Highlights
-
•
Case of 83 year old gentleman with chronic anaemia and ominous GI symptoms but unremarkable initial examination and investigations.
-
•
Leiomyosarcomas of the small bowel is extremely rare and can be differentiated from gastrointestinal stromal tumours by immunohistochemical methods.
-
•
Imaging in the form of MRE, CT colonography or WCE are crucial when faced with a differential diagnosis of small bowel malignancy.
Keywords: Leiomyosarcoma, Small bowel tumour
Abstract
INTRODUCTION
Leiomyosarcoma of the small bowel is an extremely rare form of gastrointestinal malignancy. Small bowel tumours are usually asymptomatic at the early stages, and difficult to visualise by upper and lower endoscopy.
PRESENTATION OF CASE
An 83-year-old gentleman presented in surgical outpatient clinic with chronic anaemia, abdominal discomfort and a single episode of malaena. Initial OGD and colonoscopy were both unremarkable. Subsequent CT revealed a mass in the right iliac fossa of likely small bowel origin, leading to an urgent laparotomy and resection with primary anastomosis. Histopathology showed a high grade leiomyosarcoma with no signs of metastasis and confirmatory immunological staining. Post-surgery follow up remains unremarkable.
DISCUSSION
Leiomyosarcomas of the small bowel are extremely rare entities, particularly following the advent of robust immunohistological diagnostic methods allowing differentiation from GISTs. As small bowel tumours are often not visualised by upper and lower endoscopy, further investigations to visualise the small bowel are crucial, generally in the form of magnetic resonance enterography, CT colonography or wireless capsule endoscopy.
CONCLUSION
The treatment of such tumours remains predominantly centred around surgical resection, and prognosis is dependent on tumour size and histological staging.
1. Introduction
Tumours of the small bowel account for less than 5% of all gastrointestinal malignancies.1 Of these, the majority are histologically classed as carcinoid and adenocarcinomas, with sarcomas ranking 5th (∼1.2%).2 Leiomyosarcoma is the most common sarcoma, but remains very scarce; with only 26 reported cases of primary small bowel leiomyosarcoma following robust immunohistological techniques.3,4 Small bowel tumours are usually asymptomatic at the early stages, and difficult to visualise by upper and lower endoscopy.5 Consequently, they often present late with poor prognosis. In this report, we give the account of an extremely rare case of leiomyosarcoma of the small bowel presenting in an 83-year-old gentleman with chronic anaemia and ominous GI symptoms but unremarkable initial examination and investigations.
2. Presentation of case
An 83-year-old gentleman was referred to the colorectal outpatient clinic following the discovery of chronic anaemia with a haemoglobin level of 95 g/L, mean corpuscular volume of 85.7 fL, ferritin of 20 mg/L, and normal vitamin B12 and folate levels; this was carried out under the two weeks urgent referral scheme for suspected malignancy. Further questioning revealed a single episode of malaena, some lower abdominal discomfort and a slight change in bowel habit. He was known to have diverticular disease, hypertension and ischaemic heart disease, having undergone a coronary artery bypass graft a few years before. His medications included aspirin, atorvastatin and a variety of antihypertensive agents.
On initial examination, he appeared generally well with an unremarkable abdominal examination. At this point he was transfused 3 units of red cells as the haemoglobin level declined further to 80 g/L; outpatient appointments for oesophago-gastro-duodenoscopy (OGD) and colonoscopy were arranged.
Both the OGD and colonoscopy gave no clues as to the source of anaemia; therefore a computed tomography (CT) of the chest, abdomen and pelvis was carried out (Figs. 1 and 2). The post contrast images demonstrate a lobulated, heterogeneously enhancing soft tissue mass in the right iliac fossa, likely small bowel in origin, with no overt obstruction, lymph node involvement or free fluid. No metastases were detected.
Figs. 1 and 2.
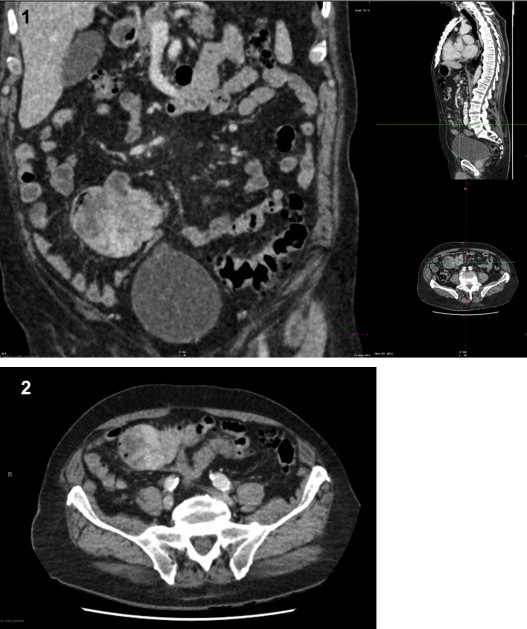
Coronal (1) and axial (2) CT post contrast images demonstrating a lobulated, heterogeneously enhancing soft tissue mass in the right iliac fossa, of likely small bowel origin. No evidence overt small bowel obstruction, lymph node involvement or free fluid.
An urgent laparotomy was performed, resulting in the resection of a small bowel mass and its surrounding tissue with primary anastomosis. The operation was without complication and post-operative management was uneventful.
Gross analysis showed a large polypoidal tumour measuring 57 mm × 55 mm × 30 mm invading into the serosa. The microscopic appearances are those of a leiomyosarcoma Trojani grade 3. A clear resection margin of 150 mm was achieved, with 10 lymph nodes recovered that showed no evidence of metastasis. Histological examination showed a submucosal malignant spindle cell tumour extending into the mucosa and muscularis propria. Tumour cells were arranged in a fascicular growth pattern and had cigar-shaped nuclei with multiple abnormal mitotic figures. Immunohistochemical stains demonstrated positive reactions for smooth muscle markers including smooth muscle actin (SMA), myosin, H-caldesmon and desmin. They were negative for CD34 (vascular marker as well as haematopoietic progenitor cell antigen and stromal cells marker), tyrosine kinase c-kit (CD117) receptor, and DOG1, which is usually expressed on the plasma membrane of gastrointestinal stromal tumours (GIST). The proliferation marker Ki-67 was variable, approximately 30% in the most active areas (Figs. 3–5).
Fig. 3.
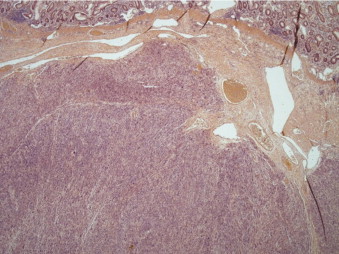
Sub-mucosal spindle cell tumour infiltrating the muscularis mucosae (H&E stain).
Fig. 4.
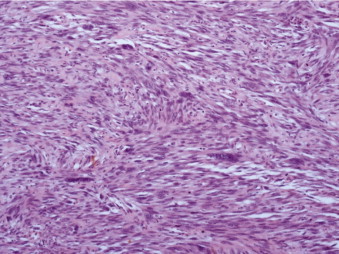
The tumour is arranged in fascicles of spindle cells with “Cigar shaped”, atypical nuclei (H&E stain).
Fig. 5.
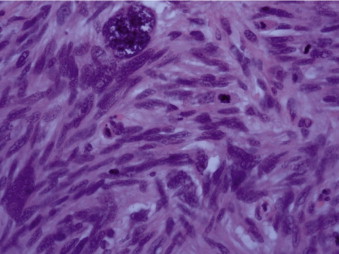
Large bizarre nuclei and multiple abnormal mitoses indicative of high-grade leiomyosarcoma (H&E stain).
This case was discussed at the local lower GI multidisciplinary meeting, with histological results confirmed at a regional tertiary centre. Regular outpatient follow up remains unremarkable.
3. Discussion and conclusion
The incidence of small bowel tumours is 22.7 cases per million, with sarcomas accounting for only 1.2% of these.1 Leiomyosarcomas of the small bowel are therefore extremely rare entities, to the extent that the World Health Organisation can provide no meaningful data on demographic or clinical features as a result. Only 26 documented cases of small bowel leiomyosarcomas were identified following the advent of its robust differentiation from gastrointestinal stromal tumours (GISTs), illustrating the importance of reporting such cases.3
The diagnosis of small bowel tumours can prove difficult, as the presentation can be delayed until metastases are present.5 Furthermore, traditional management of ominous symptoms such as weight loss, constipation and rectal bleeding consists of OGD and colonoscopy in the first instance.6 Small bowel tumours are easily missed by both modalities, and further imaging is therefore warranted. Currently, magnetic resonance enterography (MRE), CT colonography (CTC) and wireless capsule endoscopy (WCE) have all been shown to be effective in reaching the diagnosis.7 CTC and MRE are able to detect mucosal changes, whilst offering good sensitivity to any metastatic changes,8,9 as well as information with regard to any metastases. WCE on the other hand is superior in visualising small superficial lesions.10 Positron emission tomography may provide additional utility in detecting metastatic disease,11 however it is not in widespread usage.
Despite advances in imaging, the differentiation between benign and malignant tumours remains extremely difficult pre-operatively. The aetiology of many cases does not become apparent until after definitive resection, the case presented here being a prime example. Histologically, leiomyosarcomas are differentiated from GISTs by the lack of CD117 (c-KIT), DOG1 and CD34 as well as presence of smooth muscle actin (SMA) and desmin.3,12 The tumour can be graded using the Trojani13 or French14 systems for soft tissue sarcomas, denoting its aggression.
The treatment of all small bowel tumours remains centred around radical surgical resection.5 However, given the proportion of cases with late presentations, metastases are an unfortunately common finding. Whilst adjuvant chemotherapy agents are in use for GISTs12 and uterine15 leiomyosarcomas, their uses have not been proven in the case of small bowel disease.
Tumour size and histological staging are independent prognostic factors for 5 year disease-specific survival (DSS) in patients with small bowel sarcomas; these appear to be more favourable than small bowel adenocarcinomas.16 However, the overall prognosis remains poor.
In summary, leiomyosarcoma in the small bowel is a rare finding; the management relies on accurate diagnosis and a predominantly surgical approach. Given the relatively inaccessible location of the small intestine to both upper and lower GI endoscopy, this case highlights the importance of further imaging in the form of MRE, CT colonography or WCE when faced with a differential diagnosis of small bowel malignancy.
Conflict of interest
None.
Funding
None.
Ethical approval
Patient consent was obtained for this case report, and is available at the request of the publisher.
Author contributions
Joshua Luis: main writer of the manuscript; Farshid Ejtehadi: clinical case identification, case history, contributed to writing and review of manuscript; David Howlett: all sections in relation to radiology, contributed to writing and review of manuscript, acquisition of images; Imelda Donnellan: overseeing consultant of case and case report, contributed to writing and review of manuscript.
Acknowledgement
The authors would like to acknowledge Dr. Maria Bahhadi-Hardo at Frimley Park Hospital for her insight and contribution in histopathology.
Key learning points.
-
1.
Leiomyosarcomas of the small bowel can be differentiated from gastrointestinal stromal tumours by immunohistochemical methods.
-
2.
Following normal upper and lower endoscopies, the management of patients with high suspicion of gastrointestinal malignancy should include urgent second line investigations such as CT colonography, MR enterography or wireless capsule endoscopy in order to detect small bowel tumours.
References
- 1.Jemal A., Siegel R., Ward E., Hao Y., Xu J., Murray T. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(March–April (2)):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Bilimoria K.Y., Bentrem D.J., Wayne J.D., Ko C.Y., Bennett C.L., Talamonti M.S. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg. 2009;249(January (1)):63–71. doi: 10.1097/SLA.0b013e31818e4641. [DOI] [PubMed] [Google Scholar]
- 3.Aggarwal G., Sharma S., Zheng M., Reid M.D., Crosby J.H., Chamberlain S.M. Primary leiomyosarcomas of the gastrointestinal tract in the post-gastrointestinal stromal tumor era. Ann Diagn Pathol. 2012;16(December (6)):532–540. doi: 10.1016/j.anndiagpath.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Arts R., Bosscha K., Ranschaert E., Vogelaar J. Small bowel leiomyosarcoma: a case report and literature review. Turk J Gastroenterol. 2012;23(August (4)):381–384. doi: 10.4318/tjg.2012.0406. [DOI] [PubMed] [Google Scholar]
- 5.Coco C., Rizzo G., Manno A., Mattana C., Verbo A. Surgical treatment of small bowel neoplasms. Eur Rev Med Pharmacol Sci. 2010;14(April (4)):327–333. [PubMed] [Google Scholar]
- 6.Chandrasekhara V., Ginsberg G.G. Endoscopic management of gastrointestinal stromal tumors. Curr Gastroenterol Rep. 2011;13(December (6)):532–539. doi: 10.1007/s11894-011-0224-6. [DOI] [PubMed] [Google Scholar]
- 7.Jarman B.T. Small bowel imaging. Surg Clin North Am. 2011;91(February (1)):109–125. doi: 10.1016/j.suc.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Miao F., Wang M.L., Tang Y.H. New progress in CT and MRI examination and diagnosis of small intestinal tumors. World J Gastrointest Oncol. 2010;2(May (5)):222–228. doi: 10.4251/wjgo.v2.i5.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fidler J.L., Guimaraes L., Einstein D.M. MR imaging of the small bowel. Radiographics. 2009;29(October (6)):1811–1825. doi: 10.1148/rg.296095507. [DOI] [PubMed] [Google Scholar]
- 10.Jabr F.I., Skeik N. A leiomyosarcoma of the small bowels causing obscure gastrointestinal bleeding diagnosed by capsule endoscopy. J Med Liban. 2010;58(October–December (4)):238–240. [PubMed] [Google Scholar]
- 11.Punt S.E., Eary J.F., O‘Sullivan J., Conrad E.U. Fluorodeoxyglucose positron emission tomography in leiomyosarcoma: imaging characteristics. Nucl Med Commun. 2009;30(July (7)):546–549. doi: 10.1097/MNM.0b013e32832bcaec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joensuu H., Hohenberger P., Corless C.L. Gastrointestinal stromal tumour. Lancet. 2013;382(September (9896)):973–983. doi: 10.1016/S0140-6736(13)60106-3. [DOI] [PubMed] [Google Scholar]
- 13.Coindre J.M., Nguyen B.B., Bonichon F., de Mascarel I., Trojani M. Histopathologic grading in spindle cell soft tissue sarcomas. Cancer. 1988;61(June (11)):2305–2309. doi: 10.1002/1097-0142(19880601)61:11<2305::aid-cncr2820611126>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 14.Guillou L., Coindre J.M., Bonichon F., Nguyen B.B., Terrier P., Collin F. Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft tissue sarcoma. J Clin Oncol. 1997;15(January (1)):350–362. doi: 10.1200/JCO.1997.15.1.350. [DOI] [PubMed] [Google Scholar]
- 15.Hensley M.L., Wathen J.K., Maki R.G., Araujo D.M., Sutton G., Priebat D.A. Adjuvant therapy for high-grade, uterus-limited leiomyosarcoma: results of a phase 2 trial (SARC 005) Cancer. 2013;119(April (8)):1555–1561. doi: 10.1002/cncr.27942. [DOI] [PubMed] [Google Scholar]
- 16.Howe J.R., Karnell L.H., Scott-Conner C. Small bowel sarcoma: analysis of survival from the National Cancer Data Base. Ann Surg Oncol. 2001;8(July (6)):496–508. doi: 10.1007/s10434-001-0496-4. [DOI] [PubMed] [Google Scholar]


