Abstract
The mycotoxin deoxynivalenol (DON) elicits robust anorectic and emetic effects in several animal species. However, less is known about the potential for naturally occurring and synthetic congeners of this trichothecene to cause analogous responses. Here we tested the hypothesis that alterations in DON structure found in the plant metabolite deoxynivalenol-3-glucoside (D3G) and two pharmacologically active synthetic DON derivatives, EN139528 and EN139544, differentially impact their potential to evoke food refusal and emesis. In a nocturnal mouse food consumption model, oral administration with DON, D3G, EN139528, or EN139544 at doses from 2.5 to 10 mg/kg BW induced anorectic responses that lasted up to 16, 6, 6, and 3 h, respectively. Anorectic potency rank orders were EN139544>DON>EN139528>D3G from 0 to 0.5 h but DON>D3G>EN139528>EN139544 from 0 to 3 h. Oral exposure to each of the four compounds at a common dose (2.5 mg/kg BW) stimulated plasma elevations of the gut satiety peptides cholecystokinin and to a lesser extent, peptide YY3–36 that corresponded to reduced food consumption. In a mink emesis model, oral administration of increasing doses of the congeners differentially induced emesis, causing marked decreases in latency to emesis with corresponding increases in both the duration and number of emetic events. The minimum emetic doses for DON, EN139528, D3G, and EN139544 were 0.05, 0.5, 2, and 5 mg/kg BW, respectively. Taken together, the results suggest that although all three DON congeners elicited anorectic responses that mimicked DON over a narrow dose range, they were markedly less potent than the parent mycotoxin at inducing emesis.
Keywords: mycotoxin, anorexia, emesis, cholecystokinin, peptide YY3–36, 5-hydroxytryptamine, deoxynivalenol, deoxynivalenol-3-glucoside, EN139528, EN139544
Fusarium head blight in cereal grains results in contamination by the 8-ketotrichothecene deoxynivalenol (12,13-epoxy-3,4,15-trihydroxytrichothec-9-en-8-one) (DON, vomitoxin) (Fig. 1A) (Pestka, 2010). This mycotoxin has been associated with a spectrum of acute and chronic adverse effects in experimental animals that include anorexia, nausea, emesis, neuroendocrine changes, and immunotoxicity (Maresca, 2013).
FIG. 1.
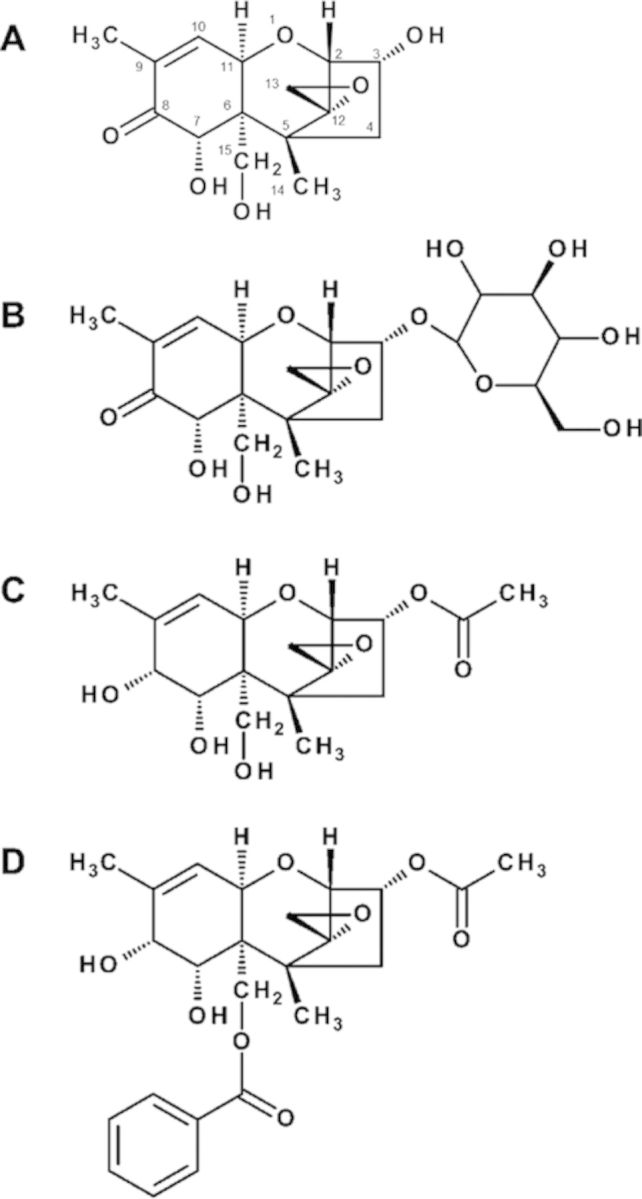
Structures of naturally occurring and synthetic DON congeners. Compounds are: (A) deoxynivalenol (DON), (B) DON-3-glucoside (D3G), (C) EN139528, and (D) EN139544.
DON's capacity to evoke anorectic responses and potential to interfere with the growth of children and young animals is of particular concern from a public health perspective (Canady et al., 2001). These effects are the basis for establishing the tolerable daily intake for DON that has defined regulatory limits for this mycotoxin in food (EFSA, 2011). The balance between anorexigenic and orexigenic signaling modulates eating behavior and this balance is influenced by various mediators of the peripheral and central nervous systems (Schwartz, 2006). Importantly, DON specifically upregulates expression of components of the anorexigenic response in hypothalamic neurons of the brain, including the pro-opiomelanocortin, melanocortin 4 receptor, and cocaine amphetamine-regulated transcript (Girardet et al., 2011). A possible upstream mechanism for the induction of these anorectic effects by DON is the release of gut satiety hormones produced by enteroendocrine cells (EECs) in the gastrointestinal tract (Moran-Ramos et al., 2012; Steinert et al., 2013).
The gut hormones cholecystokinin (CCK) and peptide YY3–36 (PYY3–36) play essential roles in regulating satiety. CCK is produced by a type of EEC called the I cell that is located in the jejunum and ileum. This hormone elicits short-term, diminished food consumption (Challis et al., 2003; Dockray, 2009) by upregulating pro-opiomelanocortin and melanocortin 4 receptor expression (Kohno et al., 2008). Both peripheral and central administration of CCK to animals decrease their consumption of food (Della-Fera and Baile, 1980; Ebenezer et al., 1990; Zhang et al., 1986). PYY3–36 is another gut satiety hormone that can regulate appetite (Batterham et al., 2002). Most PYY3–36 secreting L-cells, another subset of EEC, are found in the lower GI tract regions that include the distal ileum and colon (Hörsten et al., 2004). This hormone both upregulates anorexigenic and downregulates orexigenic signaling molecules within the brain (Challis et al., 2003), inducing satiety in many species including humans, nonhuman primates, and rodents (Karra and Batterham, 2010; le Roux et al., 2006). Previous investigations by our laboratory have established that both oral and intraperitoneal (IP) administration of DON in mice induced rapid elevations in plasma CCK and PYY3–36 (Flannery et al., 2012; Wu et al., 2014a) with concurrent food refusal. Subsequent experiments with specific pharmacologic antagonists to CCK and PYY receptors attenuated this effect. Accordingly, these findings support the contention that CCK and PYY produced by EEC are upstream mediators of DON-induced anorexigenic signaling in the hypothalamus.
The Centers for Disease Control estimates that 80% of the reported 48 million U.S. food-borne illness cases annually have an unknown etiological origin (Scallan et al., 2011). Although DON is not routinely sought as a suspected causative agent in food-borne disease outbreaks, its effects in animal models are consistent with hallmarks of gastroenteritis, leading to speculation that this mycotoxin could be, in part, contributing to some cases of undiagnosed food poisoning. The public health relevance of DON's emetogenic potential is supported by reports of large outbreaks of non-infectious gastroenteritis in many countries including China, Russia, Japan, Korea, and India over the past five decades (Pestka, 2010). Interestingly, DON was detected in the absence of other putative food poisoning agents in burritos associated with 16 large U.S. outbreaks of gastroenteritis with a characteristic rapid onset of vomiting in over 1900 schoolchildren in seven states (Steinberg et al., 2006).
Although the emetic response is a reflex that normally serves as a protective mechanism against toxic agents, severe emesis can cause disruption of normal nutrition, hydration, and electrolyte balance and therefore have the potential to adversely affect susceptible populations such as very young, infirmed, or elderly humans. The neurophysiologic basis for emesis is highly complex and involves hormones, neurotransmitters, and visceral afferent neurons (Andrews and Horn, 2006; Hornby, 2001) that are coordinated in the medulla oblongata of the brain by a neuronal network known as the vomiting center. Hormones and neurotransmitters released in the gut in response to toxic stimuli may trigger emesis by binding to their corresponding receptors located on vagal afferent neurons that project onto the area postrema of the medulla, activating the vomiting center, or by direct stimulation of the area postrema by blood-borne emetic mediators. DON-induced emesis in the pig and mink involves the monoamine neurotransmitter 5-hydroxytryptamine (5-HT, serotonin) (Prelusky and Trenholm, 1993; Wu et al., 2013b). Notably, PYY3–36 is an upstream modulator of 5-HT suggesting that both hormones act in concert to induce vomiting in DON-exposed experimental animals (Wu et al., 2013b).
We have recently demonstrated that the other 8-ketotrichothecenes related to DON and similarly found in Fusarium-infected grains can differentially induce anorectic and emetic effects in the mouse and mink models, respectively (Wu et al., 2012, 2013a). These findings demonstrate that compounds structurally related to DON can share its anorectic and emetic potential. Important to this consideration, DON can be converted in plants to the water soluble detoxification product 12,13-epoxy-3-β-D-glucopyranosyloxy-7,15-trihydroxytrichothec-9-en-8-one (DON-3-glucoside or D3G; Fig. 1B) (Berthiller et al., 2009; Lemmens et al., 2005; Poppenberger et al., 2003). D3G has been referred to as a masked mycotoxin because it can co-occur in cereals contaminated by DON but is not detectable by routine analytical methods (Berthiller et al., 2013). The increasing agricultural use of Fusarium resistance quantitative trait locus that co-localizes with the ability to efficiently convert DON to D3G is expected to increase the ratio of D3G/DON in Fusarium-infected wheat (Lemmens et al., 2005). Importantly, D3G has the potential to be ultimately hydrolyzed back to DON by gastrointestinal microbes (Berthiller et al., 2011; Gratz et al., 2013; Nagl et al., 2012). Unfortunately, despite the recognized presence of D3G in human food and its possible important toxicological significance, nothing yet is known in regard to its capacity either directly or after hydrolysis to induce anorexia or emesis in experimental animals.
DON has also been derivatized to 3α-acetoxy-12,13-epoxy-9-methyltrichothecen-9-en-7α,8α,15-triol (designated EN139528) (Fig. 1C) and 3α-acetoxy-15-benzoyloxy-7α,8α-dihydroxy-12,13-epoxytrichothec-9-ene (designated EN139544) (Fig. 1D) to explore their pharmacological potentials to alter gut motility by inducing a specific pattern of gut motor activity that signals satiety (Durst and Krantis, 2004, 2006). Specifically, a hydroxyl group replaces the C8-keto group in both compounds, whereas an acetyl group replaces the C3-hydroxyl group in EN139528, and a benzoyloxy group replaces the C15-hydroxyl in EN139544. Both derivatives have been suggested to be effective in reducing gut motility, food consumption, and weight gain in mouse and rat (Durst and Krantis, 2004, 2006), indicating that they might act in a manner similar to DON in inducing satiety. To date, there are no published data that directly compare the potential anorectic and emetic potencies of these two compounds with DON.
The goal of this investigation was to test the hypothesis that alterations in DON structure found in D3G, EN139528, and EN139544 will differentially impact their potential to evoke food refusal and emesis. Oral administration was used to mimic the ingestion of these compounds and to maximize their capacity for interaction with gut EEC. The results obtained indicate that although the congeners D3G, EN139528, and EN139544 evoked anorectic effects in the mouse with different relative kinetics and response durations, they were approximately 10–100 times less potent than DON in evoking emesis in the mink.
MATERIALS AND METHODS
Chemicals
DON was prepared by base hydrolysis of 3-acetyl-DON (3-ADON) that was isolated from Fusarium culture by a modification of a previously described method (Miller and Blackwell, 1986). DON purity (>98%) was verified by elemental analysis (Flannery et al., 2011). D3G was purified from wheat as previously reported (Berthiller et al., 2005). Briefly, wheat ears were treated with DON (1 mg/ear) at anthesis, harvested after ripening, and milled. The ears were extracted with acetonitrile/water (84/16, v/v) and silica gel 60 (Merck KGaA, Darmstadt, Germany) was added till a slurry was formed. The slurry was evaporated to dryness and the silica gel was packed on top of a glass column filled with silica gel 60. After a wash step with ethyl acetate, D3G was eluted with ethyl acetate/methanol (80/20, v/v). After a second round of normal phase chromatography, the D3G containing fractions were pooled, evaporated, and dissolved in acetonitrile/water (5/95, v/v). Subsequent reversed phase chromatography purification was performed on a PrepLC semipreparative high performance liquid chromatography (HPLC) (Waters, Milford, MA) equipped with an ultraviolet (UV) detector. A 250 mm × 15 mm i.d., 10-μm particle size, Phenomenex (Torrance, CA) Jupiter C-18 semipreparative column was used with a linear acetonitrile/water gradient to separate D3G from matrix and non-metabolized DON. Purity (>96%, not containing any detectable amounts of DON) and identity were confirmed by HPLC-UV, HPLC-tandem mass spectrometry (HPLC-MS/MS), and nuclear magnetic resonance (NMR) measurements.
EN139528 and EN139544 were prepared as previously described (Durst and Krantis, 2004, 2006). EN139528 was produced by the reduction of 3-ADON. In a typical reaction, sodium borohydride (17 mg, 0.44 mmol) was added to a cooled (0°C) solution of 3-ADON (150 mg, 0.44 mmol) and CeCl3.7H2O (82 mg, 0.22 mmol) in methanol (12 ml) was added in three portions. After stirring 20 min at the same temperature, saturated ammonium chloride solution was added, the methanol was removed, and the residual solution was extracted three times with ethyl acetate. The organic extracts were dried over Na2SO4, filtered, and concentrated in vacuo. The residue was chromatographed on silica gel to give EN139528. EN139544 was synthesized from EN139528. Briefly, benzoyl chloride (91 mg, 0.638 mmol) was added to a 25-ml round bottom flask containing EN139528 (200 mg, 0.58 mmol), triethylamine (178 mg, 1.74 mmol), and 5 mg of 4-dimethylaminopyridine in 5-ml acetonitrile at 0°C. After completion of the reaction at 0.5 h, 5 ml of saturated NaCl was added to the reaction mixture, acetonitrile was removed under reduced pressure. The residue was extracted with three times with dichloromethane and the combined organic layers were dried over Na2SO4 and concentrated. Column purification with silica gel yielded EN139544. Purities of EN139528 and EN139544 were >98% based on high field proton hydrogen-1 NMR (HNMR).DON, D3G, EN139528, and EN139544 were dissolved in filter-sterilized phosphate buffered saline for oral administration to mice and mink.
Animals
Animal experiments followed National Institutes of Health guidelines and were approved by the Michigan State University Institutional Animal Care and Use Committee (MSU-IACUC). Female B6C3F1 mice (10–12-week old) were obtained from Charles River Breeding (Portage, MI) and housed individually in polycarbonate cages in a room maintained at 21–24°C and 40–55% relative humidity under a 12-h light (6:00–18:00 h)/dark (18:00–6:00 h) cycle. Standard dark, female mink (Mustela vison) of 1 to 2 years of age (average weight = 1.18 ± 0.15 kg) were obtained from the Michigan State University (MSU) Experimental Fur Farm. Housing conditions met those specified in the Standard Guidelines for the Operation of Mink Farms in the United States (Fur Commission USA 2010). Animals were housed singly in wire cages (62 cm long × 25 cm wide × 38 cm high) and provided with a nest box (24 cm long × 24 cm wide × 29 cm high) with aspen shavings or excelsior (wood wool) within an open-sided pole barn. Temperature (daily average = 7 to 18°C), humidity, and photoperiod were dependent on the ambient environment.
Study 1. Dose response effects of DON congeners on food consumption in the mouse
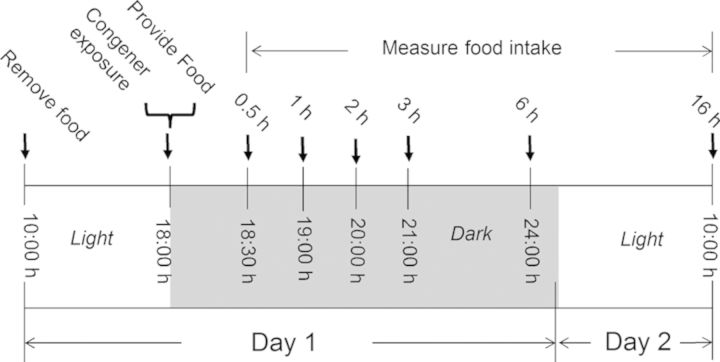
The general experimental design for the food consumption study (Fig. 2) was based on previously developed protocols (Flannery et al., 2011; Wu et al., 2012). Briefly, mice were acclimated 1 week after arriving and randomly divided into different groups according to body weight 1 day before experiment initiation. On the day of the experiment, groups of mice (n = 5) were fasted from 10:00 h to 18:00 h with water provided ad lib. At 18:00 h mice were orally gavaged with 0, 2.5, 5, and 10 mg/kg BW DON, D3G, EN139528, or EN139544 in 100-μl PBS, respectively, using a sterile 22-G 3.8-cm disposable feeding tube (Instech Solomon; Plymouth Meeting, PA). These doses were selected to compare this study with previous investigations by our laboratory (Flannery et al., 2011, 2012; Wu et al., 2012). Immediately after treatment, mice were provided with two pre-weighed food pellets (≈7 g) and food intake was measured under red light conditions during the dark cycle at 0.5 h, 1 h, 2 h, 3 h, 6 h, and 16 h post-exposure.
FIG. 2.
Experimental design for anorexia bioassay of DON in mice. Mice were gavaged with DON, D3G, EN139528, and EN139544 immediately before the dark feeding cycle. Food intake was measured at 0.5, 1, 2, 3, 6, and 16 h post administration.
Study 2. Relative effects of DON congeners on food intake and plasma CCK and PYY in the mouse
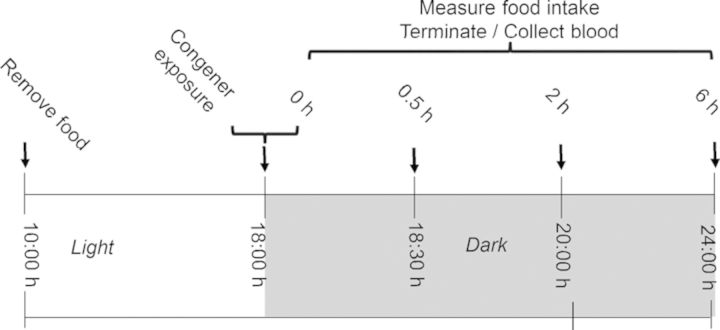
Groups of mice (n = 6) were orally gavaged with DON, D3G, EN139528, or EN139544 at 2.5-mg/kg body weight (BW) in 100-μl PBS or vehicle alone and sacrificed under red light conditions at 0, 0.5, 2, and 6 h post-exposure (Fig. 7). This dose was selected because it caused modest, reproducible inhibition of food consumption for all congeners during Study 1. At experiment termination, mice were anesthetized by IP injection with 100-μl sodium pentobarbital (56 mg/ml). Blood was then collected from the inferior vena cava into EDTA-coated tubes and mice immediately euthanized by cervical dislocation. Plasma was separated from blood by centrifugation at 3500 × g for 10 min at 4°C and frozen at −80°C until analysis. CCK and PYY3–36 concentrations were analyzed using competitive enzyme immunoassay kits from Phoenix Pharmaceuticals (Burlingame, CA).
FIG. 7.
Experimental design for relating anorectic effects of DON congeners to plasma CCK and PYY3–36 concentrations in mice. Mice were gavaged with DON immediately before the dark feeding cycle and food intake was measured over 16 h. Mice were orally gavaged with either PBS or 2.5-mg/kg bw DON congeners. Cumulative food intake was measured and plasma analyzed for CCK and PYY3–36 at 0, 0.5, 2, and 6 h post administration.
Study 3. Effects of DON congeners on emesis in mink
The general experimental design for the mink emesis bioassay (Fig. 12) was based on prior studies employing this species (Wu et al., 2012, 2013a). Mink emesis study was conducted in April through May. Beginning at 8:30 am on the day prior to the experiment, mink were fasted for 24 h with water available ad lib. On the day of the experiment, animals were provided 50 g of feed at 8:30 h and allowed to eat for 30 min to assure a constant volume of gastric contents in each animal. At 9:00 h, mink were given DON, D3G, EN139528, EN139544, or PBS in a volume of 1-ml/kg BW by oral gavage using a sterile 16-G, 5-cm stainless steel gavage tube. After dosing, animals were returned to individual cages and monitored for emesis over the subsequent 3 h. Each individual retch or vomit was counted as described previously (Wu et al., 2013b) and the counts were combined to yield total emetic events. Vomiting was characterized as rhythmic abdominal contraction with oral expulsion of either solid or liquid material, whereas retching was defined as responses that mimicked vomiting but without any material being expelled.
FIG. 12.
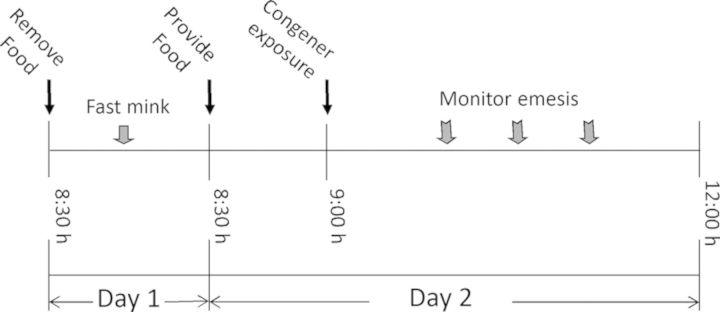
Experimental design for comparing effects of oral exposure with DON congeners on emesis in mink.
Statistics
All data were plotted and statistically analyzed using SigmaPlot 11 for Windows (Jandel Scientific; San Rafael, CA) with the exception of emetic dose (ED), which was calculated with Proc Probit using SAS (Version 9.2, SAS, Cary, NC). Means were considered significantly different at p < 0.05. Food intake at specific time points was analyzed by one-way ANOVA using Dunnett's Method to determine significant differences between treatments and the respective control. Two-way repeated ANOVA (one-factor) using the Holm-Sidak method was used to analyze significant differences in food consumption as compared with the control over time. A two-way ANOVA using the Bonferroni t-test was used to assess significant differences in kinetics of CCK and PYY3–36 in mouse plasma and cumulative emetic events in mink. Fisher's Exact Test was used for incidence, and one-way ANOVA using the Holm-Sidak method was used for latency, duration, retching, vomiting, and total emetic events. A t-test was used to assess significant differences in PYY3–36 concentrations in mink plasma induced by the different congeners (see Supplementary fig. 1).
RESULTS
Study 1: Dose Response Effects of DON Congeners on Food Intake
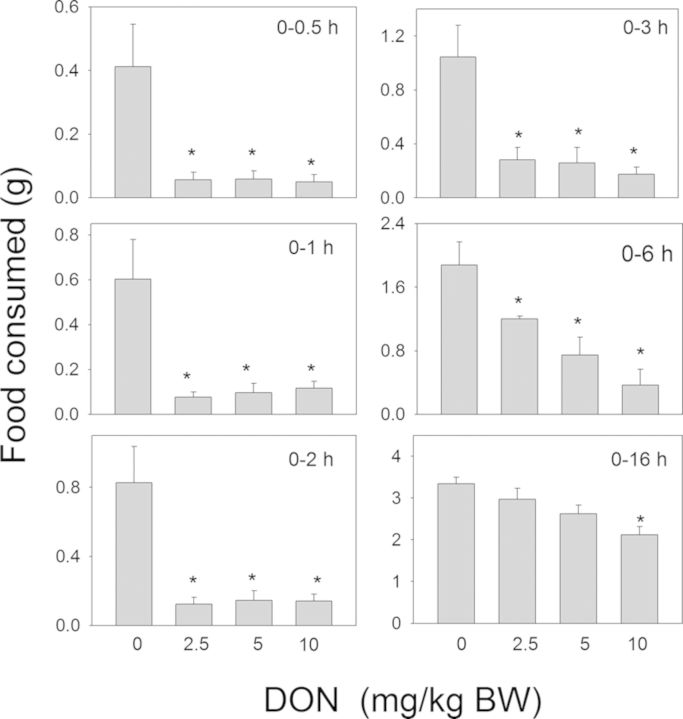
The relative effects of acute oral administration of DON at three doses (2.5, 5, and 10-mg/kg BW) on food consumption of mice during the nocturnal feeding cycle were compared with those of D3G, EN139528, and EN139544 (Fig. 2). DON evoked >80% decrease in cumulative food intake at 0.5 h, 1 h, and 2 h at all three doses when compared with vehicle controls (Fig. 3). Reductions in total food intake of 73, 75, and 83% at 3 h and 36, 60, and 80% at 6 h were observed for the 2.5, 5, and 10-mg/kg BW doses, respectively. At 16 h post-exposure, no further reductions were observed in cumulative food intake by DON-treated mice with the exception of the 10-mg/kg BW dose group (37%).
FIG. 3.
Oral exposure to DON impairs cumulative food intake for up to 16 h. Mice were gavaged with DON immediately before the dark feeding cycle and food intake was measured over 16 h. Data are mean ± SEM (n = 5/group) and analyzed by one-way ANOVA using Dunnett's Method. An asterisk indicates a statistically significant difference in cumulative food consumption between treatment and respective control (p < 0.05).
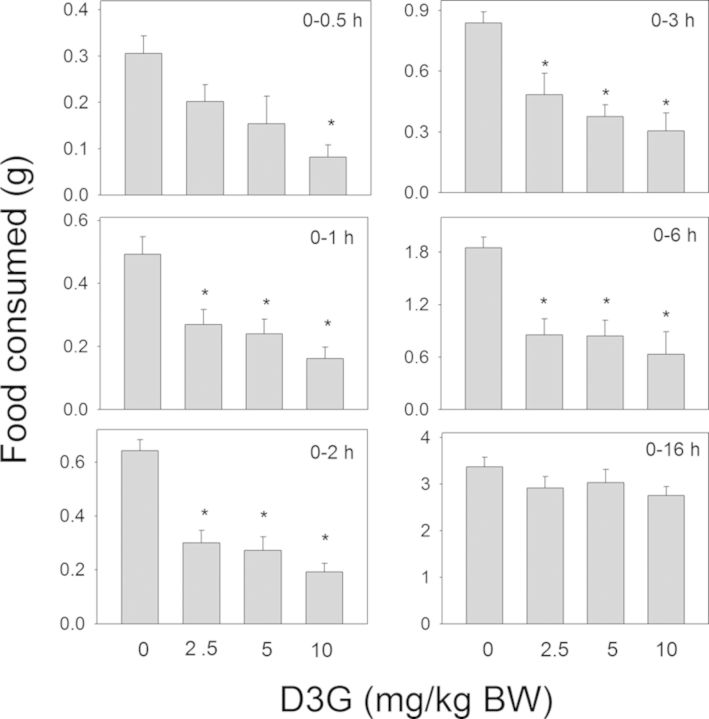
In contrast to DON, a delayed reduction in food intake was observed following oral exposure to the plant metabolite D3G at 2.5, 5, and 10-mg/kg BW (Fig. 4). Although only the 10-mg/kg BW dose reduced food intake at 0.5 h (73%), all doses reduced cumulative food intake from 1 to 6 h by 42–70%. At 16 h, differences in food intake were no longer observed between any of the D3G- and vehicle-treated groups.
FIG. 4.
Oral exposure to D3G impairs cumulative food intake for up to 6 h. Mice were gavaged with D3G immediately before the dark feeding cycle and food intake was measured over 16 h. Data are mean ± SEM (n = 5/group) and analyzed by one-way ANOVA using Dunnett's Method. An asterisk indicates a statistically significant difference in cumulative food consumption between treatment and respective control (p < 0.05).
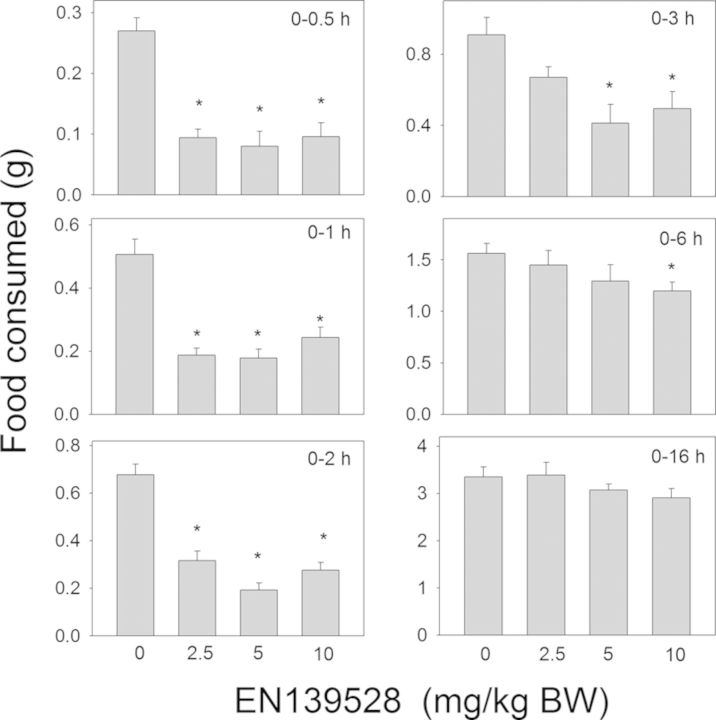
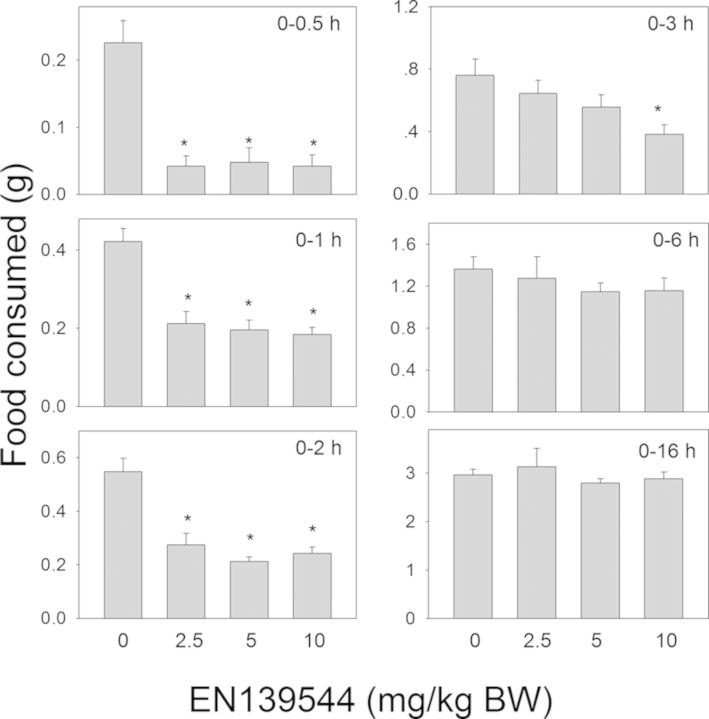
Oral exposure to the synthetic DON derivative EN139528 also evoked reductions in food consumption (Fig. 5). Cumulative food intake was reduced by 46–70% at 0.5, 1, 2, and 3 h for all three doses. At 3 or 6 h post-exposure and thereafter, differences were no longer observed between vehicle-treated mice and those treated with EN139528 at 2.5- or 5-mg/kg BW. For the 10-mg/kg BW dose, cumulative food intake was reduced by 24% at 6 h as compared with mice treated with the vehicle, but no differences were observed beyond that time point. The other synthetic DON derivative, EN139544, also inhibited food consumption in mice following exposure to 2.5, 5, and 10-mg/kg BW (Fig. 6). The 2.5-mg/kg bw dose caused an 81%, 47%, and 50% reduction in cumulative food intake at 0.5 h, 1 h, and 2 h, respectively, whereas the 5-mg/kg bw dose caused reductions of 79%, 51%, and 58%, respectively, at those time points. No differences were observed between control and the two lower dose groups at 3 h post-dosing or thereafter. The 10-mg/kg BW dose inhibited food intake by 81%, 54%, 56%, and 50% at 0.5 h, 1 h, 2 h, and 3 h, respectively, but no inhibition was observed at 6 or 16 h post-treatment. Thus, although the early anorectic effects of DON were mimicked by EN13928 and to a lower extent by EN139544, the response durations for these latter DON congeners were markedly less than DON or D3G.
FIG. 5.
Oral exposure to EN139528 impairs cumulative food intake for up to 6 h. Mice were gavaged with EN139528 immediately before the dark feeding cycle and food intake was measured over 16 h. Data are mean ± SEM (n = 5/group) and analyzed by one-way ANOVA using Dunnett's Method. An asterisk indicates a statistically significant difference in cumulative food consumption between treatment and respective control (p < 0.05).
FIG. 6.
Oral exposure to EN139544 impairs cumulative food intake for up to 3 h. Mice were gavaged with EN139544 immediately before the dark feeding cycle and food intake was measured over 16 h. Data are mean ± SEM (n = 5/group) and analyzed by one-way ANOVA using Dunnett's Method. An asterisk indicates a statistically significant difference in cumulative food consumption between treatment and respective control (p < 0.05).
Study 2: Comparative Effects of Exposure to a Common Dose of DON Congeners on Food Intake and Plasma Gut Satiety Hormones
The effects of oral administration of DON, D3G, EN139528, and EN139544 at a common dose (2.5-mg/kg BW) on food consumption over a 6-h period were related to plasma concentrations of the gut satiety hormones CCK and PYY3–36 (Fig. 7). Following oral exposure to DON, significant reductions in cumulative food consumption were observed at 0.5 h and 2 h post-exposure with an apparent trend toward an increased food consumption rate from 2 to 6 h (Fig. 8A). Consistent with its satiety-inducing effects, plasma CCK was significantly elevated by DON at 0.5 and 2 h post-exposure, but returned to vehicle control concentrations by 6 h (Fig. 8B). PYY3–36 was also significantly increased at 0.5 h but recovered to control concentrations by 2 h (Fig. 8C).
FIG. 8.
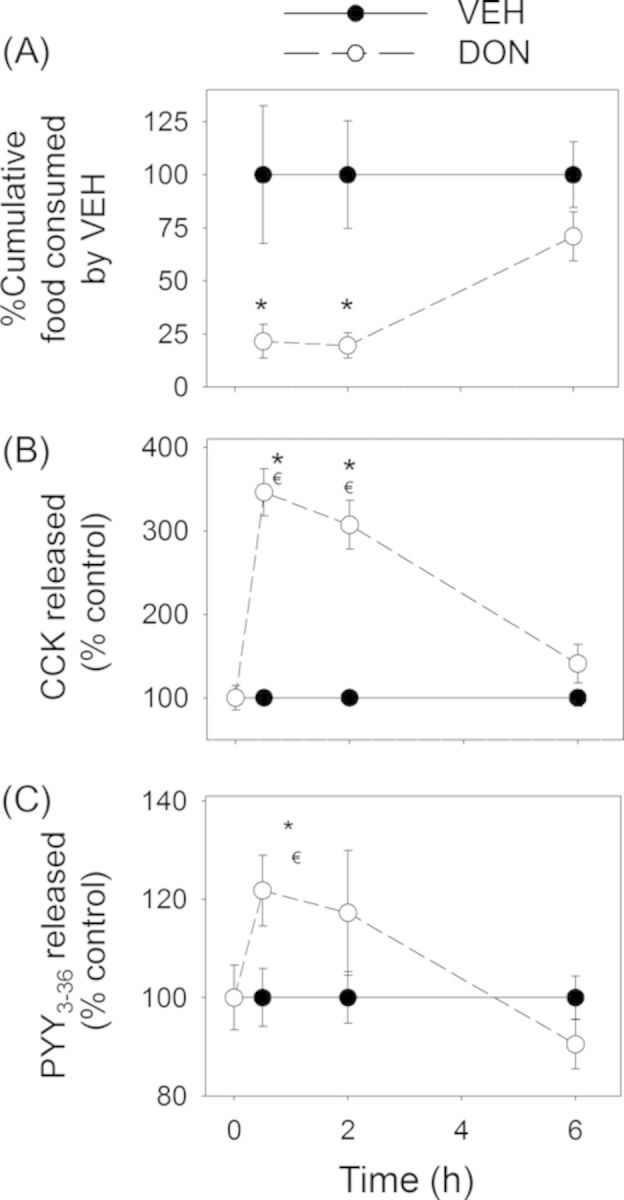
DON-induced anorectic response corresponds to plasma CCK and PYY3–36 elevation. Mice were orally gavaged with either PBS (solid lines) or 2.5-mg/kg BW DON (broken lines). (A) Rapid and transient anorectic effect following oral exposure to DON. Data are mean ± SEM (n = 5/group). Two-way repeated ANOVA (one factor) using the Holm-Sidak Method was used to analyze significant differences in food consumption as compared with the control. Symbol: * indicates a statistically significant difference in cumulative food consumption relative to the control at specific time point (p < 0.05). (B) Plasma CCK and (C) PYY3–36 elevation in mice. Data represent mean ± SEM (n = 6/group). Two-way ANOVA using Bonferroni t-test was used to assess significant differences in kinetics of CCK and PYY3–36 concentrations in plasma as compared with the control. Symbols: * indicates a statistically significant difference in plasma CCK or PYY3–36 concentration relative to the control at specific time point (p < 0.05). ŧ indicates a statistically significant difference in plasma CCK or PYY3–36 concentration relative to the 0-h time point (p < 0.05).
As observed in Study 1, the effects of D3G on food consumption were delayed in comparison with DON. Again, although food intake was unaffected at 0.5 h post administration, it was significantly reduced at 2 h and 6 h post-exposure (Fig. 9A). Consistent with this pattern, plasma CCK was not affected in D3G-treated mice at 0.5 h, however, it was significantly elevated at 2 h and returned to vehicle control concentrations by 6 h post-treatment (Fig. 9B). PYY3–36 was moderately increased 6 h after exposure but not at earlier time points (Fig. 9C).
FIG. 9.
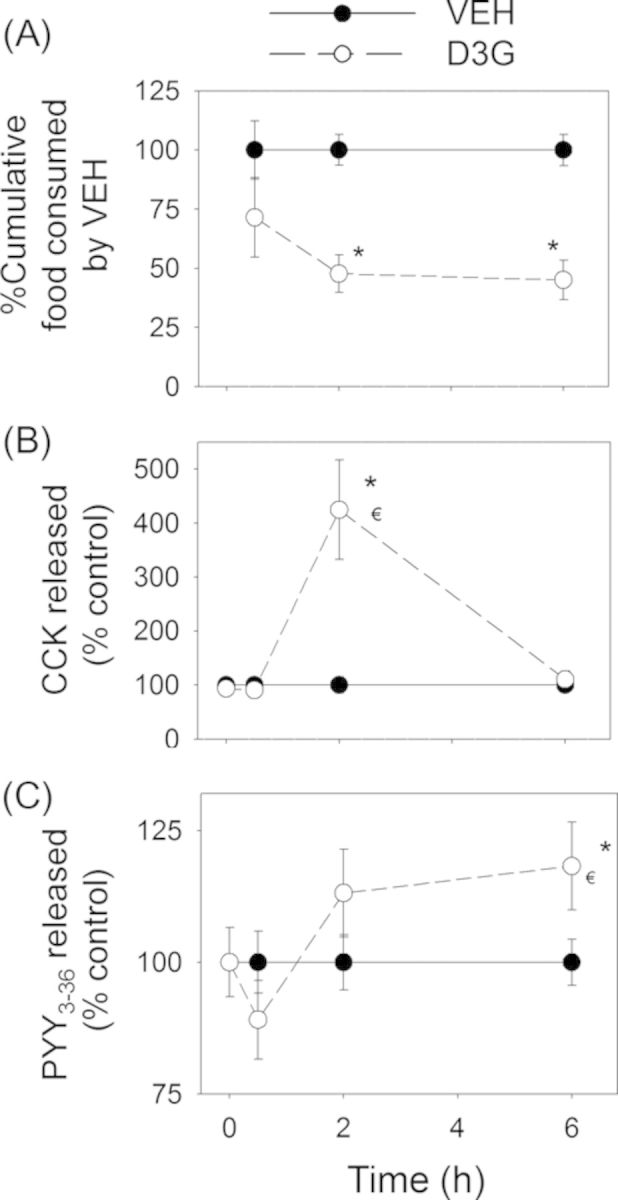
D3G-induced anorectic response corresponds to plasma CCK and PYY3–36 elevation. Mice were orally gavaged with either PBS (solid lines) or 2.5-mg/kg bw D3G (broken lines). Experiment was conducted and data were analyzed as described in the Figure 8 legend.
Significant reductions in food intake were observed at 0.5 h and 2 h but not at 6 h after dosing with EN139528 and EN139544 (Figs. 10A and 11A). Plasma CCK was also significantly increased by both DON derivatives at 0.5 and 2 h, but returned to control concentrations by 6 h post-exposure (Figs. 10B and 11B). Unlike DON and D3G, oral exposure to EN139528 and 139544 did not significantly affect plasma PYY3–36, although a modest upward trend was evident for the former compound (Figs. 10C and 11C).
FIG. 10.
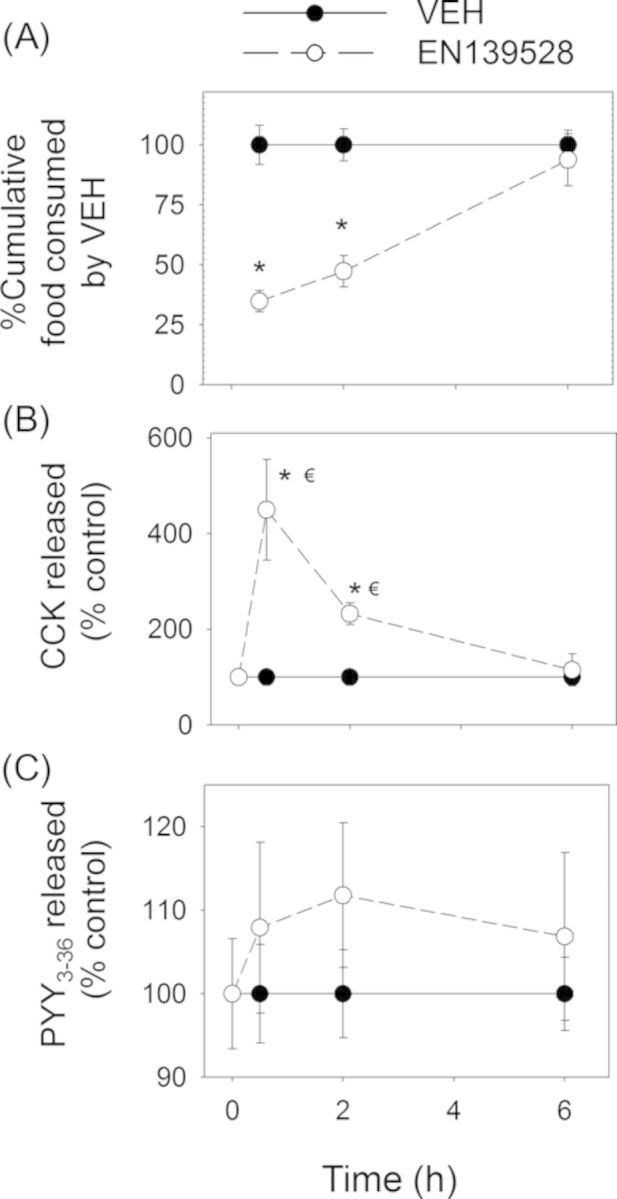
EN139528-induced anorectic response corresponds to plasma CCK elevation. Mice were orally gavaged with either PBS (solid lines) or 2.5-mg/kg BW EN139528 (broken lines).Experiment was conducted and data were analyzed as described in the Figure 8 legend.
FIG. 11.
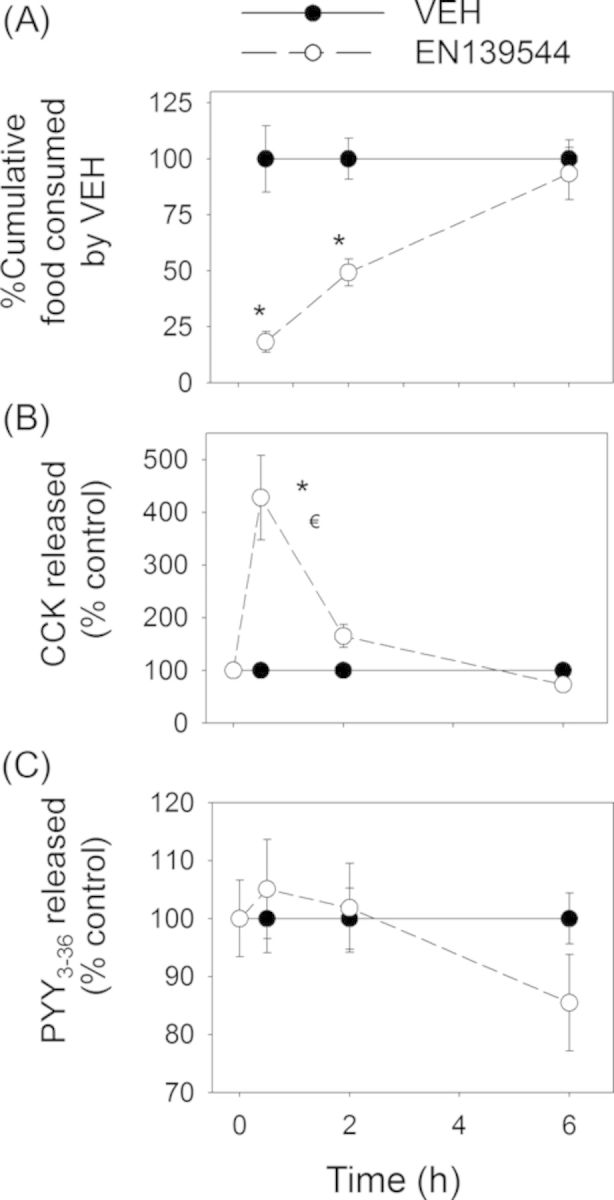
EN139544-induced anorectic response corresponds to plasma CCK elevation. Mice were orally gavaged with either PBS (solid lines) or 2.5-mg/kg BW EN139544 (broken lines). Experiment conducted and data analyzed are the same as in the Figure 8 legend.
Study 3: Effects of DON Congeners on Emesis in Mink
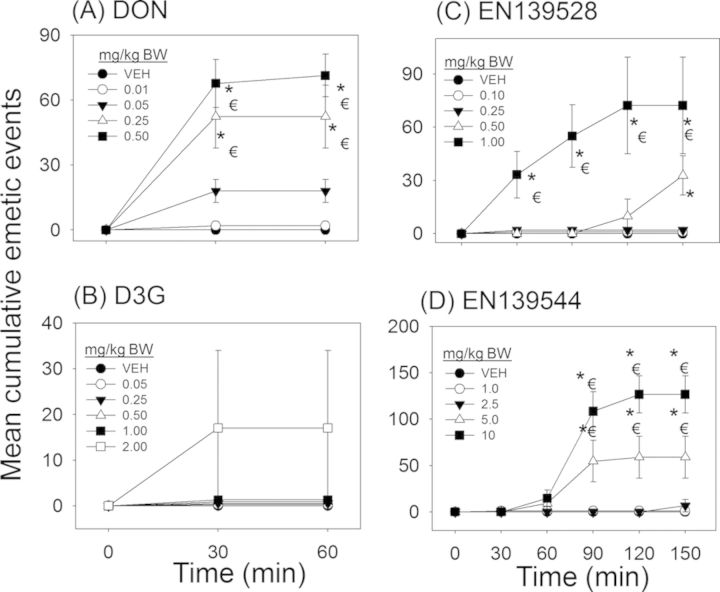
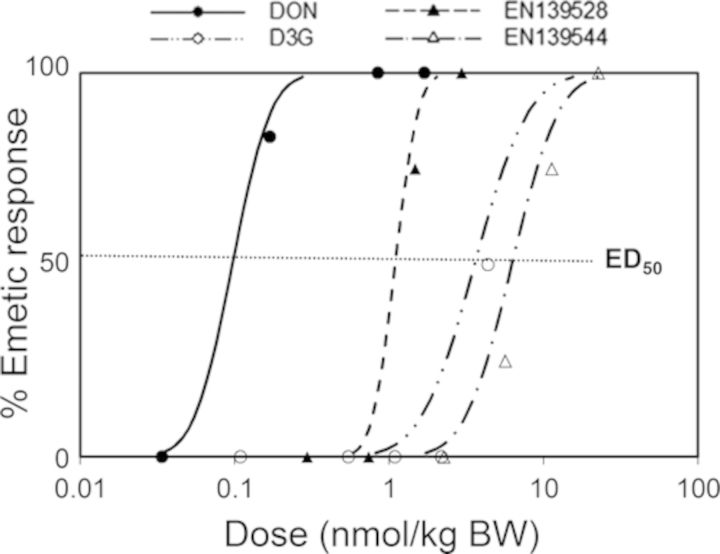
The dose response effects of oral administration with D3G, EN139528, and EN139544 on emesis by mink were measured and compared with data from a previously reported study for DON (Wu et al., 2013a) employing this animal model (Fig. 12). In the prior investigation, we found that acute oral exposure to DON at doses as low as 0.05-mg/kg BW caused emesis as early as 30 min (Fig. 13A and Table 1). In contrast, no effects were observed in the present study for D3G at 0.05–1-mg/kg BW, whereas the 2-mg/kg BW dose caused emesis in 50% (one out of two) of the exposed mink (Fig. 13B and Table 1). Additional mink and higher dosages were not employed because of the limited supply of this plant metabolite. All emetic events for DON and D3G occurred in the first 30 min with no further substantive effects being evident at later time points (Figs. 13A and B).
FIG. 13.
Differential effects of DON congeners on cumulative emetic events in mink. Mink were orally gavaged with (A) DON, (B) D3G, (C) EN139528, and (D) EN139544 at indicated doses and emesis monitored over time. Data are averages for both responders and non-responders and represent mean ± SEM (n = 4–6/group). A two-way ANOVA using Bonferroni t-test was used to assess significant differences in cumulative emetic event in mink. Symbols: * indicates statistically significant differences in cumulative emetic episodes concentrations compared with the control (p < 0.05). ŧ indicates a statistically significant difference relative to the 0-min time point within a given dose (p < 0.05).
TABLE 1. Comparison of Emetic Responses in Mink Following Oral Exposure to DON, D3G, EN139528, and EN139544.
| Toxin | Dose (mg/kg bw) | Incidence (responding/tested) | Latency to emesis (min)a,b | Emesis duration (min)a,b | Emetic eventsc | ||
|---|---|---|---|---|---|---|---|
| Retching | Vomiting | Total | |||||
| DON# | 0 | 0/6 | - | - | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 0.01 | 0/6 | - | - | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 0.05* | 5/6 | 15 ± 2 a | 1 ± 1 a | 12 ± 4 | 3 ± 1 | 15 ± 5 | |
| 0.25* | 6/6 | 17 ± 2 a | 6 ± 1 a,b | 44 ± 13 | 8 ± 1 | 52 ± 15 | |
| 0.5* | 6/6 | 11 ± 2 a | 14 ± 3 b | 62 ± 9 | 9 ± 1 | 71 ± 10 | |
| D3Gd | 0 | 0/2 | − | − | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 0.05 | 0/2 | − | − | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 0.25 | 0/2 | − | − | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 0.5 | 0/2 | − | − | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 1 | 0/2 | − | − | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 2 | 1/2 | 21 ± 0 | 8 ± 0 | 15 ± 15 | 3 ± 3 | 17 ± 17 | |
| EN139528 | 0 | 0/4 | - | - | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 0.1 | 0/4 | - | - | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 0.25 | 0/4 | - | - | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 0.5 | 3/4 | 95 ± 4 a | 4 ± 1 a | 28 ± 10 | 5 ± 2 | 33 ± 11 | |
| 1* | 4/4 | 19 ± 5 b | 40 ± 12 b | 59 ± 22 | 13 ± 5 | 72 ± 27 | |
| EN139544 | 0 | 0/4 | - | - | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 1 | 0/4 | - | - | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 2.5 | 1/4 | 133 ± 0 a | 1 ± 0 a | 6 ± 6 | 1 ± 1 | 7 ± 7 | |
| 5 | 3/4 | 61 ± 3 b | 31 ± 10 a | 50 ± 20 | 9 ± 3 | 59 ± 23 | |
| 10* | 4/4 | 58 ± 2 b | 41 ± 4 b | 112 ± 18 | 15 ± 3 | 127 ± 20 | |
#DON data have been previously reported in a prior investigation (Wu et al., 2013a).
aAverage of positive responders only.
bIf animals failed to retch or vomit, the latency and duration of emesis are shown as “-”.
cAverage of both responders and non-responders. Data represent the mean ± SEM. Values with an asterisk indicate significant difference at p < 0.05 relative to the control for incidence. Values for each congener with different superscript within a column indicate significant difference at p < 0.05.
dBecause D3G was in limited supply, a small number of experimental animals and limited dose range were used.
Following exposure to EN139528 at 0.5- and 1-mg/kg BW, 75 and 100% mink experienced emesis, respectively, whereas the 0.1- and 0.25-mg/kg BW doses had no effect (Fig. 13C and Table 1). The 0.5-mg/kg BW dose induced emesis between 120 and 150 min post-treatment, whereas 1-mg/kg BW caused emesis in 30 min with cumulative emetic events increasing until 120 min post-exposure. The lowest oral dose of EN139544 that induced emesis was 2.5-mg/kg BW with 25% of the mink responding to the treatment between 120 and 150 min post-treatment (Fig. 13D and Table 1). Doses of 5- and 10-mg/kg BW EN139544 caused emetic events in 75 and 100% of mink, respectively, within 60 min and lasted up to 120 min post-exposure.
DISCUSSION
DON is a common food-borne contaminant and therefore its anorectic and emetic effects constitute major concerns for human and animal health. As the naturally occurring metabolite D3G and synthetic DON congeners EN139528 and EN139544 are structurally similar to DON, we posited that they would likewise evoke these adverse effects. When the congeners’ anorectic and emetic potencies were compared here with DON, there were three novel findings. First, like DON, the congeners D3G, EN139528, and EN139544 caused food refusal across a narrow dose range in the mouse nocturnal feeding model. Second, equivalent doses of all four compounds in the mouse model induced increases of plasma CCK, whereas only DON and D3G caused elevations in PYY3–36. Finally, although all four compounds could elicit vomiting in mink emesis model, DON's potency was approximately 10- to 100-fold greater than that of the natural and synthetic congeners.
Any interpretations of the present data need to be considered in the context of what is known about absorption and clearance of the four compounds that were compared. Recent estimates of the intestinal transit times of food in the mouse and mink are 6–8 h and 2–3 h, respectively (Gugolek et al., 2013; Padmanabhan et al., 2013). Studies in the mouse indicate that absorption and elimination of DON is very rapid (Amuzie et al., 2008; Azcona-Olivera et al., 1995; Pestka et al., 2008). Following oral exposure, DON reaches a peak plasma level within 30 min and declines with 75–90% clearance after 3 h. The toxin appears to follow similar toxicokinetics in the mink (Wu et al., 2013b). Rat feeding studies suggest that only a small portion of intact D3G can be absorbed and excreted via the urine, however, the congener can be converted back to DON via hydrolytic enzymes produced by gastrointestinal microbiota (Berthiller et al., 2011; Gratz et al., 2013; Nagl et al., 2012). Although the kinetics of D3G biotransformation to DON is currently unknown, it is likely this transformation occurs slower than absorption of DON. In addition, it is expected that the presence of the requisite microbiota that carry out this biotransformation will be species- and strain-dependent. Finally, although little is known about the absorption and metabolism of EN139528 and EN139544, both compounds are similarly efficacious at reducing gut motility and food intake when presented in food or administered intravenously (Durst and Krantis, 2004, 2006) suggesting that they might behave similarly to DON with regard to absorption and clearance.
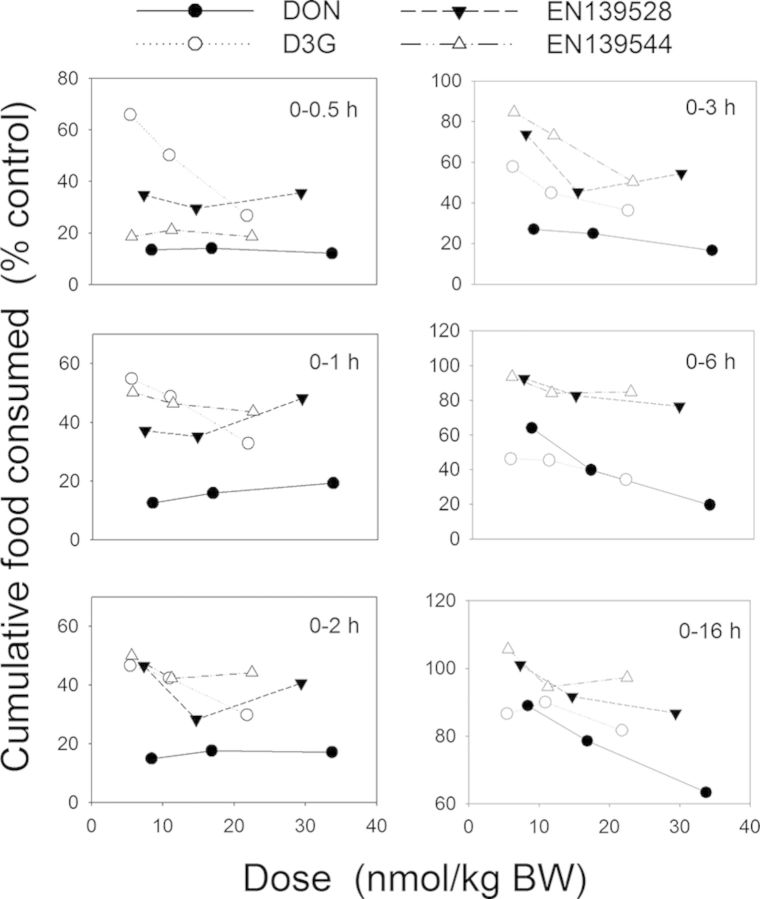
In Study 1, the kinetics and dose-dependency of the anorectic responses to DON observed were consistent with that of prior studies (Flannery et al., 2011; Wu et al., 2012). The reductions in food consumption observed in D3G-, EN139528-, and EN139544-treated mice indicated that all three DON congeners could also elicit anorectic effects. To normalize these comparative data for molecular weight, we summarized and plotted the anorectic responses over discrete time periods as a function of the molar dosage (nmol/kg BW) (Fig. 14). The results suggested that anorectic potencies followed approximate rank orders of DON > EN139544 > EN139528 > D3G from 0 to 0.5 h but DON > D3G > EN139528 > EN139544 from 0 to 3 h. Unlike DON, D3G-induced food refusal was not observed until 1 h and recovered at 16 h after oral exposure. Thus the rate of the putative D3G transformation and/or its intestinal transit time could have delayed the onset of the anorectic response in D3G-dosed mice. In contrast to D3G, the early kinetics of both EN139528- and EN139544-induced food refusal mirrored those of DON. However, the duration of these responses was 10–13 h less than that for DON. Therefore, it will be of interest in the future to more fully explore the uptake, metabolism, and clearance of D3G, EN139528, and EN139544 in the context of this mouse nocturnal feeding model.
FIG. 14.
Summary of comparative anorectic effects of DON, D3G, EN139528, and EN139544 as a function of molar dose.
Study 2 related food refusal by the congeners to plasma concentrations of CCK and PYY, two gut hormones that are secreted by EEC and are known to play an important homeostatic role in regulating appetite (Valassi et al., 2008). Our previous studies suggest that pharmacologic antagonism of the CCK1 receptor that is abundantly expressed throughout the GI tract attenuates DON-induced anorexia (Wu et al., 2014a). DON-induced anorexia is also attenuated by specific pharmacologic antagonists of CCK2 receptor (Wu et al., 2014a), which is located predominately in the brain (Crawley and Corwin, 1994). Thus DON-induced CCK can potentially act via specific receptors in the peripheral and central nervous systems. Exposure to DON, EN139528, and EN139544 increased plasma CCK within 0.5 h, a time that coincided with the initiation of significant anorectic responses. Durations of EN139528- and EN139544-induced plasma CCK elevation were markedly shorter than for DON and mirrored food consumption data. In comparison, D3G-induced plasma CCK was not evident until 2 h post-exposure, which was consistent with its anorectic effects. As discussed above, conversion of D3G to DON and/or intestinal transit time might predictably delay plasma CCK elevation in mice.
In a prior study, it was shown that that PYY3–36 also contributes to DON-induced food refusal, as antagonism of its NPY2 receptor (Y2R) attenuated the anorectic response (Flannery et al., 2012). In that study, it was further observed that PYY3–36 was robustly elevated by DON in the mouse following IP administration but less so after oral gavage (Flannery et al., 2012; Wu et al., 2013b). It is likely that IP administration could disseminate DON to both the upper and lower GI tract from the blood. In contrast, exposure by oral gavage might result in the toxin being absorbed mostly in the upper GI tract before reaching the lower GI tract, thereby limiting robust PYY3–36 secretion. As shown here in the mouse anorexia model, PYY3–36 was moderately elevated following oral exposure to 2.5-mg/kg BW DON and D3G, but not EN139528 and EN139544. Compared with DON, D3G-induced elevation of PYY3–36 was delayed until 6 h, which was consistent with its persistent anorectic response at 6 h. Again the requisite biotransformation from D3G to DON and intestinal transit times might explain the delayed effects of D3G. The inability of EN139528 and EN139544 to induce PYY3–36 elevation in mice is consistent with their capacity to evoke primarily short-term anorectic effects.
It is well-established that DON can upregulate proinflammatory cytokine expression in the spleen, liver, and lungs of mice within 2 h (Pestka, 2010) of exposure. Such a cytokine storm induced by DON could theoretically elicit a sickness response such as has been described for bacterial endotoxin that induces anorexia as a hallmark (Plata-Salaman, 1998). However, we have recently determined that, unlike DON, the congeners studied here had negligible effects on inducing innate immune genes such as IL-1β, IL-6, TNF-α, CXCL-2, CCL-2, and CCL-7 (Wu et al., 2014b). Thus, the contribution of proinflammatory cytokines and innate immune system to anorectic effects of the congeners is likely to be negligible.
Study 3 is the first report to compare emetic potencies of DON, D3G, EN139528, and EN139544 following oral exposure. Based on incidence data, we summarized each chemical's no-observed adverse effect level (NOAEL), lowest observed effect level (LOAEL), and ED20, ED50, ED80 values (Table 2). Collectively, these data show that, as compared with DON, emetic responses to D3G, EN139528, and EN139544 were 10- to 100-fold lower. When emetic responses were plotted as a function of nmol/kg BW to discern comparative molar potencies (Fig. 15), the results again indicated that D3G, EN139528, and EN139544 were markedly less emetogenic than DON. It should be noted, however, that because of a limited supply, results of D3G emetic potency are estimated based on the response in one of the two minks at the highest dose. Although not within the scope of this study, it is possible that differential emesis induction by the four compounds is caused by altered secretion of EEC hormones, notably PYY and 5-HT. Both of these have been shown to be increased in plasma of mink treated with DON by IP administration both prior to and during emesis (Wu et al., 2013b). PYY3–36 is one of the most potent peptide inducers of emesis known (Harding and McDonald, 1989). This peptide is also an upstream modulator of 5-HT in mink suggesting that both hormones act in concert to induce vomiting (Wu et al., 2013b). Interestingly, it was confirmed in a supplementary experiment (see Supplementary fig. 1) that oral exposure to DON and EN139528 caused elevations in plasma PYY3–36 that corresponded with the time of emesis induction (0–30 min) by these two compounds. In the future, it will be of interest to further relate toxicokinetics and dose response effects of DON congeners on emesis induction to the release of PYY, CCK, and 5-HT by EEC.
TABLE 2. NOAELs, LOAELs, ED20s, ED50s, and ED80s for Oral Emetic Effects of DON and Its Conjugates D3G, EN139528, and EN139544.
| (μg/kg BW) | |||||
|---|---|---|---|---|---|
| Toxin | NOAELa | LOAELb | ED20c | ED50d | ED80e |
| DON | 0.01 | 0.05 | 0.02 (0–0.03) | 0.03 (0–0.05) | 0.04 (0.01–1.1) |
| D3G | 1 | 2 | 0.96 (0–0) | 1.63 (0–0) | 2.78 (0–0) |
| EN139528 | 0.25 | 0.5 | 0.29 (0–0.41) | 0.37 (0.05–0.73) | 0.46 (0.32–40.37) |
| EN139544 | 1 | 2.5 | 1.74 (0.05–2.96) | 2.76 (0.73—6.03) | 4.39 (2.51–48.53) |
aNOAEL = no observed adverse effect level.
bLOAEL = lowest observed adverse effect level.
cED20 = dose causing emesis in 20% of the animals tested.
dED50 = dose causing emesis in 50% of the animals tested.
eED80 = dose causing emesis in 80% of the animals tested. ED20, ED50, and ED80 values were determined using a Proc Probit model.
FIG. 15.
Summary of comparative emetic effects of DON, D3G, EN139528, and EN139544 as a function of molar dose.
To summarize, the data presented herein show that although D3G, EN139528, and EN139544 rapidly elicited anorectic responses that mimicked DON over a narrow dose range, they were markedly less effective than the parent mycotoxin at inducing emesis. Thus, by modifying the structure at the C-3, C-8, and/or C-15 positions of the parent DON molecule (Fig. 1), it is possible to separate its satiety-triggering response from its emesis-inducing effect. This has practical significance from a public health perspective because it suggests that the threshold for the most severe adverse effect, vomiting, is quite high for the plant metabolite D3G compared with DON and other 8-ketotrichothecenes. From a pharmacotherapeutic perspective, it may be feasible to design structural analogs of DON such as EN139528 and EN139544 for the treatment of obesity that would have higher therapeutic indexes than the parent compound. Clearly, before application of the latter, extensive research is needed on the bioavailability of DON congeners in different experimental animal species relative to their pharmacokinetics and potential metabolism by gut microflora. Lastly, it will be essential to more fully characterize relevant EEC cells, altered gut hormone profiles and the receptors that mediate intestinal hormone exocytosis responsible for the anorectic and emetic effects of natural and synthetic DON congeners.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
USDA NIFA Award (2011-0635); USDA Wheat and Barley SCAB Initiative Award (59-0206-9-058); Public Health Service Grant, National Institutes of Health (ES03553); Priority Academic Development Program of Jiangsu Higher Education Institutions, Natural Science Foundation of Jiangsu Province of China (BK20140691 to W.W.); Vienna Science and Technology Fund (WWTF LS12-021 to G.A. and F.B.).
Supplementary Material
Acknowledgments
We would like to acknowledge the assistance of Melissa Bates, Erica Clark, and Mary Rosner. F.B. also thanks the Austrian Federal Ministry of Economy, Family and Youth and the Austrian National Foundation for Research, Technology and Development. Conflict of interest: Drs Krantis and Durst have patents for EN139528 and EN139544. They produced and provided these two compounds for this investigation but had no involvement in the design of animal experiments, conduct, or interpretation of the data for these or the other congeners. Other authors have nothing to disclose.
REFERENCES
- Amuzie C. J., Harkema J. R., Pestka J. J. Tissue distribution and proinflammatory cytokine induction by the trichothecene deoxynivalenol in the mouse: Comparison of nasal vs. oral exposure. Toxicology. 2008;248:39–44. doi: 10.1016/j.tox.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Andrews P. L., Horn C. C. Signals for nausea and emesis: Implications for models of upper gastrointestinal diseases. Auto. Neurosci. 2006;125:100–115. doi: 10.1016/j.autneu.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azcona-Olivera J. I., Ouyang Y., Murtha J., Chu F. S., Pestka J. J. Induction of cytokine mRNAs in mice after oral exposure to the trichothecene vomitoxin (deoxynivalenol): Relationship to toxin distribution and protein synthesis inhibition. Toxicol. Appl. Pharmacol. 1995;133:109–120. doi: 10.1006/taap.1995.1132. [DOI] [PubMed] [Google Scholar]
- Batterham R. L., Cowley M. A., Small C. J., Herzog H., Cohen M. A., Dakin C. L., Wren A. M., Brynes A. E., Low M. J., Ghatei M. A. Gut hormone PYY3–36 physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- Berthiller F., Crews C., Dall'Asta C., Saeger S. D., Haesaert G., Karlovsky P., Oswald I. P., Seefelder W., Speijers G., Stroka J. Masked mycotoxins: A review. Mol. Nutr. Food Res. 2013;57:165–186. doi: 10.1002/mnfr.201100764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthiller F., Dall'asta C., Corradini R., Marchelli R., Sulyok M., Krska R., Adam G., Schuhmacher R. Occurrence of deoxynivalenol and its 3-beta-D-glucoside in wheat and maize. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2009;26:507–511. doi: 10.1080/02652030802555668. [DOI] [PubMed] [Google Scholar]
- Berthiller F., Dall'Asta C., Schuhmacher R., Lemmens M., Adam G., Krska R. Masked mycotoxins: Determination of a deoxynivalenol glucoside in artificially and naturally contaminated wheat by liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2005;53:3421–3425. doi: 10.1021/jf047798g. [DOI] [PubMed] [Google Scholar]
- Berthiller F., Krska R., Domig K. J., Kneifel W., Juge N., Schuhmacher R., Adam G. Hydrolytic fate of deoxynivalenol-3-glucoside during digestion. Toxicol. Lett. 2011;206:264–267. doi: 10.1016/j.toxlet.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canady R. A., Coker R. D., Rgan S. K., Krska R., Kuiper-Goodman T., Olsen M., Pestka J. J., Resnik S., Schlatter J. Safety Evaluation of Certain Mycotoxins in Food. Fifty-Sixth Report of the Joint FAO/WHO Expert Committee on Food Additives. Geneva, Switzerland: International Programme on Chemical Safety, World Health Organization; 2001. Deoxynivalenol; pp. 420–555. [Google Scholar]
- Challis B., Pinnock S., Coll A., Carter R., Dickson S., O'rahilly S. Acute effects of PYY3–36 on food intake and hypothalamic neuropeptide expression in the mouse. Biochem. Biophys. Res. Commun. 2003;311:915–919. doi: 10.1016/j.bbrc.2003.10.089. [DOI] [PubMed] [Google Scholar]
- Crawley J. N., Corwin R. L. Biological actions of cholecystokinin. Peptides. 1994;15:731–755. doi: 10.1016/0196-9781(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Della-Fera M. A., Baile C. A. CCK-octapeptide injected in CSF decreases meal size and daily food intake in sheep. Peptides. 1980;1:51–54. doi: 10.1016/0196-9781(80)90035-2. [DOI] [PubMed] [Google Scholar]
- Dockray G. J. Cholecystokinin and gut–brain signalling. Reg. Peptides. 2009;155:6–10. doi: 10.1016/j.regpep.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Durst T., Krantis A. A multi-ring regulator of gut motility and food intake. 2004 Google Patents ( https://www.google.com/patents/WO2004009074A2?cl=en) [Google Scholar]
- Durst T., Krantis A. Multi-ring compounds for regulating gut motility, food intake, and weight gain. 2006 Google Patents ( http://www.google.com/patents/WO2006006082A3?cl=en) [Google Scholar]
- Ebenezer I., De La Riva C., Baldwin B. Effects of the CCK receptor antagonist MK-329 on food intake in pigs. Physiol. Behav. 1990;47:145–148. doi: 10.1016/0031-9384(90)90053-7. [DOI] [PubMed] [Google Scholar]
- EFSA. Scientific opinion on the risks for animal and public health related to the presence of T-2 and HT-2 toxin in food and feed. EFSA J. 2011;9:1–187. [Google Scholar]
- Flannery B. M., Clark E. S., Pestka J. J. Anorexia induction by the trichothecene deoxynivalenol (vomitoxin) is mediated by the release of the gut satiety hormone peptide YY. Toxicol. Sci. 2012;130:289–297. doi: 10.1093/toxsci/kfs255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery B. M., Wu W., Pestka J. J. Characterization of deoxynivalenol-induced anorexia using mouse bioassay. Food Chem. Toxicol. 2011;49:1863–1869. doi: 10.1016/j.fct.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardet C., Bonnet M. S., Jdir R., Sadoud M., Thirion S., Tardivel C., Roux J., Lebrun B., Wanaverbecq N., Mounien L. The food-contaminant deoxynivalenol modifies eating by targeting anorexigenic neurocircuitry. PloS ONE. 2011;6:e26134. doi: 10.1371/journal.pone.0026134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz S. W., Duncan G., Richardson A. J. The human fecal microbiota metabolizes deoxynivalenol and deoxynivalenol-3-glucoside and may be responsible for urinary deepoxy-deoxynivalenol. Appl. Environ. Microbiol. 2013;79:1821–1825. doi: 10.1128/AEM.02987-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gugolek A., Zalewski D., Strychalski J., Konstantynowicz M. Food transit time, nutrient digestibility and nitrogen retention in farmed and feral American mink (Neovison vison)—A comparative analysis. J. Anim. Physiol. Anim. Nutr. (Berl.) 2013;97:1030–1035. doi: 10.1111/jpn.12006. [DOI] [PubMed] [Google Scholar]
- Harding R., McDonald T. Identification and characterization of the emetic effects of peptide YY. Peptides. 1989;10:21–24. doi: 10.1016/0196-9781(89)90069-7. [DOI] [PubMed] [Google Scholar]
- Hornby P. J. Central neurocircuitry associated with emesis. Am. J. Med. 2001;111:106–112. doi: 10.1016/s0002-9343(01)00849-x. [DOI] [PubMed] [Google Scholar]
- Hörsten S., Hoffmann T., Alfalah M., Wrann C., Karl T., Pabst R., Bedoui S. PP, PYY and NPY: Synthesis, storage, release and degradation. Neuropeptide Y Relat. Peptides. 2004;162:23–44. [Google Scholar]
- Karra E., Batterham R. L. The role of gut hormones in the regulation of body weight and energy homeostasis. Mol. Cell. Endocrinol. 2010;316:120–128. doi: 10.1016/j.mce.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Kohno D., Nakata M., Maejima Y., Shimizu H., Sedbazar U., Yoshida N., Dezaki K., Onaka T., Mori M., Yada T. Nesfatin-1 neurons in paraventricular and supraoptic nuclei of the rat hypothalamus coexpress oxytocin and vasopressin and are activated by refeeding. Endocrinology. 2008;149:1295–1301. doi: 10.1210/en.2007-1276. [DOI] [PubMed] [Google Scholar]
- Lemmens M., Scholz U., Berthiller F., Dall'Asta C., Koutnik A., Schuhmacher R., Adam G., Buerstmayr H., Mesterházy Á., Krska R. The ability to detoxify the mycotoxin deoxynivalenol colocalizes with a major quantitative trait locus for Fusarium head blight resistance in wheat. Mol. Plant Microbe Interact. 2005;18:1318–1324. doi: 10.1094/MPMI-18-1318. [DOI] [PubMed] [Google Scholar]
- le Roux C. W., Batterham R. L., Aylwin S. J., Patterson M., Borg C. M., Wynne K. J., Kent A., Vincent R. P., Gardiner J., Ghatei M. A., et al. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinol. 2006;147:3–8. doi: 10.1210/en.2005-0972. [DOI] [PubMed] [Google Scholar]
- Maresca M. From the gut to the brain: journey and pathophysiological effects of the food-associated trichothecene mycotoxin deoxynivalenol. Toxins. 2013;5:784–820. doi: 10.3390/toxins5040784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. D., Blackwell B. A. Biosynthesis of 3-acetyldeoxynivalenol and other metabolites by Fusarium culmorum HLX 1503 in a stirred jar fermentor. Can. J. Botany. (1986;64:1–5. [Google Scholar]
- Moran-Ramos S., Tovar A. R., Torres N. Diet: Friend or foe of enteroendocrine cells—How it interacts with enteroendocrine cells. Adv. Nutr. 2012;3:8–20. doi: 10.3945/an.111.000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagl V., Schwartz H., Krska R., Moll W.-D., Knasmüller S., Ritzmann M., Adam G., Berthiller F. Metabolism of the masked mycotoxin deoxynivalenol-3-glucoside in rats. Toxicol. Lett. 2012;213:367–373. doi: 10.1016/j.toxlet.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan P., Grosse J., Asad A. B. M. A., Radda G., Golay X. Gastrointestinal transit measurements in mice with 99mTc-DTPA-labeled activated charcoal using NanoSPECT-CT. EJNMMI Res. 2013;3:60. doi: 10.1186/2191-219X-3-60. Available at: http://www.ejnmmires.com/content/pdf/2191--219X-3--60.pdf. Accessed July 30, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka J. J. Toxicological mechanisms and potential health effects of deoxynivalenol and nivalenol. World Mycotoxin J. 2010;3:323–347. [Google Scholar]
- Pestka J. J., Islam Z., Amuzie C. J. Immunochemical assessment of deoxynivalenol tissue distribution following oral exposure in the mouse. Toxicol. Lett. 2008;178:83–87. doi: 10.1016/j.toxlet.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plata-Salaman C. R. Cytokines and anorexia: A brief overview. Semin. Oncol. 1998;25:64–72. [PubMed] [Google Scholar]
- Poppenberger B., Berthiller F., Lucyshyn D., Sieberer T., Schuhmacher R., Krska R., Kuchler K., Glossl J., Luschnig C., Adam G. Detoxification of the Fusarium mycotoxin deoxynivalenol by a UDP-glucosyltransferase from Arabidopsis thaliana. J. Biol. Chem. 2003;278:47905–47914. doi: 10.1074/jbc.M307552200. [DOI] [PubMed] [Google Scholar]
- Prelusky D. B., Trenholm H. L. The efficacy of various classes of anti-emetics in preventing deoxynivalenol-induced vomiting in swine. Nat. Toxins. 1993;1:296–302. doi: 10.1002/nt.2620010508. [DOI] [PubMed] [Google Scholar]
- Scallan E., Griffin P. M., Angulo F. J., Tauxe R. V., Hoekstra R. M. Foodborne illness acquired in the United States—Unspecified agents. Emerg. Infect. Dis. 2011;17:16–22. doi: 10.3201/eid1701.P21101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. W. Central nervous system regulation of food intake. Obesity. 2006;14:1S-8S. doi: 10.1038/oby.2006.275. [DOI] [PubMed] [Google Scholar]
- Steinberg E. B., Henderson A., Karpati A., Hoekstra M., Marano N., Souza J. M., Simons M., Kruger K., Giroux J., Rogers H. S. Mysterious outbreaks of gastrointestinal illness associated with burritos supplied through school lunch programs. J. Food Protec. 2006;69:1690–1698. doi: 10.4315/0362-028x-69.7.1690. [DOI] [PubMed] [Google Scholar]
- Steinert R. E., Feinle-Bisset C., Geary N., Beglinger C. Digestive physiology of the pig symposium: Secretion of gastrointestinal hormones and eating control. J. Anim. Sci. 2013;91:1963–1973. doi: 10.2527/jas.2012-6022. [DOI] [PubMed] [Google Scholar]
- Valassi E., Scacchi M., Cavagnini F. Neuroendocrine control of food intake. Nutr. Metab. Cardiovasc. Dis. 2008;18:158–168. doi: 10.1016/j.numecd.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Wu W., Bates M. A., Bursian S. J., Link J. E., Flannery B. M., Sugita-Konishi Y., Watanabe M., Zhang H., Pestka J. J. Comparison of emetic potencies of the 8-ketotrichothecenes deoxynivalenol, 15-acetyldeoxynivalenol, 3-acetyldeoxynivalenol, fusarenon X, and nivalenol. Toxicol. Sci. 2013a;131:279–291. doi: 10.1093/toxsci/kfs286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W., Flannery B. M., Sugita-Konishi Y., Watanabe M., Zhang H., Pestka J. J. Comparison of murine anorectic responses to the 8-ketotrichothecenes 3-acetyldeoxynivalenol, 15-acetyldeoxynivalenol, fusarenon X and nivalenol. Food Chem. Toxicol. 2012;50:2056–2061. doi: 10.1016/j.fct.2012.03.055. [DOI] [PubMed] [Google Scholar]
- Wu W., He K., Zhou H. R., Berthiller F., Adam G., Sugita-Konishi Y., Watanabe M., Krantis A., Durst T., Zhang H., et al. Effects of oral exposure to naturally-occurring and synthetic deoxynivalenol congeners on proinflammatory cytokine and chemokine mRNA expression in the mouse. Toxicol. Appl. Pharmacol. 2014b;278:107–115. doi: 10.1016/j.taap.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W., Zhou H. R., He K., Pan X., Sugita-Konishi Y., Watanabe M., Zhang H., Pestka J. J. Role of cholecystokinin in anorexia induction following oral exposure to the 8-ketotrichothecenes deoxynivalenol, 15-acetyldeoxynivalenol, 3-acetyldeoxynivalenol, fusarenon X, and nivalenol. Toxicol. Sci. 2014a;138:278–289. doi: 10.1093/toxsci/kft335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W. D., Bates M. A., Bursian S. J., Flannery B., Zhou H. R., Link J. E., Zhang H. B., Pestka J. J. Peptide YY3–36 and 5-hydroxytryptamine mediate emesis induction by trichothecene deoxynivalenol (vomitoxin) Toxicol. Sci. 2013b;133:186–195. doi: 10.1093/toxsci/kft033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D.-M., Bula W., Stellar E. Brain cholecystokinin as a satiety peptide. Physiol. Behav. 1986;36:1183–1186. doi: 10.1016/0031-9384(86)90498-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.