Abstract
Plasmodium vivax is a major cause of malaria morbidity worldwide yet has remained genetically intractable. To stably modify this organism, we used zinc-finger nucleases (ZFNs), which take advantage of homology-directed DNA repair mechanisms at the site of nuclease action. Using ZFNs specific to the gene encoding P. vivax dihydrofolate reductase (pvdhfr), we transfected blood specimens from Saimiri boliviensis monkeys infected with the pyrimethamine (Pyr)–susceptible Chesson strain with a ZFN plasmid carrying a Pyr-resistant mutant pvdhfr sequence. We obtained Pyr-resistant parasites in vivo that carried mutant pvdhfr and additional silent mutations designed to confirm editing. These results herald the era of stable P. vivax genetic modifications.
Keywords: malaria, transfection, allelic modification, dihydrofolate reductase-thymidylate synthase, pyrimethamine, Saimiri boliviensis
Five species of Plasmodium parasites cause malaria in humans: P. falciparum, P. vivax, P. ovale, P. malariae, and P. knowlesi. Of these, P. falciparum and, more recently, P. knowlesi have been adapted to routine long-term cultivation in vitro [1, 2], facilitating transfection-based studies. In the case of P. falciparum, the ability to modify endogenous genes and introduce reporter sequences has generated important insights in multiple areas, including drug resistance, host cell invasion, immune evasion and antigenic variation, and mosquito susceptibility to infection [1, 3]. Transfection methods for rodent-specific Plasmodium parasites have likewise yielded a wealth of information on malaria parasite biology [4, 5]. For human parasites other than P. falciparum and P. knowlesi, the lack of in vitro culture systems has necessitated the use of in vivo infection studies in nonhuman primate models, which are expensive and often support parasite infections poorly. Efforts with P. vivax parasites are a case in point, especially as this species favors reticulocytes and typically rises to parasitemia levels of no more than 1%. To date, only transient transfection with a luciferase reporter, followed by short cultivation period, has been described for this species [6].
Recent advances in genome editing have led to dramatic improvements in the speed and efficiency of genetic manipulation in different organisms. Methods that make use of zinc-finger nucleases (ZFNs), truncated transcription activator-like effector domains for nucleases (TALENs), and clustered regulatory interspaced short palindromic repeats (CRISPRs) provide high specificity of recognition, scission, and previously unachievable rates of editing to the targeted sequences [7]. With these methods, editing extends from the double-strand break (DSB) at the nuclease cleavage site, which is subject to host cell DNA repair mechanisms. This process leads to efficient, site-specific capture of sequence provided by the plasmid donor template. ZFN-based genetic modification of P. falciparum has been highly effective in achieving rapid interruption or replacement of genes as compared to conventional transformation [8, 9]. We therefore wished to test ZFNs for their ability to modify P. vivax under in vivo conditions of low parasitemia in New World monkeys. Here we report the introduction of drug-resistance mutations into the dihydrofolate reductase (dhfr) region of the bifunctional dihydrofolate reductase-thymidylate synthase (dhfr-ts) gene of P. vivax parasites and their selection by pyrimethamine (Pyr) treatment in Saimiri boliviensis.
METHODS
Parasites, Nonhuman Primate Hosts, and Antimalarial Treatment
Samples of P. vivax Chesson and AMRU-1 lines [10, 11], obtained from the Malaria Research and Reference Reagent Resource Center (ATCC, Manassas, VA), were intravenously inoculated into S. boliviensis monkeys. Giemsa-stained thin blood smears were examined weekly or daily (depending on parasite detection), and parasitemias were determined from counts of 10 000 erythrocytes. P. vivax stocks were cryopreserved in 60% Glycerolyte 57 (Fenwal) at 0.1%–1.0% parasitemia. Infected monkeys were treated with 4 mg/kg Pyr (in sterile water, delivered via oral gavage to anesthetized animals), and parasitemias were monitored daily until recrudescence or polymerase chain reaction (PCR)–based evidence of parasite clearance (primers are indicated in Supplementary Table 1). Parasitemias were cured by 3 daily weight-adjusted doses of orally delivered atovaquone-proguanil (6.25 mg/kg and 2.5 mg/kg, respectively, using the Malarone formulation). Animals were obtained from National Institutes of Health (NIH)–approved sources and were housed in accordance with the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals (Institute for Laboratory Animal Research, 1996). All procedures were performed in accordance with the NIH guidelines under protocols approved by the Animal Care and Use Committee of the National Institute of Allergy and Infectious Diseases, NIH (animal study proposal LMVR 15).
Plasmid Constructs
Custom ZFNs targeting the pvdhfr sequence were designed, assembled, and screened by Sigma-Aldrich for activity, using a yeast proxy system [12]. The ZFN coding sequences were cloned downstream of a P. vivax calmodulin (cam; PVX_084825) 5′ untranslated region (UTR) and upstream of a heat shock protein hsp86 (PVX_87955) 3′ UTR in a pDC2-based vector [13]. The cam promoter (1072 bp) and the hsp86 3′ UTR (836 bp) were amplified from Chesson genomic DNA using primers p1 + p2 and p3 + p4 (Supplementary Table 1). For the donor sequence, a 1.37-kb region of 1.86-kb pvdhfr-ts coding sequence was amplified by PCR from Chesson genomic DNA, using primers p5 + p6 (Supplementary Table 1). We introduced 6 silent mutations at the ZFN binding sites via site-directed mutagenesis, using primers p7 + p8, and 4 mutations, at position 171, 174, 182, and 350, using primers p9 + p10 and p11 + p12. The latter 4 mutations introduced the F57L, S58R, T61M and S117T amino acid substitutions, respectively, that confer high levels of Pyr resistance [14]. The mutated Chesson pvdhfrF57L, S58R, T61M, S117T donor fragment was cloned into the ApaI and SacI sites of a pDC2-based vector [13] to generate the transfection plasmid pvDC2-ZFNpvdhfr (Figure 1).
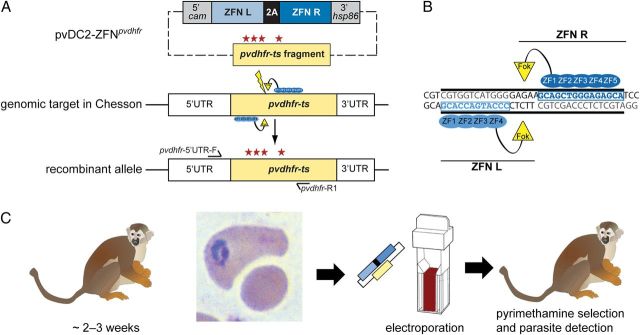
Figure 1.
Editing of the Plasmodium vivax dhfr-ts gene with zinc-finger nucleases (ZFNs). A, Schematic representation of the pvdhfr-ts allelic modification strategy. The pvDC2-ZFNpvdhfr plasmid expresses a P. vivax dhfr–specific pair of ZFNs separated by a 2A-peptide under control of the P. vivax calmodulin promoter (5′ cam) and the P. vivax hsp86 3′ untranslated regulatory element (3′ hsp86). In addition, the plasmid carries a 1.37-kb donor region from the 1.86-kb pvdhfr-ts coding sequence with 4 engineered mutations for pyrimethamine resistance (asterisks). Expression of the ZFN pair, which functions as an obligate heterodimer, in the transfected parasites induces a double-strand break (DSB) in the pvdhfr-ts sequence (thunderbolt). The donor sequence provided on the same plasmid provided a donor template for DSB repair via homologous recombination, thereby introducing pvdhfr mutations. The primer pair pvdhfr-5′UTR-F (forward) and pvdhfr-R1 (reverse) was used to confirm editing of the genomic pvdhfr sequence. For purposes of illustration, elements of the map are not drawn to scale. B, Schematic representation of ZFNs bound to the pvdhfr target. The left and right ZFNs consist of 4 and 5 ZFN modules, respectively, which bind to opposite strands of the DNA. Upon binding, the split FokI endonuclease moieties (physically proximal to each other by virtue of each binding neighboring DNA sequences) dimerize to form a functional nuclease that can then cleave the DNA and produce a DSB at the target site. C, P. vivax transfection protocol. Splenectomized Saimiri boliviensis were infected with P. vivax Chesson. When the parasitemia reached 0.5%, blood specimens were collected and depleted of white blood cells, using a Sepacell filter. The recovered erythrocyte samples were suspended in Cytomix, mixed with plasmid DNA, and electroporated. A splenectomized malaria-naive S. boliviensis was inoculated with the electroporated cells and subsequently treated with 3 weekly doses of 4 mg/kg pyrimethamine (Pyr) to select for ZFN-edited, Pyr-resistant parasites.
Plasmid Electroporation Into Parasitized S. boliviensis Erythrocytes
For each transfection experiment, a 3-mL sample of blood from a splenectomized S. boliviensis monkey infected with P. vivax Chesson parasites (parasitemia approximately 0.5%) was collected in 10% heparin and centrifuged at 800 × g for 3 minutes at room temperature. After plasma removal, the pellet was suspended in 30 mL of incomplete Roswell Park Memorial Institute (RPMI) 1640 medium (KD Medical), and white blood cells were removed by Sepacell (Fenwal) filtration. The erythrocyte pellet (approximately 1 × 1010 erythrocytes containing approximately 5 × 107 parasites) was washed once with 10 mL of Cytomix (120 mM KCl, 0.15 mM CaCl2, 2 mM EGTA, 5 mM MgCl2, 10 mM K2HPO4/KH2PO4, and 25 mM Hepes; pH 7.6) and resuspended in the same solution to a 1.4-mL volume. This suspension was combined with 200 µL of Cytomix containing 400 µg of pvDC2-ZFNpvdhfr plasmid DNA. Four electroporations were performed with 400 µL of the resulting mixture, each in a 0.2-cm electroporation cuvette (Bio-Rad), in a BioRad Gene Pulser set to 310V and 975 µF. Electroporation time constants were 14–22 milliseconds. Electroporated erythrocytes were combined, washed twice in 20 mL of incomplete RPMI 1640, resuspended in fresh incomplete RPMI 1640 medium to a final volume of 1.5 mL, and intravenously inoculated into an anesthetized and splenectomized S. boliviensis.
Selection of Transformed Parasites
Inoculated animals were treated once weekly with 4 mg/kg Pyr for 3 weeks, to select for ZFN-edited resistant parasites. Giemsa-stained blood smears were microscopically examined every other day for up to 4 weeks. If no parasites were detected, the blood was also checked weekly by a malaria-diagnostic PCR. Parasites were collected before and after Pyr selection for cryopreservation, DNA analysis, and subinoculation into an additional S. boliviensis. DNA was isolated by the Qiagen DNeasy Blood and Tissue Kit.
Microsatellite Analysis and DNA Sequencing
DNA microsatellite analysis was performed using primers listed in Supplementary Table 1. For sequencing, the pvdhfr region was amplified by PCR, using primers pvdhfr-5′UTR-F and pvdhfr-R1. PCR was performed with 30 amplification cycles of 30 seconds at 94°C, 30 seconds at 56°C, and 1 minute at 72°C. Products were purified using a Qiagen gel purification kit. DNA sequencing was performed on an Applied Biosystems 3730xl DNA Analyzer (Applied Biosystems).
RESULTS AND DISCUSSION
Previous studies showed that P. vivax infections carrying a quadruple-mutant version of pvdhfr-ts (encoding 57L/58R/61M/117T) frequently do not respond to Pyr-sulfadoxine treatment [15]. This quadruple-mutant pvdhfr-ts allele confers antifolate resistance in heterologous expression systems, including P. falciparum [14]. While Chesson parasites that carry wild-type pvdhfr-ts were reportedly cleared from Aotus monkeys treated with 4 mg/kg Pyr, AMRU-1 infections were not cured at this dose, indicating resistance in the latter strain. Our sequencing results confirmed that Chesson harbors a nonmutant (wild-type) pvdhfr-ts allele, whereas AMRU-1 carries the 57L/58R/61M/117T quadruple-mutant gene (Supplementary Table 2). To assess the course of infection and Pyr response in S. boliviensis monkeys, we infected animals with Chesson and AMRU-1 strains. A single dose of 4 mg/kg Pyr quickly reduced Chesson parasitemia; however, recrudescent parasites were detected 5 days after treatment. Complete elimination of Chesson parasites required 2–4 doses of 4 mg/kg Pyr delivered once weekly. This regimen did not eliminate AMRU-1 parasites in an infected animal (data not shown).
P. vivax Chesson parasites were transfected as illustrated in Figure 1 and detailed in Methods. For each of 4 monkeys, approximately 5 × 107 parasitized erythrocytes were electroporated ex vivo with a ZFN plasmid harboring a quadruple-mutant pvdhfr sequence and inoculated intravenously. In 3 of these experiments, parasitemias of 0.03%–0.3% developed within 3–9 days. No parasites were detected in the fourth monkey (animal 3073) during a 60-day follow-up period (Figure 2).
Figure 2.
Selection strategy of zinc-finger nuclease–transfected Plasmodium vivax parasites. Charts show courses of parasitemia for 4 different Saimiri boliviensis monkeys (animals 3848, 3073, 3904, and 4909) inoculated with electroporated parasites. The arrows indicate the time points at which the doses of 4 mg/kg pyrimethamine (Pyr) were administered. In the S. boliviensis 3848 and 3073, drug selection was started 72 hours after inoculation. Animals 3904 and 4909 were treated with Pyr as soon as parasites were detected in their blood. Time points at which blood specimens were collected for polymerase chain reaction analysis are indicated by black stars.
To select for the ZFN-edited drug-resistant parasites, the 4 monkeys were treated with weekly doses of 4 mg/kg Pyr. In 1 monkey (animal 4909), a recrudescent parasitemia was first observed on day 25 after the third Pyr dose, rising to a level of 0.05% 29 days after this treatment (Figure 2).
DNA microsatellite profiles of the transformed and nontransformed Chesson parasites were the same and were distinct from those of P. vivax AMRU-1 (Supplementary Table 1), confirming that the Pyr-selected transfected parasites originated from Chesson. Direct evidence for ZFN-based editing in the transfected Chesson transformant line in animal 4909 was provided by sequencing the PCR-amplified pvdhfr-ts gene. At the ZFN binding site, 6 engineered silent mutations from the plasmid segment had been introduced at nucleotide positions 330, 336, 346, 347, 358, and 359. The presence of codon changes for the amino acids 57L, 58R, 61M, and 117T (at nucleotide positions 171, 174, 182, and 350, respectively) demonstrated complete editing of the wild-type Chesson allele to the quadruple-mutant form (Supplementary Figure 1).
Because our amplified products for sequencing were obtained by primers external to the pvdhfr donor template carried by the pvDC2-ZFNpvdhfr plasmid, these results confirmed editing of the endogenous gene and were not an artifact of amplification from residual plasmid DNA in the monkey. The successful selection of this outcome in only 1 of 4 S. boliviensis inoculated with transfected parasites may have different possible explanations, including the stringency of drug pressure or factors affecting the probability of successful transformation and parasite expansion in the primate bloodstream. Of these explanations, stringency of drug pressure seems unlikely, as we have not observed the Pyr doses to markedly affect growth of the quadruple-mutant AMRU-1 parasites, even in animals with very low levels of parasitemia, a result in agreement with published data showing that P. falciparum transformants expressing mutant pvdhfr-ts resist very high Pyr doses [14]. A more likely possibility may be that parasitemias in the primates over several weeks are highly variable, depending on susceptibility to infection and immune responses, and can clear spontaneously without treatment. We also note that 2 different selection protocols were used in the 4 experiments (Figure 2). Additional experiments will be needed to establish whether one protocol is better than the other for selection of the modified parasites.
In conclusion, ZFN-mediated editing offers a powerful strategy to introduce genetic reporters, generate knockouts, or engineer allelic modifications for a broad range of applications in malaria research. Customized ZFNs can now be used to target other vivax loci, using quadruple-mutant pvdhfr, human dhfr [8], or other selectable markers suitable for the transformation of Plasmodium parasites. In P. falciparum, experiments have demonstrated the ready generation of ZFN-mediated gene deletions, allelic exchanges, and specific nucleotide alterations in the presence or absence of selectable markers [8, 9]. Results of the present work show that precise genome editing is now feasible for P. vivax. Introduced edits can readily extend to mutations >100 base pairs from the ZFN double-stranded break site, providing good latitude for the choices and applications of ZFN targeting. We note that, in the absence of routine in vitro P. vivax cultures for laboratory experiments, this technology continues to depend on costly nonhuman primates. The method may therefore be most usefully deployed in the context of collaborative efforts, as highlighted in recent workshops, such as the 2013 Advances in Plasmodium vivax Malaria Research workshop. Development of ZFNs and other possible approaches, such as TALEN- or CRIPSR-based genome editing, will support new advances in our understanding of the biology and pathogenesis of P. vivax.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the animal research technical support provided by Theresa Engels, Ahlin Bruce, Billeta Lewis, Autumn Roessler, Lynn Lambert, Sachy Orr-Gonzalez, and Drs Tom Thomas and John Bacher (Division of Veterinary Research, NIH).
Financial support. This work was supported by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. R. R. M. B. received partial funding from the Brazilian National Council for the Development of Science and Technology, Science Without Frontiers Program (237256/20126). D. A. F. gratefully acknowledges extramural funding support from the National Institutes of Health (AI50234 and AI109023).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Miller LH, Ackerman HC, Su XZ, Wellems TE. Malaria biology and disease pathogenesis: insights for new treatments. Nat Med. 2013;19:156–67. doi: 10.1038/nm.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gruring C, Moon RW, Lim C, Holder AA, Blackman MJ, Duraisingh MT. Human red blood cell-adapted Plasmodium knowlesi parasites: a new model system for malaria research. Cell Microbiol. 2014;16:612–20. doi: 10.1111/cmi.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flannery EL, Fidock DA, Winzeler EA. Using genetic methods to define the targets of compounds with antimalarial activity. Eur J Med Chem. 2013;56:7761–71. doi: 10.1021/jm400325j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janse CJ, Kroeze H, van Wigcheren A, et al. A genotype and phenotype database of genetically modified malaria-parasites. Trends Parasitol. 2011;27:31–9. doi: 10.1016/j.pt.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 5.Menard R, Tavares J, Cockburn I, Markus M, Zavala F, Amino R. Looking under the skin: the first steps in malarial infection and immunity. Nat Rev Microbiol. 2013;11:701–12. doi: 10.1038/nrmicro3111. [DOI] [PubMed] [Google Scholar]
- 6.Pfahler JM, Galinski MR, Barnwell JW, Lanzer M. Transient transfection of Plasmodium vivax blood stage parasites. Mol Biochem Parasitol. 2006;149:99–101. doi: 10.1016/j.molbiopara.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 7.Gaj T, Gersbach CA, Barbas CF., III ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013;31:397–405. doi: 10.1016/j.tibtech.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McNamara CW, Lee MC, Lim CS, et al. Targeting Plasmodium PI(4)K to eliminate malaria. Nature. 2013;504:248–53. doi: 10.1038/nature12782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Straimer J, Lee MC, Lee AH, et al. Site-specific genome editing in Plasmodium falciparum using engineered zinc-finger nucleases. Nat Methods. 2012;9:993–8. doi: 10.1038/nmeth.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan J, Zhang Q, O'Neil M, et al. Antimalarial activities of new pyrrolo[3,2-f]quinazoline-1,3-diamine derivatives. Antimicrob Agents Chemother. 2005;49:4928–33. doi: 10.1128/AAC.49.12.4928-4933.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt LH. Plasmodium falciparum and Plasmodium vivax infections in the owl monkey (Aotus trivirgatus). II. Responses to chloroquine, quinine, and pyrimethamine. Am J Trop Med Hyg. 1978;27:703–17. doi: 10.4269/ajtmh.1978.27.703. [DOI] [PubMed] [Google Scholar]
- 12.Doyon Y, Vo TD, Mendel MC, et al. Enhancing zinc-finger-nuclease activity with improved obligate heterodimeric architectures. Nat Methods. 2011;8:74–9. doi: 10.1038/nmeth.1539. [DOI] [PubMed] [Google Scholar]
- 13.Lee MC, Moura PA, Miller EA, Fidock DA. Plasmodium falciparum Sec24 marks transitional ER that exports a model cargo via a diacidic motif. Mol Microbiol. 2008;68:1535–46. doi: 10.1111/j.1365-2958.2008.06250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Auliff AM, Balu B, Chen N, O'Neil MT, Cheng Q, Adams JH. Functional analysis of Plasmodium vivax dihydrofolate reductase-thymidylate synthase genes through stable transformation of Plasmodium falciparum. PLoS One. 2012;7:e40416. doi: 10.1371/journal.pone.0040416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tjitra E, Baker J, Suprianto S, Cheng Q, Anstey NM. Therapeutic efficacies of artesunate-sulfadoxine-pyrimethamine and chloroquine-sulfadoxine-pyrimethamine in vivax malaria pilot studies: relationship to Plasmodium vivax dhfr mutations. Antimicrob Agents Chemother. 2002;46:3947–53. doi: 10.1128/AAC.46.12.3947-3953.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.