Highlights
-
•
A 19-year-old patient diagnosed with a rare neuroendocrine tumor is presented.
-
•
Innovative intraoperative imaging of the tumor was performed.
-
•
NIR fluorescence imaging identified an otherwise undetectable lesion during surgery.
-
•
This technique could result in more complete resections in paraganglioma surgery.
Keywords: Image-guided surgery, Near-infrared fluorescence imaging, Neuroendocrine tumor, Paraganglioma, Methylene blue
Abstract
Introduction
Intraoperative identification of tumors can be challenging. Near-infrared (NIR) fluorescence imaging is an innovative technique that can assist in intraoperative identification of tumors, which may otherwise be undetectable.
Presentation of case
A 19-year-old patient with symptoms, normetanephrine levels and radiological findings suspicious for a paraganglioma, a rare tumor arising from extra-adrenal chromaffin cells within the sympathetic nervous system, is presented. Intraoperative NIR fluorescence imaging using intravenous administration of methylene blue (MB) assisted in intraoperative detection of the tumor, and even identified a smaller second lesion, which was not identified during surgery by visual inspection.
Discussion
Although the exact mechanism of MB accumulation in neuroendocrine tumors is unclear, it is described in both preclinical and clinical studies.
Conclusion
In this report, we describe the first case of intraoperative NIR fluorescence imaging of a paraganglioma using MB, which identified an otherwise undetectable lesion.
1. Introduction
Paragangliomas are rare tumors arising from extra-adrenal chromaffin cells within the sympathetic nervous system [1–3]. They can be found anywhere from the neck to the pelvis in the vicinity of sympathetic ganglions, and belong to the family of neuroendocrine tumors. An incidence rate of 2–8 cases per million is reported, and tumors can be either functional or non-functional. When functional there can be production of catecholamine, epinephrine, or norepinephrine.
The only curative treatment of paraganglioma is complete surgical resection. To help localize all sites of disease, preoperative identification can be performed using different anatomical and functional imaging modalities [4]. However, no specific imaging modalities are available for the intraoperative identification of these tumors.
Near-infrared (NIR) fluorescence imaging is a promising technique to assist in the intraoperative identification of sentinel lymph nodes, tumors, and vital structures [5]. Advantages of this optical imaging technique include real-time imaging, high sensitivity, and high resolution, with penetration depths several millimeters into tissue.
Methylene blue (MB) is a clinically available tracer, which can be used at low dosage as a fluorescent tracer during NIR fluorescence imaging. Winer et al. [6] describe the successful identification of insulinomas, a pancreatic neuroendocrine tumor in transgenic mice, and clinically, the intraoperative identification of parathyroid adenomas was described [7].
Based on these results, we explored the possibility to identify paragangliomas during surgery, using NIR fluorescence imaging and MB.
2. Presentation of case
A 19-year-old female patient presented with complaints of fainting, nausea, vomiting, headaches, excessive sweating, flushing attacks, hand tremors, and heart palpitations. She had a negative family history for endocrine diseases or paragangliomas. While volunteering to donate blood, her systolic blood pressure was found to be 180 mm Hg. After referral, physical examination revealed a blood pressure of 180/110 mm Hg on the left arm, 190/115 mm Hg on the right arm, but no other abnormalities. Laboratory evaluation showed a normetanephrine level of 3227 umol/mol creatinine (norm. value 64–260) in the urine.
CT scan showed a 46 × 38 mm retroperitoneal mass, just caudal to the aortic bifurcation (Fig. 1). Cranial to this, a smaller lesion of 6 mm was seen, which was characterized as a para-aortic lymph node. MRI scan confirmed the retroperitoneal mass just caudal to the aortic bifurcation, with features suspicious for a paraganglioma. A slight encasement of the common iliac vein was seen. The cranially located smaller lesion was not clearly identified by MRI.
Fig. 1.
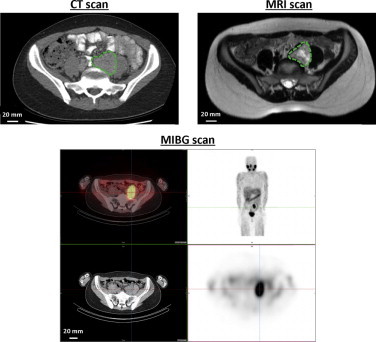
Preoperative imaging of paraganglioma: Preoperative CT, T2 weighed MRI and MIBG scan of the paraganglioma (dashed circle), retroperitoneally located, just caudal to the aortic bifurcation.
A 123I-metaiodobenzylguanidine (MIBG) scan was performed to search for lesions located elsewhere in the body. Strong accumulation of the tracer was seen in the lesion just caudal to the aortic bifurcation, but no other uptake was seen (Fig. 1).
The patient was scheduled for resection. She was successfully pretreated with doxazosine, a selective alpha1-receptor blocking sympatholytic drug, metoprolol, a beta-receptor blocking sympatholytic drug, and nifedipine, a dihydropyridine, in order to prevent a hypertensive crisis during surgery.
2.1. Intraoperative NIR fluorescence imaging
The current study was approved by the Medical Ethics Committee of the Leiden University Medical Center and was performed in accordance with the clinical standards of the Helsinki Declaration of 1975. Written informed consent was obtained.
During surgery, and directly after exposure of the lesion distal to the aortic bifurcation, MB was administered intravenously (0.5 mg/kg; 33 mg in 3.3 mL of water; 10 mg/mL final stock solution concentration) over 5 min and NIR fluorescence imaging was performed using the Mini-FLARE image-guided surgery system as described previously [8]. Directly after infusion, imaging was performed at fixed time points (0, 1, 2, 3, 4, 5, 15, 30 and 45 min).
NIR fluorescence imaging showed a strong, but patchy, fluorescent signal corresponding to the suspected lesion (Fig. 2A, lesion indicated by dashed circle). Moreover, the additional small lesion that was only identified by CT but not by MRI or by visual inspection during surgery, was clearly identified using NIR fluorescence imaging, located approximately 5 cm cranial to the main lesion (indicated by arrow). This lesion was otherwise undetectable. Already two minutes after administration, a tumor-to-background ratio (TBR) of approximately 3.5 was reached for both lesions, which slowly decreased over the next 30 min (Fig. 3).
Fig. 2.
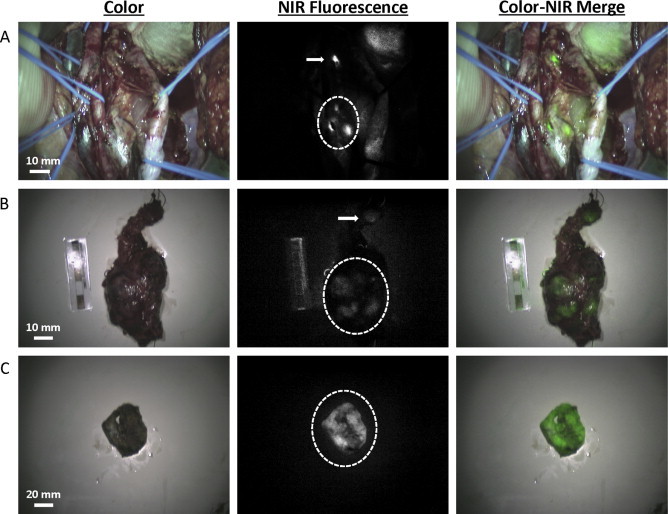
Intraoperative and ex vivo NIR fluorescence imaging of primary and metastatic paragangliomas: (A) Intraoperative NIR fluorescence imaging of the surgical field. A bright, patchy fluorescent signal was identified at the location of the tumor (dashed circle). A second, small, lesion located approximately 5 cm cranial to the main lesion, was also identified using NIR fluorescence imaging (arrow). (B) Ex vivo (T = 45 min) imaging of the resection specimens. Fluorescent signal was seen in the large (dashed circle) and small lesion (arrow). A weaker fluorescent intensity was seen than in vivo, because fluorescent signal decreased over time during surgery. (C) Ex vivo imaging of the bisected main lesion. Bright fluorescent signal was seen throughout the paraganglioma (dashed circle).
Fig. 3.
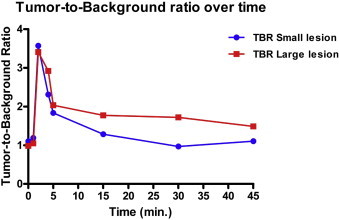
Tumor-to-background ratio of resected lesions: Tumor-to-background ratio of the large main lesion and small second lesion over time after intravenous administration of MB.
After en bloc resection of the 2 lesions, the specimen was sent to pathology for further evaluation. After surgical treatment, symptoms disappeared and normetanephrine levels normalized.
No surgical complication or adverse reactions associated with the use of MB or fluorescence imaging were observed.
2.2. Ex vivo imaging of resected lesion and sliced specimen
Ex vivo imaging of the resected specimens was performed at the Pathology department using the FLARE imaging system, which was previously described [9]. Images showed a fluorescent signal in both lesions (Fig. 2B). Of special note, the intensity of the fluorescent signal decreased significantly over time, so a much weaker fluorescence signal was measured during ex vivo imaging (i.e., T = 45 min after MB injection). The patchy fluorescent signal of the main tumor was seen both in vivo and ex vivo. This was due to fibrous connective tissue lying over the paraganglioma. After bisection of the resected main lesion, a clear fluorescent signal was seen throughout the tumor (Fig. 2C).
2.3. Histopathology
Macroscopically, a nodular lesion was seen, yellow to black on cross section and surrounded by a thin capsule. The maximum diameter was 65 mm. Cranial from this lesion, a smaller lesion of 12 mm was seen, suspicious for a tumor-positive lymph node (Fig. 2B). The excised tissue was fixed in formalin, embedded in paraffin and tissue slides were stained with hematoxylin and eosin (HE). Immunohistochemical staining was also performed (Fig. 4). Based on histopathological findings, both lesions were diagnosed as paraganglioma with diameters of 65 and 12 mm, respectively.
Fig. 4.
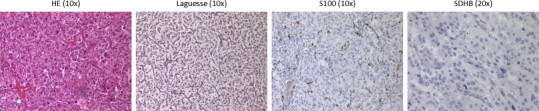
Histopathological staining of resected lesions: hematoxylin and eosin, Laguesse, S100 and SDHB staining of the resected specimen. Microscopy showed a characteristic pattern of cell nests. This pattern was accentuated by a reticulin stain. The cells had a moderate amount of eosinophilic cytoplasm and nuclei with finely clumped chromatin. Dispersed throughout the lesion, hemorrhagic foci were seen. The second, small, cranially located lesion showed the same characteristics as the larger lesion, without the presences of surrounding lymphoid tissue. Additional immunohistochemical staining for S100 showed the presence of sustentacular cells around the nests. SDHB staining was not conclusive.
3. Discussion
Despite many preoperative imaging modalities, intraoperative identification and demarcation of paragangliomas can be challenging. Because complete surgical removal of the tumor is the only curative treatment option, real-time intraoperative imaging modalities which can assist in tumor identification are desperately needed. To the best of our knowledge, this is the first report showing the intraoperative identification of paragangliomas using NIR fluorescence imaging and MB in a clinical setting.
In the presented patient, the main lesion just caudal to the aortic bifurcation was clearly identified by CT, MRI, and MIBG-scan. CT also showed a cranially located smaller lesion, which was not identified by MRI or MIBG, and not recognized as a paraganglioma, probably due to its small size and/or lack of adequate tracer photon flux from small tumors [3,10]. Importantly, intraoperative identification of all tumors is of paramount importance for the complete surgical resection and thereby curative treatment. Although not identified by MRI or MIBG, the second, smaller, lesion was intensely fluorescent and was confirmed histologically to be paraganglioma. In daily practice, there still is a discrepancy between identification of lesions during preoperative imaging and intraoperative recognition of these lesions, especially when they are smaller sized. Intraoperative NIRF imaging could potentially overcome this gap.
The pharmacokinetics of MB in the observed lesions are consistent with a perfusion effect, but not for a retention effect, because TBR decreases rapidly over time after initially high values. This makes the administration of MB for intraoperative identification of paragangliomas very useful for the direct identification of lesions, and apparently local metastases or second primary tumors.
Although the exact mechanism of MB accumulation in tumors and especially neuroendocrine tumors is mainly unclear, it has been described in several studies. Previous clinical studies show staining of neuroendocrine tumors and parathyroid adenomas using MB as a visible dye using high concentrations [11–14] and with NIR fluorescence imaging using low-dose MB [7]. Also in breast cancer, identification of tumors by NIR fluorescence imaging using MB is described [15]. The mechanism behind this accumulation is based on physicochemical similarities between MB and sestamibi, a nuclear tracer used for MIBI-scans. Reports which describe the identification of paragangliomas using MIBI-scan are rare [16,17], but strengthens the hypothesis that MB and sestamibi have a comparable biodistribution. A better understanding of the exact mechanism would be of obvious clinical importance, since this technique could potentially enhance patient cure rates.
4. Conclusion
In conclusion, we describe the successful use of NIR fluorescence imaging for the intraoperative identification of a paraganglioma using MB. This procedure is easy to perform and could result in an increased number of complete resections in paraganglioma surgery. In general, this technique could usher in a new era of optical assistance in the detection of tumors during surgery.
Conflict of interest
Q.R.J.G. Tummers, M.C. Boonstra, C.J.H. van de Velde, A.L. Vahrmeijer and B.A. Bonsing have no conflicts of interest or financial ties to disclose. Dr. J.V. Frangioni: FLARE™ technology is owned by Beth Israel Deaconess Medical Center, a teaching hospital of Harvard Medical School. Dr. Frangioni has started three for-profit companies, Curadel, Curadel ResVet Imaging, and Curadel Surgical Innovations, which has optioned FLARE™ technology for potential licensing from Beth Israel Deaconess Medical Center.
Funding
This study was performed within the framework of CTMM, the Center for Translational Molecular Medicine (MUSIS project, grant 03O-202). This work was supported by the Dutch Cancer Society grant UL2010-4732 and NIH grant R01-CA-115296.
Ethical approval
The current study was approved by the Medical Ethics Committee of the Leiden University Medical Center on the 27th of july 2012. Reference P10.001/NV/nv.
Author contribution
QT designed the study, collected and analysed the clinical data and drafted the manuscript. MB collected the clinical data and drafted the manuscript. JF participated in the design of the study and critical revision of the manuscript. CvdV participated in the design of the study and revised the manuscript. AV performed the surgery, collected the clinical data and revised the manuscript. BB participated in the design of the study, performed the surgery and critically revised the manuscript. All authors read and approved the final manuscript.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Guarantor
Q.R.J.G. Tummers and A.L. Vahrmeijer.
Key Learning Points
-
•
NIR fluorescence imaging using MB can be used for intraoperative identification of paragangliomas.
-
•
NIR fluorescence imaging identified an otherwise undetectable lesion during surgery.
-
•
This technique could result in a more complete resection of paragangliomas.
Acknowledgements
We thank Suzanne Wilhelmus (Pathology department) for her assistance with obtaining the microscopic images and David J. Burrington, Jr. for editing. This study was performed within the framework of CTMM, the Center for Translational Molecular Medicine (MUSIS project, grant 03O-202). This work was supported by the Dutch Cancer Society grant UL2010-4732 and NIH grant R01-CA-115296.
References
- 1.Erickson D., Kudva Y.C., Ebersold M.J., Thompson G.B., Grant C.S., van Heerden J.A. Benign paragangliomas: clinical presentation and treatment outcomes in 236 patients. J. Clin. Endocrinol. Metab. 2001;86(11):5210–5216. doi: 10.1210/jcem.86.11.8034. [DOI] [PubMed] [Google Scholar]
- 2.Lenders J.W., Eisenhofer G., Mannelli M., Pacak K. Phaeochromocytoma. Lancet. 2005;366(9486):665–675. doi: 10.1016/S0140-6736(05)67139-5. [DOI] [PubMed] [Google Scholar]
- 3.Brink I., Hoegerle S., Klisch J., Bley T.A. Imaging of pheochromocytoma and paraganglioma. Fam. Cancer. 2005;4(1):61–68. doi: 10.1007/s10689-004-2155-y. [DOI] [PubMed] [Google Scholar]
- 4.Timmers H.J., Brouwers F.M., Hermus A.R., Sweep F.C., Verhofstad A.A., Verbeek A.L. Metastases but not cardiovascular mortality reduces life expectancy following surgical resection of apparently benign pheochromocytoma. Endocr. Relat. Cancer. 2008;15(4):1127–1133. doi: 10.1677/ERC-08-0049. [DOI] [PubMed] [Google Scholar]
- 5.Vahrmeijer A.L., Hutteman M., van der Vorst J.R., van de Velde C.J., Frangioni J.V. Image-guided cancer surgery using near-infrared fluorescence. Nat. Rev. Clin. Oncol. 2013:507–518. doi: 10.1038/nrclinonc.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winer J.H., Choi H.S., Gibbs-Strauss S.L., Ashitate Y., Colson Y.L., Frangioni J.V. Intraoperative localization of insulinoma and normal pancreas using invisible near-infrared fluorescent light. Ann. Surg. Oncol. 2009:1094–1100. doi: 10.1245/s10434-009-0868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Vorst J.R., Schaafsma B.E., Verbeek F.P., Swijnenburg R.J., Tummers Q.R., Hutteman M. Intraoperative near-infrared fluorescence imaging of parathyroid adenomas with use of low-dose methylene blue. Head Neck. 2013:853–858. doi: 10.1002/hed.23384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mieog J.S., Troyan S.L., Hutteman M., Donohue K.J., van der Vorst J.R., Stockdale A. Towards optimization of imaging system and lymphatic tracer for near-infrared fluorescent sentinel lymph node mapping in breast cancer. Ann. Surg. Oncol. 2011;18:2483–2491. doi: 10.1245/s10434-011-1566-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Troyan S.L., Kianzad V., Gibbs-Strauss S.L., Gioux S., Matsui A., Oketokoun R. The FLARE intraoperative near-infrared fluorescence imaging system: a first-in-human clinical trial in breast cancer sentinel lymph node mapping. Ann. Surg. Oncol. 2009;16(10):2943–2952. doi: 10.1245/s10434-009-0594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campeau R.J., Garcia O.M., Correa O.A., Rege A.B. Pheochromocytoma diagnosis by scintigraphy using iodine 131 metaiodobenzylguanidine. South. Med. J. 1991;84(10):1221–1230. doi: 10.1097/00007611-199110000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Dudley N.E. Methylene blue for rapid identification of the parathyroids. Br. Med. J. 1971;3(5776):680–681. doi: 10.1136/bmj.3.5776.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fedorak I.J., Ko T.C., Gordon D., Flisak M., Prinz R.A. Localization of islet cell tumors of the pancreas: a review of current techniques. Surgery. 1993;113(3):242–249. [PubMed] [Google Scholar]
- 13.Gordon D.L., Airan M.C., Thomas W., Seidman L.H. Parathyroid Identification by methylene blue infusion. Br. J. Surg. 1975;62(9):747–749. doi: 10.1002/bjs.1800620919. [DOI] [PubMed] [Google Scholar]
- 14.Ko T.C., Flisak M., Prinz R.A. Selective intra-arterial methylene blue injection: a novel method of localizing gastrinoma. Gastroenterology. 1992;102(3):1062–1064. doi: 10.1016/0016-5085(92)90199-9. [DOI] [PubMed] [Google Scholar]
- 15.Tummers Q.R., Verbeek F.P., Schaafsma B.E., Boonstra M.C., van der Vorst J.R., Liefers G.J. Real-time intraoperative detection of breast cancer using near-infrared fluorescence imaging and methylene blue. Eur. J. Surg. Oncol. 2014;40(7):850–858. doi: 10.1016/j.ejso.2014.02.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhattacharya A., Mittal B.R., Bhansali A., Radotra B.D., Behera A. Cervical paraganglioma mimicking a parathyroid adenoma on Tc-99m sestamibi scintigraphy. Clin. Nucl. Med. 2006;31(4):234–236. doi: 10.1097/01.rlu.0000205179.73356.9c. [DOI] [PubMed] [Google Scholar]
- 17.Piga M., Farina G.P., Loi G.L., Serra A., Calia M.A., Lai L. Visualisation of a paraganglioma by technetium-99m-sestamibi scintigraphy. J. Endocrinol. Invest. 1999;22(4):296–300. doi: 10.1007/BF03343559. [DOI] [PubMed] [Google Scholar]


