Abstract
The development of advanced scaffolds that recapitulate the anisotropic mechanical behavior and biological functions of the extracellular matrix in leaflets would be transformative for heart valve tissue engineering. In this study, anisotropic mechanical properties were established in poly(ethylene glycol) (PEG) hydrogels by crosslinking stripes of 3.4 kDa PEG diacrylate (PEGDA) within 20 kDa PEGDA base hydrogels using a photolithographic patterning method. Varying the stripe width and spacing resulted in a tensile elastic modulus parallel to the stripes that was 4.1 to 6.8 times greater than that in the perpendicular direction, comparable to the degree of anisotropy between the circumferential and radial orientations in native valve leaflets. Biomimetic PEG-peptide hydrogels were prepared by tethering the cell-adhesive peptide RGDS and incorporating the collagenase-degradable peptide PQ (GGGPQG↓IWGQGK) into the polymer network. The specific amounts of RGDS and PEG-PQ within the resulting hydrogels influenced the elongation, de novo extracellular matrix deposition and hydrogel degradation behavior of encapsulated valvular interstitial cells (VICs). In addition, the morphology and activation of VICs grown atop PEG hydrogels could be modulated by controlling the concentration or micro-patterning profile of PEG-RGDS. These results are promising for the fabrication of PEG-based hydrogels using anatomically and biologically inspired scaffold design features for heart valve tissue engineering.
Keywords: Poly(ethylene glycol), Hydrogel, Heart valve tissue engineering, Anisotropy, Bioactivity
1. Introduction
Heart valve disease is prevalent worldwide, with the number of patients requiring heart valve replacement expected to increase from approximately 290,000 per year worldwide in 2003 to over 850,000 in 2050 [1]. The surgical replacement of diseased heart valves, especially aortic valves, has been widely performed using mechanical valves, bioprosthetic valves, and cryopreserved homograft valves. All these current devices, however, have limitations with risks of further morbidity and mortality. For example, mechanical valves may cause hemorrhage and thromboembolism, and thus require patients to take anticoagulation medication for the rest of their lives [2]. Bioprosthetic valves last only 10-15 years due to the leaflet mineralization and mechanical fatigue [3-5], as well as immunogenic complications. Homografts have some of the same issues as bioprosthetic valves, with the added complication of limited supply. In addition, a common problem with all these devices is their failure to grow and remodel. This limitation is especially problematic for the treatment of congenital heart diseases, which affects approximately 20,000 newborns each year in the US alone [6]. These patients will likely require valve replacement several times during their childhood and adult life. For these reasons, the prospect of a tissue engineered heart valve (TEHV) that can grow and remodel is very appealing [7-9].
Several strategies have been employed to develop TEHVs over the last 2 decades, with the intent that when the implanted engineered valve is functioning in vivo, it will demonstrate the native valve microstructure, tissue mechanics, and cell-matrix interactions. A variety of natural polymers such as collagen [10], fibrin [11] and hyaluronic acid [12], as well as synthetic polymers such as poly(lactic acid) and poly(glycolic acid) copolymers [13], polycaprolactone (PCL) [14], and polyglycolic acid and poly-4-hydroxybutyrate composite [15], have been evaluated as scaffolds for TEHVs. These previous efforts generally focused either on the overall mechanical strength of the scaffolds or on the function of the cells cultured within the scaffold, but not both. However, there is growing interest in fabricating scaffolds that can recapitulate the anisotropic behavior of the leaflets while simultaneously directing cell behavior such as degradation of the polymer scaffold matrix and de novo ECM deposition.
Native aortic valve leaflets, for example, have circumferentially oriented collagen bundles that impart anisotropic mechanical behavior [16], allowing the leaflet to extend radially while withstanding high circumferential stress during approximately 3 billion cycles of opening and closing in an average lifetime [17, 18]. Thus, the anisotropic mechanical property of the leaflets is one essential design feature for TEHV. One aspect of this present study investigated photolithography for the purpose of introducing valve-like anisotropy feature into the TEHV scaffold by mimicking the alignment of the load-bearing collagen bundles.
The leaflets also contain valvular interstitial cells (VICs), which present with two primary phenotypes. One phenotype has quiescent fibroblast characteristics, whereas the other has characteristics of myofibroblasts, staining positively for smooth muscle alpha-actin (α-SMA) and associated with the remodeling of extracellular matrix (ECM). VICs attach to the surrounding ECM at focal adhesions through integrin-receptor binding. The biological function of the scaffold, which plays a key role in the interaction between these resident cells and their surrounding ECM, including both matrix degradation and new ECM deposition, is another essential design feature that must be replicated within a TEHV [19]. Therefore, this study investigated the effect of patterning of integrin ligand RGDS on VIC phenotype and alignment to determine how VIC behavior can be modulated in a way that resembles the native valve.
Structurally and biochemically modified poly(ethylene glycol) (PEG) hydrogels were evaluated to study the effect of valve-inspired scaffold anisotropy and cell binding and alignment. PEG hydrogels are attractive for use as tissue engineering scaffolds because PEG is biocompatible, resists non-specific protein adsorption, and has been approved for internal use by the FDA [20]. The structure, mechanical behavior, and degradability of PEG hydrogels can be tuned by controlling chemistry and processing conditions [21, 22]. Moreover, the biological functions of PEG hydrogels can be modified by incorporation of bioactive molecules such as proteins [23], peptides [24] and polysaccharides [25] into the polymer network. In particular, the incorporation of small peptides derived from natural proteins can enable specific functions such as cell adhesion [26] and matrix degradation [27] and thereby improve cell-matrix interactions within PEG hydrogels.
In this study, mechanical properties and biological functions of modified PEG hydrogels were evaluated in relation to key features that are desired within a TEHV, namely anisotropy, cell adhesion and viability, VIC activation, and production of ECM. We demonstrated that PEG hydrogels can be modified according to valve-inspired design features to generate scaffolds that are suitable for the in vitro development of TEHVs.
2. Materials and Methods
2.1. PEGDA synthesis
Photocrosslinkable poly(ethylene glycol) diacrylate (PEGDA) was prepared as previously described [28]. Briefly, PEG powder (Sigma-Aldrich, St. Louis, MO) was dissolved in anhydrous dichloromethane (Sigma-Aldrich, St. Louis, MO) in a round bottom flask. One molar excess triethylamine (TEA, Sigma-Aldrich, St. Louis, MO) followed by three molar excess acryloyl chloride (Sigma-Aldrich, St. Louis, MO) were then slowly mixed into the PEG solution. The reaction was allowed to proceed under argon overnight. The resulting PEGDA was purified and lyophilized prior to further use. The degree of polymer acrylation was analyzed by 1H nuclear magnetic resonance (NMR) spectroscopy (Fig. S1) using a Bruker 400 MHz NMR Spectrometer (Bruker, USA). Acrylation percentage was calculated based on the ratio of hydrogens from the acrylate groups to hydrogens on the PEG backbone. All PEGDA samples used for experiments in this study had acrylation above 95%.
2.2. Preparation of PEG hydrogels
PEG hydrogels were prepared by crosslinking a pre-polymer solution using either UV light (BLAK-RAY UV lamp B100AP or self-assembled collimated UV equipment in The Houston Methodist Hospital Research Institute, Houston, TX) [29] or white light (Leica KL1500 LCD) [30] with different photoinitiators as previously described. First, the pre-polymer solutions were prepared by dissolving PEGDA into phosphate buffered saline (PBS, pH = 7.4) at specific concentrations, e.g. 10% and 15% w/v. For UV light crosslinking, a photoinitiator (2-Hydroxy-4′-(2-hydroxyethoxy)-2-methylpropiophenone, Irgacure 2959, Sigma-Aldrich) was added to the PEGDA solution at a final concentration of 3 mg/ml. For white light crosslinking, 1.5% (v/v) triethanolamine (TEOA, Sigma-Aldrich), 10 μM Eosin Y solution (Sigma-Aldrich) and 0.375% (v/v) N-vinylpyrrolidone (NVP, Sigma-Aldrich) were mixed into the PEGDA solution.
The pre-polymer solutions were injected into molds assembled from two glass slides pretreated with Sigmacote (Sigma-Aldrich) and a 1-mm thick polytetrafluoroethylene (PTFE) spacer. The molds were secured with metal binder clips and then exposed to the appropriate light source for specific time. Next, the crosslinked hydrogel was weighed and then soaked in fresh PBS overnight on a shaker to allow swelling and to remove any unreacted molecules. The swelling ratio was calculated by comparing the hydrogel weights before and after swelling.
2.3. Photolithographic patterning of PEG hydrogels
First, a high molecular weight (MW) PEGDA hydrogel (20 kDa, 10% w/v) was prepared as a “base slab” by crosslinking with the collimated UV light for 2 min. This base slab was then soaked in a low MW PEGDA solution (3.4 kDa, 20% w/v) for approximately 8 hours to allow the low MW PEGDA to diffuse into the high MW polymer network. The composite hydrogels were then exposed to collimated UV light for 3 min through a pre-designed stripe-pattern photomask (20,000 dpi, CAD/Art Services, Bandon, OR) to crosslink the 3.4 kDa PEGDA into the 20 kDa PEG hydrogel. The patterned hydrogels were soaked in fresh PBS for at least 6 hours to remove unreacted PEGDA and photoinitiator molecules. The photolithographic patterning was also performed under white light following similar procedures. Staining with cresyl violet acetate (2 mg/ml) allowed visual differentiation of the two regions within the hydrogels as the dye molecule preferentially remained in the denser areas of 3.4 kDa PEGDA crosslinking and washed free from the 20 kDa base PEG hydrogel.
2.4. Mechanical test of PEG hydrogels
Compression and tension tests of PEG hydrogels were conducted using a Bose Enduratec ELF 3200 system (Eden Prairie, MN). For the quasi-static compression test, cylindrical hydrogel samples, 8.0 mm (diameter) × 1.0 mm (thickness), were cut by a standard punch and placed between compression plates. The maximum displacement of the crosshead was 0.3 mm with a speed of 0.02 mm/s. Gauge length was 1.0 mm.
For the uniaxial tension test, PEG hydrogels were cut into rectangle shapes, 30.0 mm (length) × 5.0 mm (width) × 1.0 mm (thickness), using a lab-machined mold. The ends of the hydrogels were glued between two pieces of paper, which were gripped by the clamps. The shortest distance measured between the two pieces of paper was used as the gauge length. Tension tests were performed at a speed of 0.1 mm/s until either a maximum displacement of 12 mm or sample failure was reached.
In all compression and tension analyses, 6 replicates of each hydrogel sample were tested. The loading force and sample displacement were recorded in each test, and divided by the sample cross-sectional area and gauge lengths, respectively to obtain stress and strain values. In both compression and tension data analysis, the elastic modulus was determined as the slope of the stress-strain curve (0-20% strain for compression, 0-fracture for tension) using the linear regression tool in Excel software.
2.5. Incorporation of bioactive peptides to the PEG network
The cell-adhesive peptide RGDS was obtained from American Peptide Company (Sunnyvale, CA) and incorporated into the PEG network to allow cell adhesion to the hydrogel [26]. In addition, a matrix metalloproteinase (MMP)-sensitive peptide sequence, GGGPQG↓IWGQGK (herein referred as PQ; ↓marks the cleavable bonding site), was prepared by the solid phase peptide synthesis using an APEX 396 synthesizer (Aapptec, Louisville, KY) as previously described [31]. PQ was incorporated into the PEG network to provide hydrogel degradability.
For preparation of Acrl-PEG-RGDS (hereafter abbreviated as PEG-RGDS), a mixture of Acrl-PEG-SVA (MW 3400, Laysan Bio, Arab, AL) and RGDS in PBS at a molar ratio of 1:1.2 was adjusted to pH 8.0 using 0.1 N NaOH, and reacted overnight on an orbital shaker. For synthesis of Acrl-PEG-PQ-PEG-Acrl (hereafter abbreviated as PEG-PQ-PEG), a PBS solution with Acrl-PEG-SVA (MW 3400, Laysan Bio, Arab, AL) and PQ in the molar ratio of 2.1:1 was used for the reaction. PEG-RGDS and PEG-PQ-PEG solutions were then dialyzed against ultrapure H2O and lyophilized to obtain dry powder. Peptide conjugations were confirmed by the gel permeation chromatography (GPC).
2.6. Micro-patterning of RGDS on PEG hydrogels
Coverslips were exposed to Piranha solution for 3-5 min, and then soaked in ethanol with 85 mM 3-(Trimethoxysilyl)propyl methacrylate (Sigma-Aldrich, St. Louis, MO) overnight, to obtain acrylated coverslips. To pattern cell adhesive ligands, a thin 10% (w/v) 20 kDa PEG hydrogel (thickness ~160 μm) was first created on an acrylated coverslip by white light crosslinking for 30 seconds as described in section 2.2. The hydrogel was then soaked in the white light photoinitiator solution for a specific amount of time (at least 2 hours for bulk patterning of RGDS inside of the hydrogel and ~5 min for surface patterning of RGDS atop of the hydrogel). Next, a pre-designed photomask was aligned on the hydrogel and then assembled in a photo-crosslinking mold as previously described. The hydrogel was further crosslinked through the photomask by exposure to white light for 3 min. These gels were then rinsed with sterile-filtered PBS three times to remove unpolymerized PEG-RGDS. To confirm the results of the initial patterning study, fluorescently-tagged PEG-RGDS-Alexa Fluor 633 was prepared by reacting PEG-RGDS with Alexa Fluor succinimidyl ester (Invitrogen, Carlsbad, CA) at a 10:1 molar ratio overnight as previously described [32]. PEG-RGDS-Alexa Fluor 633 was mixed in the pre-polymer solution to demonstrate the efficiency of the micro-patterning of RGDS ligands as it could be visualized under a fluorescence microscope. Three different aspect ratios (1:1, 1:2 and 1:3) of rectangles (25 × 25 μm, 25 × 50 μm, 25 × 75 μm) were micro-patterned in this confirmation study.
2.7. Cell culture experiments
Aortic valve leaflets were dissected from porcine hearts obtained fresh from a commercial abattoir (Fisher Ham and Meats, Spring, TX). VICs were isolated from the dissected leaflets following a two-step digestion method [33]. The leaflets were first digested with collagenase II for 30 min at 37°C, then the surfaces were swabbed to remove the valvular endothelial cells. The leaflets were then finely minced and digested with collagenase III for 4 hours at 37°C to isolate the VICs. These VICs were cultured with DMEM/F12 supplemented with 10% (v/v) bovine growth serum (Hyclone, Logan, UT), 1% (v/v) 1M HEPES (Lonza, Walkersville, MD) and 1% (v/v) Antibiotic-Antimycotic (Mediatech, Manassas, VA), and the culture media were changed every 2 days. Cells at passages 3-5 with confluency above 90% were used in the following experiments.
For two-dimensional (2D) cell culture, VICs were cultured atop PEG-RGDS hydrogels with various RGDS concentrations (0.1-5 mM), as well as on glass coverslips, at an initial density ~10,000 cells/cm2. The cellular area and circularity were analyzed using Image J (NIH, Bethesda, MD) to study the effect of the amount of cell-adhesive peptide RGDS on cell morphology. Moreover, VICs were cultured on PEG-RGDS micro-patterns to investigate influence of these patterns to cell size and morphology.
For three-dimensional (3D) cell encapsulation, VICs were suspended in the pre-polymer solution, which was then crosslinked to form hydrogels on the acrylated coverslips. To study the effect of the PQ amount on VIC activity, 5% (w/v) pre-polymer solutions of 3.4 kDa PEGDA and PEG-PQ-PEG were prepared with the following weight percentages of PEG-PQ-PEG in the mixture: 0 (pure PEGDA), 20%, 60% and 100% (pure PEG-PQ-PEG). All pre-polymer solutions contained 1 mM RGDS. To study the effect of the RGDS amount on VIC activity, cells were encapsulated in 5% (w/v) PEG-PQ-PEG hydrogels with three different concentrations of RGDS: 0.3, 1, 5 mM. A cell density of ~106 cells/ml was used. Cell viability and morphology were evaluated by staining of filamentous actin using phalloidin and α-SMA for VIC activity as described in the next section.
In addition, media with/without serum collected from cell culture on PEG-peptide hydrogels (5% (w/v) PEG-PQ-PEG with 5 mM RGDS) were used to determine MMP activity through a zymography test using a 10% precast polyacrylamide gel with gelatin (BioRad, Hercules, CA) following a Millipore protocol as previously described [31]. Compression tests of PEG-peptide hydrogels (4% (w/v) PEG-PQ-PEG with 5 mM RGDS) prepared either without cells or with cells (3×107 cells/ml) and cultured for 1, 14, 21, or 28 days were performed to evaluate the integrity and mechanical strength of these hydrogels.
2.8. Cell viability and immunofluorescence staining
The viability/cytotoxicity kit from Molecular Probes (Eugene, OR) was used to evaluate cell viability in different PEG hydrogels crosslinked using white light (Section 2.2).
To identify new ECM deposition and characterize cell phenotype, VICs were cultured in 5% (w/v) PEG-PQ-PEG hydrogels with 5 mM RGDS for 14 days and 10% (w/v) PEG-PQ-PEG hydrogels with 5 mM RGDS for 21 days. Immunofluorescence staining of type I collagen and fibronectin was then performed following common protocols. For the negative controls, the primary antibodies were omitted. Antibodies used for immunofluorescence analysis included primaries: rabbit anti-alpha smooth muscle actin, rabbit anti-type I collagen, mouse anti-fibronectin, mouse anti-vimentin, rabbit anti-calponin, mouse anti-caldesmon and rabbit anti-smooth muscle myosin heavy chain from Abcam (Cambridge, UK), and secondaries: Alexa Fluor 488 phalloidin, Alexa Fluor 488 goat anti-mouse IgG, Alexa Fluor 488 chicken anti-rabbit IgG, Alexa Fluor 555 donkey anti-mouse IgG, and Alexa Fluor 594 goat anti-rabbit IgG from Invitrogen (Grand Island, NY). DAPI from KPL (Gaithersburg, MD) was used to visualize cell nuclei. All samples were fixed with 4% (w/v) paraformaldehyde solution, permeabilized with 0.2% (v/v) Triton X-100, and blocked with 1% (w/v) bovine serum albumin (BSA) solution. The primary and secondary antibodies were incubated with samples overnight at 4°C. Samples were imaged using either a Nikon Eclipse TE300 microscope for 2D cell culture, or a Zeiss 5 Live confocal microscope with a z-interval of 1 μm for 3D cell culture.
2.9. Data analysis
All tests were conducted with at least triplicate samples. All data are expressed as means ± standard deviation. Statistical analysis was performed using a one-way ANOVA with post-hoc Tukey testing. Statistical significance was accepted with p values of less than 0.05.
3. Results
3.1. Characterization of PEGDA hydrogels
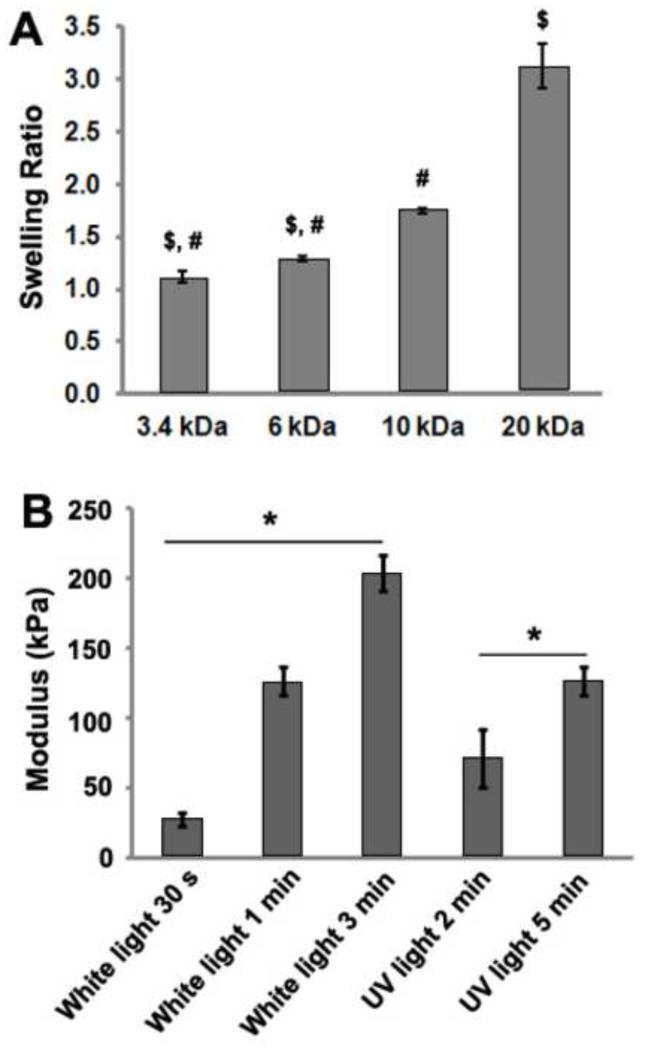
The swelling ratios of 10% (w/v) PEGDA hydrogels crosslinked with UV light were dependent upon PEGDA MW (Fig. 1A). For example, the 10% (w/v) 20 kDa PEGDA hydrogels swelled about 3 times, while the 10% (w/v) 3.4 kDa PEGDA hydrogels barely swelled. Furthermore, the compressive elastic moduli of PEGDA hydrogels were dependent on crosslinking time (Fig. 1B). There was a significant increase in elastic modulus with greater crosslinking time when either white light or UV light was used. Previous studies also demonstrated that the physical and mechanical properties of PEGDA hydrogels correlated with the MW of PEGDA molecules and crosslinking time [34-36].
Fig. 1.
(A) Swelling ratios of 10% (w/v) PEGDA hydrogels varied across different molecular weights (3.4, 6, 10, 20 kDa); (B) Elastic moduli of 10% 3.4 kDa PEGDA hydrogels increased with longer crosslinking time using either white light or UV light (from non-collimated UV lamp). $p < 0.001 of sample versus the 10 kDa sample, #p < 0.001 of sample versus the 20 kDa sample, *p < 0.001 among groups denoted by the horizontal line.
3.2. Anisotropic mechanical behavior of patterned hydrogels
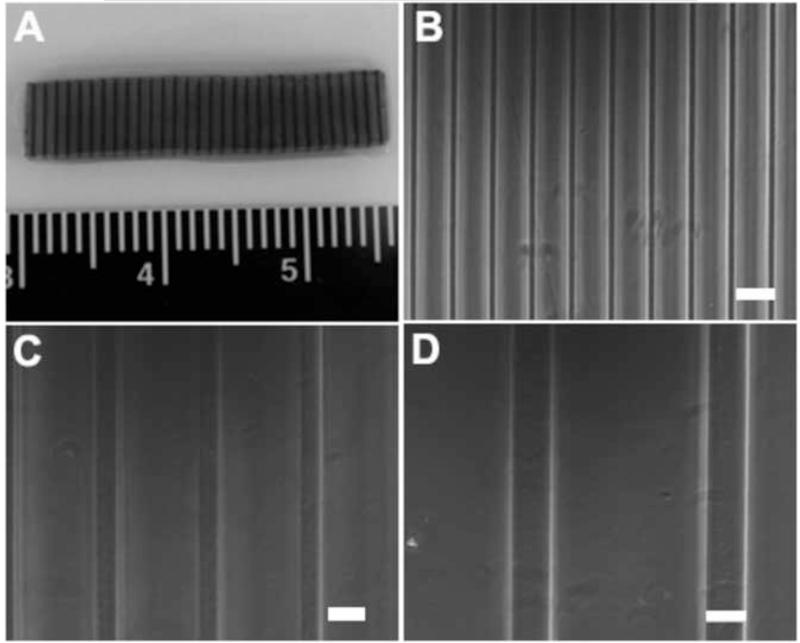
We next attempted to create anisotropic PEG hydrogels by photolithographic patterning of the stiffer, lower MW (3.4 kDa) PEGDA into weaker, higher MW (20KDa) PEGDA network. Stripe-patterned hydrogels were prepared using collimated UV light (Fig. 2). The dark stripes in Fig. 3A indicated the area where 3.4 kDa PEGDA was crosslinked within the 20 kDa PEGDA hydrogels, while the grey portion was 20 kDa PEGDA base gel. These stripe-patterned hydrogels were also prepared using white light (Fig. 3B-D). Fig. 3B showed that a resolution of 20 μm for the stripe pattern could be obtained by patterning with white light.
Fig. 2.
A schematic drawing of the photolithographic patterning process used to create stripe-patterned PEG hydrogels: (A) 10% 20 kDa PEGDA hydrogel was crosslinked with collimated UV light for 2 min as a “base slab”; (B) the “base slab” was soaked in a 20% 3.4 kDa PEGDA solution; (C) the 3.4 kDa PEGDA was crosslinked into the 20 kDa PEGDA hydrogel through a pre-designed stripe-pattern photo mask; (D) the patterned hydrogel was soaked in fresh PBS to remove unreacted PEGDA and photoinitiator molecules; (E) the patterned hydrogels were stained with cresyl violet acetate for visualization of patterns.
Fig. 3.
Stripe-patterned PEGDA hydrogels can be prepared by crosslinking 3.4 kDa PEGDA within a 20 kDa PEGDA base gel: (A) 100 μm stripe (dark lines) patterns with 15% area coverage created by collimated UV light patterning; (B) 20 μm, (C) 50 μm and (D) 100 μm stripe patterns with 20% area coverage created by white light patterning. For (B-D), scale bar = 100 μm.
The tensile elastic modulus of these patterned hydrogels was significantly increased (relative to the base material alone) by incorporating 3.4 kDa PEGDA polymer in a pattern within the 20 kDa PEGDA base material (Fig. 4). Further, for the three partially stripe-patterned hydrogels with pattern area coverage of 10%, 15% and 20%, the elastic modulus of the samples tested parallel to the stripe patterns was significantly different from the elastic modulus of those tested perpendicular to the patterns.
Fig. 4.
Tensile elastic modulus of PEGDA hydrogels created with or without anisotropic stripe-patterns. The stripe patterns were formed by crosslinking 3.4 kDa PEGDA within a 20 kDa PEGDA base gel, with area coverage of 10%, 15% and 20%. The sample denoted as 100% 3.4 kDa indicates full crosslinking (i.e., no stripe pattern was present) of the 20 kDa base gel with the added 3.4 kDa PEGDA. $p < 0.01 of sample versus the 20 kDa sample, &p < 0.001 of sample versus the 100%3.4kDa sample, %p < 0.001 of sample versus the 10%3.4kDa para sample, +p < 0.01 of sample versus the 10%3.4kDa perp sample, *p < 0.001 among groups denoted by the horizontal line.
Our results show that stripe patterns of different width and area coverage can be formed using pre-designed photomasks with either UV light or white light patterning. The stripe-patterned generally demonstrate anisotropic mechanical behavior. The ratio of tensile elastic modulus in the parallel to perpendicular directions (relative to the stripe patterns) was approximately 4.1-6.8, which is a range comparable to that previously reported for aortic valve leaflets [37-40]. Therefore, PEG hydrogels that mimic the anisotropic behavior of valve leaflets can be fabricated by this photolithographic patterning method.
3.3. Modulation of cell morphology and activation using bioactive peptides
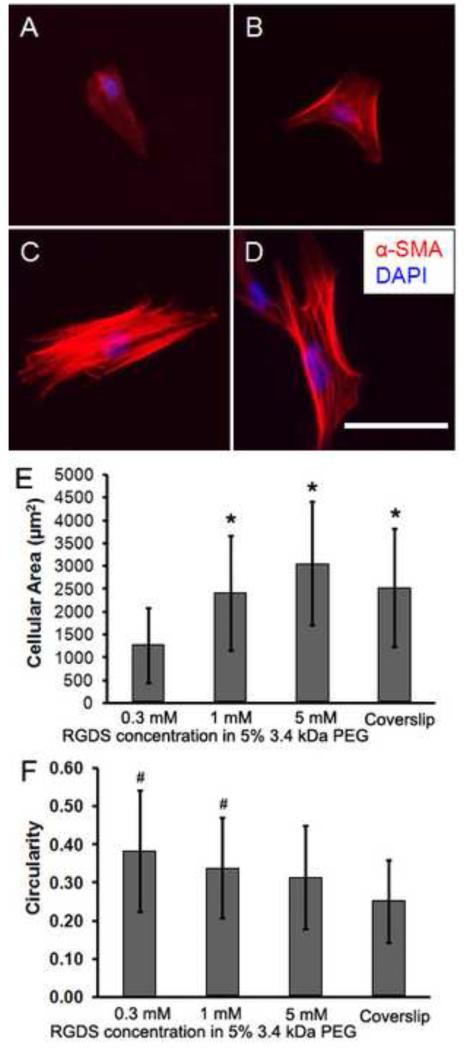
In our work, the peptide RGDS, derived from fibronectin [26], was incorporated into the PEG network to encourage adhesion and spreading of VICs. VICs cultured on PEG-RGDS hydrogels with varying amounts of RGDS (Fig. 5A-C) showed different adhesion characteristics from VICs seeded on glass coverslips (Fig. 5D). Upon increasing the amount of RGDS in the PEG network, the average cell area increased (Fig. 5E) while the average cell circularity decreased (Fig. 5F), indicating cell spreading to a greater degree. Fig. 5E showed that the average area of cells cultured on PEGDA hydrogels with 1 mM RGDS (~2.41 × 103 μm2) was similar to that of VICs cultured on the coverslip (~2.53 × 103 μm2).
Fig. 5.
Immunocytochemical staining of α-SMA (red) and nuclei (blue DAPI) for cells cultured on PEG hydrogels for 1 day with different amounts of RGDS: (A) 0.3 mM, (B) 1 mM and (C) 5 mM, as well as on the coverslip as a control (D). Cell spreading was significantly affected by the RGDS concentration. Images were processed using Image J to calculate the cellular area (E) and circularity (F). Scale bar = 100 μm. *p < 0.001 of sample versus the 0.3 mM RGDS sample, #p < 0.01 of sample versus the coverslip.
We also found that VIC activation as characterized by α-SMA staining was correlated with the cell size (area), which was influenced by the concentration of RGDS incorporated into the PEG network (Fig. 5A-C and Fig. 6). VICs were found at higher levels of activation when they were cultured on PEG hydrogels that had larger amounts of RGDS in the polymer network (Fig. 6A-C). VICs were also immunostained for vimentin (D-F), smooth muscle myosin heavy chain (G-I), caldesmon (J-L), and calponin (M-O) on different concentrations of RGDS to further characterize VIC phenotypes. As expected, all VICs expressed similar levels of vimentin. VIC expressed fairly strong levels of caldesmon and weak levels of calponin, with the RGDS concentration of the hydrogel having little effect on these proteins. VIC expression of smooth muscle myosin appeared same trend with α-SMA, showing low levels in cells cultured atop low RGDS concentration and higher levels in cells cultured on top of the higher RGDS concentration. These results showed that higher RGDS concentrations can influence VICs toward a myofibroblast phenotype, which is characterized by these contractile proteins [41, 42].
Fig. 6.
VICs cultured on 5% (w/v) 3.4 kDa PEGDA hydrogels for 2 days demonstrated phenotypic differences depending on the amount of RGDS: (A, D, G, J, M) 0.1 mM (quiescent status), (B, E, H, K, N) 1 mM (low degree of activation) and (C, F, I, L, O) 5 mM (high degree of activation), characterized by immunocytochemical staining of α-SMA (A-C), vimentin (D-F), smooth muscle myosin heavy chain (G-I), caldesmon (J-L), calponin (M-O) and DAPI for nuclei (blue). Scale bar = 20 μm.
To investigate further the effect of cell morphology and orientation on VIC activation, we prepared micro-patterns (rectangular or oval) of PEG-RGDS on PEGDA hydrogels by photolithographic patterning using pre-designed photomasks (Fig. S2). Single cells could be confined at certain size and morphology on PEG-RGDS micro-patterns after culture for 1 day (Fig. 7A, B). Staining for α-SMA in these cells (Fig. 7E, F) demonstrated that the α-SMA stress fibers were constrained to the edges of the micro-patterns, likely due to adhesive forces forming along the edges at the initiation of adhesion. Cells were also able to migrate from one patterned section to another (Fig. 7C, G). Alpha-SMA stress fibers in these cells showed aligned orientation spanning these two patterns (Fig. 7G), compared with the random orientation in cells cultured on PEGDA hydrogels with RGDS in a bulk presentation (Fig. 7H).
Fig. 7.
Phase contrast images of VICs cultured for 1 day on micro-patterns of PEG-RGDS (A-C) created by the photolithographic patterning, and the immunocytochemical staining (E-G) of α-SMA (red) and DAPI (blue), as well as cells cultured atop the PEGDA hydrogel with 1 mM RGDS in a bulk condition as control (D, H). VICs that were restricted to the single micro-patterns had their α-SMA stress fibers aligned around the pattern periphery. Images C and G show cells spanning two micro-patterns. Scale bar = 50 μm.
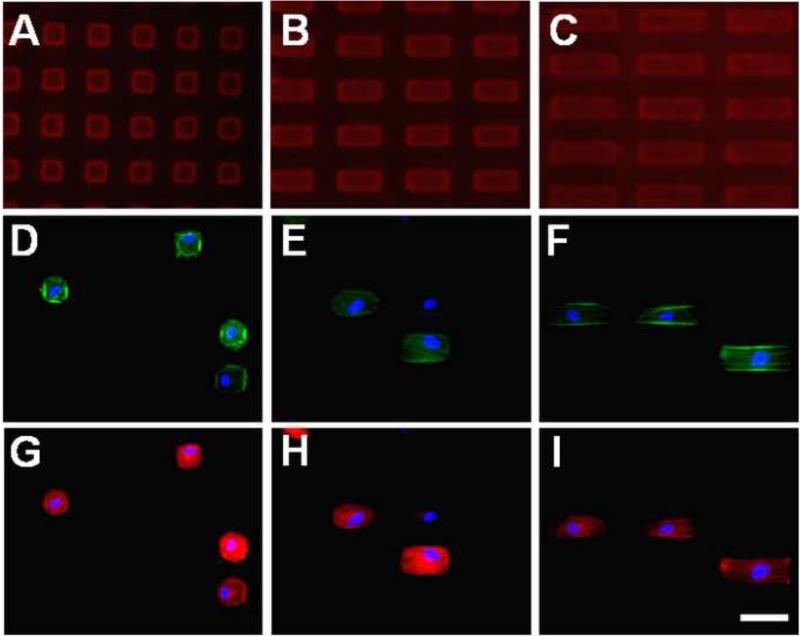
In the follow-up study employing different aspect ratios, the use of fluorescently-tagged PEG-RGDS-Alexa Fluor 633 in the photolithographic patterning confirmed that the PEG-RGDS was localized to the micro-patterns (Fig. 8A-C). Again, VICs were found to be confined to the micro-patterns for all different pattern aspect ratios. Phalloidin staining (Fig. 8D-F) showed a greater abundance of filamentous actin along the edges compared to the pattern interior. As in the first micro-pattern study, positive staining of α-SMA (Fig. 8G-I) indicated that VICs cultured on these patterns were activated.
Fig. 8.
Fluorescent images of rectangular micro-patterns of PEG-RGDS and VICs cultured on these patterns at different aspect ratios: (A, D, G) 25 × 25 μm (1:1), (B, E, H) 25 × 50 μm (1:2) and (C, F, I) 25 × 75 μm (1:3) for 1 day. Fluorescently-tagged PEG-RGDS-Alexa Fluor 633 was localized to the micro-patterns (A-C). Staining of filamentous actin (phalloidin/green, D-F), α-SMA (red, G-I) and cell nuclei (DAPI/blue, D-I) was performed after the VICs were cultured for 1 day. Scale bar = 50 μm.
3.4. Effect of peptide composition in PEGDA hydrogels on cellular activity
Because PEG hydrogels are bioinert, they are not able to support cell adhesion without further modification. For example, VICs encapsulated in 5% (w/v) 3.4 kDa PEG hydrogels were found to exhibit rounded morphologies after 2 days in culture (Fig. 9A). In 2D culture, incorporation of RGDS in the PEG network promoted VIC adhesion and influenced cell morphology and activation. In 3D culture, however, degradation of the scaffold is a necessity for cell-mediated remodeling of the TEHV. Thus, the PQ peptide was incorporated into the PEG network to improve VIC activity for matrix remodeling in 3D cultures. PQ is derived from collagen motifs [27] and has previously been used to form MMP-degradable polymer networks [43-45]. Here, VICs were found to elongate after 2 days of culture in 5% (w/v) PEG-PQ-PEG with 1 mM RGDS (Fig. 9B). Additionally, some VICs became activated, as evidenced by positive staining for α-SMA, after 4 days in culture (Fig. 9C).
Fig. 9.
Fluorescent staining showed that the VICs were rounded after culture in 5% (w/v) 3.4 kDa PEG hydrogel for 2 days (A), but spread and activated after culture in 5% (w/v) PEG-PQ-PEG with 1 mM RGDS for 2 days (B) and 4 days (C). Green: phalloidin/filamentous actin, Red: α-SMA, Blue: DAPI/nuclei; (D) VICs cultured in 5% (w/v) PEG hydrogels (with 1 mM RGDS) composed of different amounts of PEG-PQ-PEG and PEGDA for 5 days. There was greater VIC viability in the hydrogels containing a greater proportion of PQ. (E) Viability of cells varied when cultured in 5% (w/v) PEG-PQ-PEG hydrogels with different amounts of RGDS. Scale bar = 50 μm. NS (no significant difference) and *p < 0.001 among groups denoted by the horizontal line. #p < 0.05 of sample versus the 0.3 mM RGDS sample. &p < 0.01 of sample versus the 0.3 mM RGDS sample. +p < 0.001 of sample versus the 1 mM RGDS sample.
Our results indicated that cell viability significantly decreased with lower amounts of PQ peptide in the hydrogels (Fig. 9D), suggesting that the ability of cells to spread and extend 3D processes into the polymer network was critical for VICs to survive in the otherwise bioinert PEG hydrogels. The highest cell viability (~80%) was found in the hydrogels containing solely PEG-PQ-PEG (Fig. 9D, 5% w/v) compared to mixtures of PEG-PQ-PEG and PEGDA. This 5% PEG-PQ-PEG hydrogel was then used to investigate the effect of RGDS concentration on cell viability and 3D process (elongation with dendritic extensions). Although cell viability was significantly greater for hydrogels containing 0.3 mM RGDS (Fig. 9E), viability was high (> 80%) for all concentrations tested (0.3-5 mM). Overall, it was confirmed that the PEG-peptide hydrogels, containing both PQ and RGDS derived from natural proteins, were able to provide a biomimetic microenvironment for VIC culture in vitro.
Loss of structural integrity was observed in some of the PEG-peptide hydrogels undergoing 3D cell culture for 14 days or longer, suggesting that the hydrogels were being degraded by the VICs, as intended. As previously noted, the PQ in the polymer network can be cleaved by MMPs [43-45] to allow hydrogel degradation. It has been reported that VICs can express matrix-degrading enzymes including MMPs and their tissue inhibitors (TIMPs) [46, 47]. Therefore, a zymography assay was performed to confirm the presence of MMPs in different media samples collected from VIC cultures (Fig. S3). This assay demonstrated that VICs cultured in the PEG-peptite hydrogel secreted MMP2 (~68 kDa) into serum-free media (Fig. S3C), which corresponds with the remodeling of PEG-peptide hydrogels by these cells.
3.5. Extracellular matrix deposition from 3D cell culture in PEG-peptide hydrogels
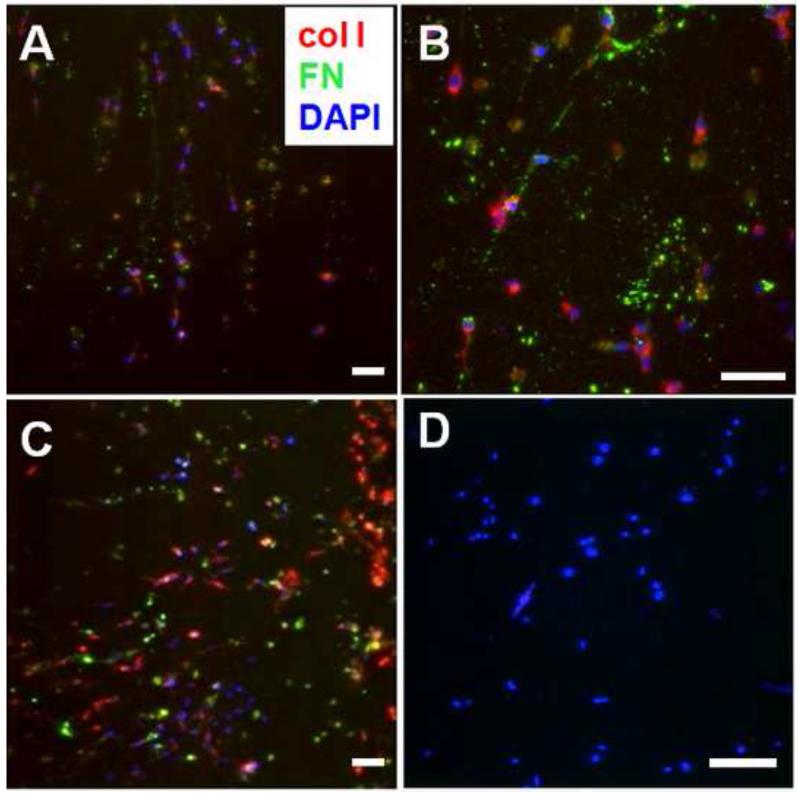
To identify new ECM deposition by VICs encapsulated in PEG-peptide hydrogels, fluorescent staining of type I collagen and fibronectin proteins was performed. Both type I collagen and fibronectin were secreted by VICs in the hydrogel scaffold after culture for 14 days (Fig. 10A, B) and 21 days (Fig. 10C). A negative control processed without the primary antibodies demonstrated a lack of background staining (Fig. 10D). The 3D views of two of the stained samples (Fig. S4) demonstrated that new ECM was secreted to fill the space within the PEG-peptide hydrogel scaffolds. Furthermore, it was found that elastic moduli of PEG-peptide hydrogels with VICs cultured for 21 and 28 days significantly increased compared to those cultured for 1 and 14 days (Fig. S5, the PEG-peptide hydrogels without cells were also included for comparison), which indicated that ECMs deposited by VICs reinforced the hydrogels.
Fig. 10.
ECM deposition in 3D culture of VICs in PEG-PQ-PEG and PEG-RGDS hydrogels: (A, B) 5% PEG-PQ-PEG with 5 mM RGDS for 14 days and (C) 10% PEG-PQ-PEG with 5 mM RGDS for 21 days stained for type I collagen (red), fibronectin (green) and cell nuclei (DAPI/blue), and (D) negative control processed without primary antibodies for type I collagen and fibronectin. Cell density = 106 cells/ml. Scale bar = 50 μm.
4. Discussion
The development of TEHVs is appealing due to the limitations of current devices for heart valve replacement. Advanced scaffold design features modeled upon those of native valves are crucially important for fabrication of a functional TEHV that can fulfill requisite tissue mechanics and cell-matrix interactions in vivo. Anisotropic mechanical behavior is typical for aortic valve leaflets, which experience different loading along their circumferential and radial axes. Moreover, the interaction between valvular cells and the ECMs plays a critical role in maintaining homeostasis of the resident cells, the ECM structures, and the mechanical behavior of the leaflets. Therefore, scaffolds used for TEHVs should mimic the mechanical properties and cell-matrix interactions of leaflets. However, no current polymer scaffold meets these criteria. In this study, we attempted to fabricate advanced PEGDA hydrogels to recapitulate key features of aortic valve leaflets, namely anisotropic mechanical properties, cell-to-ECM-like integrin binding sites, and proteolytically degradable matrix. By employing photolithographic patterning, we created stripes of 3.4 kDa PEGDA in 20 kDa PEGDA hydrogels, which endowed these hydrogels with a degree of anisotropy similar to that of aortic valve leaflets. Furthermore, the biological functionality of PEG hydrogels was modified by incorporation of bioactive peptides into the polymer network, including RGDS for cell adhesion and the PQ peptide (GGGPQG↓IWGQGK) to allow proteolytic degradation of the hydrogels.
The elastic moduli of PEG hydrogels were previously summarized as approximately 0.02-3.5 MPa in tension and 0.055-42.9 MPa in compression [48]. Indeed, the tunable mechanics of PEG scaffolds has resulted in the proposal for a variety of tissue engineering and drug delivery applications [48-50]. The hydrogels developed in the present study varied in strength with tensile elastic moduli of ~5-200 kPa depending on PEGDA MW and crosslinking time. Based on literature [37, 38, 40], the elastic moduli of aortic valve leaflets varies from approximately 3 MPa to 15 MPa in the circumferential direction and approximately 1 MPa to 2 MPa in the radial direction. The hydrogels used in this study were not intended to replicate the native valve stiffness, but in future work PEGDA hydrogels for heart valve tissue engineering should be reinforced to obtain mechanical strength close to that of native leaflets.
Considering the range of hydrogel mechanical properties that can be generated using different MW PEG [34-36], we created stripe-patterns of stiffer 3.4 kDa PEGDA within softer 20 kDa PEGDA base hydrogels using photolithographic patterning (Fig. 3) to render anisotropic behavior. Several partially patterned PEG hydrogels showed anisotropic mechanical behavior, with the average tensile modulus along the orientation parallel to the stripe patterns approximately 4-7 times greater than that in the perpendicular orientation (Fig. 4). This level of anisotropy was comparable to that of aortic valve leaflets (human or porcine), in which the ratios of tensile elastic moduli between the circumferential and the radial directions were reported as between 2-8 [37-40]. For TEHVs, the scaffold material would not only need to withstand the dynamic, anisotropic mechanical loading in vivo after implantation, but would also need to convey appropriate mechanical stimulation to the cells within the scaffold to promote matrix remodeling [17]. Anisotropic scaffolds for TEHVs have been developed previously, although not using hydrogels. Courtney et al. [51] developed electrospun poly(ester urethane) urea scaffolds and obtained anisotropic mechanical behavior that resembled that of native pulmonary valve leaflets. Additionally, Masoumi et al. [52] microfabricated poly(glycerol sebacate) scaffolds by laser ablation following a computational design to mimic anisotropy of native bovine aortic valve leaflets. Our demonstration that photolithographic stripe-patterned PEG hydrogels can mimic the anisotropy of aortic valve leaflets adds a new class of materials to the array of mechanically suitable TEHV scaffolds. A particular advantage of our method is that the degree of anisotropy of the scaffold can be easily modulated by controlling the stripe width and spacing.
In addition, we confirmed that incorporation of the fibronectin-derived, cell adhesive peptide RGDS into the PEG network can support adhesion and migration of VICs in 2D culture. Bioactive moieties in the scaffold play a key role in signal transduction between matrix and cells. A variety of peptides have been incorporated into the bioinert PEG network in order to elicit specific responses of resident or recruited cells by mimicking their microenvironment [43, 53, 54]. In particular, RGDS, which serves as a ligand for several integrins [55], has been widely used to support adhesion of a variety of cells to different substrates [56-59]. In the present work, RGDS was incorporated into the PEG network to support adhesion of VICs and modulate cell morphology. As the concentration of RGDS within the PEG hydrogel was increased, there was an increase in cellular area and a corresponding decrease in circularity, indicating greater spreading of VICs. This RGDS-driven cell spreading also resulted in greater activation of VICs cultured atop the PEG hydrogels. Moreover, we demonstrated that VIC morphology and orientation can be modulated using micro-patterns of RGDS created by photolithographic methods. This technique allowed us to restrict the size and aspect ratios of single VICs, and thus modulate the degree of VIC activation.
VIC activation involves a transition from the fibroblast to myofibroblast phenotype, and activated VICs play a key role in matrix remodeling in physiological and pathological conditions of heart valves [60-62]. For in vitro tissue engineering, VIC activation facilitates the production of new ECM proteins as well as matrix degradation. Previous studies have shown that VIC activation in vitro can be influenced by cell density [63], growth factor availability [64], matrix composition [64, 65] and substrate stiffness [66, 67]. However, the underlying mechanism for VIC activation in general remains poorly understood. Our study in which we confined VICs with micro-patterns of RGDS provided a proof-of-concept demonstration that VIC activation in vitro can be controlled by restricting cell size and morphology. In the future, PEG hydrogels that have RGDS patterned or present in varying concentrations could be used in TEHV designs to modulate VIC activation.
For 3D VIC cultures, biomimetic PEG hydrogels were developed by tethering two ECM-derived peptides, RGDS and PQ, into the polymer network. There was a significant influence of the amount of PQ and RGDS in the PEG network on the viability of VICs in 3D. As noted above, incorporation of RGDS into the PEG network was important for VIC adhesion on 2D culture, and has been previously shown to support VIC adhesion and migration in 3D culture [45]. Because scaffold degradation is a critical facet of remodeling in tissue engineered constructs, MMP-sensitive hydrogels were developed to render the network proteolytically degradable by encapsulated cells [43]. Indeed, incorporation of the PQ peptide was previously shown to support in vitro degradation of PEG hydrogels by a variety of cells [43-45]. In this work, VIC viability was increased when greater amounts of PQ were present in the network. Upon the addition of 1 mM RGDS to the PEG-PQ-PEG hydrogels, a high percentage of VICs within the hydrogels showed pronounced elongation with dendritic extensions and activation of these cells indicated by the positive staining of α-SMA.
Activation of VICs cultured in these biomimetic PEG-peptide hydrogels was also borne out in the longer term by early degradation of the polymer matrix (likely modulated by VIC-secreted MMP2) and de novo deposition of type I collagen and fibronectin. Degradation decreases the integrity and strength of the scaffold, whereas deposition of new ECMs into the 3D structure increases the mechanical strength of the scaffold. Therefore, it is crucially important to obtain a balance between these two processes in order to maintain the overall mechanical integrity of tissue engineered constructs. In future work, the amount of RGDS, PQ, or other bioactive peptides incorporated into the PEG network should be further investigated to fine-tune this balance.
Conclusions
Recapitulating the critical features of leaflets such as the anisotropic mechanical behavior and biological functions is a critical step in developing a tissue engineered heart valve (TEHV). In this study, PEG hydrogels with tunable mechanical properties and tailored biological functions were evaluated as TEHV scaffolds. First, stripe-patterned structures were created in PEG hydrogels by photolithographic patterning leading to anisotropic mechanical characteristics mimicking that of aortic valve leaflets. Furthermore, the adhesion, morphology, protein secretion and activation state of VICs encapsulated in these materials was modulated by the specific amounts of bioactive peptides incorporated into the polymer network. Secretion of MMP2 was identified in the culture of VICs on the PEG-peptide hydrogel, corresponding with the degradation of the PEG-PQ-PEG hydrogel scaffold by these cells. In addition, we showed that VIC activation correlated with cell size and morphology and could be modulated by RGDS concentration or the presence of micro-patterns in the hydrogel matrix. Taken together, this study lays the basis for the fabrication of advanced PEG hydrogels utilizing valve-inspired design features for heart valve tissue engineering, as well as for investigation of the mechanisms of VIC activation.
Supplementary Material
Acknowledgements
This work was supported by NIH R01HL107765. The authors thank Dr. Lidong Qin and Dr. Yuanqing Zhang at Department of Nanomedicine, The Methodist Hospital Research Institute, Houston, Texas, for use of their collimated UV equipment. The authors are also grateful to Dr. Melissa McHale for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Yacoub MH, Takkenberg JJ. Will heart valve tissue engineering change the world? Nat Clin Pract Cardiovasc Med. 2005;2:60–1. doi: 10.1038/ncpcardio0112. [DOI] [PubMed] [Google Scholar]
- [2].Cannegieter SC, Rosendaal FR, Briët E. Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation. 1994;89:635–41. doi: 10.1161/01.cir.89.2.635. [DOI] [PubMed] [Google Scholar]
- [3].Fann JI, Miller DC, Moore KA, Mitchell RS, Oyer PE, Stinson EB, Robbins RC, Reitz BA, Shumway NE. Twenty-year clinical experience with porcine bioprostheses. Ann Thorac Surg. 1996;62:1301–11. doi: 10.1016/0003-4975(96)00629-7. discussion 1311-2. [DOI] [PubMed] [Google Scholar]
- [4].Schoen FJ, Levy RJ. Tissue heart valves: current challenges and future research perspectives. J Biomed Mater Res. 1999;47:439–65. doi: 10.1002/(sici)1097-4636(19991215)47:4<439::aid-jbm1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- [5].Schoen FJ, Levy RJ. Calcification of tissue heart valve substitutes: progress toward understanding and prevention. Ann Thorac Surg. 2005;79:1072–80. doi: 10.1016/j.athoracsur.2004.06.033. [DOI] [PubMed] [Google Scholar]
- [6].Sacks MS, Schoen FJ, Mayer JE. Bioengineering challenges for heart valve tissue engineering. Annu Rev Biomed Eng. 2009;11:289–313. doi: 10.1146/annurev-bioeng-061008-124903. [DOI] [PubMed] [Google Scholar]
- [7].Murphy SV, Atala A. Organ engineering - combining stem cells, biomaterials, and bioreactors to produce bioengineered organs for transplantation. BioEssays: news and reviews in molecular, cellular and developmental biology. 2012:1–10. doi: 10.1002/bies.201200062. [DOI] [PubMed] [Google Scholar]
- [8].Mol A, Smits AI, Bouten CV, Baaijens FP. Tissue engineering of heart valves: advances and current challenges. Expert Rev Med Devices. 2009;6:259–75. doi: 10.1586/erd.09.12. [DOI] [PubMed] [Google Scholar]
- [9].Apte SS, Paul A, Prakash S, Shum-Tim D. Current developments in the tissue engineering of autologous heart valves: moving towards clinical use. Future Cardiol. 2011;7:77–97. doi: 10.2217/fca.10.120. [DOI] [PubMed] [Google Scholar]
- [10].Shi Y, Iyer R, Soundararajan A, Dobkin D, Vesely I. Collagen-based tissue engineering as applied to heart valves. Conf Proc IEEE Eng Med Biol Soc. 2005;5:4912–5. doi: 10.1109/IEMBS.2005.1615574. [DOI] [PubMed] [Google Scholar]
- [11].Barsotti MC, Felice F, Balbarini A, Di Stefano R. Fibrin as a scaffold for cardiac tissue engineering. Biotechnol Appl Biochem. 2011;58:301–10. doi: 10.1002/bab.49. [DOI] [PubMed] [Google Scholar]
- [12].Ramamurthi A, Vesely I. Evaluation of the matrix-synthesis potential of crosslinked hyaluronan gels for tissue engineering of aortic heart valves. Biomaterials. 2005;26:999–1010. doi: 10.1016/j.biomaterials.2004.04.016. [DOI] [PubMed] [Google Scholar]
- [13].Stock UA, Mayer JE. Tissue engineering of cardiac valves on the basis of PGA/PLA Co-polymers. J Long Term Eff Med Implants. 2001;11:249–60. [PubMed] [Google Scholar]
- [14].Del Gaudio C, Bianco A, Grigioni M. Electrospun bioresorbable trileaflet heart valve prosthesis for tissue engineering: in vitro functional assessment of a pulmonary cardiac valve design. Ann Ist Super Sanita. 2008;44:178–86. [PubMed] [Google Scholar]
- [15].Weber B, Emmert MY, Behr L, Schoenauer R, Brokopp C, Drögemüller C, Modregger P, Stampanoni M, Vats D, Rudin M, Bürzle W, Farine M, Mazza E, Frauenfelder T, Zannettino AC, Zünd G, Kretschmar O, Falk V, Hoerstrup SP. Prenatally engineered autologous amniotic fluid stem cell-based heart valves in the fetal circulation. Biomaterials. 2012;33:4031–43. doi: 10.1016/j.biomaterials.2011.11.087. [DOI] [PubMed] [Google Scholar]
- [16].Sauren AA, van Hout MC, van Steenhoven AA, Veldpaus FE, Janssen JD. The mechanical properties of porcine aortic valve tissues. J Biomech. 1983;16:327–37. doi: 10.1016/0021-9290(83)90016-7. [DOI] [PubMed] [Google Scholar]
- [17].Simionescu DT, Chen J, Jaeggli M, Wang B, Liao J. Form follows function: Advances in trilayered structure replication for aortic heart valve tissue engineering. J Healthc Eng. 2012;3:179–202. doi: 10.1260/2040-2295.3.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mendelson K, Schoen FJ. Heart valve tissue engineering: concepts, approaches, progress, and challenges. Ann Biomed Eng. 2006;34:1799–819. doi: 10.1007/s10439-006-9163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Brown RA, Phillips JB. Cell responses to biomimetic protein scaffolds used in tissue repair and engineering. Int Rev Cytol. 2007;262:75–150. doi: 10.1016/S0074-7696(07)62002-6. [DOI] [PubMed] [Google Scholar]
- [20].Harris JM. Poly(ethylene glycol) chemistry: Biotechnical and biomedical applications. xxi. Plenum Press; New York: 1992. p. 385. [Google Scholar]
- [21].Zustiak SP, Leach JB. Hydrolytically degradable poly(ethylene glycol) hydrogel scaffolds with tunable degradation and mechanical properties. Biomacromolecules. 2010;11:1348–57. doi: 10.1021/bm100137q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Truong V, Blakey I, Whittaker AK. Hydrophilic and amphiphilic polyethylene glycol-based hydrogels with tunable degradability prepared by “click” chemistry. Biomacromolecules. 2012;13:4012–21. doi: 10.1021/bm3012924. [DOI] [PubMed] [Google Scholar]
- [23].Lee SH, Moon JJ, Miller JS, West JL. Poly(ethylene glycol) hydrogels conjugated with a collagenase-sensitive fluorogenic substrate to visualize collagenase activity during three-dimensional cell migration. Biomaterials. 2007;28:3163–70. doi: 10.1016/j.biomaterials.2007.03.004. [DOI] [PubMed] [Google Scholar]
- [24].Lee HJ, Lee JS, Chansakul T, Yu C, Elisseeff JH, Yu SM. Collagen mimetic peptide-conjugated photopolymerizable PEG hydrogel. Biomaterials. 2006;27:5268–76. doi: 10.1016/j.biomaterials.2006.06.001. [DOI] [PubMed] [Google Scholar]
- [25].Benoit DS, Durney AR, Anseth KS. The effect of heparin-functionalized PEG hydrogels on three-dimensional human mesenchymal stem cell osteogenic differentiation. Biomaterials. 2007;28:66–77. doi: 10.1016/j.biomaterials.2006.08.033. [DOI] [PubMed] [Google Scholar]
- [26].Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309:30–3. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- [27].Netzel-Arnett S, Fields GB, Birkedal-Hansen H, Van Wart HE. Sequence specificities of human fibroblast and neutrophil collagenases. J Biol Chem. 1991;266:6747–55. [PubMed] [Google Scholar]
- [28].DeLong SA, Moon JJ, West JL. Covalently immobilized gradients of bFGF on hydrogel scaffolds for directed cell migration. Biomaterials. 2005;26:3227–34. doi: 10.1016/j.biomaterials.2004.09.021. [DOI] [PubMed] [Google Scholar]
- [29].Hahn MS, Taite LJ, Moon JJ, Rowland MC, Ruffino KA, West JL. Photolithographic patterning of polyethylene glycol hydrogels. Biomaterials. 2006;27:2519–24. doi: 10.1016/j.biomaterials.2005.11.045. [DOI] [PubMed] [Google Scholar]
- [30].Bahney CS, Lujan TJ, Hsu CW, Bottlang M, West JL, Johnstone B. Visible light photoinitiation of mesenchymal stem cell-laden bioresponsive hydrogels. Eur Cell Mater. 2011;22:43–55. doi: 10.22203/ecm.v022a04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Saik JE, Gould DJ, Watkins EM, Dickinson ME, West JL. Covalently immobilized platelet-derived growth factor-BB promotes angiogenesis in biomimetic poly(ethylene glycol) hydrogels. Acta biomaterialia. 2011;7:133–43. doi: 10.1016/j.actbio.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Culver JC, Hoffmann JC, Poché RA, Slater JH, West JL, Dickinson ME. Three-dimensional biomimetic patterning in hydrogels to guide cellular organization. Adv Mater. 2012;24:2344–8. doi: 10.1002/adma.201200395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Stephens EH, Carroll JL, Grande-Allen KJ. The use of collagenase III for the isolation of porcine aortic valvular interstitial cells: rationale and optimization. J Heart Valve Dis. 2007;16:175–83. [PubMed] [Google Scholar]
- [34].Nguyen QT, Hwang Y, Chen AC, Varghese S, Sah RL. Cartilage-like mechanical properties of poly (ethylene glycol)-diacrylate hydrogels. Biomaterials. 2012;33:6682–90. doi: 10.1016/j.biomaterials.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Drira Z, Yadavalli VK. Nanomechanical measurements of polyethylene glycol hydrogels using atomic force microscopy. J Mech Behav Biomed Mater. 2013;18:20–8. doi: 10.1016/j.jmbbm.2012.09.015. [DOI] [PubMed] [Google Scholar]
- [36].Durst CA, Cuchiara MP, Mansfield EG, West JL, Grande-Allen KJ. Flexural characterization of cell encapsulated PEGDA hydrogels with applications for tissue engineered heart valves. Acta Biomater. 2011;7:2467–76. doi: 10.1016/j.actbio.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Clark RE. Stress-strain characteristics of fresh and frozen human aortic and mitral leaflets and chordae tendineae. Implications for clinical use. J Thorac Cardiovasc Surg. 1973 Aug;66(2):202–8. [PubMed] [Google Scholar]
- [38].Missirlis YF, Chong M. Aortic valve mechanics-Part I: material properties of natural porcine aortic valves. J Bioeng. 1978;2:287–300. [PubMed] [Google Scholar]
- [39].Lee JM, Boughner DR, Courtman DW. The glutaraldehyde-stabilized porcine aortic valve xenograft. II. Effect of fixation with or without pressure on the tensile viscoelastic properties of the leaflet material. J Biomed Mater Res. 1984 Jan;18(1):79–98. doi: 10.1002/jbm.820180109. [DOI] [PubMed] [Google Scholar]
- [40].Stradins P, Lacis R, Ozolanta I, Purina B, Ose V, Feldmane L, Kasyanov V. Comparison of biomechanical and structural properties between human aortic and pulmonary valve. Eur J Cardiothorac Surg. 2004;26(3):634–9. doi: 10.1016/j.ejcts.2004.05.043. [DOI] [PubMed] [Google Scholar]
- [41].Taylor PM, Batten P, Brand NJ, Thomas PS, Yacoub MH. The cardiac valve interstitial cell. Int J Biochem Cell Biol. 2003;35(2):113–8. doi: 10.1016/s1357-2725(02)00100-0. [DOI] [PubMed] [Google Scholar]
- [42].Thayer P, Balachandran K, Rathan S, Yap CH, Arjunon S, Jo H, Yoganathan AP. The effects of combined cyclic stretch and pressure on the aortic valve interstitial cell phenotype. Ann Biomed Eng. 2011;39(6):1654–67. doi: 10.1007/s10439-011-0273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB, Hubbell JA. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. Proc Natl Acad Sci USA. 2003;100:5413–8. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Moon JJ, Saik JE, Poché RA, Leslie-Barbick JE, Lee SH, Smith AA, Dickinson ME, West JL. Biomimetic hydrogels with pro-angiogenic properties. Biomaterials. 2010;31:3840–7. doi: 10.1016/j.biomaterials.2010.01.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Benton JA, Fairbanks BD, Anseth KS. Characterization of valvular interstitial cell function in three dimensional matrix metalloproteinase degradable PEG hydrogels. Biomaterials. 2009;30:6593–603. doi: 10.1016/j.biomaterials.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Dreger SA, Taylor PM, Allen SP, Yacoub MH. Profile and localization of matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs) in human heart valves. J Heart Valve Dis. 2002;11:875–80. [PubMed] [Google Scholar]
- [47].Schoen FJ. Evolving concepts of cardiac valve dynamics: the continuum of development, functional structure, pathobiology, and tissue engineering. Circulation. 2008;118:1864–80. doi: 10.1161/CIRCULATIONAHA.108.805911. [DOI] [PubMed] [Google Scholar]
- [48].Nguyen QT, Hwang Y, Chen AC, Varghese S, Sah RL. Cartilage-like mechanical properties of poly (ethylene glycol)-diacrylate hydrogels. Biomaterials. 2012;33:6682–90. doi: 10.1016/j.biomaterials.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Turturro MV, Sokic S, Larson JC, Papavasiliou G. Effective tuning of ligand incorporation and mechanical properties in visible light photopolymerized poly(ethylene glycol) diacrylate hydrogels dictates cell adhesion and proliferation. Biomed Mater. 2013;8:025001. doi: 10.1088/1748-6041/8/2/025001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ashley GW, Henise J, Reid R, Santi DV. Hydrogel drug delivery system with predictable and tunable drug release and degradation rates. Proc Natl Acad Sci USA. 2013;110:2318–23. doi: 10.1073/pnas.1215498110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Courtney T, Sacks MS, Stankus J, Guan J, Wagner WR. Design and analysis of tissue engineering scaffolds that mimic soft tissue mechanical anisotropy. Biomaterials. 2006;27:3631–8. doi: 10.1016/j.biomaterials.2006.02.024. [DOI] [PubMed] [Google Scholar]
- [52].Masoumi N, Jean A, Zugates JT, Johnson KL, Engelmayr GC. Laser microfabricated poly(glycerol sebacate) scaffolds for heart valve tissue engineering. J Biomed Mater Res A. 2013;101:104–14. doi: 10.1002/jbm.a.34305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Miller JS, Shen CJ, Legant WR, Baranski JD, Blakely BL, Chen CS. Bioactive hydrogels made from step-growth derived PEG-peptide macromers. Biomaterials. 2010;31:3736–43. doi: 10.1016/j.biomaterials.2010.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Schukur L, Zorlutuna P, Cha JM, Bae H, Khademhosseini A. Directed Differentiation of Size-Controlled Embryoid Bodies Towards Endothelial and Cardiac Lineages in RGD-Modified Poly(Ethylene Glycol) Hydrogels. Adv Healthc Mater. 2013;2:195–205. doi: 10.1002/adhm.201200194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J Cell Sci. 2006;119:3901–3. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Lei Y, Rémy M, Labrugère C, Durrieu MC. Peptide immobilization on polyethylene terephthalate surfaces to study specific endothelial cell adhesion, spreading and migration. J Mater Sci Mater Med. 2012;23:2761–72. doi: 10.1007/s10856-012-4736-x. [DOI] [PubMed] [Google Scholar]
- [57].Patel D, Vandromme SE, Reid ME, Taite LJ. Synergistic activity of αvβ3 integrins and the elastin binding protein enhance cell-matrix interactions on bioactive hydrogel surfaces. Biomacromolecules. 2012;13:1420–8. doi: 10.1021/bm300144y. [DOI] [PubMed] [Google Scholar]
- [58].Goubko CA, Majumdar S, Basak A, Cao X. Hydrogel cell patterning incorporating photocaged RGDS peptides. Biomed Microdevices. 2010;12:555–68. doi: 10.1007/s10544-010-9412-7. [DOI] [PubMed] [Google Scholar]
- [59].Xu FJ, Wang ZH, Yang WT. Surface functionalization of polycaprolactone films via surface-initiated atom transfer radical polymerization for covalently coupling cell-adhesive biomolecules. Biomaterials. 2010;31:3139–47. doi: 10.1016/j.biomaterials.2010.01.032. [DOI] [PubMed] [Google Scholar]
- [60].Sainger R, Grau JB, Branchetti E, Poggio P, Seefried WF, Field BC, Acker MA, Gorman RC, Gorman JH, Hargrove CW, Bavaria JE, Ferrari G. Human myxomatous mitral valve prolapse: role of bone morphogenetic protein 4 in valvular interstitial cell activation. J Cell Physiol. 2012;227:2595–604. doi: 10.1002/jcp.22999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Durbin AD, Gotlieb AI. Advances towards understanding heart valve response to injury. Cardiovasc Pathol. 2002;11:69–77. doi: 10.1016/s1054-8807(01)00109-0. [DOI] [PubMed] [Google Scholar]
- [62].Stephens EH, Shangkuan J, Kuo JJ, Carroll JL, Kearney DL, Carberry KE, Fraser CD, Jr, Grande-Allen KJ. Extracellular matrix remodeling and cell phenotypic changes in dysplastic and hemodynamically altered semilunar human cardiac valves. Cardiovasc Pathol. 2011;20:e157–67. doi: 10.1016/j.carpath.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Xu S, Liu AC, Kim H, Gotlieb AI. Cell density regulates in vitro activation of heart valve interstitial cells. Cardiovasc Pathol. 2012;21:65–73. doi: 10.1016/j.carpath.2011.01.004. [DOI] [PubMed] [Google Scholar]
- [64].Cushing MC, Liao JT, Anseth KS. Activation of valvular interstitial cells is mediated by transforming growth factor-beta1 interactions with matrix molecules. Matrix Biol. 2005;24:428–37. doi: 10.1016/j.matbio.2005.06.007. [DOI] [PubMed] [Google Scholar]
- [65].Gould ST, Darling NJ, Anseth KS. Small peptide functionalized thiol-ene hydrogels as culture substrates for understanding valvular interstitial cell activation and de novo tissue deposition. Acta Biomater. 2012;8:3201–9. doi: 10.1016/j.actbio.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Quinlan AM, Billiar KL. Investigating the role of substrate stiffness in the persistence of valvular interstitial cell activation. J Biomed Mater Res A. 2012;100:2474–82. doi: 10.1002/jbm.a.34162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Stephens EH, Durst CA, West JL, Grande-Allen KJ. Mitral valvular interstitial cell responses to substrate stiffness depend on age and anatomic region. Acta Biomater. 2011;7:75–82. doi: 10.1016/j.actbio.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.