjean P., Weihe P., Needham L. L., Burse V. W., Patterson D. G., Jr, Sampson E. J., Jorgensen P. J., Vahter M. Relation of a seafood diet to mercury, selenium, arsenic, and polychlorinated biphenyl and other organochlorine concentrations in human milk. Environ. Res. 1995;71:29–38. doi: 10.1006/enrs.1995.1064. [DOI] [PubMed] [Google Scholar]
- Granum B., Haug L. S., Namork E., Stolevik S. B., Thomsen C., Aaberge I. S., van Loveren H., Lovik M., Nygaard U. C. Pre-natal exposure to perfluoroalkyl substances may be associated with altered vaccine antibody levels and immune-related health outcomes in early childhood. J. Immunotoxicol. 2013;10:373–379. doi: 10.3109/1547691X.2012.755580. [DOI] [PubMed] [Google Scholar]
- Gu J. Y., Qian C. H., Tang W., Wu X. H., Xu K. F., Scherbaum W. A., Schott M., Liu C. Polychlorinated biphenyls affect thyroid function and induce autoimmunity in Sprague-Dawley rats. Hormone Metabolic Res. 2009;41:471–474. doi: 10.1055/s-0029-1220768. [DOI] [PubMed] [Google Scholar]
- Gump B. B., Wu Q., Dumas A. K., Kannan K. Perfluorochemical (PFC) exposure in children: Associations with impaired response inhibition. Environ. Sci. Technol. 2011;45:8151–8159. doi: 10.1021/es103712g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada K. H., Koizumi A. Environmental and biological monitoring of persistent fluorinated compounds in Japan and their toxicities. Environ. Health Prev. Med. 2009;14:7–19. doi: 10.1007/s12199-008-0058-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug L. S., Thomsen C., Becher G. A sensitive method for determination of a broad range of perfluorinated compounds in serum suitable for large-scale human biomonitoring. J. Chromatogr. A. 2009;1216:385–393. doi: 10.1016/j.chroma.2008.10.113. [DOI] [PubMed] [Google Scholar]
- Hong Y. S., Kim Y. M., Lee K. E. Methylmercury exposure and health effects. J. Prevent. Med. Public Health. 2012;45:353–363. doi: 10.3961/jpmph.2012.45.6.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q., Franklin J. N., Bryan I., Morris E., Wood A., DeWitt J. C. Does developmental exposure to perflurooctanoic acid (PFOA) induce immunopathologies commonly observed in neurodevelopmental disorders. Neurotoxicology. 2012;33:1491–1498. doi: 10.1016/j.neuro.2012.10.016. [DOI] [PubMed] [Google Scholar]
- Igarashi T., Osuga U., Tsutsumi O., Momoeda M., Ando K., Matsumi H., Takai Y., Okagaki R., Hiroi H., Fujiwara O., et al. Expression of Ah receptor and dioxin-related genes in human uterine endometrium in women with or without endometriosis. Endocr. J. 1999;46:765–772. doi: 10.1507/endocrj.46.765. [DOI] [PubMed] [Google Scholar]
- Kato K., Wong L. Y., Jia L. T., Kuklenyik Z., Calafat A. M. Trends in exposure to polyfluoroalkyl chemicals in the U.S. population: 1999–2008. Environ. Sci. Technol. 2011;45:8037–8045. doi: 10.1021/es1043613. [DOI] [PubMed] [Google Scholar]
- Lanner J. T., Georgiou D. K., Joshi A. D., Hamilton S. L. Ryanodine receptors: Structure, expression, molecular details, and function in calcium release. Cold Spring Harbor Perspect. Biol. 2010;2:a003996. doi: 10.1101/cshperspect.a003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C., Anitole K., Hodes C., Lai D., Pfahles-Hutchens A., Seed J. Perfluoroalkyl acids: A review of monitoring and toxicological findings. Toxicol. Sci. 2007;99:366–394. doi: 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- Lindstrom A. B., Strynar M. J., Libelo E. L. Polyfluorinated compounds: Past, present, and future. Environ. Sci. Technol. 2011;45:7954–7961. doi: 10.1021/es2011622. [DOI] [PubMed] [Google Scholar]
- Llop S., Guxens M., Murcia M., Lertxundi A., Ramon R., Riano I., Rebagliato M., Ibarluzea J., Tardon A., Sunyer J., et al. Prenatal exposure to mercury and infant neurodevelopment in a multicenter cohort in Spain: Study of potential modifiers. Am. J. Epidemiol. 2012;175:451–465. doi: 10.1093/aje/kwr328. [DOI] [PubMed] [Google Scholar]
- Marchi N., Bazarian J. J., Puvenna V., Janigro M., Ghosh C., Zhong J., Zhu T., Blackman E., Stewart D., Ellis J., et al. Consequences of repeated blood-brain barrier disruption in football players. PLoS One. 2013;8:e56805. doi: 10.1371/journal.pone.0056805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariussen E. Neurotoxic effects of perfluoroalkylated compounds: Mechanisms of action and environmental relevance. Arch. Toxicol. 2012;86:1349–1367. doi: 10.1007/s00204-012-0822-6. [DOI] [PubMed] [Google Scholar]
- Mason M. E., Okey A. B. Cytosolic and nuclear binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin to the Ah receptor in extra-hepatic tissues of rats and mice. Eur. J. Biochem. 1982;123:209–215. doi: 10.1111/j.1432-1033.1982.tb06518.x. [DOI] [PubMed] [Google Scholar]
- Matus A., Ackermann M., Pehling G., Byers H. R., Fujiwara K. High actin concentrations in brain dendritic spines and postsynaptic densities. Proc. Natl. Acad. Sci. U.S.A. 1982;79:7590–7594. doi: 10.1073/pnas.79.23.7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane B. M., Bridger C. B., Smith H. M., Antonov K. A., Naoumov N., Williams R., McFarlane I. G. Autoimmune mechanisms in chronic hepatitis B and delta virus infections. Eur. J. Gastroenterol. Hepatol. 1995;7:615–621. [PubMed] [Google Scholar]
- Merbl Y., Zucker-Toledano M., Quintana F. J., Cohen I. R. Newborn humans manifest autoantibodies to defined self molecules detected by antigen microarray informatics. J. Clin. Invest. 2007;117:712–718. doi: 10.1172/JCI29943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima K., Fujii Y., Tsukamoto T., Nukuzuma S., Satoh M., Fujita M., Fujioka Y., Akagi H. Apoptotic process of cerebellar degeneration in experimental methylmercury intoxication of rats. Acta Neuropathol. 1996;91:72–77. doi: 10.1007/s004010050394. [DOI] [PubMed] [Google Scholar]
- Nagele E. P., Han M., Acharya N. K., DeMarshall C., Kosciuk M. C., Nagele R. G. Natural IgG autoantibodies are abundant and ubiquitous in human sera, and their number is influenced by age, gender, and disease. PLoS One. 2013;8:e60726. doi: 10.1371/journal.pone.0060726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natter S., Seiberler S., Hufnagl P., Binder B. R., Hirschl A. M., Ring J., Abeck D., Schmidt T., Valent P., Valenta R. Isolation of cDNA clones coding for IgE autoantigens with serum IgE from atopic dermatitis patients. FASEB J. 1998;12:1559–1569. doi: 10.1096/fasebj.12.14.1559. [DOI] [PubMed] [Google Scholar]
- Navratil J. S., Liu C. C., Ahearn J. M. Apoptosis and autoimmunity. Immunol. Res. 2006;36:3–12. doi: 10.1385/IR:36:1:3. [DOI] [PubMed] [Google Scholar]
- Nichols B. R., Hentz K. L., Aylward L., Hays S. M., Lamb J. C. Age-specific reference ranges for polychlorinated biphenyls (PCB) based on the NHANES 2001–2002 survey. J. Toxicol. Environ. Health A. 2007;70:1873–1877. doi: 10.1080/15287390701457688. [DOI] [PubMed] [Google Scholar]
- Nielsen J. B., Andersen O., Grandjean P. Evaluation of mercury in hair, blood and muscle as biomarkers for methylmercury exposure in male and female mice. Arch. Toxicol. 1994;68:317–321. doi: 10.1007/s002040050075. [DOI] [PubMed] [Google Scholar]
- Paulin D., Li Z. Desmin: A major intermediate filament protein essential for the structural integrity and function of muscle. Exp. Cell Res. 2004;301:1–7. doi: 10.1016/j.yexcr.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Peng S. L., Szabo S. J., Glimcher L. H. T-bet regulates IgG class switching and pathogenic autoantibody production. Proc. Natl. Acad. Sci. U.S.A. 2002;99:5545–5550. doi: 10.1073/pnas.082114899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post G. B., Cohn P. D., Cooper K. R. Perfluorooctanoic acid (PFOA), an emerging drinking water contaminant: A critical review of recent literature. Environ. Res. 2012;116:93–117. doi: 10.1016/j.envres.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Quintana F. J., Basso A. S., Iglesias A. H., Korn T., Farez M. F., Bettelli E., Caccamo M., Oukka M., Weiner H. L. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- Rodrigues J. L., Serpeloni J. M., Batista B. L., Souza S. S., Barbosa F., Jr Identification and distribution of mercury species in rat tissues following administration of thimerosal or methylmercury. Arch. Toxicol. 2010;84:891–896. doi: 10.1007/s00204-010-0538-4. [DOI] [PubMed] [Google Scholar]
- Samoylikov P., Gervazieva V., Kozhevnikov S. Association between autoimmune reactions and severity of atopic dermatitis in children with herpes virus infection. Wld Allergy Org. J. 2013;6:8. doi: 10.1186/1939-4551-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegrist C. A., Aspinall R. B-cell responses to vaccination at the extremes of age. Nat. Rev. Immunol. 2009;9:185–194. doi: 10.1038/nri2508. [DOI] [PubMed] [Google Scholar]
- Slotkin T. A., MacKillop E. A., Melnick R. L., Thayer K. A., Seidler F. J. Developmental neurotoxicity of perfluorinated chemicals modeled in vitro. Environ. Health Perspect. 2008;116:716–722. doi: 10.1289/ehp.11253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usuki F., Yasutake A., Matsumoto M., Umehara F., Higuchi I. The effect of methylmercury on skeletal muscle in the rat: A histopathological study. Toxicol. Lett. 1998;94:227–232. doi: 10.1016/s0378-4274(98)00022-8. [DOI] [PubMed] [Google Scholar]
- Vanden Heuvel J. P., Thompson J. T., Frame S. R., Gillies P. J. Differential activation of nuclear receptors by perfluorinated fatty acid analogs and natural fatty acids: A comparison of human, mouse, and rat peroxisome proliferator-activated receptor-alpha, -beta, and -gamma, liver X receptor-beta, and retinoid X receptor-alpha. Toxicol. Sci. 2006;92:476–489. doi: 10.1093/toxsci/kfl014. [DOI] [PubMed] [Google Scholar]
- Vyshkina T., Kalman B. Autoantibodies and neurodegeneration in multiple sclerosis. Laboratory Invest. 2008;88:796–807. doi: 10.1038/labinvest.2008.53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.