Abstract
Background
In Plasmodium falciparum–infected patients treated with artemisinins, parasitemia declines through so-called pitting, an innate splenic process that transforms infected red blood cells (iRBCs) into once-infected RBCs (O-iRBCs).
Methods
We measured pitting in 83 French travelers and 42 Malian children treated for malaria with artesunate.
Results
In travelers, O-iRBCs peaked at 107.7% initial parasitemia. In Malian children aged 1.5–4 years, O-iRBCs peaked at higher concentrations than in children aged 9–13 years (91.60% vs 31.95%; P = .0097). The parasite clearance time in older children was shorter than in younger children (P = .0001), and the decline in parasitemia in children aged 1.5–4 years often started 6 hours after treatment initiation, a lag phase generally absent in infants and older children. A 6-hour lag phase in artificial pitting of artesunate-exposed iRBCs was also observed in vitro. The proportion of iRBCs recognized by autologous immunoglobulin G (IgG) correlated with the parasite clearance time (r = −0.501; P = .0006) and peak O-iRBC concentration (r = −0.420; P = .0033).
Conclusions
Antimalarial immunity correlates with fast artemisinin-induced parasite clearance and low pitting rates. In nonimmune populations, artemisinin-induced P. falciparum clearance is related to pitting and starts after a 6-hour lag phase. In immune populations, passively and naturally acquired immune mechanisms operating faster than pitting may exist. This mechanism may mitigate the emergence of artemisinin-resistant P. falciparum in Africa.
Keywords: malaria, Plasmodium falciparum, parasite clearance, artemisinin, pitting, acquired immunity, spleen
Artemisinin and its derivatives (artemisinins) are first-line treatments for severe and uncomplicated Plasmodium falciparum malaria worldwide [1]. In patients with malaria, the clearance of blood-stage parasites is faster after treatment with artemisinins than with other antimalarial drugs (eg, quinine) [2]. Since artemisinins act very early in the parasite's asexual life cycle, young parasite forms (rings) are cleared before they mature to potentially pathogenic cytoadherent forms [2]. These 2 pharmacodynamics effects, which are not achieved by quinine, are believed to reduce the morbidity and mortality of severe malaria in Southeast Asia and sub-Saharan Africa [3, 4].
In all patients treated with artemisinins, parasite clearance from the bloodstream has been related to so-called pitting [5–7], a spleen-specific process whereby a particulate intraerythrocytic body is expelled from a red blood cell (RBC) as it crosses narrow slits in the endothelial wall of red pulp sinuses [8, 9]. Because pitting occurs exclusively in the spleen, nuclear remnants from erythroblasts (Howell–Jolly bodies) persist in mature RBCs in individuals with anatomical or functional asplenia [10, 11]. The proportion of erythrocytes carrying Howell–Jolly bodies in the circulation is related to the intensity of splenic dysfunction.
P. falciparum rings exposed to artemisinins appear morphologically similar to Howell–Jolly bodies and are expelled from their host RBC in the same way [5–7, 12]. Consistent with these similarities, pitting of infected RBCs (iRBCs) does not occur in splenectomized patients or in in vitro culture [13]. iRBCs that are deparasitized by pitting return to circulation as once-infected RBCs (O-iRBCs), which can be detected by an erythrocyte membrane immunofluorescence (EMIF) test that visualizes parasite proteins deposited at the internal aspect of the RBC membrane very soon after parasite invasion [14]. In Southeast Asia, pitting accounts for a substantial fraction of parasite clearance in artesunate (AS)–treated patients but not in quinine-treated patients [5–7]. Whether pitting operates at the same level in other epidemiological contexts, particularly in highly malaria-endemic regions of Africa, is not known.
Parasite clearance rates in response to artemisinin treatment are now generally quantified by measuring the slope of the central portion of the parasite clearance curve [2] and then calculating the parasite clearance half-life (in hours) from this slope [15]. Some patients exhibit parasite clearance curves with an initial lag phase, a terminal tail phase, or both [2]. Lag phases are generally believed to reflect some combination of biological processes rather than poor drug action. For example, lag phases may represent apparent steady states in parasitemia, produced by the simultaneous clearance of drug-exposed rings and appearance of new rings from mature schizonts or resulting from a clearance mechanism that is not triggered instantaneously. Evidence for these and other mechanisms of lag-phase formation in patients treated with artemisinins has not been reported.
The kinetics and mechanisms of P. falciparum clearance in artemisinin-treated patients have recently come under intense scrutiny. Reduced parasite sensitivity to artemisinins has indeed emerged in multiple areas of Southeast Asia [16–19], where initial evidence of resistance manifested as delayed parasite clearance. A better understanding of artemisinin-induced parasite clearance may help assess whether reduced sensitivity to artemisinin has spread to or independently emerged in Africa, a threatening situation with potentially catastrophic consequences for global malaria control efforts. Only 2 studies have recently measured parasite clearance half-lives after artemisinin treatment in Africa. In Malian children with uncomplicated P. falciparum malaria, parasite clearance rates were found to be much faster than in Southeast Asian patients [16, 17]. In Kenyan children treated with artemisinin-based combination therapy, the proportion of cases showing complete parasite clearance by 24 hours were observed to be moderately decreased [20, 21]. The mechanisms underlying these observations have not been defined.
Using newly optimized methods to quantify iRBCs and O-iRBCs in patient blood, we sought to investigate the kinetics and mechanisms of parasite clearance after AS treatment in Africa.
METHODS
Patients and Samples
All patients with imported severe malaria provided consent according to a procedure common to all National Reference Centers (CNR; http://www.invs.sante.fr/Espace-professionnels/Centres-nationaux-de-reference/Textes-reglementaires) in France. Forms and data from all patients were collected as part of an observational program implemented by the CNR for Malaria. Intravenous AS (Guilin Pharmaceuticals, Shanghai, China) has been available in France since May 2011 through a named-patient program for imported severe malaria cases. Quinine (Sanofi Aventis, Paris, France) remained available for treating severe malaria in 40% of patients. After attending physicians identified at least 1 criterion of severe malaria, AS was released by hospital pharmacists and the indication confirmed by the French National Agency for Drug Safety (ANSM) [22]. Adult patients meeting at least 1 criterion for severe P. falciparum malaria [23] were admitted to public hospitals from May 2011 to May 2013 and treated intravenously with 2.4 mg/kg of AS at 0, 12, 24, and 48 hours. Blood samples were obtained at the time of admission and on days 3, 7, 14, 21, and 28 for most patients, as recommended by the French High Committee for Public Health. At each time point, thick and thin blood smears were prepared and O-iRBC concentrations were quantified by the EMIF test or flow cytometry (see below).
During the 2011 malaria transmission season in Mali, 215 children with uncomplicated P. falciparum malaria were enrolled in a parasite clearance rate study reported previously [24]. Briefly, inclusion criteria were age 0.5–15 years, a screening parasite density of 10 000–100 000 parasites/μL, a negative result of a pregnancy test, and no previous antimalarial therapy for present symptoms. Patients were treated orally with 4 mg/kg of AS at 0, 24, and 48 hours, followed by 10 mg/kg of amodiaquine daily for 3 days. A blood sample was obtained by finger prick before treatment and every 6 hours thereafter until 2 successive thick blood smears showed undetectable parasitemia or until 48 hours elapsed, whichever occurred first. For pitting analysis, a subset of 42 consecutively enrolled children with at least 0.5% parasitemia was selected from the main cohort of 215 patients. Thick and thin blood smears and indirect fluorescent antibody (IFA) slides for erythrocyte membrane immunofluorescence (EMIF) were prepared at each time point. This study is registered at Clinicaltrials.gov (NCT00669084) and was approved by the Institutional Review Board of the National Institute of Allergy and Infectious Diseases, National Institutes of Health, and by the Ethics Committee of the University of Bamako, Mali. The parents of all children gave written informed consent.
Measurement of Parasite Clearance and Pitting Rates
Venous blood samples from French travelers were washed with Tris-buffered Hanks' solution (TBH) before fixation with phosphate-buffered saline (PBS)/1% glutaraldehyde. Samples were incubated with primary antibody (polyclonal hyperimmune serum) at 1:10 in PBS/1% Albumax/0.1% Triton X-100 for 30 minutes, and then washed twice with PBS before incubation with secondary antibody (AlexaFluor 488–conjugated goat anti-human immunoglobulin G [IgG] at 1:500 plus Hoechst dye at 1:1000) in PBS/1% Albumax/0.1% Triton X100 for 30 minutes. Finally, samples were washed twice with PBS and analyzed with a BD FACS Fortessa-LSRII to quantify O-iRBC concentration and parasitemia. Giemsa-stained thin blood films and IFA slides for EMIF tests were prepared from the same samples for quality control.
Capillary blood samples from Malian children and venous blood samples from French travelers were processed and EMIF tests performed as described elsewhere [25]. Briefly, RBCs were washed 4 times with TBH and deposited on 8-well IFA slides pretreated with coating buffer (pH 9.6; 1.59 g/L Na2CO3, 2.93 g/L NaHCO3, and 200 mg/L NaN3). The slides were then washed, fixed with 1% glutaraldehyde, and kept at −20°C until staining as described above. Images were acquired on a Leica DMI3000 microscope, using a Leica DFC310FX camera controlled by LAS Superposition Images software (Leica Microsystèmes, Nanterre, France).
In Malian children, parasite densities were estimated from thick blood smears and parasite clearance half-lives and lag phases calculated using the Parasite Clearance Estimator (http://www.wwarn.org/research/parasite-clearance-estimator) as described previously [24].
Surface IgG Reactivity Assay
Each child's plasma IgG reactivity to their own iRBCs was measured as described elsewhere [24]. Briefly, plasma samples were heated at 56°C for 30 minutes and then stored at −80°C until use. iRBCs were cultured ex vivo for about 24 hours until rings matured to trophozoites, incubated with diluted (1:5, 1:10, 1:20, and 1:40) plasma samples, and then washed before staining with AlexaFluor 488–conjugated goat anti-human IgG (2 mg/mL) and SYTO 61 nucleic acid stain (5 mM in dimethyl sulfoxide; Life Technologies, Grand Island, NY) for 30 minutes. After 3 washes, iRBCs were analyzed by an Accuri C6 Flow Cytometer.
In Vitro Pitting Assay
The FUP/CB P. falciparum line was cultured in Roswell Park Memorial Institute (RPMI) 1640 medium containing 25 mM HEPES, 25 mM NaHCO3, 0.3 g/L glutamine, 10 mg/L gentamicin, and 10% human serum at 2.4%–5% hematocrit in an atmosphere of 5% CO2, 10% O2, and 85% N2 and a temperature of 37°C. Cultures were exposed to sequential sorbitol lysis (Sigma Aldrich, Saint-Quentin, France) and to cycles of differential gelatin flotation to obtain synchronized populations.
To achieve pitting of iRBCs in vitro, synchronized parasites at 4% hematocrit and 2%–6% parasitemia were exposed to 1 µM AS for 2 or 6 hours and then washed twice with RPMI 1640 medium and filtered by a microsphiltration system as described previously [12]. Briefly, metal microspheres (96.5% tin, 3% silver, and 0.5% copper; Industrie des Poudres Sphériques, Annemasse, France) with a diameter of 5–15 µm or 15–25 µm were mixed at a 70:30 ratio, respectively. Two grams of this mixture was suspended in 5 mL of PBS/1% Albumax II (Life Technologies, Saint Aubin, France). Glucose (7 µM) and calcium (1.2 mM) were added to the PBS/1% Albumax II solution. The microsphere suspension was poured into an inverted 1000-µL pipette tip (Gentaur, Paris, France) on top of the tip's antiaerosol filter. RBCs (600 µL and 2% hematocrit) were then introduced upstream from the microsphere layer and perfused through it at a flow rate of 60 mL/hour, using an electric pump (Syramed _sp6000, Arcomed'Ag, Regensdorf, Switzerland). The concentrations of iRBCs and O-iRBCs in upstream and downstream samples were quantified by EMIF.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 6 software. All reported P values are 2-tailed, and differences with a P value <.05 are considered statistically significant. Variables are expressed as medians and ranges or as means and standard errors of the mean (SEMs), as appropriate. Qualitative variables are expressed as percentages. Correlations were performed using the Spearman method. Differences among continuous variables were analyzed with the Kruskal–Wallis test. Artificial pitting rates after AS exposure were compared using the paired t test.
RESULTS
Kinetics of Parasite Clearance and Pitting in French Travelers and Malian Children After AS Treatment
From May 2011 through May 2013, we measured the concentration of circulating iRBCs and O-iRBCs in 48 AS-treated patients and 35 quinine-treated patients. From each patient, we obtained at least 3 venous blood samples between hour 0 (H0) and day 3 (D3) after treatment initiation. The median initial parasitemias were 4.1% and 7.0% and the parasite clearance times (PCTs) ranged from 24–48 hours and 24–72 hours in AS-treated patients and quinine-treated patients, respectively (Table 1). The concentration of O-iRBCs peaked at D2 in both groups, but the normalized peak was higher in the AS group (107.7% vs 30.82% initial parasitemia; Figure 1A and 1B). In some patients, it appeared that essentially all parasites were removed by pitting (Figure 1C). These data suggest that pitting was the predominant mechanism of parasite clearance in AS recipients but not in quinine recipients in our sample set.
Table 1.
Parasitemia, Parasite Clearance, and Parasite Pitting Parameters in French Travelers Treated With Intravenous Artesunate or Quinine for Severe Plasmodium falciparum Malaria
| Parameter | Artesunate (n = 48) | Quinine (n = 35) |
|---|---|---|
| Parasitemia, % | 4.10 (0.80–23.9) | 7.00 (0.30–33.8) |
| Parasite clearance time, h | 24 (24–48) | 48 (24–72) |
| Peak O-iRBC concentration at day 2, % | 107.7 (66.66–195.0) | 30.82 (19.54–42.10) |
Data are median (interquartile range).
Abbreviation: O-iRBC, once-infected red blood cell.
Figure 1.
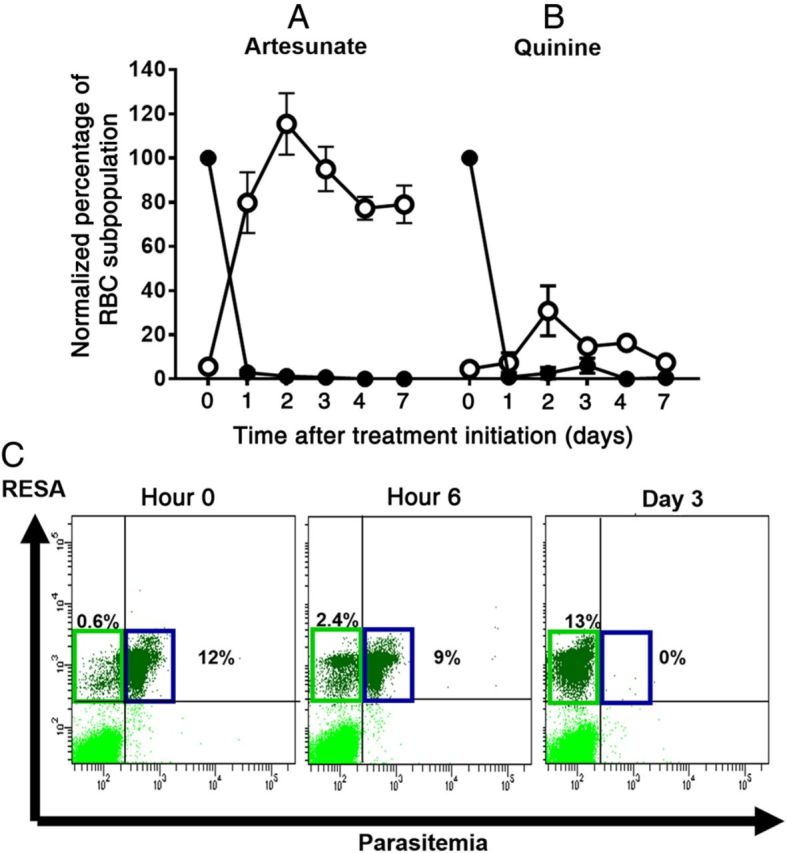
Kinetics of parasite clearance and pitting in French travelers. Mean concentrations (±standard error of the mean) of infected red blood cells (iRBCs; filled circles) and once-infected red blood cells (O-iRBCs; empty circles) were measured over 7 days in peripheral blood specimens from French travelers treated with intravenous artesunate (A; n = 48) or quinine (B; n = 35) for severe Plasmodium falciparum malaria. iRBC and O-iRBC concentrations were estimated by flow cytometry, using the DNA-staining Hoechst or SYBR green I dyes (which detect iRBCs only) and a RESA-staining protocol (which detects both iRBCs and O-iRBCs). iRBCs are identified as DNA-positive/RESA-positive cells, while O-iRBCs are identified as DNA-negative/RESA-positive cells. C, Monitoring of parasite clearance over 3 days in a French traveler. Flow cytometry analysis of blood samples collected 0, 6, and 72 hours after initiating artesunate treatment shows progressive reductions in the concentration of iRBCs (upper right quadrants) and simultaneous increases in the concentration of O-iRBCs (upper left quadrants). In this patient, parasite clearance was completely pitting dependent.
In 2011 and 2012, we performed a similar analysis in 42 Malian children (age range, 0.5–13 years) who were treated orally for uncomplicated malaria with AS at 0, 24, and 48 hours. From each patient, we obtained blood samples by finger prick every 6 hours until parasitemia was undetectable. The median PCT was 18 hours (range, 6–42 hours), and the median parasite clearance half-life was 1.52 hours (range, 0.88–3.48 hours; Table 2). The proportion of O-iRBCs peaked at H42 at 88.92% of initial parasitemia (Figure 2A) and ranged from 3.10% to 151.0% across all time points. These data indicate that pitting-dependent clearance also occurs in AS-treated Malian children but is associated with wide variation in PCTs and peak O-iRBC concentrations.
Table 2.
Parasitemia, Parasite Clearance, Parasite Pitting, and Immune Parameters in Malian Children Treated With Oral Artesunate for Uncomplicated Plasmodium falciparum Malaria, Stratified by Age
| Parameter | All |
0.5–4 y |
5–8 y |
9–13 y |
||||
|---|---|---|---|---|---|---|---|---|
| Children, No. | Value, Median (IQR) | Children, No. | Value, Median (IQR) | Children, No. | Value, Median (IQR) | Children, No. | Value, Median (IQR) | |
| Parasitemia, % | 42 | 2.37 (1.30–5.00) | 11 | 6.77 (2.54–7.58) | 21 | 1.82 (1.22–4.04) | 10 | 1.98 (1.24–3.76) |
| Parasite clearance time, h | 42 | 18 (12–25.5) | 11 | 24 (24–30) | 21 | 18 (12–24) | 10 | 12 (10.5–19.5) |
| Parasite clearance half-life, h | 42 | 1.52 (1.20–1.90) | 11 | 1.88 (1.45–2.15) | 10 | 1.49 (1.24–1.88) | 10 | 1.21 (0.98–1.62) |
| Peak O-iRBC concentration, % | 40 | 58.8 (31.2–91.7) | 11 | 91.6 (86.1–119) | 19 | 61.0 (24.9–91.7) | 10 | 32.0 (12.4–42.0) |
| Proportion of IgG + iRBCs, % | 37 | 25.4 (12.0–38.2) | 10 | 11.6 (8.12–31.3) | 18 | 25.4 (12.2–34.5) | 9 | 45.7 (21.6–64.3) |
Abbreviations: IgG, immunoglobulin G; IQR, interquartile range; iRBC, infected red blood cell; O-iRBC, once-infected red blood cell.
Figure 2.
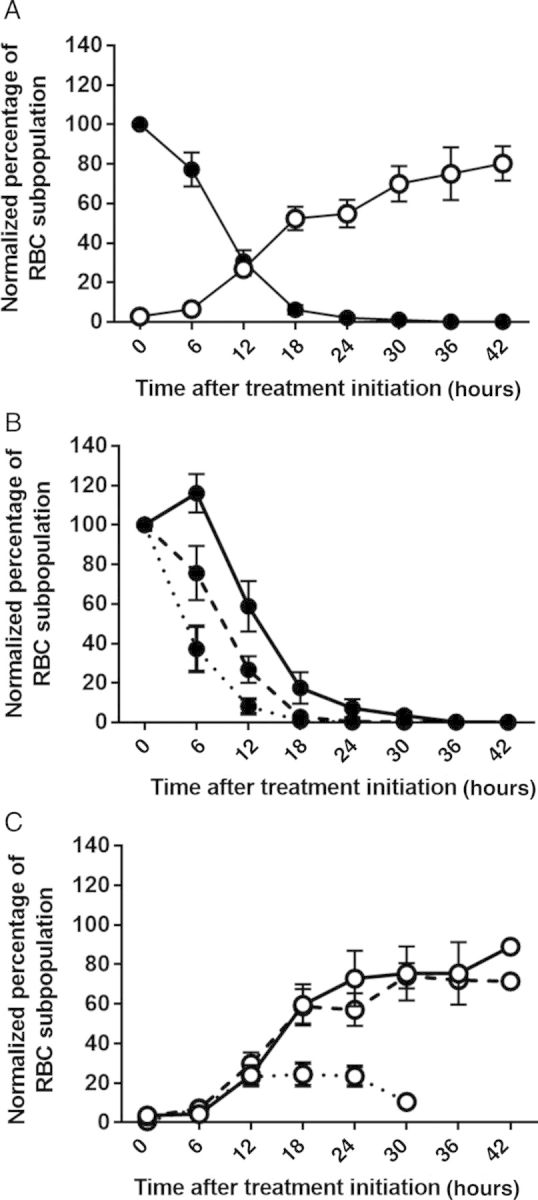
Kinetics of parasite clearance and pitting in Malian children. A, Mean concentrations (±standard error of the mean [SEM]) of infected red blood cells (iRBCs; filled circles) and once-infected red blood cells (O-iRBCs; empty circles) were measured every 6 hours in peripheral blood specimens from 42 children treated with oral artesunate for uncomplicated Plasmodium falciparum malaria. iRBC and O-iRBC concentrations were estimated by the erythrocyte membrane immunofluorescence method, using the DNA-staining Hoechst dye (which detects iRBCs only) and a RESA-staining protocol (which detects both iRBCs and O-iRBCs). iRBCs are identified as DNA-positive/RESA-positive cells, while O-iRBCs are identified as DNA-negative/RESA-positive cells. These cells were quantified among 10 000 RBCs, and their concentrations were normalized to the initial parasitemia set at 100%. B, iRBC clearance curves for the 42 children in panel A, stratified by age, as follows: 1.5–4 years (solid line), 5–8 years (dashed line), and 9–13 years (dotted line). Mean concentrations (±SEM) of iRBCs over time are shown. C, O-iRBC appearance curves of the 42 children in panel A, stratified by age, as follows: 1.5–4 years (solid line), 5–8 years (dashed line), and 9–13 years (dotted line). Mean concentrations (±SEM) of O-iRBCs over time are shown.
In Older Malian Children, Parasitemia Declines Rapidly, but Few O-iRBCs Appear in Circulation
As previously observed in Malian children [24, 26], PCT significantly and inversely correlated with age (n = 42; r = −0.65; P = .0001). To further study the relationship between age and variability in parasite clearance kinetics, we stratified the parasite clearance and pitting profiles into 3 age categories (1.5–4, 5–8, and 9–13 years). Parasite clearance curves showed distinct patterns in each age category (Figure 2B), with faster clearance in children aged 9–13 years than in those aged 1.5–4 years (median PCT, 12 vs 24 hours; median half-life, 1.21 vs 1.88 hours; Table 2). In children aged 1.5–4 years, but not in older children, parasite clearance was delayed by about 6 hours (lag phase; Figure 2B). Indeed, most parasite clearance curves in children aged 9–13 years have no lag phase, showing a mean >60% decline in parasitemia at H6.
In 40 Malian children, the peak O-iRBC concentration correlates positively with both PCT (r = 0.587; P = .0001) and half-life (r = 0.523; P = .001); that is, children with higher pitting rates had paradoxically slower parasite clearance rates. Children aged 1.5–4 years displayed the highest pitting rates, with a peak O-iRBC concentration of 91.60% at H42, compared with a peak of 31.95% at H18 in children aged 9–13 years (Figure 2C and Table 2). Pitting rates correlated inversely with age (n = 42; r = −0.41; P = .004), and the peak O-iRBC concentration decreased significantly with age: median, 91.60%, 61.00%, and 31.95% for children aged 0.5–4, 5–8, and 9–13 years, respectively (P = .0025; Table 2). These data indicate that faster parasite clearance rates are not accompanied by increased parasite pitting rates, suggesting that age-related, pitting-independent mechanisms contribute to parasite clearance in Malian children.
Artificial Pitting of AS-Exposed iRBCs In Vitro Starts After 6 Hours
To study the kinetics of pitting independently from complex in vivo processes, we exposed in vitro–cultured iRBCs to 1 µM AS for 2 or 6 hours and then filtered them through microsphere layers, a process that mimics the mechanical sensing of RBCs by the human spleen (Figure 3A) [12]. The pitting rate of iRBCs after 6 hours of AS exposure was significantly greater than after 2 hours of exposure (20.8 vs 3.82%; P = .0189) and greater than in unexposed controls (6.63%; P = .0360; Figure 3B). Since in vitro pitting occurred in the absence of cell-cell interactions, these findings indicate that the pitting process is triggered at least partially by mechanical interactions. It also suggests that, while parasite killing occurs very rapidly, additional modifications of the dying parasite or host RBC (or both) are required for pitting to occur. This 6-hour delay in in vitro pitting is reminiscent of the 6-hour lag phase in the parasite clearance curve observed in young Malian children with uncomplicated malaria (Figure 2B).
Figure 3.
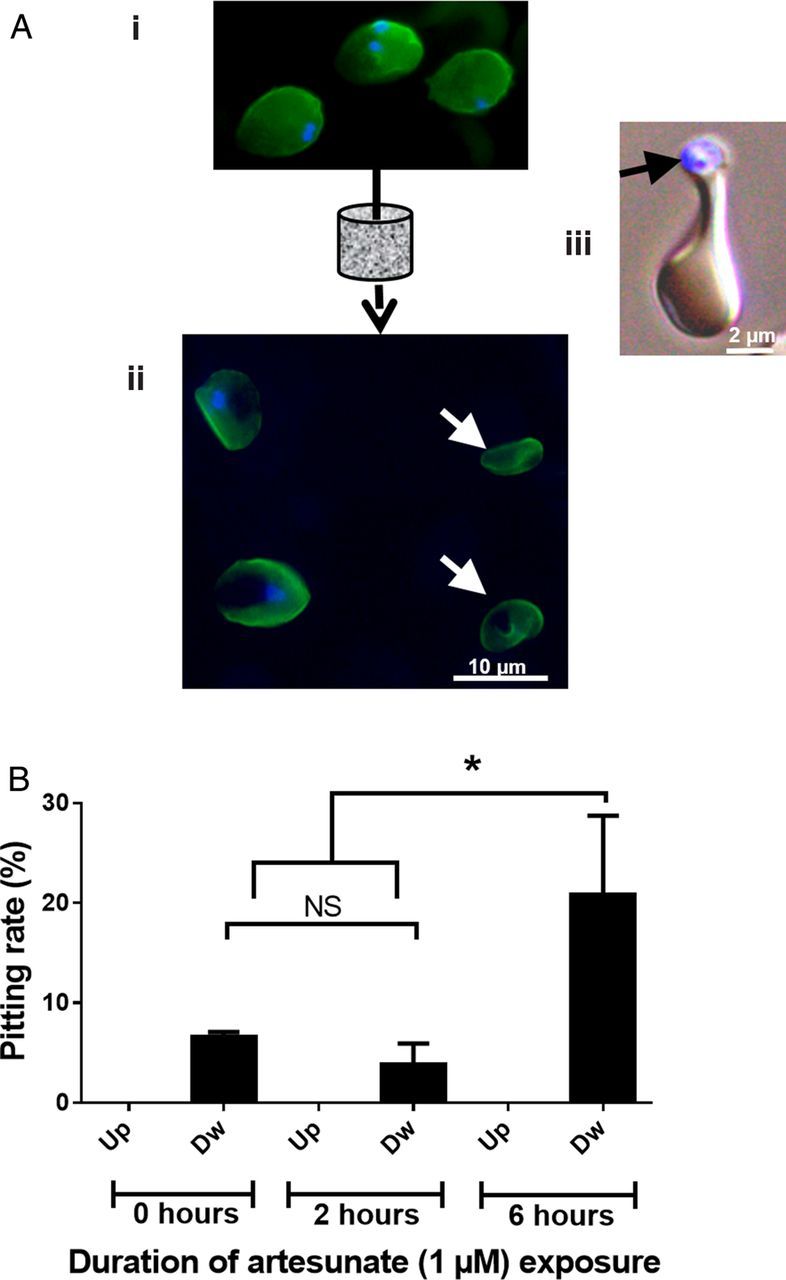
Artificial pitting in vitro, using microsphiltration. A, Infected red blood cells (iRBCs; containing ring-stage parasites) were left unexposed or exposed to 1 µM artesunate for 2 or 6 hours (i) and then filtered through microsphere layers as described elsewhere (see Methods) [12]. After being stained for DNA (blue) and RESA (green) using the erythrocyte membrane immunofluorescence (EMIF) method, iRBCs (blue and green) and once-infected red blood cells (O-iRBCs; green only [white arrows]) are easily identified by conventional fluorescence microscopy (ii). Some iRBCs in the microsphere layer contained parasite remnants (black arrow) in the process of being pitted (iii). B, Samples upstream (Up) and downstream (Dw) from the filters were stained using the EMIF method, and the concentrations of O-iRBCs were measured. Mean pitting rates (±standard error of the mean) are shown. iRBCs exposed to artesunate for 6 hours were pitted at a significantly higher rate than those exposed to artesunate for 2 hours (P = .0189) or left untreated (P = .0360). Abbreviation: NS, not significant.
Differences in Lag-Phase Duration Contribute in Part to Age-Dependent Differences in Parasite Clearance Kinetics
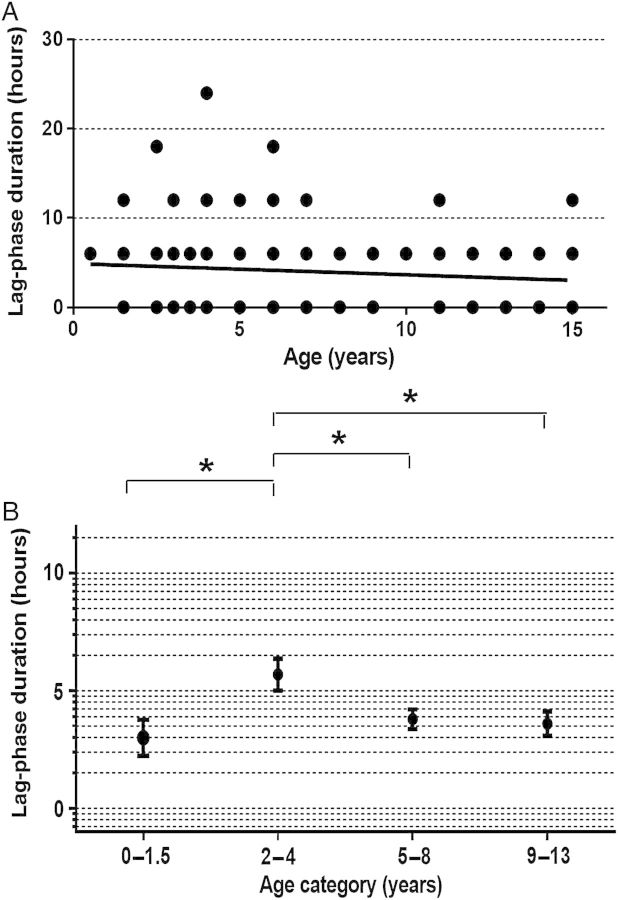
To explore the pitting process further, we retrospectively analyzed the different phases of parasite clearance curves in a cohort study of 215 Malian children aged 0.5–15 years [24]. While this previous investigation focused on analyzing parasite clearance half-lives, our observations suggested that age-dependent differences in PCTs may in part relate to differences in lag-phase duration. Among all 215 children, we found no correlation between age and lag-phase duration (r = −0.097; P = .155; Figure 4A). However, when we removed infants aged 0.5–1.5 years from the analysis, age and lag-phase duration correlated inversely and significantly (r = −0.205; P = .004). This finding suggests that a nonlinear association of age and lag-phase duration between infants and young children confounded our correlation analysis of the entire cohort.
Figure 4.
Relationship between age and lag-phase duration in the parasite clearance curve. A, Correlation between age and lag-phase duration was investigated in 215 Malian children treated with oral artesunate for uncomplicated Plasmodium falciparum malaria. The lag phase was defined as the length of time parasitemia remained >75% of its initial level. Using data from all 215 children, no correlation was found (r = −0.097; P = .155; many data points overlap). B, Mean lag-phase durations (±standard error of the mean), stratified by age category, are shown. Lag-phase duration was significantly longer in children aged 2–4 years, compared with children aged 0–1.5 (P = .007), 5–8 (P = .008), and 9–15 (P = .013) years.
Assuming the existence of differences between passively and naturally acquired immune responses in infants and young children, we stratified the entire cohort into 4 age categories (0.5–1.5, 2–4, 5–8, and 9–15 years). We found that children aged 2–4 years had a significantly longer lag phase than children in the other 3 age groups (Figure 4B). Also, the proportion of children with no lag phase was lower in children aged 2–4 years (30%) than in children aged 9–15 years (45%; P = .051) or infants (58%; P = .039). We thus observed accelerated parasite clearance (ie, short or absent lag phases) in infants and in children aged ≥5 years. This suggests that the age-related heterogeneity in parasite clearance and pitting kinetics in Malian children is not related to a continuous linear effect of age on innate clearance mechanisms, but rather to adaptive immune responses—acquired either passively in infants or actively through repeated parasite exposure in older children.
Adaptive Immunity and Parasite Clearance in AS-Treated Malian Children
To explore whether adaptive immunity contributes to the age-dependent heterogeneity in parasite clearance rates in Malian children, we measured the reactivity of a child's plasma IgG (collected at the time of diagnosis) to its own iRBCs (cultivated ex vivo from rings to trophozoites expressing variant surface antigens). In a subset of 36 children aged 1.5–13 years, for whom plasma reactivity assay results were available, we found that the proportion of iRBCs recognized by plasma IgG correlated positively with age (n = 36; r = 0.463; P = .0028) and negatively with peak pitting rate (r = −0.350; P = .038). These data suggest that immune IgG responses may contribute to the clearance of circulating iRBCs.
DISCUSSION
Patients treated with artemisinins for P. falciparum malaria clear their parasitemias faster that those treated with quinine and other antimalarial drugs [2]. Here we have confirmed previous observations [5–7, 12] that pitting contributes substantially to artemisinin-induced parasite clearance. Indeed, the peak concentration of O-iRBCs approximates the initial concentration of circulating iRBCs in some patients, indicating that almost all iRBCs can be deparasitized by pitting and then released back into circulation as O-iRBCs. In nonimmune French travelers with severe malaria, we found that pitting rates were significantly higher after treatment with AS than with quinine. While our data indicate that pitting contributes substantially to parasite clearance in Africa, as it does in Asia [5–7], they also suggest that the mechanisms of artemisinin-induced parasite clearance are more diverse in African children than previously thought.
Depending on the age of Malian children, P. falciparum clearance was predominantly pitting dependent or pitting independent. Specifically, in children aged 5–15 years, parasite clearance was very fast, often starting within 6 hours after treatment initiation. Paradoxically, this rapid parasite clearance was associated with the release of relatively few O-iRBCs into circulation (eg, only one third of the initial number of iRBCs). In line with this observation, the initial lag phase in the parasite clearance curve was longer in children aged 2–4 years than in infants or children aged 5–8 years, and a 6-hour exposure to AS was required for significant levels of artificial pitting to occur in vitro. These findings suggest a model in which distinct, age-dependent mechanisms contribute to parasite clearance in African children treated with artemisinins. In this model, pitting is the predominant, almost exclusive mechanism of parasite clearance in younger children, in which the lag phase corresponds to the time required to initiate pitting. In contrast, the model predicts that the very rapid parasite clearance in older children is partially due to another mechanism that does not involve pitting. Larger studies that are specifically designed for age-stratified analysis of pitting dynamics are needed to assess the robustness of this proposed model.
The reactivity of plasma IgG to the surface of trophozoite-infected RBCs correlated significantly and negatively with peak pitting rates. This confirms and extends previous observations in Malian children treated with artemisinins [24, 26] and strongly suggests that very rapid parasite clearance in children aged 5–15 years involves acquired immunity. It seems reasonable to speculate that the lag-less parasite clearance in infants and children aged 5–15 years relates to adaptive humoral immune factors. Immune IgG, for example, is passively transferred from mother to infant [27] and is progressively acquired after repeated P. falciparum infections in older children [28, 29]. Additional field and laboratory studies are warranted to identify such factors and investigate the mechanisms by which they clear artemisinin-exposed, ring-iRBCs from circulation. We have previously observed intense phagocytosis of AS-exposed iRBCs in an ex vivo human spleen model [9]. Such a mechanism may be triggered almost instantaneously in the spleen and other macrophage-rich organs and so may occur within the 6-hour lag phase before pitting occurs. While macrophages can innately phagocytose trophozoite-infected RBCs expressing PfEMP1 [30], the removal of young ring-iRBCs lacking PfEMP1 likely occurs by a different mechanism.
To achieve parasite clearance in the bloodstream, adaptive immune factors presumably target antigens on the surface of artemisinin-exposed, ring-infected RBCs. One previous investigation, in which IgG reactivity to the surface of trophozoite-infected RBCs correlated with parasite clearance rates, was not designed to identify specific antigens on AS-exposed, ring-infected RBCs [6]. Using laboratory-adapted P. falciparum strains, we have thus far been unable to observe differences in the surface seroreactivity of ring-infected RBCs exposed or not to artemisinins [31] (P. A. N. and C. R., unpublished data). Further studies using paired parasite isolates and immune plasma from African patients with rapid, pitting-independent clearance profiles may be more informative. Alternatively, age-dependent processes not associated with adaptive immunity may explain our observations. Whatever the mechanisms involved, identifying them may improve our understanding of emerging artemisinin resistance in P. falciparum. Slow parasite clearance rates have been reported in Southeast Asia, where antimalarial immunity levels are low and artemisinin-induced parasite clearance is predominantly pitting dependent. The very fast, pitting-independent parasite clearance we observe in older Malian children may mitigate the spread or delay the emergence of artemisinin-resistant parasites in Africa. Identifying parasite clearance mechanisms that involve adaptive immune responses may help to discover novel vaccine antigens.
Notes
Acknowledgments. We thank all patients and their families for participating in this study.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by the DIM Mal Inf Région Ile de France; the Worldwide Antimalarial Resistance Network; the Intramural Research Program, National Institute of Allergy and Infectious Diseases, National Institutes of Health; the Bill and Melinda Gates Foundation (to P. A. N.); the Follereau Foundation (to L. C.); INSERM-APHP France (to S. J.); and the University of Oxford.
Potential conflicts of interest. S. J. is engaged in a collaboration with Guilin Pharmaceutical. P. B. is engaged in a collaboration with Guilin Pharmaceutical and has provided expertise to Sigma Tau and Sanofi. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.WHO. The treatment of malaria. 2nd ed. Geneva, Switzerland: WHO: 2010. [Google Scholar]
- 2.White NJ. The parasite clearance curve. Malar J. 2011;10:278. doi: 10.1186/1475-2875-10-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dondorp A, Nosten F, Stepniewska K, Day N, White N. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet. 2005;366:717–25. doi: 10.1016/S0140-6736(05)67176-0. [DOI] [PubMed] [Google Scholar]
- 4.Dondorp AM, Fanello CI, Hendriksen IC, et al. Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial. Lancet. 2010;376:1647–57. doi: 10.1016/S0140-6736(10)61924-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angus BJ, Chotivanich K, Udomsangpetch R, White NJ. In vivo removal of malaria parasites from red blood cells without their destruction in acute falciparum malaria. Blood. 1997;90:2037–40. [PubMed] [Google Scholar]
- 6.Chotivanich K, Udomsangpetch R, Dondorp A, et al. The mechanisms of parasite clearance after antimalarial treatment of Plasmodium falciparum malaria. J Infect Dis. 2000;182:629–33. doi: 10.1086/315718. [DOI] [PubMed] [Google Scholar]
- 7.Newton PN, Chotivanich K, Chierakul W, et al. A comparison of the in vivo kinetics of Plasmodium falciparum ring-infected erythrocyte surface antigen-positive and -negative erythrocytes. Blood. 2001;98:450–7. doi: 10.1182/blood.v98.2.450. [DOI] [PubMed] [Google Scholar]
- 8.Schnitzer B, Sodeman T, Mead ML, Contacos PG. Pitting function of the spleen in malaria: ultrastructural observations. Science. 1972;177:175–7. doi: 10.1126/science.177.4044.175. [DOI] [PubMed] [Google Scholar]
- 9.Buffet PA, Milon G, Brousse V, et al. Ex vivo perfusion of human spleens maintains clearing and processing functions. Blood. 2006;107:3745–52. doi: 10.1182/blood-2005-10-4094. [DOI] [PubMed] [Google Scholar]
- 10.Rogers ZR, Wang WC, Luo Z, et al. Biomarkers of splenic function in infants with sickle cell anemia: baseline data from the BABY HUG Trial. Blood. 2011;117:2614–7. doi: 10.1182/blood-2010-04-278747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buffet PA, Safeukui I, Deplaine G, et al. The pathogenesis of Plasmodium falciparum malaria in humans: insights from splenic physiology. Blood. 2011;117:381–92. doi: 10.1182/blood-2010-04-202911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deplaine G, Safeukui I, Jeddi F, et al. The sensing of poorly deformable red blood cells by the human spleen can be mimicked in vitro. Blood. 2011;117:e88–95. doi: 10.1182/blood-2010-10-312801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chotivanich K, Udomsangpetch R, McGready R, et al. Central role of the spleen in malaria parasite clearance. J Infect Dis. 2002;185:1538–41. doi: 10.1086/340213. [DOI] [PubMed] [Google Scholar]
- 14.Berzins K. Pf155/RESA is not a surface antigen of Plasmodium falciparum-infected erythrocytes. Parasitol Today. 1991;7:193–4. doi: 10.1016/0169-4758(91)90136-c. [DOI] [PubMed] [Google Scholar]
- 15.Flegg JA, Guerin PJ, White NJ, Stepniewska K. Standardizing the measurement of parasite clearance in falciparum malaria: the parasite clearance estimator. Malar J. 2011;10:339. doi: 10.1186/1475-2875-10-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dondorp AM, Nosten F, Yi P, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–67. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amaratunga C, Sreng S, Suon S, et al. Artemisinin-resistant Plasmodium falciparum in Pursat province, western Cambodia: a parasite clearance rate study. Lancet Infect Dis. 2012;12:851–8. doi: 10.1016/S1473-3099(12)70181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tran TH, Nguyen TT, Nguyen HP, et al. In vivo susceptibility of Plasmodium falciparum to artesunate in Binh Phuoc Province, Vietnam. Malar J. 2012;11:355. doi: 10.1186/1475-2875-11-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kyaw MP, Nyunt MH, Chit K, et al. Reduced susceptibility of Plasmodium falciparum to artesunate in southern Myanmar. PLoS One. 2013;8:e57689. doi: 10.1371/journal.pone.0057689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beshir KB, Sutherland CJ, Sawa P, et al. Residual Plasmodium falciparum parasitemia in Kenyan children after artemisinin-combination therapy is associated with increased transmission to mosquitoes and parasite recurrence. J Infect Dis. 2013;208:2017–24. doi: 10.1093/infdis/jit431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borrmann S, Sasi P, Mwai L, et al. Declining responsiveness of Plasmodium falciparum infections to artemisinin-based combination treatments on the Kenyan coast. PLoS One. 2011;6:e26005. doi: 10.1371/journal.pone.0026005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ANSM. Protocole d'utilisation thérapeutique et de receuil d'informations Malacef (artésunate) 60 mg, poudre et solvant pour solution injectable laboratoire ACE Pharmaceuticals BV // ARTECEF BV. 2013. http://ansm.sante.fr/var/ansm_site/storage/original/application/01d138a64031bcf9f37fa2737308caa5.pdf . Accessed 18 August 2014.
- 23.WHO. Severe falciparum malaria. Trans R Soc Trop Med Hyg. 2000;94(suppl):1–90. [PubMed] [Google Scholar]
- 24.Lopera-Mesa TM, Doumbia S, Chiang S, et al. Plasmodium falciparum clearance rates in response to artesunate in Malian children with malaria: effect of acquired immunity. J Infect Dis. 2013;207:1655–63. doi: 10.1093/infdis/jit082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perlmann H, Berzins K, Wahlgren M, et al. Antibodies in malarial sera to parasite antigens in the membrane of erythrocytes infected with early asexual stages of Plasmodium falciparum. J Exp Med. 1984;159:1686–704. doi: 10.1084/jem.159.6.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maiga AW, Fofana B, Sagara I, et al. No evidence of delayed parasite clearance after oral artesunate treatment of uncomplicated falciparum malaria in Mali. Am J Trop Med Hyg. 2012;87:23–8. doi: 10.4269/ajtmh.2012.12-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moormann AM. How might infant and paediatric immune responses influence malaria vaccine efficacy? Parasite Immunol. 2009;31:547–59. doi: 10.1111/j.1365-3024.2009.01137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tetteh KK, Osier FH, Salanti A, et al. Analysis of antibodies to newly described Plasmodium falciparum merozoite antigens supports MSPDBL2 as a predicted target of naturally acquired immunity. Infect Immun. 2013;81:3835–42. doi: 10.1128/IAI.00301-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richards JS, Arumugam TU, Reiling L, et al. Identification and prioritization of merozoite antigens as targets of protective human immunity to Plasmodium falciparum malaria for vaccine and biomarker development. J Immunol. 2013;191:795–809. doi: 10.4049/jimmunol.1300778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan CL, Renia L, Tan KS. A simplified, sensitive phagocytic assay for malaria cultures facilitated by flow cytometry of differentially-stained cell populations. PLoS One. 2012;7:e38523. doi: 10.1371/journal.pone.0038523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Safeukui I, Correas JM, Brousse V, et al. Retention of Plasmodium falciparum ring-infected erythrocytes in the slow, open microcirculation of the human spleen. Blood. 2008;112:2520–8. doi: 10.1182/blood-2008-03-146779. [DOI] [PubMed] [Google Scholar]