Highlights
-
•
Choriocarcinoma is a malignant germ cell that occurs in gonadal and extra-gonadal sites.
-
•
Choriocarcinoma is classified into gestational and non-gestational forms.
-
•
Non-gestational choriocarcinoma has been reported in extra-placental and extra-gonadal sites.
-
•
Choriocarcinoma rarely involves the adrenal glands.
-
•
We report the first case of an ectopic primary adrenal choriocarcinoma.
Abstract
Introduction
Non-gestational, extragonadal choriocarcinoma is a rare clinical entity.
Presentation of case
Herein, we report a 56 year old woman who presented with an incidental adrenal mass and was diagnosed with a non-gestational choriocarcinoma of the adrenal gland as the sole site of disease.
Discussion
To our knowledge, this is the first case of an ectopic primary adrenal choriocarcinoma. A metastasis from a primary tumor that completely regressed or that could not be identified is an alternate explanation.
Conclusion
It should be recognized that choriocarcinoma can affect the adrenal gland and it should be considered as a rare cause for an adrenal incidentaloma.
1. Introduction
Choriocarcinoma is a malignant germ cell tumor that occurs in gonadal and extragonadal sites. In women, choriocarcinoma is classified into gestational and non-gestational forms. In the gestational form, choriocarcinoma develops as a result of invasive growth and erosion of blood vessels in placental tissue, often resulting in hemorrhage and necrosis. Choriocarcinoma occurs in approximately 1 in 30,000 pregnancies, two thirds developing after a normal delivery and one third following a molar gestation.
Non gestational choriocarcinoma has been reported in extraplacental and extragonadal sites. The vast majority represents systemic metastases, with the most common sites being lung, liver and brain [1]. Occasionally the primary tumor regresses, leaving only metastatic lesions to be detected on imaging [2]. Extragonadal choriocarcinoma may occur as a result of abnormal migration of endodermal-derived germ cells along the urogenital ridge and localization of migrating cells in an ectopic site before reaching the gonads [3]. Extragonadal sites include the brain, lungs, stomach and liver. Herein, we report a case of an adrenal choriocarcinoma.
2. Case presentation
A 56 year old gravida 0, para 0 postmenopausal woman presented to an Emergency Department with abdominal pain and bloating. She had known hypertension and hypothyroidism from a total thyroidectomy performed for treatment of Graves disease at age 12. She had no prior history of malignancy and no abdominal surgeries. There was no family history of endocrinopathies. She smoked one-half of a pack of cigarettes per day for 20 years. On physical examination her blood pressure and heart rate were 122/72 mm Hg and 101 beats per minute. Her abdominal examination was normal.
She had computed tomography of the abdomen and pelvis completed and was found to have an incidental 4.4 × 3.5 × 5.3 cm right adrenal mass (Fig. 1) that was not present on imaging from two years prior. On a follow-up magnetic resonance imaging study, the mass was found to have heterogenous signal intensity on both T1 and T2-weighted pulse sequences. There was a 2.3 cm focal area of hemorrhage, adjacent stranding and edema and no fat component.
Fig. 1.

Computed tomography of right adrenal tumor, A axial, B coronal.
The patient was referred to our institution and underwent an evaluation to exclude a functional adrenal tumor. Physical examination revealed a normotensive woman in no acute distress with a body mass index of 28. She had bilateral proptosis. She had a well-healed scar in her lower neck with no palpable thyroid tissue, no cervicodorsal hump or prominence of the supraclavicular fat pads. Her abdomen was soft and nontender with no violaceous striae. She had no bruises and her skin turgor was normal. She had a normal neurologic examination. Laboratory studies included serum electrolytes, a 24-h urine collection for metanephrines, serum aldosterone, plasma renin activity and a 1 mg overnight dexamethasone suppression test, all of which were normal. She was diagnosed with a nonfunctioning tumor of the right adrenal gland that was 5.3 cm in maximum dimension.
Due to the increased risk of malignancy for adrenal tumors larger than 4.0 cm, she underwent a laparoscopic right adrenalectomy. Exploration revealed extensive peritoneal adhesions in the right upper quadrant of her abdomen. The mass was confined to the adrenal gland without local invasion. She recovered well and was discharged the following day.
Pathologic examination of the specimen demonstrated a tumor mass measuring 5.0 × 3.0 × 7.0 cm with a friable lobulated yellow–tan cut surface. The tumor was comprised of irregular sheets and nests of large undifferentiated trophoblast-like cells admixed with multinucleated syncytiotrophoblasts. Foci of extensive hemorrhage and necrosis were present and replaced the native adrenal architecture (Fig. 2). There was no evidence of local invasion. The tumor cells were positive for keratin, epithelial membrane antigen, and human choriogonadotropin (hCG). Based on the morphological and immunohistochemical features, the patient was diagnosed with a choriocarcinoma.
Fig. 2.
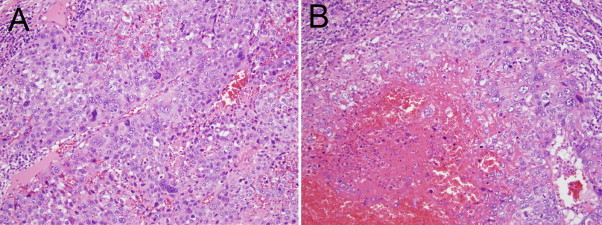
Hematoxylin and eosin stain of tumor, demonstrating irregular sheets and nests of large undifferentiated trophoblast-like cells admixed with multinucleated syncytiotrophoblasts (A) and foci of extensive hemorrhage and necrosis (B).
Postoperatively, a gynecologic oncologist further evaluated the patient. A pelvic exam was normal. Computed tomography of the chest, abdomen and pelvis revealed a normal uterus and ovaries with no evidence for additional sites of cancer. Serum β-hCG was 7.1 mIU/mL. Positron emission tomography in combination with computed tomography of the chest, abdomen and pelvis showed no focal area of abnormal metabolic activity. A follow up magnetic resonance imaging study of the pelvis revealed an incidental uterine leiomyoma and no adnexal masses. Adjuvant chemotherapy and hysteroscopy with dilation and curettage were recommended, both of which the patient had declined.
Six months following her initial evaluation for surgery, the patient presented to the Emergency Department with a three day history of nausea, dizziness and lightheadedness. She had computed tomography of the head, which showed two new enhancing cerebellar masses likely representing metastatic disease. Repeat serum β-hCG was 19.8 mIU/mL. She followed up with her gynecologic oncologist and after a long discussion, was agreeable to whole brain radiation. She is currently undergoing radiation and has not received additional treatment.
3. Discussion
Choriocarcinoma rarely involves the adrenal gland. Our case is unique in that it may represent an ectopic primary adrenal choriocarcinoma, which has not been previously reported. It was a nongestational choriocarcinoma that occurred in a patient who had never been pregnant. A review of the literature reveals primary choriocarcinoma reported in extragonadal sites such as the brain, lungs, stomach and liver, but not in the adrenal gland [4,5]. Although bilateral adrenal metastases have been reported in patients with choriocarcinoma [3], the bulk of evidence in our patient supports that the choriocarcinoma was a primary adrenal tumor with subsequent development of brain metastases. Alternative explanations for the choriocarcinoma in the patient's right adrenal gland include a metastasis from a primary tumor that has not yet been identified or a primary tumor that has undergone complete regression.
Treatment of choriocarcinoma includes tumor resection and systemic chemotherapy with methotrexate. In patients with gestational choriocarcinoma, evacuation of uterine contents by dilation and curettage is completed. Serial serum hCG levels are monitored and patients are evaluated for metastatic disease. The 5-year survival for choriocarcinoma exceeds 85%. Fertility is not impaired; however, there is a 2% risk of developing trophoblastic disease in subsequent pregnancies.
Conflict of interest
We have none.
Sources of funding
There were no source of funding for our research.
Consent
Ok. Consent obtained.
Authors contribution
Study design: Christopher R. McHenry.
Data collections: Christopher R.McHenry, Hannah Y. Zhou, Jane K. Nguyen, Santhi Ganesan.
Data analysis: Christopher R. McHenry, Hannah Y. Zhou, Jane K. Nguyen, Santhi Ganesan.
Writing: Christopher R. McHenry, Hannah Y. Zhou.
References
- 1.L.J. Copeland, Gestational trophoblastic neoplasia, in: W.B. Saunders, Textbook of Gynecology, 2nd ed. Philadelphia, 2000, 1418-1427.
- 2.Ibi T., Hirai K., Bassho R., Kawamoto M., Koizumi K., Shimizu K. Choriocarinoma of the lung: report of a case. Gen. Thoracic Cardiovas. Surg. 2012;60:377–380. doi: 10.1007/s11748-012-0009-3. [DOI] [PubMed] [Google Scholar]
- 3.Trastour C., Rahili A., Chevallier A., Bernard J., Bongain A., Sadoul J., Thyss A. Isolated bilateral adrenal choriocarcinoma. Lancet Oncol. 2005;6:905–907. doi: 10.1016/S1470-2045(05)70426-5. [DOI] [PubMed] [Google Scholar]
- 4.J.A. Cowan, B.G. Thompson, in: Doherty GM, ed. Current Diagnosis & Treatment,13th ed. 2010.
- 5.Noguchi T., Takeno S., Sato T., Takahashi Y., Uchida Y., Yokoyama S. A patient with primary gastric choriocarcinoma who received a correct preoperative diagnosis and achieved prolonged survival. Gastric Cancer. 2002;5:112–117. doi: 10.1007/s101200200019. [DOI] [PubMed] [Google Scholar]


