Highlights
-
•
This manuscript raises the issue of optimal treatment of isolated visceral metastases in thyroid cancer.
-
•
This patient was treated unsuccessfully with multiple bouts of radioiodine before definite successful surgery to resect the metastasis.
-
•
This manuscript makes physicians aware that surgery is a viable option in oligometastases of thyroid cancer.
Keywords: Thyroid cancer, Liver metastases, Liver surgery, Rare thyroid metastases
Abstract
Introduction
Papillary (PTC) and follicular (FTC) thyroid carcinomas, together known as differentiated thyroid carcinomas (DTC), are among the most curable of cancers. Sites of metastases from FTC are usually osseous and those from PTC are in regional nodal basins and the lungs. Visceral metastases are rare and when they do occur, they tend do so in multiple sites. We present the case of a patient with a follicular variant of PTC and a solitary metastasis to the liver then review the relevant literature.
Presentation of case
An otherwise healthy 68-year-old woman was diagnosed with follicular variant papillary thyroid cancer in 2003 and subsequently underwent thyroidectomy. The patient’s endocrinologist conducted surveillance of her thyroid cancer. In 2012, due to rise in thyroglobulin, a whole body radioiodine scan was obtained which revealed an iodine-avid left liver lobe mass. Three cycles of radioiodine ablation therapy were unsuccessful and eventually the patient was referred for surgical resection. Metastatic evaluation including a PET scan was negative with the exception of an isolated enhancing 4 cm mass in segment 4B of the liver. Anatomic segmental resection of liver was performed without complications. Intraoperative ultrasonography was used to guide resection of the liver mass. Pathology reports confirmed metastatic follicular variant of PTC. Surgical margins were free of tumor. Patient was discharged home and is doing well one year after surgery. The latest thyroglobulin level was undetectable.
Discussion
Post-operative surveillance by PCP, endocrinologist or surgeon for patients with thyroid carcinoma should be performed routinely. If identified, a solitary liver metastasis from primary thyroid carcinoma should be considered for surgical resection. Due to sparse data available in literature, collecting more data to establish algorithms for treatment of such rare metastatic cancers may be able to aid physicians to achieve better outcomes.
Conclusion
Rare distant sites of metastases from DTC include eyes, pharynx, skin, muscle, ovaries, adrenal glands, kidneys, esophagus, pancreas and liver. Isolated, resectable liver metastases from PTC are exceedingly rare. Literature review revealed only 10 reported cases of liver metastases from DTC. As in our patient, solitary liver metastasis from PTC should be considered for surgical resection which offers the best chance for prolonged survival.
1. Introduction
Papillary and follicular thyroid cancers, together, are referred to as differentiated thyroid cancer (DTC) [1]. Differentiated thyroid carcinomas are relatively rare despite common incidence of thyroid nodules [2]. Furthermore, thyroid carcinomas constitute less than 1% of all human cancers. The annual incidence world-wide ranges from 0.5 to 10 cases per 100,000 population [1]. The median age at diagnosis is 45–50 years with two to four times more frequent in women than men [1]. Fortunately, both papillary and follicular (differentiated) thyroid carcinomas are among the most curable cancers. However, some patients are at higher risk for recurrent disease or even death depending on the age at diagnosis, stage, capsular involvement, nodal involvement, size and histological type. Several factors influence pathogenesis of these cancers. Previous studies report a high frequency (70%) of activating somatic alterations of genes encoding effectors in the mitogen-activated protein kinase (MAPK) signaling pathway, including point mutations of BRAF and the RAS genes [14–18]. Rearrangements of the tyrosine kinase domains of the RET and TRK genes with the amino-terminal sequence of an unlinked gene are found in some papillary carcinomas [1]. Additionally, activating point mutations of the RAS genes are found with a similarly high frequency in thyroid adenomas and follicular carcinomas, suggesting that RAS mutations represent an early event in thyroid tumorigenesis [1]. Also, activating mutations of the genes encoding the thyrotropin receptor and the α subunit of the stimulatory G (Gs) protein have been reported in some follicular carcinomas [1,3]. And inactivating point mutations of the p53 tumor-suppressor gene are rare in patients with differentiated thyroid carcinomas, but common in those with undifferentiated (anaplastic) thyroid carcinomas [3]. From an environmental stand point, external irradiation to the neck during childhood increases the risk of papillary thyroid carcinoma. A major risk factor is a young age at the time of irradiation; after the age of 15 or 20 years, the risk is not increased [1]. Lastly, in countries where iodine intake is adequate, differentiated cancers account for more than 80% of all thyroid carcinomas, with the papillary histologic type being the more frequent (accounting for 60–80% of cases) [1]. There is no increase in the incidence of thyroid carcinomas in countries where iodine intake is low, but there is a relative increase in follicular and anaplastic carcinomas [2,3]. From a genetic perspective, in non-sporadic cases, a higher incidence of papillary carcinomas has been reported in patients with adenomatous polyposis coli and Cowden’s disease (the multiple hamartoma syndrome) [3]. About 3% of cases of papillary carcinoma are familial. In this case report, the primary focus will be papillary carcinoma which is an unencapsulated tumor with papillary and follicular structures that is characterized by overlapping nuclei that have a ground-glass appearance and longitudinal grooves, with invaginations of cytoplasm [1–3]. Encapsulated, follicular, tall-cell, columnar-cell, clear-cell, and diffuse sclerosing carcinomas are recognized histologic variants; they are classified as papillary carcinomas because of their characteristic nuclear features.
Isolated metastasis to the liver from thyroid cancer is a rare event with a reported frequency of less than 0.5% [3]. Metastatic liver involvement from differentiated thyroid cancer, both follicular and papillary, is nearly always multiple or diffuse and usually found along with other distant metastases including the lungs, bones and the brain) [4–12]. Locations with very rare incidence of metastases include eyes, pharynx, skin, muscle, ovaries, adrenal glands, kidneys, esophagus, pancreas and liver metastasis. A review of the literature revealed that only ten cases of all liver metastases from DTC have been documented, with a rate of 0.5% or less. Three patients were men and seven were women. Their average age was 63 years (range from 32 to 85 years). Histologically, the primary tumor was identified as papillary in four patients, follicular in five and Hürthle cell thyroid cancer in one patient [3]. In two cases, the metastatic histological type was inconsistent with the primary tumor. The primary tumors were FTC and PTC, while both their metastatic lesions were a FV-PTC [3]. What makes the case presented here in an interesting one is the fact that thyroid cancer metastases to liver are rare, and even more so is an isolated and resectable solitary liver metastases from thyroid cancer.
2. Methods
This is a retrospective case report and a review of the literature. Our patient’s evaluation and surgical intervention were performed at Scottsdale Healthcare, in Scottsdale, Arizona, USA.
3. Results
The patient is an otherwise healthy 68-year-old woman with a history of follicular variant papillary thyroid cancer diagnosed in 2003 and breast cancer diagnosed in 2005. Patient had no cardiac, pulmonary or renal diseases. Patient had no history of diabetes or hypertension. In 2003, the patient had a total thyroidectomy for thyroid cancer. In 2005, she had a modified radical mastectomy for stage I breast cancer and did not receive radiation therapy or chemotherapy. The patient was under the care of an endocrinologist for surveillance of her thyroid cancer. In 2012, a whole body radioiodine scan done to monitor the patient for thyroid cancer recurrence or metastases revealed a radioiodine-avid mass in the medial left lobe of the liver, presumably a solitary hematogenous metastasis. Patient then underwent three separate bouts of radioiodine ablation therapy for a total of 425 mCi. The patient received ablation post RAI ablation after her original thyroid surgery and a subsequent RAI treatment due to persistent low level thyroglobulin elevation. Her thyroglobulin level remained undetectable until the current illness, during which it rose dramatically. Accordingly, a third RAI therapy was administered and the post-RAI scan revealed only the very radioiodine avid lesion in the liver. Because this was so intense, isolated and could possibly have obscured imaging of other foci of metastatic tumor, a PET CT scan was performed, that revealed only the single liver metastasis. A fourth 131I dose was administered and while it resulted in a reduced the thyroglobulin level, the solitary lesion in the liver remained. Consultations with medical oncology, radiation oncology, interventional radiology and surgery obtained with the resultant consensus that the best option was surgical resection Fig. 1. The patient underwent an anatomic segment 4B liver resection for a 4 cm isolated tumor mass without complications. Intraoperative ultrasonography was used to assess the liver mass (Fig. 2). Fig. 3(a–d) describes the pathological findings of the metastasis. The gross hepatic metastasis seen intraopratively is shown in Fig 3a. The hepatic resection specimen (Fig. 3b) showed a bulging ovoid subserosal nodule measuring 4.3 cm in greatest dimension. The mass was well-demarcated from the surrounding hepatic tissue and showed a variegated tan to red–brown cut surface with punctate areas of yellow discoloration. Microscopic evaluation of routine hematoxylin and eosin-stained sections showed a neoplasm with a follicular architecture consistent with metastatic thyroid carcinoma (Fig 3c). Tumor nuclei were enlarged and frequently showed irregular contours. Chromatin was fine and, in some cells, inconspicuous. Many cells showed nuclear grooves. Nuclear inclusions were not identified. The microscopic features were consistent with metastatic well-differentiated thyroid carcinoma and in particular, appeared most consistent with the follicular variant of papillary thyroid carcinoma. Immunohistochemical stains showed expression of TTF-1 (Fig. 3d) and thyroglobulin (not shown), supporting the diagnosis of metastatic thyroid carcinoma. Surgical margins were free of involvement with tumor. Patient was discharged home and has been doing well for 12 months after her operation. At this last clinic visit, her TSH-stimulated thyroglobulin and thryglobulin antibodies levels remain undetectable. The prognostic significance of the presence of RAS or BRAF mutations remains controversial so testing was not done. Presently, the authors would perform mutational analysis of BRAF as an adjunct to either diagnose or confirm PTC on equivocal FNA and if a patient has unresectable metastases that are refractory-to-radioiodine in order to discern whether or not the treatment with a BRAF inhibitor would be indicated.
Fig. 1.
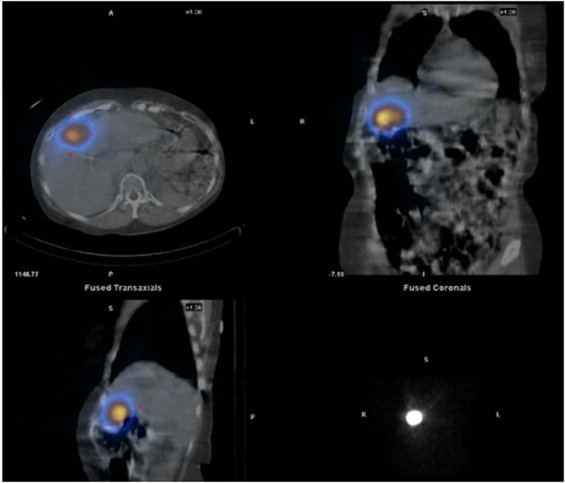
Positron emission tomography (PET) scan demonstrates an isolated focus of FDG uptake in the liver indicating an isolated metastatic focus of tumor.
Fig. 2.
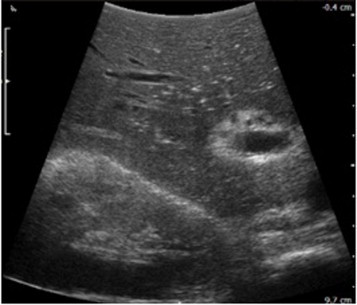
Intraoperative ultrasonography was used to assess the liver mass.
Fig. 3.
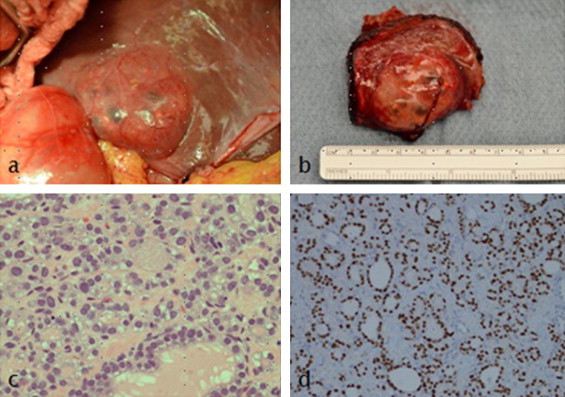
(a–d) Describes the pathological findings of the metastasis.
The gross appearance of the hepatic metastasis seen intraopratively is shown in Fig. 3a.
The hepatic resection specimen (Fig. 3b) showed a bulging ovoid subserosal nodule, with in the liver, measuring 4.3 cm in greatest dimension.
Microscopic evaluation of routine hematoxylin and eosin-stained sections showed a neoplasm with a follicular architecture (Fig. 3c). The cells were cuboidal with pleomorphic nuclei which frequently showed deep indentations or grooves. Nuclear chromatin was fine and nucleoli were inconspicuous. Intranuclear pseudoinclusions were not seen. Within the follicles was homogeneous eosinophilic material. The morphologic features were highly suggestive of metastatic papillary thyroid carcinoma (follicular variant). This impression was confirmed by immunohistochemical stains showing nuclear staining for TTF-1 (Fig. 3d) and cytoplasmic positivity for thyroglobulin (not shown).
4. Discussion
Liver metastasis from differentiated thyroid cancer are quite rare, with a reported frequency of 0.5% [3,13]. Even more unusual are isolated resectable liver metastases from papillary thyroid cancer. The patient presented herein, fits well the typical epidemiological descriptors of previously reported cases: our patient was female, 68 years old, and had a follicular variant of papillary thyroid carcinoma. As noted in a recent study in 2011 by Hong-Jun Song et al. “…only ten cases have been documented in the literature; three were males and seven were females, with an average age of about 63 years (range from 32 to 85 years). Histologically, the primary tumor was identified as papillary in four patients, follicular in five patients, and Hürthle cell thyroid cancer in one patient.” Post-operative surveillance by PCP, endocrinologist or surgeon for patients with thyroid carcinoma should be complete and include appropriate imaging modalities when metastasis is suspected. PET, CT and ultrasonography remain the standard imaging options. Once identified, a solitary liver metastasis from primary thyroid carcinoma should be considered for surgical resection in the appropriate clinical scenario. In addition, a compilation of the reported cases of solitary visceral metastasis from thyroid carcinoma should be studied and evaluated for success of surgical resection, prognosis, rate of survival, and size of metastatic lesions in each histological type of thyroid carcinoma. Collecting more data to establish algorithms for treatment of such rare cancers may be able to aid physicians to better utilize diagnostic tests, surveillance and ultimately to provide more definitive care for those who suffer from rare diseases.
Conflicts of interest
No conflicts.
Funding
No relevant funding.
Author contribution
Dr Brano Djenic was the primary author and did data collection.
Dr Jim Newell did the analysis of pathology and figures. He also reviewed and approved the manuscript.
Dr Dan Duick treated patient and provided historical clinical information and followup. He reviewed and did critical editorial review.
Dr Demeure was the primary surgeon, wrote much of the manuscript and provided critical review of data and manuscript.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
References
- 1.Schlumberger M.J. Papillary and follicular thyroid carcinoma. N. Engl. J. Med. 1998;338:297–306. doi: 10.1056/NEJM199801293380506. [DOI] [PubMed] [Google Scholar]
- 2.Keeston Jones M. Management of papillary and follicular thyroid cancer. J. R. Soc. Med. 2002;95(July (7)):325–326. doi: 10.1258/jrsm.95.7.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song H.J., Xue Y.L., Qiu Z.L. Uncommon metastases from differentiated thyroid carcinoma. Hell. J. Nucl. Med. 2012;15(3):233–240. doi: 10.1967/s002449910059. [DOI] [PubMed] [Google Scholar]
- 4.Bakheet S.M., Powe J., Hammami M.M. Isolated porta hepatis metastasis of papillary thyroid cancer. J. Nucl. Med. 1996;37:993–994. [PubMed] [Google Scholar]
- 5.Tur G.E., Asanuma Y., Sato T. Resection of metastatic thyroid carcinomas to the liver and the kidney: report of a case. Surg. Today. 1994;24:844–848. doi: 10.1007/BF01636320. [DOI] [PubMed] [Google Scholar]
- 6.Kraft O. Hepatic metastasis of differentiated thyroid carcinoma. Nucl. Med. Rev. Cent. East Eur. 2005;8:44–46. [PubMed] [Google Scholar]
- 7.Malhotra G., Upadhye T.S., Sridhar E. Unusual case of adrenal and renal metastases from papillary carcinoma of thyroid. Clin. Nucl. Med. 2010;35:731–736. doi: 10.1097/RLU.0b013e3181ea342b. [DOI] [PubMed] [Google Scholar]
- 8.Niederle B., Roka R., Schemper M. Surgical treatment of distant metastases in differentiated thyroid cancer: indication and results. Surgery. 1986;100:1088–1097. [PubMed] [Google Scholar]
- 9.Young R.H., Jackson A., Wells M. Ovarian metastasis from thyroid carcinoma 12 years after partial thyroidectomy mimicking struma ovarii: report of a case. Int. J. Gynecol. Pathol. 1994;13:181–185. doi: 10.1097/00004347-199404000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Guglielmi R., Pacella C.M., Dottorini M.E. Severe thyrotoxicosis due to hyperfunctioning liver metastasis from follicular carcinoma: treatment with 131I and interstitial laser ablation. Thyroid. 1999;9:173–177. doi: 10.1089/thy.1999.9.173. [DOI] [PubMed] [Google Scholar]
- 11.Kondo T., Katoh R., Omata K. Incidentally detected liver metastasis of well-differentiated follicular carcinoma of the thyroid, mimicking ectopic thyroid. Pathol. Int. 2000;50:509–513. doi: 10.1046/j.1440-1827.2000.01065.x. [DOI] [PubMed] [Google Scholar]
- 12.Kelessis N.G., Prassas E.P., Dascalopoulou D.V. Unusual metastatic spread of follicular thyroid carcinoma: report of a case. Surg. Today. 2005;35:300–303. doi: 10.1007/s00595-004-2922-2. [DOI] [PubMed] [Google Scholar]
- 13.Salvatori M., Perotti G., Rufini V. Solitary liver metastasis from Hürthle cell thyroid cancer: a case report and review of the literature. J. Endocrinol. Invest. 2004;27(January (1)):52–56. doi: 10.1007/BF03350911. [DOI] [PubMed] [Google Scholar]
- 14.Cohen Y., Xing M., Mambo E., Guo Z., Wu G., Trink B., Beller U., Westra W.H., Ladenson P.W., Sidransky D. BRAF mutation in papillary thyroid carcinoma. J. Natl. Cancer Inst. 2003;95:625–627. doi: 10.1093/jnci/95.8.625. [DOI] [PubMed] [Google Scholar]
- 15.Kimura E.T., Nikiforova M.N., Zhu Z., Knauf J.A., Nikiforov Y.E., Fagin J.A. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC–RAS–BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003;63:1454–1457. [PubMed] [Google Scholar]
- 16.Lemoine N.R., Mayall E.S., Wyllie F.S., Farr C.J., Hughes D., Padua R.A., Thurston V., Williams E.D., Wynford-Thomas D. Activated ras oncogenes in human thyroid cancers. Cancer Res. 1988;48:4459–4463. [PubMed] [Google Scholar]


