Abstract
Human immunodeficiency virus (HIV) accesses the brain early in infection and can lead to neurocognitive disorders. The brain can also serve as a viral reservoir, but how virus is controlled in the brain is unknown. To examine this, CD8-depleting monoclonal antibody was injected into the cerebrospinal fluid of rhesus monkeys with chronic simian immunodeficiency virus (SIV) infection. This treatment led to the rapid increase of SIV in the brain. Virus in the brain is maintained by active suppression from the host immune system. This dynamic interaction can be manipulated in efforts to control and eradicate virus from the brain and other reservoirs.
Keywords: SIV, HIV, brain, cerebrospinal, CD8, immune
Human immunodeficiency virus (HIV) enters the central nervous system (CNS) early after infection and results in immune activation in the CNS, which continues during the course of disease [1]. These initial events can ultimately lead to HIV-associated neurocognitive disorder (HAND), and infection of the CNS can provide a sanctuary and reservoir for the virus. This can be modeled by simian immunodeficiency virus (SIV) infection in monkeys, where it was found that virus enters the brain and initiates a series of changes, including infiltration of CD8+ T cells [2]. CD8+ T cells are also found in the brains of HIV-infected individuals [3]. The role of these CD8+ T cells remains relatively unexplored. Here we tested the hypothesis that CD8+ T cells play a key role in the maintenance of viral control in the CNS.
MATERIALS AND METHODS
Monkeys, SIV Infection, and Antibody Administration
Animal work was performed under institutional animal care and use committee approval, following National Institutes of Health and US Department of Agriculture guidelines. Rhesus monkeys were infected intravenously with a stock derived from SIVmac251. Longitudinal characterization of infection is provided in Supplementary Figure 1. Biofluid sampling and necropsy were performed, with cell isolation from the blood, lymph nodes, and brain as described elsewhere [2]. Intra-CSF administration was performed in anesthetized animals by injection into the cisterna magna [4].
Antibodies and Primers/Probes
Information on antibodies and oligonucleotides used in these experiments is given in Supplementary Table 1.
Viral and Gene Quantitation
SIV RNA was measured using the branched DNA assay by Siemens (Emeryville, CA). For graphing and statistical analysis, undetectable values were replaced by the minimum detectable value (125 copies/mL in biofluids and <54 copies/µg for RNA). Quantitative real-time polymerase chain reaction (PCR) analysis was performed on complementary DNA produced from tissue RNA, using TaqMan chemistry, and relative SIV RNA expression was quantified using the 2−ΔΔCt method, with the average of GAPDH and TBP serving as reference.
Flow Cytometry
Cells were isolated and surface antigens stained as previously described [2]. Intracellular staining was performed on cells stimulated with PMA (10 ng/mL; Sigma) and ionomycin (200 ng/mL; Sigma) in the presence of GolgiBlock and GolgiStop (BD Biosciences) for 6 hours. Following the above stimulation, cells were stained with a Live/Dead kit (Invitrogen), then for surface antigens, followed by fixation and permeabilization according to manufacturer's recommendations (eBioscience), and intracellular staining. Analysis was performed using FlowJo 6.2.1 (Tree Star, San Carlos, CA).
Immunohistochemistry and In Situ Hybridization
These were performed on formalin-fixed, paraffin-embedded tissue sections as described elsewhere [5].
Statistics
Tests were performed using Prism software (GraphPad Software, San Diego, CA).
RESULTS
Effector Memory CD8+ T Cells Dominate in the Chronically Infected Brain
Using 3 monkeys chronically infected with SIV (mean infection duration [±SD], 11.8 ± 0.5 months) and without signs of disease, we characterized the CD8+ T cells that accumulated in the brain. Flow cytometric analysis revealed that CD8+ T cells from the brain were divided between an effector memory phenotype (ie, CD28−CD197−; mean percentage [±SD], 50.9% ± 3.8%) and a transitional population (ie, CD28+CD197−; 46.6% ± 3.2% ), with a small percentage exhibiting a central memory phenotype (ie, CD28+CD197+; 1.6% ± 0.2%; Supplementary Figure 2A). These CD8+ T cells were further assessed for their ability to express interferon γ (mean percentage [±SD], 76.3% ± 25.6%), granzyme B (59.7% ± 10.0%; Supplementary Figure 2B), and tumor necrosis factor α (54.3% ± 25.0%), with 51.4% ± 24.3% positive for all 3 molecules. In conjunction with our earlier studies using cytotoxic T lymphocytes and tetramer studies [6, 7], these CD8+ T cells in the brain likely comprised anti-SIV effector cells.
Intra-CSF Antibody Treatment Depletes CNS CD8+ T Cells
Administration of a CD8-depleting antibody in doses capable of eliminating CD8+ T cells from the blood and lymphoid organs to chronically SIV-infected monkeys results in a transient increase in plasma viral load [8, 9]. However, peripherally administered antibodies do not penetrate into the CNS, whereas antibodies infused into the CSF can penetrate the brain parenchyma [10]. Therefore, to assess the effect of CD8 depletion in the CNS, we administered a depleting anti-CD8 antibody into the CSF and examined both the CNS and periphery for effects on the virus and immune cells.
Six animals with chronic SIV infection (mean infection duration [±SD], 10.9 ± 1.6 months) were studied in CNS antibody administration experiments; all were without signs of disease. We administered CD8-depleting antibody into the CSF of 4 animals. The other 2 animals served as controls and received immunoglobulin G (IgG) in the CSF to assess the effect of nonspecific antibody.
In the blood, all animals receiving the CD8-depleting antibody showed a decrease in the CD8+ T cell count (Figure 1A), whereas both of the animals treated with IgG showed a slight increase. The decreased level of CD8+ T cells in the blood of animals in the experimental group was expected because antibodies exit the CSF through the arachnoid granulations and enter the bloodstream. To assess whether this level of antibody could affect blood levels of CD8+ T cells, a seventh animal with chronic SIV infection was given this same dose of CD8-depleting antibody intravenously and had a similar decrease (from 1523 to 11 cells/mm3) in the number of CD8+ T cells in the blood.
Figure 1.
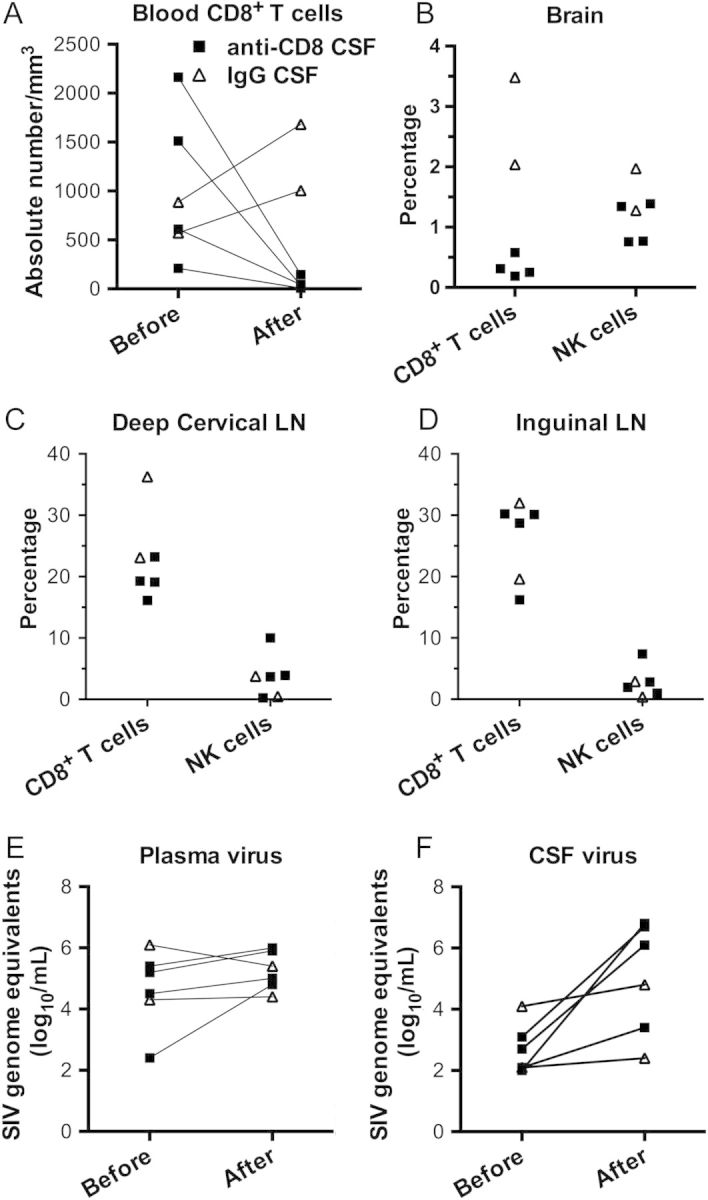
Effect of anti-CD8 treatment in the cerebrospinal fluid (CSF) on immune cells and virus. A–D, Quantification of cells in individual animals, with the indicated treatment protocol, as determined by flow cytometry. A, CD8+ T-cell counts in blood are shown before and after treatment. B, Brain CD8+ T cell and natural killer (NK) cell proportions are shown at necropsy. C and D, CD8+ T cell and NK cell proportions in deep cervical and inguinal lymph nodes (LNs) are shown at necropsy. E and F, Quantification of simian immunodeficiency virus (SIV) load in plasma and CSF specimens obtained from animals before and after the indicated treatment.
At the time animals were euthanized, they received a perfusion of PBS to clear blood-borne cells, and the brain and lymphoid tissues were collected for study. Animals receiving CD8-depleting antibody in the CSF showed a paucity of CD8+ T cells in the brain (average proportion, 0.33% of cells) but not in lymph nodes (average proportion, 19.4% and 26.3% of cells in the deep cervical and inguinal lymph nodes, respectively; Figure 1B–D). CD8+ T cell levels were maintained in the animals given IgG in the CSF (average proportion, 2.76% of cells in the brain and 29.7% and 25.8% of cells in the deep cervical and inguinal lymph nodes, respectively; Figure 1B–D). This was also seen in the additional (seventh) animal that received the same dose of antibody intravenously (proportion, 5.3% of cells in the brain and 31.1% and 21.7% of cells in the deep cervical and inguinal lymph nodes, respectively). Natural killer (NK) cells, which, in rhesus monkeys, express CD8, were maintained at the expected low proportions in the brain and lymph nodes (Figure 1B–D). Thus, administration of CD8-depleting antibody into the CSF is effective in eliminating CD8+ T cells from the brain (as well as from the blood) without affecting those in the lymph nodes or affecting the proportion of NK cells.
Intra-CSF Antibody Treatment Increases CNS Viral Load
In the animals receiving the CD8-depleting antibody in the CSF, while an increase in the plasma viral load was found (1.05 log10 copies), it did not reach statistical significance; in the control animals, which received IgG in the CSF, the plasma viral load decreased slightly (by 0.50 log10 copies; Figure 1E). In contrast, an increase in CSF viral load was found in all animals receiving intra-CSF antibody treatment. While the magnitude of the increase was mild in those receiving control IgG (0.50 log10 copies), in those receiving the intra-CSF CD8-depleting antibody the CSF viral load increased profoundly, by >1000-fold (3.25 log10 copies; P < .02; Figure 1F).
To assess the effects of brain CD8 cell depletion on viral load in the brain parenchyma, in situ hybridization was performed to visualize SIV-infected cells within the brain. In the animals that received anti-CD8 in the CSF, SIV-positive cells could be found along the leptomeninges, as well as perivascularly and within the parenchyma (Figure 2A and B). SIV-positive cells could not be found in the brains of animals treated with intra-CSF IgG (Figure 2C) or untreated control animals, although positive cells could be found in lymphoid tissue (data not shown). To quantify viral expression, RNA was prepared from 5 different regions of the brain in each monkey, and the SIV RNA load was measured. When compared to the control monkeys that did not receive intra-CSF administration of the CD8-depleting antibody (either the 3 untreated animals or the 2 that received IgG), those that did receive this antibody had a considerably elevated SIV load in the brain parenchyma (Figure 2D). In addition to the overall effect of treatment, significant increases in viral load (compared with control untreated animals) were found in the frontal and occipital lobes, as well as in the hippocampus and cerebellum. Thus, we found that elimination of CD8+ T cells from the brain leads to productive expression of SIV within the brain.
Figure 2.
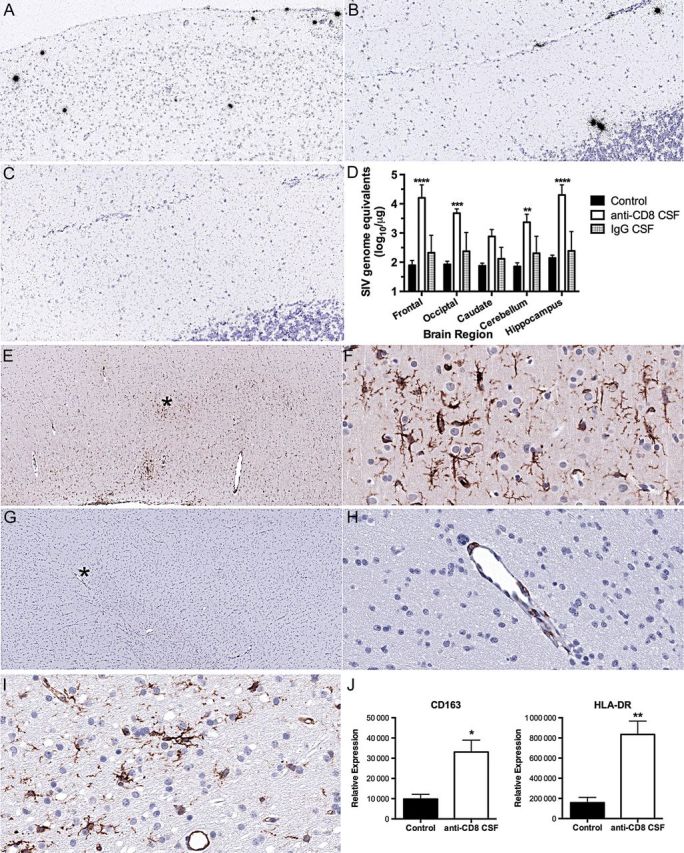
Receipt of anti-CD8 treatment in the cerebrospinal fluid (CSF) induces viral expression and microglial activation. A–C, In situ hybridization of brain sections. Antisense simian immunodeficiency virus (SIV) probe identifies SIV-positive cells (black silver grains) in animals treated intra-CSF with the CD8-depleting antibody but not in animals treated intra-CSF with control immunoglobulin G (IgG). A, Photomicrograph (10× original magnification) from frontal lobe of anti-CD8 treated monkey. B, Photomicrograph (20× original magnification) from the cerebellum of an anti-CD8–treated monkey. C, Photomicrograph (20× original magnification) from the cerebellum of control IgG–treated monkey. D, SIV load measured in RNA from different regions of the brain. Mean and standard deviation are indicated for the 3 untreated animals (black columns), the 4 receiving intra-CSF anti-CD8 (unfilled columns), and the 2 receiving intra-CSF IgG (gray columns; not used in statistical analysis). Samples below the limit of detection were assigned the value of the limit of detection (1.73 log10/µg RNA). Two-way analysis of variance revealed a significant effect of treatment (P = .0018). Comparisons between groups were performed using the Bonferroni multiple comparisons test, with significant differences between the intra-CSF anti-CD8–treated group and the control untreated group indicated. Values indicate mean and standard error of the mean (SEM). ****P < .0001, ***P < .001, and **P < .01. E–I, Immunohistochemical staining of frontal lobe brain sections for microglial activation markers. E and F, CD163 reactivity in an animal treated intra-CSF with the CD8-depleting antibody. The image in panel E is at low (4× original) magnification. The asterisk indicates the area shown in panel F at high (40× original) magnification, revealing reactive ramified microglia. G and H, CD163 reactivity in animal treated intra-CSF with control IgG. The image in panel G is at low (4× original) magnification. The asterisk indicates the area shown in panel H at high (40× original) magnification, revealing only reactive perivascular macrophages. I, HLA-DR reactivity in an animal treated intra-CSF with the CD8-depleting antibody, revealing reactive ramified microglia, as well as endothelial cells (note the blood vessel). The photomicrograph is at high (40× original) magnification. J, Quantification by real-time polymerase chain reaction of microglia activation markers in RNA from the frontal lobe of untreated SIV-infected (control) and intra-CSF anti-CD8–treated animals. Values indicate mean and SEM. **P < .01, *P < .05.
Productive HIV/SIV infection of the brain is associated with activation of microglia, and such cells are linked to neuronal damage in HAND. We therefore examined sections of the frontal lobe that were remote from the site of antibody administration but showed a significant increase in viral load in the monkeys that received anti-CD8 in the CSF. CD163 is expressed on macrophages as well as on activated microglia in HIV/SIV encephalitis [11]. Indeed CD163 expression was present in ramified microglia in SIV-infected monkeys that received CD8-depleting antibody in the CSF (Figure 2E and F), but it was limited to occasional perivascular macrophages in those that received IgG in the CSF (Figure 2G and H). Expression of HLA-DR was similarly increased in microglia of the monkeys that received anti-CD8 in the CSF (Figure 2I). Quantification was again performed by analysis of RNA expression. Real-time PCR revealed that the levels of both CD163 and HLA-DR were significantly increased (Figure 2J). The activation of microglia is consistent with the recrudescence of viral replication in the brains of animals in which anti-CD8 was administered in the CSF.
DISCUSSION
We found that depletion of CD8+ T cells from the brain of monkeys with chronic SIV infection greatly increases viral load and microglial activation. Thus, CD8+ T cells in the CNS function to suppress viral replication and protect the brain from neuropathogenic factors present in viral proteins and the products of activated microglia. The brain acts as a reservoir and has the potential to produce relatively large amounts of virus, the level of which is kept repressed by CD8+ T cells. These CD8+ T cells are poised to provide immediate effector function in response to activation of the virus in the brain. Our experiments reveal that they play an active role, rather than an anticipatory role, since their targeted depletion leads to a rapid rise in brain viral load.
It is important to point out the limitations of our study. First, no attempt was made to optimize the amount of antibody administered. Second, our control for intra-CSF administration consisted of presumptive nontargeting antibodies (ie, purified IgG). Thus, it is possible that the antigen-antibody reaction itself activates microglial cells and leads to SIV reactivation. We do not believe this is the case, because experimental attempts to activate brain microglia in SIV-infected monkeys with an intracerebral infusion of proinflammatory cytokines revealed no activation of virus production [12]. Third, while the sample size was relatively small, the effect was robust and consistent in the 4 anti-CD8–treated animals,. Finally, a comparison can be made to situations in which the CD8-depleting antibody is given systemically. In such protocols, the antibody is administered in much larger amounts. When it is given to chronically infected animals, the magnitude of the increase in plasma viral load is greater [8, 9]; when given at the time of acute infection, immune control of viremia is not achieved, and rapid development of terminal simian AIDS with a high incidence of encephalitis occurs [13, 14], quite distinct from our experiments.
In summary, our findings have relevance to the control of HIV within the CNS and during HAND. While the incidence of frank HIV-associated dementia and encephalitis have greatly decreased, HAND unfortunately persists in the current era of therapy [15]. It is possible that the CD8+ T cells that infiltrate the brain during HIV infection act as a double-edged sword, protecting from the virus initially but contributing to damage over the greatly prolonged course of infection. The balance between protective and pathologic immune responses in the brain is crucial, and there is growing appreciation for the role of immune activation in HAND and other neurodegenerative disorders.
Eradication of HIV remains a current goal of research and treatment and requires elimination of virus from reservoirs such as the brain. While there are many unique aspects of the immune response to HIV in the CNS, our experiments indicate that CD8+ T cells play a critical role. Knowledge of this key role of CD8+ T cells in controlling virus in the brain is essential in devising strategies to rid the CNS, and the body, of virus.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Debbie Watry, Kelly Barnett, Shannon Callen, and Drs Elizabeth Ford, Susan Spray, Karen Clingerman, and Kent Osborne, for excellent assistance with the experiments, and Dr Susan Swindells, for critical comments on the manuscript.
Financial support. This work was supported by the National Institutes of Health (grants MH073490 and MH062261).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed
References
- 1.Spudich S, Gisslen M, Hagberg L, et al. Central nervous system immune activation characterizes primary human immunodeficiency virus 1 infection even in participants with minimal cerebrospinal fluid viral burden. J Infect Dis. 2011;204:753–60. doi: 10.1093/infdis/jir387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marcondes MC, Burudi EM, Huitron-Resendiz S, et al. Highly activated CD8(+) T cells in the brain correlate with early central nervous system dysfunction in simian immunodeficiency virus infection. J Immunol. 2001;167:5429–38. doi: 10.4049/jimmunol.167.9.5429. [DOI] [PubMed] [Google Scholar]
- 3.Petito CK, Torres-Munoz JE, Zielger F, McCarthy M. Brain CD8+ and cytotoxic T lymphocytes are associated with, and may be specific for, human immunodeficiency virus type 1 encephalitis in patients with acquired immunodeficiency syndrome. J Neurovirol. 2006;12:272–83. doi: 10.1080/13550280600879204. [DOI] [PubMed] [Google Scholar]
- 4.Clingerman KJ, Spray S, Flynn C, Fox HS. A technique for intracisternal collection and administration in a rhesus macaque. Lab Anim (NY) 2010;39:307–11. doi: 10.1038/laban1010-307. [DOI] [PubMed] [Google Scholar]
- 5.Fox HS, Weed MR, Huitron-Resendiz S, et al. Antiviral treatment normalizes neurophysiological but not movement abnormalities in simian immunodeficiency virus-infected monkeys. J Clin Invest. 2000;106:37–45. doi: 10.1172/JCI9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcondes MC, Burdo TH, Sopper S, et al. Enrichment and persistence of virus-specific CTL in the brain of simian immunodeficiency virus-infected monkeys is associated with a unique cytokine environment. J Immunol. 2007;178:5812–9. doi: 10.4049/jimmunol.178.9.5812. [DOI] [PubMed] [Google Scholar]
- 7.von Herrath M, Oldstone MB, Fox HS. Simian immunodeficiency virus (SIV)-specific CTL in cerebrospinal fluid and brains of SIV-infected rhesus macaques. J Immunol. 1995;154:5582–9. [PubMed] [Google Scholar]
- 8.Jin X, Bauer DE, Tuttleton SE, et al. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999;189:991–8. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmitz JE, Kuroda MJ, Santra S, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–60. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 10.Chauhan NB, Siegel GJ, Lichtor T. Distribution of intraventricularly administered antiamyloid-beta peptide (Abeta) antibody in the mouse brain. J Neurosci Res. 2001;66:231–5. doi: 10.1002/jnr.1215. [DOI] [PubMed] [Google Scholar]
- 11.Roberts ES, Masliah E, Fox HS. CD163 identifies a unique population of ramified microglia in HIV encephalitis (HIVE) J Neuropathol Exp Neurol. 2004;63:1255–64. doi: 10.1093/jnen/63.12.1255. [DOI] [PubMed] [Google Scholar]
- 12.Joag SV, Adams RJ, Pinson DM, Adany I, Narayan O. Intracerebral infusion of TNF-alpha and IL-6 failed to activate latent SIV infection in the brains of macaques inoculated with macrophage-tropic neuroadapted SIVmac. J Leukoc Biol. 1994;56:353–7. doi: 10.1002/jlb.56.3.353. [DOI] [PubMed] [Google Scholar]
- 13.Roberts ES, Zandonatti MA, Watry DD, et al. Induction of pathogenic sets of genes in macrophages and neurons in NeuroAIDS. Am J Pathol. 2003;162:2041–57. doi: 10.1016/S0002-9440(10)64336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams K, Westmoreland S, Greco J, et al. Magnetic resonance spectroscopy reveals that activated monocytes contribute to neuronal injury in SIV neuroAIDS. J Clin Invest. 2005;115:2534–45. doi: 10.1172/JCI22953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heaton RK, Clifford DB, Franklin DR, Jr., et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–96. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


