Modest voucher incentives were associated with significantly better linkage to and retention in care but not with virologic suppression among human immunodeficiency virus (HIV)-infected drug users in Chennai, India, a population with high HIV disease burden and low access to antiretroviral therapy.
Keywords: HIV, drug users, linkage to care, contingency management, India
Abstract
Background. Drug users (DUs), a population that accounts for some of the fastest-growing human immunodeficiency virus (HIV) epidemics globally, lag behind other populations with regard to HIV-related outcomes. We evaluated the role of voucher incentives on linkage and retention in care among DUs in India.
Methods. In this randomized clinical trial, 120 DUs who were aged ≥18 years, HIV-infected, antiretroviral therapy (ART) naive, and ART eligible and who reported drug use in the prior month were randomized to incentive (INC) or control (CTL) conditions for 12 months. Participants randomized to the INC arm received incentives (redeemable for food/household goods) ranging in value from USD4 to USD8 for achieving prespecified targets (eg, ART initiation, visits to ART center). Subjects in the CTL group could win vouchers in prize-bowl drawings, but HIV care behaviors were not incentivized. The primary endpoint was time to ART initiation.
Results. Sixty participants each were randomized to the INC and CTL arms between December 2009 and September 2010. Participants in the INC arm were more likely to visit the government ART center (49 vs 33; P = .002); 27 participants in the INC and 16 participants in the CTL arm initiated ART (P = .04; hazard ratio for ART = 2.33 [95% confidence interval, 1.15–4.73]). Participants in the INC arm also had significantly more visits to the ART center (median number of visits, 8 vs 3.5; P = .005). However, no difference in viral suppression was observed.
Conclusions. Modest voucher incentives improved linkage to and retention in HIV care, but did not significantly impact viral suppression among DUs in India, a disenfranchised and difficult-to-treat population.
Clinical Trials Registration. NCT01031745.
“Seek, test, treat, and retain” has been posited as the key strategy to end the human immunodeficiency virus (HIV) epidemic [1, 2], and entails (1) identifying persons at high risk for HIV infection, (2) testing and identifying those who are HIV infected, (3) linking HIV-infected persons with care centers and initiating antiretroviral therapy (ART) in those eligible, (4) retaining HIV-infected persons in care, and (5) ensuring that HIV-infected persons on ART suppress virus [3]. To be successful, this strategy must encompass all populations including those hardest to reach (eg, drug users [DUs]). DUs account for some of the fastest-growing HIV epidemics globally; ART coverage is significantly lower in countries with epidemics driven by drug use, particularly in low- and middle-income countries [4].
India is home to the largest number of opiate users globally (approximately 3 million) [5]. We have previously demonstrated high HIV prevalence among DUs in Chennai (approximately 25%) [6, 7], coupled with high AIDS-related mortality, especially among those with advanced disease [8]. These data collectively suggest poor access to ART and an urgency to develop interventions to improve linkage to care and ART initiation in this vulnerable population.
A key barrier to ART initiation, particularly among DUs, is that it requires a delayed gratification perspective. It has been theorized that individuals disproportionately prioritize current issues over future ones (present-biased). Thus, they overvalue present inconveniences or losses of time as those experienced when visiting ART centers compared with the future benefits of ART (eg, prolonged survival). Behavioral incentives are intended to overcome this barrier [9]. Behavioral incentives have been demonstrated to be effective in promoting favorable outcomes including reducing illicit drug use [10], smoking cessation [11], weight loss [12], and returning to collect HIV test results [13]. Pilot studies have also suggested that incentive-based strategies can improve ART adherence in the United States [14–16]. However, to date, no published studies have examined the impact of incentives on linkage and retention to care and treatment outcomes among HIV-infected DUs.
Accordingly, we conducted a pilot randomized clinical trial (RCT) to examine the impact of voucher incentives on time to ART initiation among ART-naive, ART-eligible DUs in Chennai, India.
METHODS
Study Setting
This study was conducted at the YR Gaitonde Centre for Substance Abuse Research (YRGCSAR), established in November 2004 [7]. YRGCSAR provides HIV counseling, testing and basic medical services to DUs. Patients requiring ART are referred to government facilities where ART is available free of charge [17].
Study Population
The study population comprised individuals receiving care at YRGCSAR and participants referred to the clinic by outreach workers/organizations working with DUs. Screening procedures included an interviewer-administered questionnaire, HIV serologic testing, and CD4+ testing among HIV-positive subjects. To be eligible, participants had to (1) be aged ≥18 years, (2) provide written informed consent, (3) report injection or noninjection drug use in the prior 30 days, (4) have documented evidence of HIV-1 infection, (5) be ART naive (self-report), and (6) satisfy Indian national guidelines for initiation of ART at the time [18]. This study was approved by the YRGCARE and Johns Hopkins Medicine institutional review boards.
Randomization
Eligible participants who provided consent were randomly assigned to the incentive (INC) arm or the control (CTL) arm. Blocked randomization with randomly varying blocks of 2, 4, and 6 at a 1:1 allocation ratio between the INC and CTL arms was used to generate a randomization list. Opaque envelopes with study arm assignments were numbered and opened sequentially when subjects were enrolled.
Study Procedures
Study visits occurred at baseline and at 3, 6, 9, and 12 months. Demographic information was collected at baseline. Government ART centers provide HIV-infected patients with treatment registers, which patients keep in their possession. Government physicians/nurses document information including visit dates, ART initiation date, medications prescribed, refill dates, and selected clinical and laboratory results in these registers and sign the register at every visit. Study participants were instructed to bring their registers to study visits, where relevant data were abstracted. Blood samples were collected at baseline and at 6 and 12 months. Samples were tested for CD4+ cell count (FlowCARE PLG, Beckman Coulter) and HIV RNA (COBAS HIV-1 Monitor, Version 1.5, Roche Molecular Diagnostics) according to manufacturers' instructions. Government ART centers prescribed 3-drug regimens containing zidovudine or stavudine plus lamivudine plus nevirapine or efavirenz, according to standard care in India at the time.
Intervention and Control Conditions
Participants in both arms were referred to a government ART center with a referral letter. Participants in the INC arm were given voucher incentives for achieving specific targets (Table 1). Over 12 months, INC subjects could earn up to 15 vouchers: 12 for attending monthly (required by government) clinical/medication refill visits at government ART centers, 1 for initiating ART, and 2 for achieving viral suppression (HIV RNA <400 copies/mL). Vouchers could be traded for groceries (rice, lentils, etc) or household items (toothpaste, soap, etc). The incentives for visit attendance and ART initiation were worth 200 Indian rupees (INR; equal to USD4) each and the incentives for HIV RNA suppression were INR 400 (USD8). INR 200 is approximately the daily wage for an average DU in Chennai [19].
Table 1.
Design of the Intervention Condition
| Incentive Target | No. of Times Outcome Possible During Study (Visits Where Incentives Were Given) | Value of Voucher Incentive in INR (USD) | Maximum Possible Incentive During Study in INR (USD) |
|---|---|---|---|
| Initiate ART | 1 | 200 (4) | 200 (4) |
| HIV clinic visit for clinical care/refilla | 12 (3-, 6-, 9-, 12-mo) | 200 (4) | 2400 (48) |
| HIV RNA suppression | 2 (6- and 12-mo study visits) | 400 (8) | 800 (16) |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; INR, Indian rupee; USD, United States dollar.
a Government ART centers require participants to visit the center on a monthly basis; therefore, at each quarterly study visit (3, 6, 9, and 12 months), participants could have visited the ART centers a maximum of 3 times in the preceding 3 months.
Participants in the CTL arm were not offered incentives for treatment targets. However, because complete absence of vouchers might be perceived as unfair and influence behaviors in unanticipated ways or lead to differential dropout [16], we offered CTL participants the opportunity to win vouchers in “prize-bowl” drawings unlinked to target behaviors. There was one prize bowl for INR 200 prizes (equivalent to base incentive in the INC arm) and a second prize bowl for INR 400 prizes (equivalent to HIV RNA suppression incentive) (Table 2). Each prize bowl had 100 cards. Fifty said “Congratulations! You have won!” and 50 said “Sorry! Better luck next time!” Cards were replaced in the bowl after each drawing, such that each bowl always contained 100 cards and the probability of drawing a winning card each time was 0.50. The prize-bowl drawings were designed to mimic incentive opportunities in the INC arm. For example, at the 3-month visit, each CTL subject was given 4 drawings from the INR 200 prize bowl, corresponding to the 4 opportunities an INC subject would have to earn INR 200 incentive vouchers during the first 3 months following randomization (ie, 3 monthly visits to the government ART center and 1 for ART initiation). Details on incentives and prize-bowl drawings were provided in the consent form.
Table 2.
Design of the Control Conditiona
| Study Visit | No. of Drawingsb |
Maximum Possible Bonus in INR (USD) | |
|---|---|---|---|
| Prize Bowl A (INR 200 [USD4]) | Prize Bowl B (INR 400 [USD8]) | ||
| 3 mo | 4 | 0 | 800 (16) |
| 6 mo | 3 | 1 | 1000 (20) |
| 9 mo | 3 | 0 | 600 (12) |
| 12 mo | 3 | 1 | 1000 (20) |
Abbreviations: INR, Indian rupee; USD, United States dollar, ART, Antiretroviral Therapy.
a Schedule of “prize-bowl” drawing for individuals randomized to control arm.
b For example, at the 6-month visit, each participant in the control (CTL) arm would draw from prize bowl A 3 times corresponding to the potential for incentive (INC) participants to earn INR 600 for attending all 3 monthly visits at the ART center in the preceding 3 months. Also, the CTL participants would draw once from prize bowl B corresponding to the INR 400 that INC participants could earn for achieving viral suppression at the 6-month visit.
Statistical Analyses
The primary outcome was time to ART initiation at a government ART center. Participants' HIV treatment registers were reviewed to identify whether ART had been initiated. Study staff confirmed data on clinic visits and ART use from participants' registers with the records maintained at the government centers to minimize manipulation of the treatment registers by study participants to earn incentives. Secondary outcomes included number of visits made to the government ART center (within visit windows) for routine clinical follow-up and/or ART refills; median change from baseline in CD4+ count, and proportion with HIV RNA <400 copies/mL at the 6- and 12-month visits.
Primary analyses used an intention-to-treat approach. Mann–Whitney U test and χ2 tests were used to compare continuous and categorical variables between the study arms, respectively. Kaplan-Meier curves and log-rank tests were used to compare time to ART initiation between the study arms. For viral suppression, missing values were considered detectable. Cox regression was used to identify factors associated with ART initiation; coefficients were expressed as hazard ratios (HRs). Factors that were associated with the outcome at P < .1 in the univariate analysis or deemed important a priori (eg, sex) were included in the multivariate model.
This pilot RCT was designed as a feasibility study to determine the effect size of voucher incentives on ART initiation. Assuming that overall 50% of participants would initiate ART, a 2-sided α = .05 and 80% follow-up, we determined that a sample size of 120 would have 80% power to detect a relative hazard of ≥2.2 for ART initiation. For viral suppression, we had 80% power to detect a difference of 27% between study arms assuming the prevalence of viral suppression in the CTL arm ranged from 10% to 60%.
All statistical analyses were conducted in Intercooled Stata version 11.1 (StataCorp, College Station, Texas).
RESULTS
Study Participants
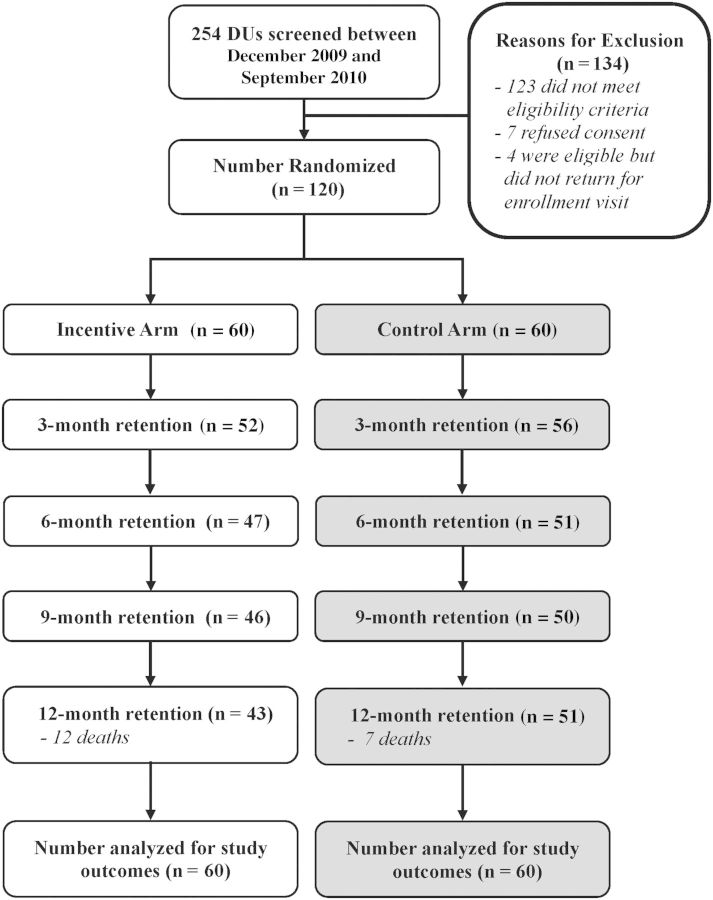
Two hundred fifty-four participants were screened between December 2009 and September 2010 to recruit 120 participants, and 94 participants returned for the 12-month visit (Figure 1). Nineteen deaths were observed (mortality rate, 17.9 per 100 person-years [py]; 95% confidence interval [CI], 11.5–28.0]), and no statistical difference was observed between arms. Seven deaths were tuberculosis related and 3 were non–AIDS related based on verbal autopsy.
Figure 1.
Study flowchart. Abbreviation: DUs, drug users.
The median age was 38 years, and 91% were male. All participants who had hepatitis C virus serology data (n = 53) were seropositive. The median baseline CD4+ counts in the INC and CTL arms were 248 and 268 cells/µL, respectively, and the median HIV RNA values were 5.06 and 4.75 log10 copies/mL, respectively. The INC and CTL arms were similar with respect to demographic, clinical, and laboratory characteristics at baseline (Table 3).
Table 3.
Demographic and Clinical Characteristics of Study Participants at Baseline (n = 120)
| Characteristic | Control Arm (n = 60) | Intervention Arm (n = 60) |
|---|---|---|
| Median age, y (IQR) | 38 (32.5–44) | 38.5 (34–43) |
| Male, No. (%) | 55 (91.7) | 54 (90) |
| Self-identified as heterosexual, No. (%) | 54 (90) | 58 (96.7) |
| Current marital status, No. (%) | ||
| Unmarried and single | 17 (28.3) | 13 (21.7) |
| Married | 32 (53.3) | 31 (51.7) |
| Widowed | 7 (11.7) | 6 (10) |
| Employment status, No. (%) | ||
| Unemployed | 13 (21.7) | 5 (8.3) |
| Daily wage earners | 36 (60) | 45 (75) |
| Weekly or monthly wage earners | 11 (18.3) | 10 (16.7) |
| Current living situation, No. (%) | ||
| Homeless | 3 (5) | 8 (13.3) |
| Rent an apartment/house | 36 (60) | 37 (61.7) |
| Own house | 15 (25) | 14 (23.3) |
| Anti-HCV antibody positivea, No. (%) | 24 (100) | 29 (100) |
| Median CD4 count at enrollment, cells/µL (IQR) | 268 (188.5–319) | 248 (179–305) |
| Median HIV RNA at enrollment, log10 copies/mL (IQR) | 4.75 (2.30–5.44) | 5.06 (3.19–5.52) |
| Median follow up, d (IQR) | 357 (350.5–365) | 355 (265.5–361.5) |
Abbreviations: HCV, hepatitis C virus; HIV, human immunodeficiency virus; IQR, interquartile range.
a Information available for only 53 participants; no significant differences were observed between the 2 arms.
Implementation of Incentive and Control Conditions
The median value of voucher incentives earned by participants in the INC arm was INR 2200 (approximately USD44; interquartile range [IQR], INR 1200–2600), and the median value of voucher prizes won by participants in the CTL arm was INR 1900 (approximately USD38; IQR, INR 1600–2400).
Linkage to Care, ART Initiation, and Retention
Significantly more participants from the INC arm visited a government ART center (49 vs 33; P = .002; Figure 2A). Twenty-seven (45%) participants in the INC arm and 16 participants (26.7%) in the CTL arm initiated ART (P = .04). Participants in the INC arm initiated ART sooner than participants in the CTL arm (Figure 2B; P for log-rank test statistic = .015).
Figure 2.
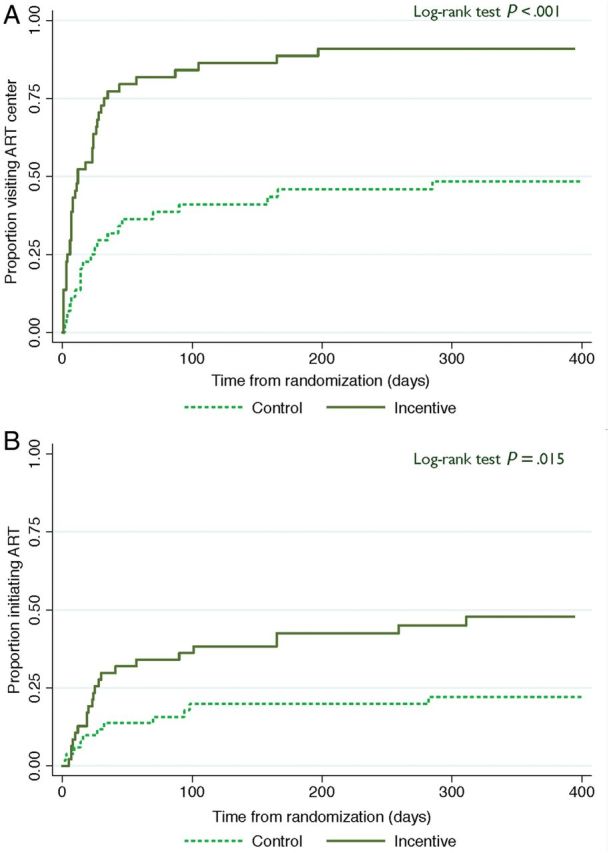
Kaplan-Meier curves of time to visiting a government antiretroviral therapy (ART) center and time to initiation of ART. A, Time to visiting a government ART center from randomization. B, Time to initiation of ART from randomization.
In univariate analyses, the only factor significantly associated with ART initiation was study arm (HR for INC vs CTL arm, 2.33; 95% CI, 1.15–4.73). In multivariate analyses, adjusting for sex and CD4+ count and viral load at baseline, randomization to the INC arm remained significantly associated with ART initiation (HR, 2.93; 95% CI, 1.39–6.20). Additionally, participants with CD4+ count ≤200 cells/µL were twice as likely to initiate ART (HR, 2.05; 95% CI, 1.01–4.13) as those with CD4 >200 cells/µL; increasing viral load was negatively associated (HR per unit log10 increase in HIV RNA, 0.72; 95% CI, .54–.95) (Table 4).
Table 4.
Factors Associated With Antiretroviral Therapy Initiation Among 120 Drug Users Enrolled in a Randomized Clinical Trial in Chennai, India
| Unadjusted HR (95% CI) | Adjusted HR (95% CI) | |
|---|---|---|
| Study arm | ||
| Control | 1 | 1 |
| Intervention | 2.33 (1.15–4.73) | 2.93 (1.39–6.20) |
| Sex | ||
| Female | 1 | 1 |
| Male | 0.85 (.30–2.42) | 0.95 (.33–2.70) |
| Baseline absolute CD4+ cell count | ||
| 201–350 cells/µL | 1 | 1 |
| ≤200 cells/µL | 1.91 (.95–3.82) | 2.05 (1.01–4.13) |
| Baseline HIV load | ||
| Per log10 increase in HIV RNA | 0.83 (.64–1.09) | 0.72 (.54–.95) |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; HR, hazard ratio.
INC participants also had more monthly follow-up visits to the government center compared with CTL participants (median, 8 [IQR, 3–11] vs 3.5 [IQR, 0–9]; P = .005).
Viral Suppression and Immunological Outcomes
No differences were observed between the INC and CTL arms in CD4+ gains or viral suppression. At 6 months, 16 (26.7%) and 21 (35%) in the INC and CTL arms, respectively, had HIV RNA <400 copies/mL. At 12 months, 19 participants (31.7%) in the INC arm and 20 (33.3%) in the CTL arm had HIV RNA <400 copies/mL. At the 6-month visit, the median change in CD4+ count in the INC and CTL arms was 11 cells/µL (IQR, −44 to 60) and 12 cells/µL (IQR, −50 to 64), respectively. The median change in CD4+ count at the 12-month visit compared with baseline was 23 cells/µL (IQR, −39 to 71) and 9 cells/µL (IQR, −25 to 64) in the INC and CTL arms, respectively.
In sensitivity analyses restricted to the 43 participants who initiated ART, viral suppression at the 6-month and 12-month visits was 62.5% and 66.7% in the CTL arm and 60.9% and 68.2% in INC arm, respectively, showing no statistical difference.
DISCUSSION
In this pilot randomized trial, we demonstrated that modest nonmonetary voucher incentives were associated with higher rates of linkage to care, ART initiation, and retention in care among DUs in India. However, no differences were observed in HIV RNA or CD4 outcomes. While improved rates of linkage and retention in this hard-to-reach population are a promising sign of the potential of voucher incentives, additional study is needed to determine whether the lack of clinical impact is the result of unintended negative consequences of the voucher incentives or the need to more directly target behaviors leading to viral suppression.
HIV-infected DUs are a disenfranchised, vulnerable population with high levels of mortality primarily due to delayed access to care [20]. Despite the rapid scale-up of ART in low- and middle-income countries, ART uptake has lagged behind in countries with drug use–driven epidemics. For example, in sub-Saharan Africa (predominantly heterosexual), 49% of ART-eligible individuals were receiving ART compared with those in Eastern Europe or Central Asia (predominantly drug use), where only 23% of eligible persons were receiving ART [4].
In this trial, the incentive was designed to compensate a day's wage of the average DU in Chennai [19]. Moreover, incentives were provided as food and household goods, providing benefit for participants' families as well. This modest incentive had a meaningful impact on linkage to care, ART initiation, and retention in care; DUs in the INC arm also had more visits to government ART centers. These findings illustrate the potential of voucher incentives to improve multiple steps along the HIV care continuum.
This is not the first study to evaluate incentives to improve HIV-related outcomes. Studies from Africa demonstrated that modest incentives increased rates at which individuals returned for HIV test results [13] and were associated with lower HIV prevalence among school girls [21]. Studies have also evaluated incentives for medication adherence (using electronic pill dispensing devices) [16] and viral load reductions [22]. To our knowledge, there are no completed studies reporting impact of incentives on linkage and retention. However, there are large ongoing studies, such as the HIV Prevention Trials Network 065 (TLC-Plus) [23] and the Clinical Trials Network 0049 (Project Hope) [24] protocols, which incorporate incentives to improve treatment engagement and outcomes.
It is important to note that despite significantly better retention in care, our study failed to detect differences in viral suppression to corroborate differences observed in ART uptake. There are multiple possible explanations for this. It is possible that some participants in the INC arm obtained ART prescriptions to earn the incentive, but did not take medications as prescribed. This is particularly worrisome, as suboptimal adherence results in the emergence of drug-resistant variants that could compromise efficacy of the ART regimen [25] and also result in the transmission of drug resistance. We intended the incentives for viral suppression to motivate adherence, but this outcome may have been too remote from the day-to-day adherence required to achieve viral suppression. Behavioral research suggests that immediate incentives are more effective than delayed ones [26].
Attending a government ART center, initiating ART (ie, having ART prescribed), and refilling ART on schedule are discrete, verifiable events that lend themselves as behavioral targets for incentives. In contrast, viral suppression requires consistent daily adherence with medications. Alternative approaches to fostering optimal adherence may be needed, including patient education, outreach or peer-support systems, or incentives that more directly capture medication adherence such as incentivizing directly observed therapy or presence of antiretroviral drugs in blood/urine. Future studies should explore both incentivizing more directly adherence and potentially combining incentives with other interventions to promote adherence to avoid unintended consequences of incentives (eg, emergence of drug resistance).
Another possible explanation for the lack of difference in viral suppression is that participants did not take any of their doses and sold prescriptions to pharmacies (as is possible in India) or on the street. Although this scenario would be less likely lead to drug resistance, it would be associated with higher levels of mortality as observed in this trial. Preexisting antiretroviral drug resistance may have also limited the virologic and immunologic responses to ART. However, this seems unlikely given the low prevalence of pretreatment HIV drug resistance [27], as among DUs it would be expected to be even lower, given low penetration of ART.
Even with the positive impact of the voucher incentives on linkage to and retention in care in this trial, we observed an extremely high mortality rate (MR) of 17.9 per 100 py, the strongest predictor being lower CD4+ counts. However, it is important to note that a prior analysis in this same population in 2005–2006 demonstrated even higher mortality among DUs with advanced HIV disease (MR, 34.5 per 100 py). Moreover, a third of the participants never visited a government center despite being referred. Common reasons cited for nonattendance were “not interested to visit ART center” and “don't want to initiate ART,” suggesting that treatment literacy interventions should also be included as part of any intervention package.
Last, the relatively higher retention rate observed in the CTL arm at all study visits was an unexpected finding but may have been a function of the study design, reflecting that participants in the INC arm were not incentivized for attending study visits. Thus, participants in the INC arm who did not achieve their targets might have opted not to attend study visits because no incentive would have been received. By contrast, participants in the CTL arm had opportunities to win prizes at all study visits regardless of their health-seeking behaviors.
This study was limited by small sample size, which restricted our ability to examine differences in biological outcomes. The generalizability of these findings to DUs in other parts of the world or other populations in India is also limited. However, the use of a randomized design and an active control arm reinforce the validity of these findings. Another limitation was the lack of a comparator arm where no incentives were given. Such an arm would have permitted us to observe the impact of incentives not linked to achieving any targets (CTL arm) on uptake of ART; however, our design was intended to provide a conservative estimate of the potential benefit of an incentive intervention because the control condition included nonspecific benefits for participating in a study where random prize drawings were incorporated into study visits.
In conclusion, although this pilot RCT failed to detect differences in viral suppression, it demonstrated that modest voucher incentives played a role in modifying health-seeking behaviors by improving linkage and retention in care (essential first steps in the pathway to viral suppression) among HIV-infected DUs in low- and middle-income countries, a population with high disease burden and low ART uptake. Additional studies will be needed to characterize the steps in the care continuum where incentives may be most beneficial and to develop integrated multifaceted interventions to engage and retain marginalized populations of HIV-infected persons in care.
Notes
Acknowledgments. The authors thank the staff at YR Gaitonde Centre for Substance Abuse Research and YR Gaitonde Centre for AIDS Research and Education who helped with the implementation of this study.
Disclaimer. The funding sources for this study did not play any role in the design, conduct, or analysis of the data.
Financial support. This study was supported in part by the National Institute on Drug Abuse, National Institutes of Health (grant number DA018577).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.El-Sadr WM, Serwadda DM, Sista N, Cohen MS. HIV prevention: great achievements, more challenges ahead. J Acquir Immune Defic Syndr. 2013;63(suppl 2):S115–6. doi: 10.1097/QAI.0b013e318299c3d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fauci AS, Folkers GK. Toward an AIDS-free generation. JAMA. 2012;308:343–4. doi: 10.1001/jama.2012.8142. [DOI] [PubMed] [Google Scholar]
- 3.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52:793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.United Nations Joint Programme on HIV/AIDS. Global report: UNAIDS report on the global AIDS epidemic. Available at: http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2012/gr2012/20121120_UNAIDS_Global_Report_2012_en.pdf. Accessed 10 July 2013.
- 5.United Nations Office on Drugs and Crime. World drug report 2007. Available at: http://www.unodc.org/pdf/research/wdr07/WDR_2007.pdf. Accessed 10 August 2010.
- 6.Solomon SS, Srikrishnan AK, Mehta SH, et al. High prevalence of HIV, HIV/hepatitis C virus coinfection, and risk behaviors among injection drug users in Chennai, India: a cause for concern. J Acquir Immune Defic Syndr. 2008;49:327–32. doi: 10.1097/QAI.0b013e3181831e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solomon SS, Celentano DD, Srikrishnan AK, et al. Low incidences of human immunodeficiency virus and hepatitis C virus infection and declining risk behaviors in a cohort of injection drug users in Chennai, India. Am J Epidemiol. 2010;172:1259–67. doi: 10.1093/aje/kwq288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solomon SS, Celentano DD, Srikrishnan AK, et al. Mortality among injection drug users in Chennai, India (2005–2008) AIDS. 2009;23:997–1004. doi: 10.1097/QAD.0b013e32832a594e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loewenstein G, Brennan T, Volpp KG. Asymmetric paternalism to improve health behaviors. JAMA. 2007;298:2415–7. doi: 10.1001/jama.298.20.2415. [DOI] [PubMed] [Google Scholar]
- 10.Hser YI, Li J, Jiang H, et al. Effects of a randomized contingency management intervention on opiate abstinence and retention in methadone maintenance treatment in China. Addiction. 2011;106:1801–9. doi: 10.1111/j.1360-0443.2011.03490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volpp KG, Troxel AB, Pauly MV, et al. A randomized, controlled trial of financial incentives for smoking cessation. N Engl J Med. 2009;360:699–709. doi: 10.1056/NEJMsa0806819. [DOI] [PubMed] [Google Scholar]
- 12.Kullgren JT, Troxel AB, Loewenstein G, et al. Individual- versus group-based financial incentives for weight loss: a randomized, controlled trial. Ann Intern Med. 2013;158:505–14. doi: 10.7326/0003-4819-158-7-201304020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thornton RL. The demand for, and impact of, learning HIV status. Am Econ Rev. 2008;98:1829–63. doi: 10.1257/aer.98.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rigsby MO, Rosen MI, Beauvais JE, et al. Cue-dose training with monetary reinforcement: pilot study of an antiretroviral adherence intervention. J Gen Intern Med. 2000;15:841–7. doi: 10.1046/j.1525-1497.2000.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosen MI, Dieckhaus K, McMahon TJ, et al. Improved adherence with contingency management. AIDS Patient Care STDS. 2007;21:30–40. doi: 10.1089/apc.2006.0028. [DOI] [PubMed] [Google Scholar]
- 16.Sorensen JL, Haug NA, Delucchi KL, et al. Voucher reinforcement improves medication adherence in HIV-positive methadone patients: a randomized trial. Drug Alcohol Depend. 2007;88:54–63. doi: 10.1016/j.drugalcdep.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solomon SS, Hawcroft CS, Narasimhan P, et al. Comorbidities among HIV-infected injection drug users in Chennai, India. Indian J Med Res. 2008;127:447–52. [PMC free article] [PubMed] [Google Scholar]
- 18.Pujari S, Patel A, Joshi SR, et al. Guidelines for use of antiretroviral therapy for HIV infected individuals in India (ART guidelines 2008) J Assoc Phys India. 2008;56:339–48. 353–71. [PubMed] [Google Scholar]
- 19.Solomon SS, Desai M, Srikrishnan AK, et al. The profile of injection drug users in Chennai, India: identification of risk behaviours and implications for interventions. Subst Use Misuse. 2010;45:354–67. doi: 10.3109/10826080903452447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucas GM, Cheever LW, Chaisson RE, Moore RD. Detrimental effects of continued illicit drug use on the treatment of HIV-1 infection. J Acquir Immune Defic Syndr. 2001;27:251–9. doi: 10.1097/00126334-200107010-00006. [DOI] [PubMed] [Google Scholar]
- 21.Baird S, Chirwa E, McIntosh C, Ozler B. The short-term impacts of a schooling conditional cash transfer program on the sexual behavior of young women. Health Econ. 2010;19(suppl):55–68. doi: 10.1002/hec.1569. [DOI] [PubMed] [Google Scholar]
- 22.Javanbakht M, Prosser P, Grimes T, Weinstein M, Farthing C. Efficacy of an individualized adherence support program with contingent reinforcement among nonadherent HIV-positive patients: results from a randomized trial. J Int Assoc Physicians AIDS Care (Chic) 2006;5:143–50. doi: 10.1177/1545109706291706. [DOI] [PubMed] [Google Scholar]
- 23.HIV Prevention Trials Network. HPTN 065 (TLC-PLUS): a study to evalauate the feasibility of an enhanced test, link to care, plus treat approach for HIV prevention in the United States. Available at: http://www.hptn.org/research_studies/hptn065.asp. Accessed 10 July 2013. [DOI] [PMC free article] [PubMed]
- 24.Clinical Trials Network. Protocol NIDA-CTN-0049 (Project Hope): hospital visit as opportunity for prevention and engagement for HIV-infected drug users. Available at: http://ctndisseminationlibrary.org/protocols/ctn0049.htm. Accessed 10 July 2013.
- 25.Gardner EM, Hullsiek KH, Telzak EE, et al. Antiretroviral medication adherence and class- specific resistance in a large prospective clinical trial. AIDS. 2010;24:395–403. doi: 10.1097/qad.0b013e328335cd8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stitzer M, Petry N. Contingency management for treatment of substance abuse. Annu Rev Clin Psychol. 2006;2:411–34. doi: 10.1146/annurev.clinpsy.2.022305.095219. [DOI] [PubMed] [Google Scholar]
- 27.Balakrishnan P, Kumarasamy N, Kantor R, et al. HIV type 1 genotypic variation in an antiretroviral treatment-naive population in southern India. AIDS Res Hum Retroviruses. 2005;21:301–5. doi: 10.1089/aid.2005.21.301. [DOI] [PubMed] [Google Scholar]