Abstract
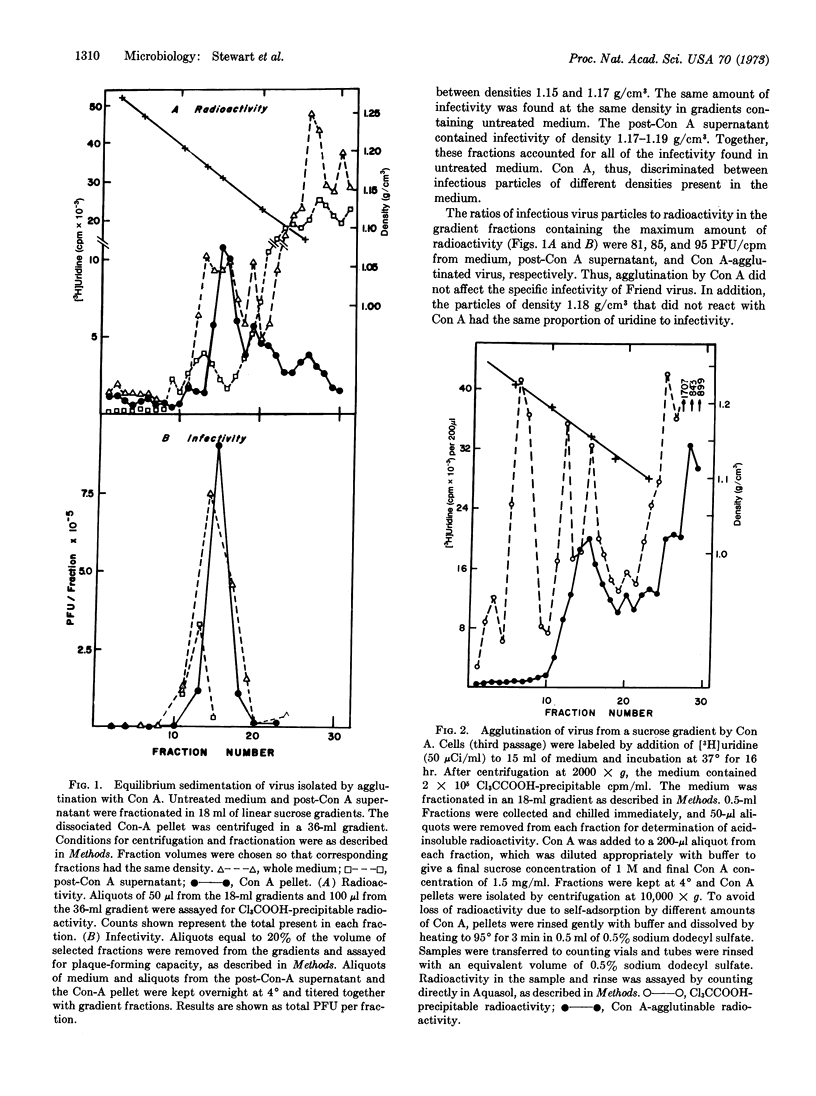
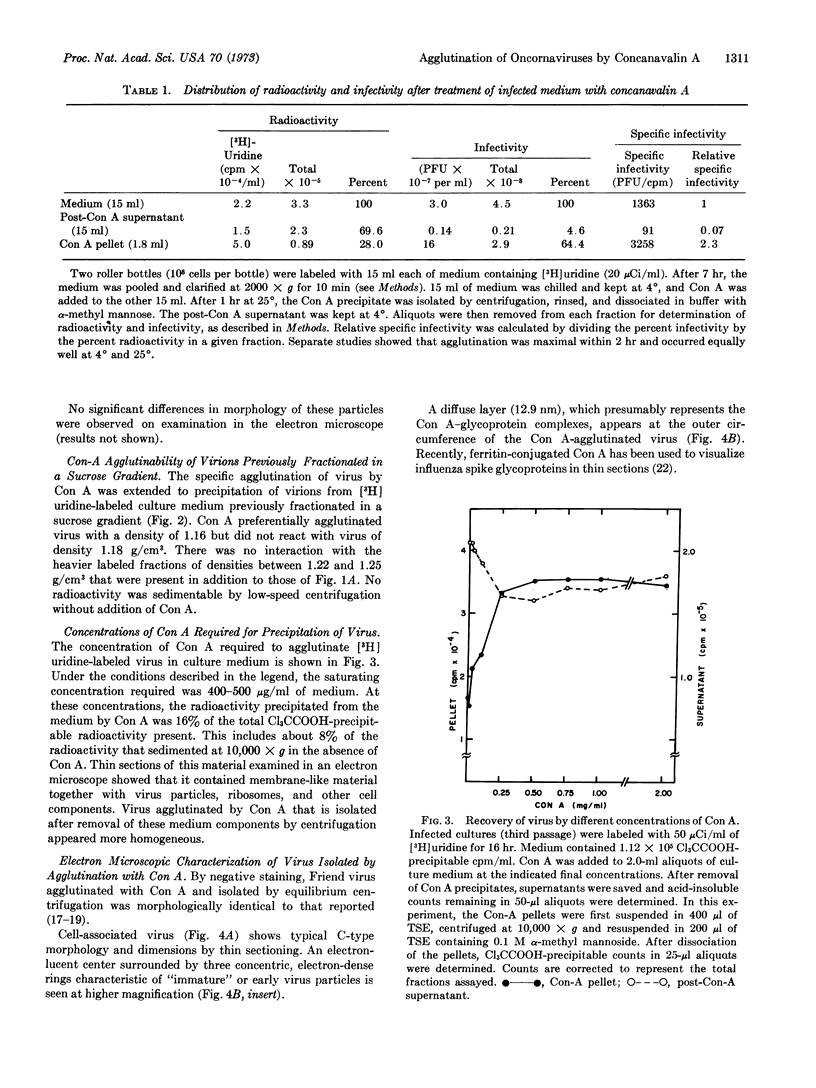
Concanavalin A (Con A) has been used to rapidly and selectively agglutinate murine and avian oncornavirions from culture medium or plasma. The agglutinated virus was concentrated rapidly and gently by low-speed centrifugation and solubilization with α-methyl mannoside. Infectious virus was purified 2.3 times with respect to nucleic-acid content, and more than 60% of its infectivity was recovered. Infectious particles of densities 1.18 and 1.16 g/cm3 were found in mouse cells infected with Friend virus. Con A reacted only with particles of density 1.16 g/cm3, indicating heterogeneity with respect to carbohydrate content or structure as well as buoyant density. Electron microscopy of virus agglutinated with Con A showed a zone of Con A-glycoprotein complexes averaging 12-15 nm in thickness.
Keywords: murine leukemia virus, phytohemagglutinin, Friend virus
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassin R. H., Tuttle N., Fischinger P. J. Rapid cell culture assay technic for murine leukaemia viruses. Nature. 1971 Feb 19;229(5286):564–566. doi: 10.1038/229564b0. [DOI] [PubMed] [Google Scholar]
- Becht H., Rott R., Klenk H. D. Effect of Concanavalin A on cells infected with enveloped RNA viruses. J Gen Virol. 1972 Jan;14(1):1–8. doi: 10.1099/0022-1317-14-1-1. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P., Bauer H., Gelderblom H., Hüper G. Polypeptides of avian RNA tumor viruses. IV. Components of the viral envelope. Virology. 1972 Mar;47(3):551–566. doi: 10.1016/0042-6822(72)90545-4. [DOI] [PubMed] [Google Scholar]
- De-Thé G., O'Connor T. E. Structure of a murine leukemia virus after disruption with tween-ether and comparison with two myxoviruses. Virology. 1966 Apr;28(4):713–728. doi: 10.1016/0042-6822(66)90256-x. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Martin G. S., Vogt P. K. Glycoprotein components of avian and murine RNA tumor viruses. Virology. 1970 Aug;41(4):631–646. doi: 10.1016/0042-6822(70)90428-9. [DOI] [PubMed] [Google Scholar]
- Eagle H. Buffer combinations for mammalian cell culture. Science. 1971 Oct 29;174(4008):500–503. doi: 10.1126/science.174.4008.500. [DOI] [PubMed] [Google Scholar]
- Feller U., Dougherty R. M., Di Stefano H. S. Comparative morphology of avian and murine leukemia viruses. J Natl Cancer Inst. 1971 Dec;47(6):1289–1298. [PubMed] [Google Scholar]
- Fleissner E. Chromatographic separation and antigenic analysis of proteins of the oncornaviruses. I. Avian leukemia-sarcoma viruses. J Virol. 1971 Nov;8(5):778–785. doi: 10.1128/jvi.8.5.778-785.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDSTEIN I. J., HOLLERMAN C. E., MERRICK J. M. PROTEIN-CARBOHYDRATE INTERACTION. I. THE INTERACTION OF POLYSACCHARIDES WITH CONCANAVALIN A. Biochim Biophys Acta. 1965 Jan 4;97:68–76. doi: 10.1016/0304-4165(65)90270-9. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P., Huebner R. J. Host-range restrictions of murine leukemia viruses in mouse embryo cell cultures. J Virol. 1970 Feb;5(2):221–225. doi: 10.1128/jvi.5.2.221-225.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein P. A., Adams W. R. Location of ferritin-labeled concanavalin A binding to influenza virus and tumor cell surfaces. J Virol. 1972 Oct;10(4):844–854. doi: 10.1128/jvi.10.4.844-854.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEIF R. C., VINOGRAD J. THE DISTRIBUTION OF BUOYANT DENSITY OF HUMAN ERYTHROCYTES IN BOVINE ALBUMIN SOLUTIONS. Proc Natl Acad Sci U S A. 1964 Mar;51:520–528. doi: 10.1073/pnas.51.3.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luftig R. B., Kilham S. S. An electron microscope study of Rauscher leukemia virus. Virology. 1971 Nov;46(2):277–297. doi: 10.1016/0042-6822(71)90030-4. [DOI] [PubMed] [Google Scholar]
- Nermut M. V., Frank H., Schäfer W. Properties of mouse leukemia viruses. 3. Electron microscopic appearance as revealed after conventional preparation techniques as well as freeze-drying and freeze-etching. Virology. 1972 Aug;49(2):345–358. doi: 10.1016/0042-6822(72)90487-4. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Fleissner E., Sarkar N. H., Aoki T. Chromatographic separation and antigenic analysis of proteins of the oncornaviruses. II. Mammalian leukemia-sarcoma viruses. J Virol. 1972 Feb;9(2):359–366. doi: 10.1128/jvi.9.2.359-366.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowinski R. C., Old L. J., Sarkar N. H., Moore D. H. Common properties of the oncogenic RNA viruses (oncornaviruses). Virology. 1970 Dec;42(4):1152–1157. doi: 10.1016/0042-6822(70)90367-3. [DOI] [PubMed] [Google Scholar]
- Okada Y., Kim J. Interaction of concanavalin A with enveloped viruses and host cells. Virology. 1972 Nov;50(2):507–515. doi: 10.1016/0042-6822(72)90401-1. [DOI] [PubMed] [Google Scholar]
- Oram J. D., Ellwood D. C., Appleyard G., Stanley J. L. Agglutination of an arbovirus by concanavalin A. Nat New Biol. 1971 Sep 8;233(36):50–51. doi: 10.1038/newbio233050a0. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- SABATINI D. D., BENSCH K., BARRNETT R. J. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol. 1963 Apr;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar N. H., Nowinski R. C., Moore D. H. Helical nucleocapsid structure of the oncogenic ribonucleic acid viruses (oncornaviruses). J Virol. 1971 Oct;8(4):564–572. doi: 10.1128/jvi.8.4.564-572.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer W., Fischinger P. J., Lange J., Pister L. Properties of mouse leukemia viruses. I. Characterization of various antisera and serological identification of viral components. Virology. 1972 Jan;47(1):197–209. doi: 10.1016/0042-6822(72)90252-8. [DOI] [PubMed] [Google Scholar]
- Sumner J. B., Howell S. F. Identification of Hemagglutinin of Jack Bean with Concanavalin A. J Bacteriol. 1936 Aug;32(2):227–237. doi: 10.1128/jb.32.2.227-237.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine R. C., Shapiro B. M., Stadtman E. R. Regulation of glutamine synthetase. XII. Electron microscopy of the enzyme from Escherichia coli. Biochemistry. 1968 Jun;7(6):2143–2152. doi: 10.1021/bi00846a017. [DOI] [PubMed] [Google Scholar]




