Highlights
-
•
Patients with severe liver injury may as part of the initial damage control operation, concurrently with intermittent Pringle maneuver, and intra- and perihepatic packing.
-
•
For patient unstable to undergo embolization in the intervention radiology suite, or if there is no such service in the hospital, selective vascular isolation of various liver vessels performed by the trauma surgeon should be an option.
-
•
Trauma surgeons should be able and trained to perform selective vascular isolation for liver injuries.
Keywords: Severe liver injury, Selective vascular isolation for liver injury, Embolization, Damage control
Abstract
Background
Severe liver trauma (grade 4 and 5) carries mortality greater than 40%. It represents a major surgical challenge in patients with hemodynamic instability who require an immediate exploratory laparotomy. Perihepatic packing and damage control can sometimes work, but for severe liver injuries, adjunct maneuvers might be needed (such as early embolization or hepatic artery ligation). During a patient’s first operation for severe liver trauma, anatomic resection is rarely tolerated.
Materials and methods
We managed a 31 year-old male with a blunt grade 5 right-lobe liver injury in severe hypovolemic shock.
Results
As part of the initial damage control operation, concurrently with intermittent Pringle maneuver, he underwent intra- and perihepatic packing; selective isolation and ligation of the right portal vein, right hepatic artery, and right hepatic vein; and repair of the retrohepatic inferior vena cava. Then, 36 h later, the patient underwent a right hepatectomy.
Conclusion
For patients with severe liver injuries, selective vascular isolation and ligation may be considered as part of damage control (in addition to intermittent Pringle maneuver) and might enable anatomic resection at a later stage.
1. Background
In recent decades, management of liver injuries has undergone significant changes. Even patients with severe liver injuries (grades 4 and 5) do not require an exploratory laparotomy, as long as they are hemodinamically stable. With advances in the use of massive blood transfusion protocols [1], along with the ability of intervention radiologists to perform selective embolization of arterial bleeds of the liver [2], the need for major operative intervention in liver trauma has diminished significantly [3–6]. As trauma surgeons, most of us now rely on perihepatic packing, abbreviated surgery or damage control, and intervention radiology for the management of these important injuries.
We report the case of a young man with a blunt grade 5 right-lobe liver injury who came in severe hypovolemic shock. As part of the initial damage control operation, he underwent intermittent Pringle maneuver; intra- and perihepatic packing; selective vascular isolation with ligation of the right portal vein, right hepatic artery, and right hepatic vein; and repair of the retrohepatic inferior vena cava. Then, 36 h later, he successfully underwent a right hepatectomy. All of this was done after intrahepatic packing with all available clotting materials failed to halt the bleeding. To our knowledge, such a case has not previously appeared in the literature.
2. Our patient
A 31-year-old man was hit by a high-speed SUV, while sitting in his office at a construction site; the wall collapsed on him and caused major injuries to the right side of the chest and abdomen. He was brought to trauma resuscitation room of the level I trauma center, in severe hypovolemic shock, with barely palpable pulses. He was taken directly to the operating room without any delay. Simultaneously, he was intubated, and the abdomen was opened through a midline incision from the xiphoid process to the pubic bone. About 4 l of blood was encountered in the abdomen. Once intubated, on release of the abdominal tamponade, he promptly lost his peripheral pulses, requiring aortic clamping and packing to allow the anesthesia team to catch up with blood and blood product transfusion.
On re-examination, it was revealed massive destruction of the right lobe of the liver, as well as other injuries, including right lower rib fractures. We placed several rolls of Kerlix gauze and any available hemostatic gauze inside the dome of the right lobe. With intrahepatic and perihepatic packing, intermittent finger clamping of the upper abdominal aorta, and intermittent Pringle maneuver, we were able to obtain temporary hemostasis and a recordable blood pressure, while the massive blood transfusion protocol was being implemented. Attempts to terminate the operation and take the patient to the intervention radiology suite or to the intensive care unit (ICU) for further resuscitation were unsuccessful, because of welling up of the blood from inside the liver that was coming from behind the gallbladder, as well as from the suprahepatic region. During the operation, the patient was given three shipment of massive blood transfusion with a ratio 1:1:1 of PRB:FFP:Platelets.
At this stage, we extended the incision transversely on the right side to obtain easier exposure to the retrohepatic portion of the liver and to be able to deliver the liver into the operative field. We completed the cholecystectomy, which revealed communication of the destructive area between the anterior and posterior portion of the right lobe. Faced with this revelation, we followed the cystic duct remnant to expose the portal triad. After identifying the left portal vein, artery, and duct, we identified— and placed large clips on— the right portal vein, right hepatic artery, and right hepatic duct. With intrahepatic packing, suturing of the liver with large liver sutures (Fig. 1), and selective vascular isolation of the right lobe, we obtained hemodynamic normalcy, although the patient’s acidosis was still significant. We packed the abdomen with wet Kerlix gauze, closed the skin with continuous 1.0 nylon sutures to maintain tamponade, and brought him to the ICU.
Fig. 1.
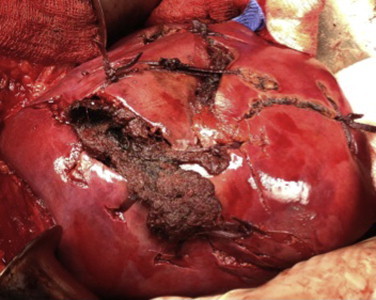
Massive liver destruction packed and sutured at the end of damage control still oozing.
In the ICU, the patient required acute dialysis. Then, 36 h later, we took him back to the operating room for a formal right hepatectomy (Fig. 2). Most of the right lobe was necrotic (Fig. 3a–c). During the second operation, we transected the right portal vein, and transected, and suture-ligated the right hepatic artery and duct, and transected the right hepatic vein. We preserved the middle hepatic vein. Once we removed the packing and removed the right lobe of the liver we recognized a small laceration (3–4 mm) on the retrohepatic vena cava, which was repaired with 5 prolene continuous suture. The remaining liver lobe was well perfused, although we had to use the Pringle maneuver for 17 min. We placed 2 large drains (19 French) and closed the abdominal wall. We left the skin open, but approximated closure with 2.0 nylon sutures, delaying definitive closure.
Fig. 2.
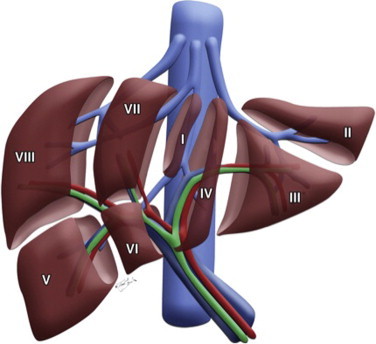
Cartoon of the liver lobes depicting vasculararization. See operative description for details of selective vascular ligation.
Fig. 3.
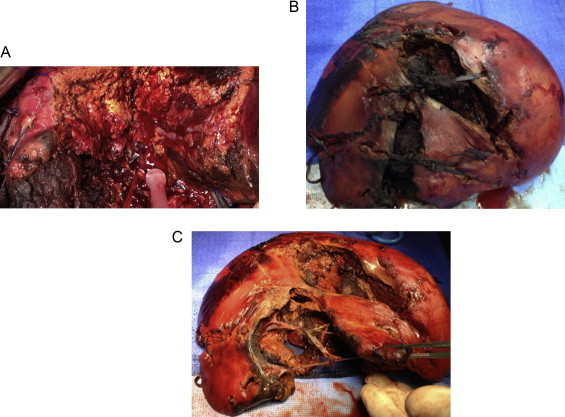
(a) View from resection of the right lobe. Left lobe of the liver well perfused. (b) Right lobe of the liver at the back table with packs in. (c) Right lobe of the liver after the packs were removed.
The patient’s postoperative course was complicated by bacteremia and fungemia. A computed tomography (CT) revealed dilatation of the left intrahepatic duct. On postoperative day (POD) #8, he underwent magnetic resonance cholangiopancreatography (MRCP), which revealed no ductal obstruction. It was thought that periportal edema and inflammation were responsible for the intrahepatic dilatation. He was extubated on POD# 14 and resumed oral feeding; for enteral nutrition, we prescribed a special formula enriched with branched-chain amino acids and with additional taurine. He continued on daily dialysis.
On POD# 25, he again developed sepsis and was found to have a necrotizing myositis of bilateral rectus muscles (Fig. 4a,b). He underwent resection of that dead tissue; his remaining liver was cholestatic (Fig. 5). He recovered from this episode nicely and was undergoing abdominal wound treatment with wound VAC dressing, and was extubated. On POD# 33, he developed another episode of septic shock and did not response to aggressive intensive care therapy. He died on POD# 37.
Fig. 4.
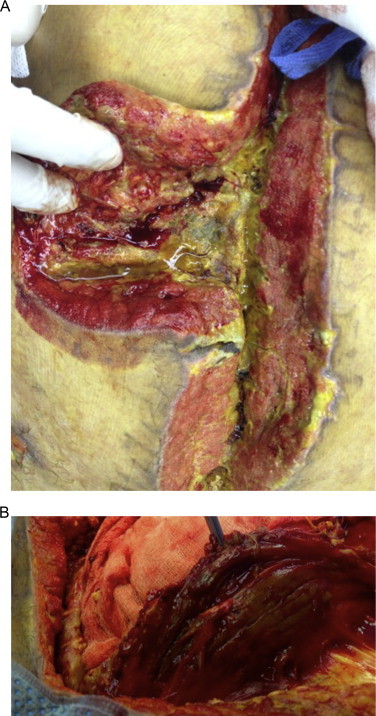
(a) Abdominal wall wound infections, requiring massive debridment. (b) Myositis of left rectus muscle.
Fig. 5.
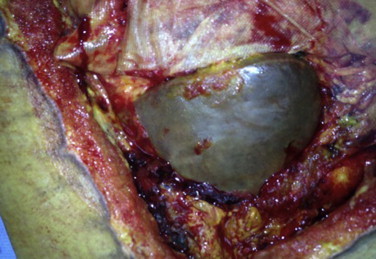
Intraoperative left lobe of the liver at the time of excision of necrotic abdominal wall.
3. Discussion
Various techniques to arrest severe bleeding from the liver or from the retrohepatic vena cava have been described. The Schrock shunt [7,8], total vascular occlusion [9], venovenous bypass [10,11], and even liver transplants [12] have each been deployed, with varied success. However, most of us do not have enough experience with any of those surgical techniques, so we rely on packing. Typically, by the time we think of any of those techniques, patient is already on a downhill slope toward the triangle of death with severe acidosis, coagulopathy, and hypothermia.
Total or selective vascular occlusion for liver resection has been popularized in patients undergoing elective liver surgery, mainly for tumor resection [13,14], yet it remains controversial. In patients with liver trauma, most published reports have been on selective hepatic artery ligation [15,16]; however, with the advent of embolization done by intervention radiologists or perhaps vascular surgeons [17,18], selective hepatic artery ligation is now rarely reported these days.
Despite all advances, the management of severe liver trauma represents a significant challenge [19–21]. Most patients with blunt trauma [22,23], and even some with penetrating trauma [24], are now cared for nonoperatively, yet those with acute massive injuries may still need a liver resection. For such injuries, damage control and intervention radiology embolization have become standard. Many countries in the world, however, do not have resources nor expertise for embolization. Clearly, attempting to resect major portions of the liver in an acidotic, hypothermic patient who is in severe hypovolemic shock would kill that patient. Thus, adjunct techniques and initial maneuvers are needed. Total vascular occlusion can be used to repair major bleeds in a short time, but massive resuscitation is required; too often, patients with massive liver injuries do not tolerate it. Moreover, to safely obtain suprahepatic control, the surgeon may have to enter the right chest, exposing the patient to further surgery and potential risks. Selective vascular isolation or ligation should be used when peri- and intrahepatic packing does not arrest a patient's bleeding. When the bleeding is arterial and stops with a Pringle maneuver [25], then ligation of the hepatic artery (after its bifurcation) is a very good choice. On occasion, the main trunk of the hepatic artery needs to be ligated. However, in our patient, the bleeding decreased yet then continued, despite the clamping of the porta hepatis; in such situations, the surgeon must identify and ligate the bleeding vessels, as part of damage control. After the bleeding has been stopped, the patient can be resuscitated in the ICU, with care taken to not flood the patient with crystalloid solution. Central venous pressure should be maintained at less than 10 cm H20, in order to avoid excessive congestion of the remaining vascularized liver. The hepatic veins should be ligated to eliminate the backflow of bleeding from the suprahepatic inferior vena cava.
Liver resections and liver operations are rarely performed in trauma patients anymore, thanks to the advances mentioned above, so trauma surgeons are being exposed to complex liver resections less and less. The decision not to operate at all, or to just pack the liver, has become routine. When trauma surgeons do attempt a liver resection, the results may not be very favorable. Thus, the need for trauma surgeons to be trained in hepatobiliary surgery and in complex liver resections has become acute. The advent of the acute care surgery fellowship might help. Trauma surgeons also need to consider collaborating more closely with colleagues who have real experience in liver surgery, including those in transplantation [26]. Involving the transplant team at an early stage in a patient’s course is important for another reason, namely, to help avoid long-term complications posttransplant. Unfortunately our patient died from septic shock and we were unable to identify and treat the cause. His kidney failure never resolved and he required daily dialysis. His liver enzymes become normal, but bilirubin level was elevated throughout the entire post-operative period. He was eating regular diet until he developed the last episode of sepsis that cascaded into irreversible septic shock.
4. Conclusion
Although our patient died from septic shock on POD# 37, we believe that in patients with severe liver injuries, selective vascular isolation and ligation should be part of damage control (in addition to intermittent Pringle maneuver). In particular this may be applicable for patient that cannot be moved to interventional radiology suite, or when this modality is not available, as in many developing countries. This might enable anatomic resection at a later stage. Of crucial importance, trauma surgeons need to be trained in hepatobiliary surgery and in complex liver resections.
Conflicts of interest
No conflict of interst.
Funding
None.
Author contribution
Dr. Latifi and Dr. Khalaf performed the surgery.
Dr. Latifi designed the study, reviewed the literature and wrote the manuscript.
Dr. Khalaf revieed critically the manuscript.
Footnotes
Presented as Oral Presentation at the 2nd World Congress of Emergency Surgery, July, 2013, Bergamo, Italy.
References
- 1.Malone D., Hess L., John R., Fingerhut A. Massive transfusion practices around the globe and a suggestion for a common massive transfusion protocol. J. Trauma. 2006;60(6):S91–S96. doi: 10.1097/01.ta.0000199549.80731.e6. [DOI] [PubMed] [Google Scholar]
- 2.Mohr A.M., Lavery R.F., Barone A., Bahramipour P. Angiographic embolization for liver injuries: low mortality, high morbidity. J. Trauma. 2003;55(6):1077–1082. doi: 10.1097/01.TA.0000100219.02085.AB. [DOI] [PubMed] [Google Scholar]
- 3.Cogbill T.H., Moore E.E., Jurkovich G.J., Feliciano D.V. Severe hepatic trauma: a multi-center experience with 1335 liver injuries. J. Trauma. 1988;28(10):1433–1438. [PubMed] [Google Scholar]
- 4.Feliciano D.V., Pachter H.L. Hepatic trauma revisited. Curr. Prob. Surg. 1989;26(7):453–524. doi: 10.1016/0011-3840(89)90014-2. [DOI] [PubMed] [Google Scholar]
- 5.Pachter H.L., Spencer F.C., Hofstetter S.R. Significant trends in the treatment of hepatic trauma experience with 411 injuries. Ann. Surg. 1992;215(5):492–502. doi: 10.1097/00000658-199205000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feliciano D.V., Mattox K.L., Jordan G.L., Jr. Management of 1000 consecutive cases of hepatic trauma (1979–1984) Ann. Surg. 1986;204(4):438–445. doi: 10.1097/00000658-198610000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schrock T., Blaisdell F.W., Mathewson C., Jr. Management of blunt trauma to the liver and hepatic veins. Arch. Surg. 1968;96(5):698–704. doi: 10.1001/archsurg.1968.01330230006002. [DOI] [PubMed] [Google Scholar]
- 8.Burch J.M., Feliciano D.V., Mattox K.L. The atriocaval shunt. Facts and fiction. Ann. Surg. 1988;207(5):555–568. doi: 10.1097/00000658-198805000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du D.-Y., Zhao X.-J., Liu G.-L. Liver trauma: experience in 348 cases. World J. Surg. 2003;27(6):703–708. doi: 10.1007/s00268-003-6573-z. [DOI] [PubMed] [Google Scholar]
- 10.Baumgartner F., Scudamore C., Nair C. Venovenous bypass for major hepatic and caval trauma. J. Trauma. 1995;39(4):671–673. doi: 10.1097/00005373-199510000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Biffl W., Moore E.E., Franciose R.J. Venovenous bypass and hepatic vascular isolation as adjuncts in the repair of destructive wounds to the retrohepatic inferior vena cava – case reports. J. Trauma. 1998;45(2):400–403. doi: 10.1097/00005373-199808000-00038. [DOI] [PubMed] [Google Scholar]
- 12.Tucker O.N., Marriott P., Rela M., Heaton N. Emergency liver transplantation following severe liver trauma. Liver Transpl. 2008;14(8):1204–1210. doi: 10.1002/lt.21555. [DOI] [PubMed] [Google Scholar]
- 13.Zhou W., Li A., Pan Z., Fu S., Yang Y., Tang L., Hou Z., Wu M. Selective hepatic vascular exclusion and Pringle maneuver: a comparative study in liver resection. Eur. J. Surg. Oncol. 2008;34(1):49–54. doi: 10.1016/j.ejso.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Bismuth H., Castaing D., Garden O.J. Major hepatic resection under total vascular exclusion. Ann. Surg. 1989;210(July (1)):13–19. doi: 10.1097/00000658-198907000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gurusamy K.S., Kumar Y., Sharma D., Davidson B.R. Methods of vascular occlusion for elective liver resection. Cochrane Database Syst. Rev. 2009;1:CD006409. doi: 10.1002/14651858.CD006409.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Flint L.M., Jr., Polk H.C., Jr. Selective hepatic artery ligation: limitations and failures. J. Trauma. 1979;19(5):319–323. doi: 10.1097/00005373-197905000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Lopera J.E. Embolization in trauma: principles and techniques. Semin. Intervent. Radiol. 2010;27(1):14–28. doi: 10.1055/s-0030-1247885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jason Bauer R., Ray C.E., Jr. Transcatheter arterial embolization in the trauma patient: a review. Semin. Intervent. Radiol. 2004;21(1):11–22. doi: 10.1055/s-2004-831401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beal S.L. Fatal hepatic hemorrhage: an unresolved problem in the management of complex liver injuries. J. Trauma. 1990;30(2):163–169. [PubMed] [Google Scholar]
- 20.Fabian T.C., Croce M.A., Stanford G.G. Factors affecting morbidity following hepatic trauma. A prospective analysis of 482 injuries. Ann. Surg. 1991;213(6):540–548. doi: 10.1097/00000658-199106000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asensio J.A., Petrone P., García-Núñez L. Multidisciplinary approach for the management of complex hepatic injuries AAST-OIS grades IV-V: a prospective study. Scand J. Surg. 2007;96(3):214–220. doi: 10.1177/145749690709600306. [DOI] [PubMed] [Google Scholar]
- 22.Polanco P., Leon S., Pineda J., Puyana J.C. Hepatic resection in the management of complex injury to the liver. J. Trauma. 2008;65(6):1264–1269. doi: 10.1097/TA.0b013e3181904749. discussion 1269–70. [DOI] [PubMed] [Google Scholar]
- 23.Malhotra A.K., Latifi R., Fabian T.C. Multiplicity of solid organ injury: influence on management and outcomes after blunt abdominal trauma. J. Trauma. 2003;54(5):925–929. doi: 10.1097/01.TA.0000066182.67385.86. [DOI] [PubMed] [Google Scholar]
- 24.Demetriades D., Gomez H., Chahwan S., Charalambides K. Gunshot injuries to the liver: the role of selective nonoperative management. JACS. 1999;188(4):343–348. doi: 10.1016/s1072-7515(98)00315-9. [DOI] [PubMed] [Google Scholar]
- 25.Pringle J.H.V. Notes on the arrest of hepatic hemorrhage due to trauma. Ann. Surg. 1908;48(4):541–549. doi: 10.1097/00000658-190810000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Petri S., Gruttadauria S., Pagano D. Surgical management of complex liver trauma: a single liver transplant center experience. Am. Surg. 2012;78(January (1)):20–25. [PubMed] [Google Scholar]


