Abstract
Background. The time to acquisition of simian immunodeficiency virus (SIV) infection following low-dose repeated rectal challenge correlated inversely with the number of transmitted/founder strains among macaques vaccinated with ALVAC-SIV/gp120 or gp120 alone. We determined if the ability of postvaccination, prechallenge sera to enhance SIVmac251 transcytosis across epithelial cells was associated with transmitted/founder strain number.
Methods. Transcytosis was carried out by exposing sera and SIVmac251 to the apical surface of human endometrial carcinoma (HEC-1A) cells at pH 6.0 and 12 hours later quantifying virus in fluid bathing the basolateral cell surface (maintained at pH 7.4). These conditions allow Fc neonatal receptor (FcRn)-dependent shuttling of virus across cells.
Results. There was a strong correlation between the amount of virus transcytosed and number of transmitted variants (R = 0.86, P < .0001). We also found that 4 animals who remained uninfected after repeated rectal challenges had lower serum transcytosis activity than did 19 animals who subsequently became infected (P = .003). Using immunohistochemistry, we demonstrated FcRn on columnar epithelial cells facing the lumen of the macaque rectum.
Conclusions. Vaccine-induced antibody capable of enhancing transcytosis in vitro via FcRn may play a role in determining transmitted/founder strain number and infection outcomes following in vivo challenge.
Keywords: SIV, transcytosis, antibody, Fc neonatal receptor, vaccine
The RV144 vaccine trial demonstrated limited but significant protection from primarily heterosexually transmitted human immunodeficiency virus (HIV) infection among Thai men and women [1]. The vaccine regimen consisted of priming with 4 injections of ALVAC expressing human immunodeficiency virus type 1 (HIV-1) Gag-Pro and gp120 and 2 booster injections with recombinant gp120. Two other trials, VAX003 and VAX004, employing repeated injections of gp120 without ALVAC priming, failed to show efficacy [2, 3]. In an attempt to model the findings of the RV144 and AIDSVAX trials, Pegu et al used a low-dose, repeated SIVmac251 rectal challenge of rhesus macaques immunized with either ALVAC-SIV/gp120 in alum, SIV gp120 in alum, or alum alone (controls) [4]. Three of 11 macaques in the ALVAC-SIV/gp120 group were protected as was 1 of 12 gp120-vaccinated animals. Although there were no significant differences in the time to acquisition of SIVmac251 infection between the groups, antibody in the prechallenge sera of the 4 vaccinated, uninfected macaques had significantly higher avidity to gp120 than did the sera of animals that became infected. Neither serum neutralizing nor antibody-dependent cell-mediated cytotoxicity (ADCC) activity was associated with acquisition of infection. Unexpectedly, the investigators observed an inverse correlation between the number of transmitted SIVmac251 variants that established infection and the time to infection acquisition in both the ALVAC-SIV/gp120 group (R = −0.84, P = .0025) and the gp120 group (R = −0.65, P = .028) but not in the control group (R = −0.31, P = .42). This finding presents an interesting conundrum because whereas some vaccinated animals might have been protected by the vaccine (particularly the ALVAC-SIV/gp120 vaccine), vaccination also appeared to be associated with the early transmission of a higher number of virus variants.
We recently described the effect of immunoglobulin G (IgG) antibodies and acidic pH on transcytosis of HIV-1 across polarized epithelial cells [5]. Our findings demonstrated that virus transcytosis is enhanced in the presence of Env-specific IgG antibodies and acidic pH at the apical surface, as compared to transcytosis with no or nonspecific IgG or with Env-specific IgG at neutral pH. Moreover, we established that this enhanced transcytosis was dependent on the Fc neonatal receptor (FcRn), an MHC class I-like molecule that binds IgG and immune complexes at low pH and releases them at neutral pH [6, 7]. Because the pH of the macaque rectal luminal surface can be acidic and the subepithelial mucosal tissue where infection is likely to occur is neutral, we tested the possibility that FcRn-mediated enhanced transcytosis, due to vaccine-elicited antibody, might be associated with the number of transmitted SIVmac251 variants in ALVAC-SIV/gp120- and gp120-immunized macaques [8].
METHODS
Animal Studies
The study design has been described elsewhere [4]. Briefly, Indian rhesus macaques were immunized intramuscularly with ALVAC-SIV and gp120 in alum (n = 11), gp120 in alum (n = 12), or alum alone (n = 11). ALVAC-SIV (which expressed simian immunodeficiency virus [SIV] Gag, Pol, and Env) was given at 0, 4, 12, and 24 weeks, and the gp120 was given at 12 and 24 weeks. The control group received alum at 12 and 24 weeks. Starting at week 28, all macaques were challenged weekly per rectum with a 1:500 dilution of SIVmac251 (120 50% tissue culture infective doses). Blood and other specimens were collected at intervals for SIV RNA and DNA determinations and for various immunological assays.
Transmitted/founder variants were identified by single-genome amplification and direct sequencing of the env gene using SIV RNA from plasma as part of the published macaque vaccine study [4]. Briefly, SIV RNA was extracted, and limiting-dilution polymerase chain reaction (PCR) of synthesized complementary DNA (cDNA) was conducted. Transmitted/founder lineages were identified by phylogenetic analysis within the context of inoculum sequences as described previously [9]. The number of variants used for analyses were reported in Pegu et al [4].
Transcytosis Assay
Transcytosis of SIVmac251 was conducted by modifying methods described in detail for HIV-1 [5]. Briefly, human endometrial carcinoma (HEC-1A) cell monolayers were created on hanging transwell inserts. Electrical resistance across the wells, which ranged from 400 to 450 Ohms (1200–1500 Ohms/cm2) at the start of the transcytosis assay, confirmed monolayer integrity. SIVmac251 and postvaccination, prechallenge serum (1:100 dilution) or IgG was added to the apical surface of the monolayers in media buffered to pH 6.0. After 12 hours, fluid in the lower chamber (“subnatant fluid”), maintained at pH 7.4, was collected and used to measure viral RNA copy number and infectivity on TZM-bl cells. For most experiments, the inoculum of SIVmac251 was 2 ng of p27; however, due to subsequent loss of infectivity in virus aliquots, 10 ng was used in 2 of 8 assays where transcytosis was quantified by reverse-transcription polymerase chain reaction (RT-PCR) and in 1 of 3 assays where infectivity of transcytosed virus was determined. The results of analyses that excluded the higher-inoculum assays did not differ substantially from the results reported herein (data not shown), which include all assays. In one set of experiments, the pH was varied to determine the range of pH values within which enhanced transcytosis occurs.
Immunohistochemical Staining for FcRn Expression
Tissue was fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) and embedded in paraffin. Slides were subjected to an antigen retrieval step consisting of incubation in Reveal Decloaker (Biocare Medical Inc, Concord, CA) for 2 minutes at 125°C in the Digital Decloaking Chamber (Biocare Medical Inc, Concord, CA) followed by cooling to 90°C before rinsing in running water and Tris-buffered saline (TBS; 50 mM Tris and 150 mM NaCl). Endogenous peroxidase was blocked by incubating slides in 1% hydrogen peroxide for 20 minutes at room temperature before the antigen retrieval. Staining was carried out using rabbit anti-FcRn serum (a gift from N. Simister, Brandeis University) at 1:100 in antibody diluent (Dako Inc, Carpinteria, CA) or using normal rabbit control serum (Life Technologies, Grand Island, NY). Nonspecific binding sites were blocked by a 10-minute incubation in protein block (Dako Inc, Carpenteria, CA) before the primary antibody (ie, rabbit serum) incubation. The EnVision + system (Dako, Inc, Carpinteria, CA) was used according to the manufacturer's instructions to detect labeling of the primary antibody. TBS with 0.05% Tween-20 (Dako Inc, Carpenteria, CA) was used for all wash steps after the first antibody and the EnVision polymer. 3, 3′-diaminobenzidine (DAB) was used as the chromogen. Counterstaining with Harris hematoxylin (American MasterTech, Lodi, CA) was applied after the development of DAB. Slides were visualized with a Zeiss Imager microscope (Carl Zeiss Inc, Thornwood, NY), and digital images were captured using a Zeiss Axiocam System (Carl Zeiss Inc, Thornwood, NY).
Statistics
Median values of repeated runs of transcytosis results were analyzed by Spearman rho to evaluate correlations with transmitted variant number, time-to-infection, and immunological parameters measured as part of the published animal study [4]. Median values were used to mitigate the effect of assay-to-assay variability (Table 1). However, when mean values were used, results were similar (not shown). Moreover, given the interassay variability (likely due to small variations in electrical resistance across the monolayer), we determined correlations between T/F variants and fold-increase in transcytoses for each assay separately. For each individual assay (8 assays for RT-PCR-based transcytosis quantification, including 3 in which infectious transcytosed virus was quantified), there was a significant correlation with T/F variant number (not shown). The relationship between serum transcytosis activity and infection status was evaluated by the Mann–Whitney U test. No corrections were made for multiple comparisons.
Table 1.
Quantity and Infectivity of SIVmac251 Transcytosed at pH 6.0 in the Presence of Postvaccination, Prechallenge Serum
| Animal | Vaccine Group | Outcome | No. of Challenges | T/F Variants | Transcytosisa |
|||
|---|---|---|---|---|---|---|---|---|
| RNA Copies (log10) | % Transcytosis | RLUs | % of Infectious Virus Transcytosis | |||||
| P062 | ALVb | Infected | 4 | 3 | 5.96 | 1.02 | 61 554 | 5.46 |
| P063 | ALV | Infected | 2 | 3 | 5.98 | 1.02 | 82 251 | 7.29 |
| P067 | ALV | Infected | 1 | 4 | 6.10 | 1.42 | 96 216 | 8.53 |
| P112 | ALV | Infected | 2 | 5.67 | 0.51 | 27 878 | 2.30 | |
| P147 | ALV | Infected | 4 | 1 | 5.42 | 0.28 | 24 525 | 1.90 |
| P150 | ALV | Infected | 1 | 6.05 | 1.34 | 52 918 | 4.11 | |
| P172 | ALV | Infected | 5 | 1 | 5.53 | 0.46 | 26 978 | 2.09 |
| P254 | ALV | Infected | 1 | 1 | 5.57 | 0.43 | 29 013 | 2.25 |
| P148 | ALV | Uninfected | 5 | 0 | 5.53 | 0.35 | 24 968 | 1.94 |
| P250 | ALV | Uninfected | 5 | 0 | 5.54 | 0.35 | 22 070 | 1.71 |
| M624 | ALV | Uninfected | 5 | 0 | 5.48 | 0.33 | 28 818 | 2.24 |
| P065 | gp120 | Infected | 1 | 6 | 6.14 | 1.44 | 60 790 | 4.72 |
| P122 | gp120 | Infected | 3 | 1 | 5.62 | 0.50 | 29 378 | 2.28 |
| P123 | gp120 | Infected | 2 | 3 | 5.90 | 0.95 | 51 505 | 4.00 |
| P124 | gp120 | Infected | 1 | 6 | 5.92 | 1.06 | 50 918 | 3.95 |
| P126 | gp120 | Infected | 4 | 1 | 5.63 | 0.53 | 29 373 | 2.28 |
| P127 | gp120 | Infected | 1 | 3 | 6.06 | 1.13 | 46 928 | 3.64 |
| P132 | gp120 | Infected | 2 | 1 | 5.59 | 0.52 | 30 780 | 2.39 |
| P133 | gp120 | Infected | 5 | 4 | 5.93 | 0.91 | 45 920 | 3.57 |
| P135 | gp120 | Infected | 1 | 5 | 6.16 | 1.63 | 61 713 | 4.79 |
| P137 | gp120 | Infected | 1 | 3 | 5.90 | 1.09 | 47 695 | 3.70 |
| P139 | gp120 | Infected | 2 | 5 | 6.10 | 1.50 | 69 953 | 5.43 |
| M927 | gp120 | Uninfected | 5 | 0 | 5.51 | 0.34 | 29 625 | 2.27 |
a % transcytosis is based on the inoculum (RNA copy number) of SIVmac251 added to the apical surface of HEC-1A cells (median = 7.98 log10 RNA copies) and results are median values from 8 independent experiments; % of infectious virus transcytosis is based on TZM-bl cell infectivity (relative light units [RLUs]) of the SIVmac251 inoculum added to the apical surface of HEC-1A cells (median = 6.15 log10 relative light units) and results are median values from 3 independent experiments.
b ALVAC-SIV/gp120.
RESULTS
Enhanced Transcytosis by Serum at pH 6.0 is Associated With the Number of Transmitted SIVmac251 Variants Following Immunization With ALVAC-SIV/gp120 or gp120
In order to investigate the role of antibody-enhanced transcytosis in determining the number of transmitted/founder strains after low-dose repeated rectal challenge, SIVmac251 transcytosis mediated by individual postvaccination, prechallenge macaque sera (diluted 1:100) at pH 6.0 was measured. Using results from only those animals that became infected (N = 19), there was a strong correlation between fold-increase in transcytosis and the number of transmitted variants (R = 0.86, P < .0001; Figure 1A). This correlation was maintained when ALVAC-SIV/gp120-vaccinated animals (R = 0.94, P = .0036, N = 8; Figure 1B) or gp120-vaccinated animals (R = 0.75, P = .011, N = 11; Figure 1C) were analyzed separately. Moreover, when results of all vaccinated animals, including those that remained uninfected (considered to have 0 variants) were analyzed (N = 23), these correlations were as strong or stronger (R = ≥0.81 in all cases) as those observed with only infected animals. Similar associations were observed when transcytosis was expressed as the percentage of the virus transcytosed relative to the inoculum of virus applied to the apical surface, a percentage that ranged from a median of 0.1 (with preimmune sera, not shown) to 1.63 with postvaccine, prechallenge sera (Table 1).
Figure 1.
Transcytosis of SIVmac251 mediated by prechallenge sera at pH 6.0 correlates with the number of transmitted SIVmac251 variants. Prechallenge sera (1:100 dilution) from animals in both vaccine groups (A), from ALVAC-SIV/gp120-vaccinated animals (B) or from gp120-vaccinated animals (C) that subsequently became infected were incubated with SIVmac251 and applied to the apical surface of HEC-1A cells at pH 6.0. Twelve hours later, the amount of transcytosed virus was determined by RT-PCR in the subnatant fluid (pH 7.4). Data are reported as the fold-increase in transcytosis compared to preimmune serum and are median values from 8 independent experiments. D, Enhanced transcytosis of SIVmac251 occurs with IgG and is pH dependent. SIVmac251 and 100 µg/mL of IgG from an SIV-infected rhesus macaque were added to the apical surface of HEC-1A cells at the indicated pH. Twelve hours later, SIVmac251 in the subnatant fluid (pH 7.4) was quantified by RT-PCR. Data are reported as the fold-increase in transcytosis compared to no antibody controls. Bars represent averages of 2 experiments (± standard error). Abbreviations: IgG, immunoglobulin G; RT-PCR, reverse-transcription polymerase chain reaction; SIV, simian immunodeficiency virus.
The phenomenon of enhanced transcytosis of HIV-1-immune complexes at low pH was previously reported by us [5]. Similar to HIV-1, enhanced SIVmac251 transcytosis, using IgG purified from a separate group of animals infected with SIV, occurs between pH 4.5 and 6.5, with a maximum occurring between pH 5.5 and 6.0 (Figure 1D). The use of IgG confirms that enhanced transcytosis of SIVmac251, as is the case with HIV-1, is dependent on antibody [5].
Infectivity of Transcytosed Virus Correlates With the Number of Transmitted Variants
Both the ALVAC-SIV/gp120 and the gp120 vaccines elicited low level neutralizing activity against SIVmac251 measured on TZM-bl cells, although neutralizing activity did not correlate with SIVmac251 infection or with the number of transmitted variants [4]. We found that virus transcytosed at pH 6.0 in the presence of serum remained infectious (Figure 2, Table 1), consistent with the low neutralizing activity of the sera. Moreover, the amount of transcytosed infectious virus (as fold-increase compared with preimmune serum) correlated with the number of transmitted variants (R = 0.87, P < .0001; Figure 2). Again, this correlation was observed in both ALVAC-SIV/gp120-vaccinated animals (R = 0.94, P = .0036) and gp120-vaccinated animals (R = 0.88, P = .0007; data not shown). Similar correlations were obtained when data were analyzed as the percentage of transcytosed infectious virus compared to the infectious inoculum (not shown). Thus, postvaccination, prechallenge sera from monkeys with higher numbers of transmitted variants were better able to mediate the transcytosis of infectious virus across epithelial cells than were sera from monkeys with fewer variants.
Figure 2.
Transcytosis of infectious SIVmac251 mediated by prechallenge sera at pH 6.0 correlates with the number of transmitted variants. Serum (1:100) and SIVmac251 were applied to the apical surface of HEC-1A cells at pH 6.0, and 12 hours later, the amount of transcytosed virus was determined by adding the subnatant fluid (pH 7.4) to TZM-bl cells. Data are reported as the fold-increase in infectivity compared to preimmune serum and are median values from 3 independent experiments.
Transcytosis is Inversely Correlated With the Number of Rectal Challenges
Because animals with higher numbers of transmitted variants were infected sooner [4], we analyzed the relationship between the number of challenges and the serum transcytosis activity. In these analyses, we included animals that became infected and those that did not become infected after the last (fifth) challenge. Faster acquisition of SIVmac251 infection (ie, fewer challenges) was associated with transcytosis measured by PCR of subnatant fluid virus (R = −0.75, P < .0001, Figure 3A) or by the quantity of infectious virus in subnatant fluid (R = −0.68, P = .0005, Figure 3B).
Figure 3.
Transcytosis of SIVmac251 mediated by prechallenge sera at pH 6.0 correlates inversely with the number of rectal challenges required for infection. Prechallenge sera (1:100 dilution) from animals in both vaccine groups were incubated with SIVmac251 and applied to the apical surface of HEC-1A cells at pH 6.0. Twelve hours later, the amount of transcytosed virus was determined in the subnatant fluid (pH 7.4) by RT-PCR (A) or by infecting TZM-bl cells (B). Data are reported as the fold-increase in transcytosis or infectivity compared to preimmune serum and are median values from 8 (A) or 3 (B) independent experiments. For plotting purposes, animals uninfected after 5 challenges were assigned the value 6 as their number of challenges. Abbreviation: RT-PCR, reverse-transcription polymerase chain reaction.
Serum From Animals That Were Vaccinated and Became Infected Had Greater Transcytosis Activity Than Those That Remained Uninfected
The challenge study revealed that 3 of 11 ALVAC-SIV/gp120- and 1 of 12 gp120-vaccinated animals remained uninfected after repeated low dose rectal SIVmac251 challenges [4]. We analyzed the serum transcytosis activity to determine its relationship with protection. We found that animals that remained uninfected (n = 4) had lower serum transcytosis activity determined either by PCR (P = .003, Mann–Whitney U; Figure 4) or by infectivity of transcytosed virus (P = .002; not shown). Sera from all 4 uninfected and only 1 of 19 infected animals fell below a cutoff of 3-fold increase in transcytosis (P = .0006, Fisher exact test).
Figure 4.
Prechallenge sera from uninfected animals mediate less transcytosis of SIVmac251 than prechallenge sera of animals who subsequently became infected. Virus and sera were applied to the apical surface of HEC-1A cells at pH 6.0, and subnatant fluid (pH7.4) was sampled 12 hours later by RT-PCR. Data are reported as the fold-increase in transcytosis compared to preimmune serum and are median values from 8 independent experiments. Abbreviation: RT-PCR, reverse-transcription polymerase chain reaction.
Transcytosis and Other Immunological Assays
We also determined if serum transcytosis activity was associated with other immunological parameters measured in sera or secretions of study animals (Table 2). We found modest inverse correlations between gp140 binding levels measured in rectal secretions (n = 17) and serum transcytosis activity measured by RT-PCR (R = −0.54, P = .026) or measured by virus infectivity (R = −0.66, P = .0052). Lymphoproliferative responses to Env (n = 20) correlated inversely with transcytosis measured by RT-PCR (R = −0.60, P = .0065) or by virus infectivity (R = −0.49, P = .029); transcytosis activity also correlated inversely with Gag-specific lymphoproliferation and at a similar magnitude to that described above for Env. Other parameters measured, including gp120 binding activity, gp120 avidity, neutralizing activity, and ADCC activity were not significantly associated with serum transcytosis activity (P > .05; Table 2).
Table 2.
Correlations Between Serum Transcytosis Activity and Results of Other Immunological Testsa
| Test | R valueb | P value |
|---|---|---|
| gp140 binding in rectal secretions | −0.54 | .026 |
| % Env-specific memory B cells | 0.05 | .85 |
| Serum gp120 binding titer | −0.34 | .12 |
| gp120 avidity | −0.20 | .36 |
| Serum neutralizing titer (M7-luc cells) | −0.25 | .24 |
| Serum neutralizing titer (TZM-bl cells) | 0.03 | .90 |
| Serum maximum granzyme B activity | 0.09 | .69 |
| Serum ADCC titer | −0.21 | .34 |
| Lymphoproliferation to Env | −0.60 | .0065 |
| Lymphoproliferation to Gag | −0.50 | .026 |
Abbreviation: ADCC, antibody-dependent cell-mediated cytotoxicity.
a Correlations are between indicated tests and fold-increase in transcytosis; results are similar using fold-increase in transcytosis of infectious virus.
b Spearman rho.
Expression of FcRn on Rectal Tissue of Macaques
A biological role for antibody-enhanced transcytosis in determining the number of transmitted variants after rectal SIVmac251 challenge would likely require the presence of FcRn in rectal tissues. Using anti-FcRn rabbit serum to visualize FcRn, we found positive staining in the rectal columnar epithelium of all four healthy rhesus macaques studied. Only the fully mature columnar epithelial cells forming the luminal surface of the rectal mucosa were FcRn-positive, whereas the immature epithelial cells lining the crypts and goblet cells were FcRn-negative (Figure 5). In general, FcRn staining was orientated toward the basolateral surface of the mature epithelial cells; however, some staining was also found in the apical portion of the cells (Figure 5). FcRn-positive cells were also located in the lamina propria of the rectum, especially subjacent to the surface mucosal FcRn-positive epithelial cells (Figure 5). Many of these FcRn-positive cells had the bean-shaped eccentric nucleus and abundant cytoplasm characteristic of tissue macrophages. The remaining FcRn-positive cells were flattened cells lining vascular spaces, a morphology and location consistent with endothelial cells. Similar FcRn staining was found in all four macaques studied.
Figure 5.
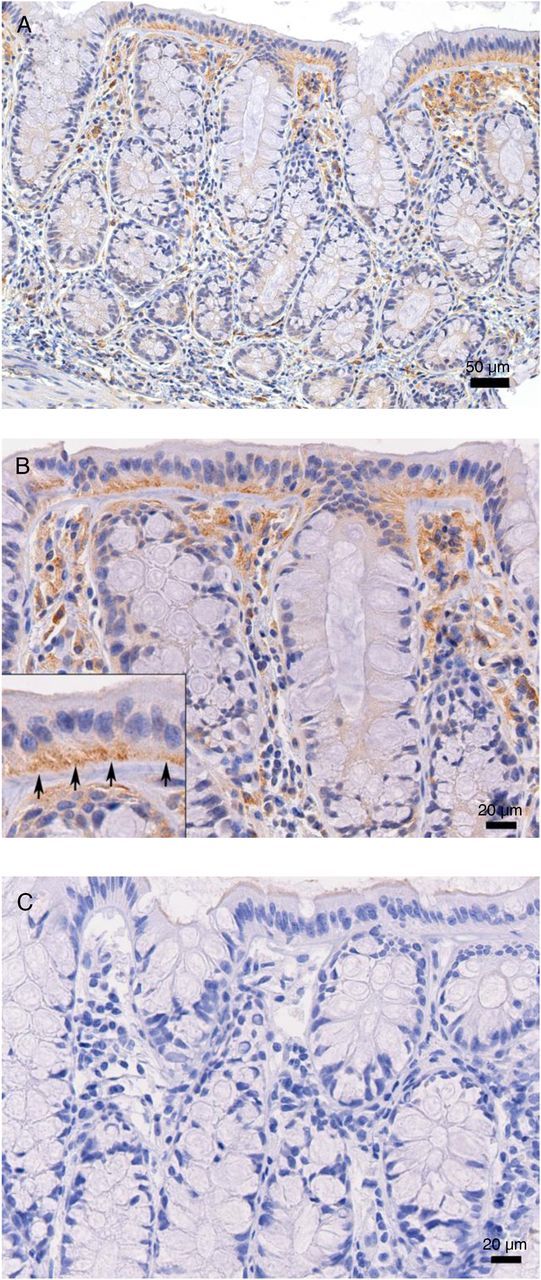
FcRn is expressed by epithelial cells on the surface of the rectal mucosa of rhesus macaques. Tissue obtained by biopsy was stained with anti-FcRn rabbit serum (A, low magnification or B, high magnification) or with normal rabbit serum (C). Note that FcRn staining is limited to the surface epithelial cells (inset, arrows) and to mononuclear cells (presumptive macrophages and endothelial cells) in the lamina propria of the rectum. DAB label (brown) and hematoxylin nuclear stain were used. Scale bars denote the degree of magnification. Abbreviation: FcRn, Fc neonatal receptor.
DISCUSSION
We have explored the possibility that antibody-enhanced transcytosis of SIVmac251 is associated with the number of transmitted/founder strains acquired after rectal challenge of vaccinated rhesus macaques. In the previously published rectal challenge study, there was an inverse correlation between the time-to-infection and the number of transmitted variants among animals vaccinated with either ALVAC-SIV/gp120 or with gp120 alone; no such correlation was observed among control animals [4]. Our principal finding is that the ability of postvaccination, prechallenge serum to mediate transcytosis of SIVmac251 in vitro is highly predictive of the number of variants that established infection. Moreover, the sera of animals that remained uninfected after vaccination and challenge mediated significantly less transcytosis than did the sera of animals that became infected. We also demonstrate the presence of FcRn in columnar epithelial cells facing the rectal lumen of healthy macaques.
Enhanced transcytosis in our in vitro system is observed in the presence of antibody and when the pH of the apical surface of the epithelial cells is maintained at 6.0 and the basolateral surface at 7.4. These conditions allow IgG-SIVmac251 complexes to form, to engage FcRn at the apical surface of the cells and to be released at the basolateral surface into the subnatant fluid [5]. Using the same in vitro conditions, we recently showed that FcRn is required for enhanced transcytosis of HIV-1 mediated by both neutralizing and nonneutralizing monoclonal and polyclonal antibodies [5]. As with HIV-1, SIVmac251 that is transcytosed in vitro can remain infectious [5].
The strong correlation between SIVmac251 transcytosis, quantified either by RT-PCR or by virus infectivity, and the number of transmitted/founder variants suggests a role for vaccine-induced antibody in determining transmitted variant numbers after rectal challenge in vivo. In this regard, no other immunological parameter examined correlated with transmitted variant number in the challenge study [4]. In addition, the inverse association between variant number and time-to-infection occurred only among vaccinated animals, strongly suggesting that the vaccination itself is a determining factor [4]. It is important to underscore the fact that the enhanced transcytosis measured in our study is dependent on acidic pH at the apical epithelial cell surface. Therefore, if similarly enhanced transcytosis were to occur across the rectal mucosa after challenge in vivo, the mucosal surface should have a pH < 6.5. We did not measure the pH of rectal mucosa or secretions in the study animals. However, the normal pH range in the pigtail macaque rectum has been reported to be 5.5–8.0, and a similar range would be expected in rhesus macaques [8]. Thus, although quite variable, animals may have rectal pH values in the range where FcRn engagement and enhanced transcytosis is likely to occur [8]. We also documented, for the first time, FcRn expression in rhesus macaque columnar epithelial cells of the rectum, although colonic epithelial FcRn has been demonstrated in cynomologous monkeys (Macaca fascicularis) [10]. Human colonic epithelial cells also express FcRn [10, 11]; to our knowledge, rectal FcRn expression has not been reported in humans. We propose that upon challenge, SIVmac251-immune complexes are formed with vaccine-induced antibody in a slightly acidic luminal milieu that allows engagement of FcRn on rectal columnar epithelial cells. Following FcRn binding, immune complexes are shuttled across the epithelium into subepithelial tissue containing SIVmac251-susceptible target cells. This model is consistent with studies showing FcRn-dependent immune complex shuttling in mice and with our own studies showing FcRn-dependent enhanced transcytosis of HIV-1 across HEC-1A cells in vitro [5, 12, 13]. It is also possible that in vivo, infectious immune complexes enter epithelial cells through a mechanism independent of FcRn; FcRn might then engage with the complex within endosomes resulting in shuttling to the subepithelial tissue. In either case, our findings provide the first in vivo evidence of a novel mechanism of antibody-dependent enhancement of infection (ADE), wherein immune complex binding to FcRn might result in an increased number of transmitted/founder virus strains. On the other hand, we recognize that our assay results may be serving as a surrogate for some other antibody-mediated effect that is in turn associated with the number of transmitted founder strains.
We also found that sera from animals that remained uninfected after vaccination and challenge had lower antibody-enhanced transcytosis than did those animals that became infected. This finding again suggests that the antibody response to vaccination can be involved deleteriously with infection outcome. However, our results present a conundrum because, on the one hand, it appears that one measure of vaccine-induced antibody, avidity, is associated with protection [4], whereas another, antibody-mediated enhanced transcytosis, is associated with worse outcomes. Moreover, serum transcytosis activity correlates inversely, though modestly (and with P values that would be marginal or nonsignificant after correction for multiple comparisons), with other potentially beneficial antibody measurements, such as gp140 binding levels in rectal secretions and lymphoproliferative responses to Env and Gag. We have previously shown that the ability of antibody to capture infectious virus correlates with enhanced transcytosis [5]; whether virus transcytosed in the presence of antibody is better able to infect susceptible target cells or not depends on the neutralizing activity of the antibody. In the macaque challenge study, neither neutralizing nor ADCC activity correlated with protection [4]. Thus, it remains unclear how avidity or capture measurements relate to functional antibody responses. It is also possible that Env-specific IgA or IgM, which were not measured, could influence FcRn-mediated transcytosis by competing with IgG for virion epitopes. More importantly, it is unclear how potentially deleterious functions, such as enhanced transcytosis, relate to beneficial activities and how vaccine strategies can be designed to separate good and bad functions.
It should be emphasized that the macaque rectal challenge study upon which our findings are based utilized direct application of SIVmac251 on the rectal mucosa. During receptive anal intercourse, HIV would be contained in semen, which would rapidly elevate the pH above the threshold at which FcRn engagement might occur [14]. In addition, rectal secretions have low levels of IgG, although virus is likely to be complexed with IgG from the semen [15]. Thus, our study may not be directly applicable to receptive anal intercourse. Nonetheless, our results suggest an in vivo role for FcRn-mediated transcytosis in the outcome of infection; such a role would more likely be applicable to male-to-female or female-to-male HIV transmission, which occur in the presence of acidic cervicovaginal secretions with IgG levels higher than those found in rectal secretions [5, 15, 16].
In summary, we have shown a strong correlation between vaccine-induced serum transcytosis activity and the number of transmitted/founder variants acquired after rectal challenge of rhesus macaques. In addition, we have demonstrated the presence of FcRn in rectal columnar epithelial cells. These findings, together with our observation that prechallenge serum transcytosis activity is highest in animals that become infected, hint at the possibility that ADE, through an FcRn-mediated mechanism, could contribute to infection outcomes. Finally, these results add to a growing body of evidence suggesting a role for ADE in the outcomes of some monkey and human lentiviral infection studies [4, 17–21].
Notes
Financial support. This work was supported by US Public Health Service grant AI102715 from the National Institutes of Health (NIH) and by the Intramural Research Program of the NIH under NCI contract HHSN2662004000088C.
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–20. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 2.Pitisuttithum P, Gilbert P, Gurwith M, et al. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis. 2006;194:1661–71. doi: 10.1086/508748. [DOI] [PubMed] [Google Scholar]
- 3.Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005;191:654–65. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- 4.Pegu P, Vaccari M, Gordon S, et al. Antibodies with high avidity to the gp120 envelope protein in protection from simian immunodeficiency virus SIV(mac251) acquisition in an immunization regimen that mimics the RV-144 Thai trial. J Virol. 2013;87:1708–19. doi: 10.1128/JVI.02544-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta S, Gach JS, Becerra JC, et al. The neonatal Fc receptor (FcRn) enhances human immunodeficiency virus type 1 (HIV-1) transcytosis across epithelial cells. PLoS Pathog. 2013;9:e1003776. doi: 10.1371/journal.ppat.1003776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuo TT, Baker K, Yoshida M, et al. Neonatal Fc receptor: from immunity to therapeutics. J Clin Immunol. 2010;30:777–89. doi: 10.1007/s10875-010-9468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raghavan M, Gastinel LN, Bjorkman PJ. The class I major histocompatibility complex related Fc receptor shows pH-dependent stability differences correlating with immunoglobulin binding and release. Biochemistry. 1993;32:8654–60. doi: 10.1021/bi00084a037. [DOI] [PubMed] [Google Scholar]
- 8.Patton DL, Cosgrove Sweeney YT, Rabe LK, Hillier SL. Rectal applications of nonoxynol-9 cause tissue disruption in a monkey model. Sex Transm Dis. 2002;29:581–7. doi: 10.1097/00007435-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Keele BF, Li H, Learn GH, et al. Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. J Exp Med. 2009;206:1117–34. doi: 10.1084/jem.20082831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hornby PJ, Cooper PR, Kliwinski C, et al. Human and non-human primate intestinal FcRn expression and immunoglobulin G transcytosis. Pharm Res. 2014;31:908–22. doi: 10.1007/s11095-013-1212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah U, Dickinson BL, Blumberg RS, Simister NE, Lencer WI, Walker WA. Distribution of the IgG Fc receptor, FcRn, in the human fetal intestine. Pediatr Res. 2003;53:295–301. doi: 10.1203/01.PDR.0000047663.81816.E3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshida M, Claypool SM, Wagner JS, et al. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity. 2004;20:769–83. doi: 10.1016/j.immuni.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Paveglio S, Puddington L, Rafti E, Matson AP. FcRn-mediated intestinal absorption of IgG anti-IgE/IgE immune complexes in mice. Clin Exp Allergy. 2012;42:1791–800. doi: 10.1111/j.1365-2222.2012.04043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox CA, Meldrum SJ, Watson BW. Continuous measurement by radio-telemetry of vaginal pH during human coitus. J Reprod Fertil. 1973;33:69–75. doi: 10.1530/jrf.0.0330069. [DOI] [PubMed] [Google Scholar]
- 15.Wright PF, Kozlowski PA, Rybczyk GK, et al. Detection of mucosal antibodies in HIV type 1-infected individuals. AIDS Res Hum Retroviruses. 2002;18:1291–300. doi: 10.1089/088922202320886334. [DOI] [PubMed] [Google Scholar]
- 16.Mestecky J, Jackson S, Moldoveanu Z, et al. Paucity of antigen-specific IgA responses in sera and external secretions of HIV-type 1-infected individuals. AIDS Res Hum Retroviruses. 2004;20:972–88. doi: 10.1089/aid.2004.20.972. [DOI] [PubMed] [Google Scholar]
- 17.Gorlani A, Forthal DN. Antibody-dependent enhancement and the risk of HIV infection. Curr HIV Res. 2013;11:421–6. doi: 10.2174/1570162x113116660062. [DOI] [PubMed] [Google Scholar]
- 18.Forthal DN, Gabriel EE, Wang A, Landucci G, Phan TB. Association of Fcgamma receptor IIIa genotype with the rate of HIV infection after gp120 vaccination. Blood. 2012;120:2836–42. doi: 10.1182/blood-2012-05-431361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brouwer KC, Lal RB, Mirel LB, et al. Polymorphism of Fc receptor IIa for IgG in infants is associated with susceptibility to perinatal HIV-1 infection. AIDS. 2004;18:1187–94. doi: 10.1097/00002030-200405210-00012. [DOI] [PubMed] [Google Scholar]
- 20.Onyango-Makumbi C, Omer SB, Mubiru M, et al. Safety and efficacy of HIV hyperimmune globulin for prevention of mother-to-child HIV transmission in HIV-1-infected pregnant women and their infants in Kampala, Uganda (HIVIGLOB/NVP STUDY) J Acquir Immune Defic Syndr. 2011;58:399–407. doi: 10.1097/QAI.0b013e31822f8914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burton DR, Hessell AJ, Keele BF, et al. Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody. Proc Natl Acad Sci U S A. 2011;108:11181–6. doi: 10.1073/pnas.1103012108. [DOI] [PMC free article] [PubMed] [Google Scholar]






