Abstract
Facioscapulohumeral muscular dystrophy (FSHD) is an autosomal-dominant disease with no effective treatment. The genetic cause of FSHD is complex and the primary pathogenic insult underlying the muscle disease is unknown. Several disease candidate genes have been proposed including DUX4 and FRG1. Expression analysis studies of FSHD report the deregulation of genes which mediate myoblast differentiation and fusion. Transgenic mice overexpressing FRG1 recapitulate the FSHD muscular dystrophy phenotype. Our current study selectively examines how increased expression of FRG1 may contribute to myoblast differentiation defects. We generated stable C2C12 cell lines overexpressing FRG1, which exhibited a myoblast fusion defect upon differentiation. To determine if myoblast fusion defects contribute to the FRG1 mouse dystrophic phenotype, this strain was crossed with skeletal muscle specific FHL1-transgenic mice. We previously reported that FHL1 promotes myoblast fusion in vitro and FHL1-transgenic mice develop skeletal muscle hypertrophy. In the current study, FRG1 mice overexpressing FHL1 showed an improvement in the dystrophic phenotype, including a reduced spinal kyphosis, increased muscle mass and myofiber size, and decreased muscle fibrosis. FHL1 expression in FRG1 mice, did not alter satellite cell number or activation, but enhanced myoblast fusion. Primary myoblasts isolated from FRG1 mice showed a myoblast fusion defect that was rescued by FHL1 expression. Therefore, increased FRG1 expression may contribute to a muscular dystrophy phenotype resembling FSHD by impairing myoblast fusion, a defect that can be rescued by enhanced myoblast fusion via expression of FHL1.
Introduction
FSHD region gene 1 (FRG1) is an evolutionarily conserved protein [1], associated with the inherited muscle disease Facioscapulohumeral muscular dystrophy (FSHD) [2]. The role of FRG1 in skeletal muscle is not fully understood, however it has reported roles in mRNA splicing [2–4] and actin-bundling [5,6]. Maintenance of FRG1 expression levels are important for normal skeletal muscle. In Xenopus laevis both FRG1 overexpression and morpholino-mediated inhibition result in muscle abnormalities [7].
FSHD is an autosomal-dominant inherited disease with a prevalence ranging from 1:14,000–20,000 [8–11] However, the frequency of FSHD can be underestimated due to the high degree of clinical variability and the large proportion of patients with only mild symptoms. A recent population study reported the incidence as high as ∼1:8,500 (12/100,000) [12] FSHD is characterized by the progressive wasting of muscles, frequently commencing with weakening of facial muscles, and eventually progressing to the pelvic-girdle muscles affecting the ability to walk. Individuals with the most prevalent form of FSHD (FSHD Type 1) have contractions of a 3.3kb macrosatellite repeat array, D4Z4, located in the subtelomeric region of chromosome 4 (4q35) [13]. The most widely accepted FSHD disease gene, DUX4, resides within each D4Z4 repeat and encodes the double-homeodomain transcription factor DUX4 [14]. Contractions of the D4Z4 repeat result in chromatin relaxation and de-repression of DUX4 expression [15]. Multiple DUX4-target genes have been identified [16–18] and their potential involvement in the pathogenesis of FSHD examined [19]. In zebrafish, expression of DUX4 results in muscle abnormalities [20], however, although mice carrying human FSHD D4Z4 arrays recapitulate the important epigenetic profiles for FSHD, they do not develop a muscular dystrophy phenotype [21]. A recently developed X-linked inducible-DUX4-transgenic mouse resulted in embryonic lethality in hemizygous male mice. Surviving male DUX4-transgenic mice exhibited muscle weakness (with the absence of dystrophic pathology) and reduced myoblast differentiation, but did not recapitulate a FSHD phenotype [22].
The FRG1 gene maps approximately 100 kb proximal to the D4Z4 repeat array on chromosome 4 [23]. Individuals with larger deletions at the 4q35 locus including the D4Z4 repeat and loss of the FRG1 gene, do not develop FSHD, supporting the potential involvement of FRG1 in this disease [24,25]. The molecular pathogenesis of FSHD is complex, contentious and not yet fully elucidated. Studies have suggested that FSHD may result from a complex inter-play of genetic and epigenetic events including the possible de-repression of a number of genes proximal to the D4Z4 repeat, including FRG1 [26]. This lead to the hypothesis that FSHD may result from the collaborative effects of multiple genes including FRG1, DUX4 and others (FRG2 and ANT1), which determines the final dystrophic phenotype. Many studies have addressed the deregulation of proximally located genes with inconclusive results. An initial study reported that FRG1 expression was increased in FSHD muscle [27]. However, follow-up studies by different groups and using different techniques have failed to confirm the upregulation of FRG1 in FSHD affected muscle [28–32]. Despite the inconclusive results showing increased FRG1 expression in FSHD patient muscle, overexpression of FRG1 in animal models shows remarkable and reproducible similarity to the FSHD phenotype. FRG1-transgenic mice develop a muscular dystrophy phenotype that shares key histological and physiological features with FSHD including, abnormal curvature of the spine (kyphosis) and skeletal muscle atrophy involving characteristic FSHD affected muscles [2]. Gene expression profiling studies in muscle from FRG1-transgenic mice have also revealed a remarkably similar gene expression profile to FSHD patient muscle [33]. In Xenopus laevis the skeletal muscle abnormalities caused by FRG1 overexpression are accompanied by defects in vasculature [34], reminiscent of the retinal vasculopathy that can develop in FSHD [28]. Interestingly, a recent study presented a possible unifying model for the pathogenesis of FSHD by showing a direct interplay between two key FSHD disease genes, DUX4 and FRG1 [25]. The transcription factor DUX4 was shown to promote FRG1 expression by binding to putative enhancer elements within the human FRG1 gene. FSHD is also a highly heterogeneous disease showing marked variability in age of onset, clinical severity and disease progression. This may be a consequence of the complex interplay between multiple genes coupled with significant variability in disease gene expression As such, it is imperative to characterize the individual functions of each candidate gene.
Recent studies have identified defects in muscle stem cells (satellite cells) [33] and myoblast fusion [35], which may contribute to disease pathogenesis in the dystrophic FRG1 mouse model. Defects in myogenesis may also be an important pathological hallmark of FSHD as multiple expression analyses studies have documented the deregulation of genes that regulate myoblast differentiation and fusion [30,36–39]. Defects in myoblast fusion contribute to the pathogenesis of an increasing number of muscular dystrophies [40–42] compromising muscle growth and regeneration. In this regard, the identification of novel factors that enhance myoblast fusion may provide a therapeutic strategy for muscle diseases associated with myoblast fusion defects.
Here we addressed two key questions; does a myoblast fusion defect contribute to the pathogenesis of muscular dystrophy observed in FRG1-transgenic mice and if so, does enhanced myoblast fusion rescue the dystrophic phenotype. Four and a half LIM protein 1 (FHL1) is a protein that is highly expressed in skeletal muscle [43] and significant recent interest has focused on FHL1 and its role in the maintenance of healthy muscle since the discovery of FHL1 mutations as the cause of human muscle disease [44–47]. We have previously reported wild type FHL1 promotes myoblast fusion in vitro and skeletal muscle-specific FHL1-transgenic mice exhibit enhanced muscle growth (hypertrophy) via activation of calcineurin/NFAT signaling [48]. Therefore, we investigate here whether FHL1 could enhance myoblast fusion in diseased muscle.
We report that FRG1 overexpression causes a defect in the myogenic pathway by impairing myoblast fusion. Critically, transgenic FHL1 expression in FRG1 mice reduces muscle wasting and improves the dystrophic phenotype by driving enhanced myoblast fusion. These studies reveal that FRG1 overexpression contributes to dystrophy pathogenesis by impairing myoblast fusion and provides evidence that targeting enhanced myoblast fusion can reduce disease severity.
Materials and Methods
Ethics Statement
Ethics approval was obtained from the Monash Animal Research Platform Animal Ethics Committee (SOBSB/2008/61), Monash University, Melbourne, Australia, under the guidelines of the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes.
Animal strains and breeding
FRG1-transgenic mice and FHL1-transgenic mice were both generated using human FRG1 or FHL1 cDNA respectively. FRG1-transgenic mice [2] were obtained from Professor Rossella Tupler (University of Massachuetts Medical School, Worcester, MA) and maintained by breeding to C57BL6/J mice. Skeletal muscle-specific FHL1-transgenic mice were generated as previously described [48] using the human skeletal actin (HSA) promoter and were maintained by breeding to FVB/N wild type mice. FRG1 mice over-expressing FHL1 were generated by breeding male FRG1 mice with female FHL1-transgenic mice to generate wild type, FRG1 and FRG1/FHL1 littermates for analysis. Mice carrying FRG1 and FHL1 transgenes were identified by PCR amplification from genomic tail DNA as previously reported [2,48]. All mice colonies were maintained at the Monash Animal Research Platform, Monash University, Australia, with a 12-hour day/night cycle with access to food and water ad libitum. All experiments used male transgenic mice and sex-matched wild type littermates at 6 and 12 weeks of age.
Antibodies
A list of antibodies used for experiments is included as S1 Table.
Growth and stable transfection of C2C12 cells
The C2C12 mouse myoblast cell line [49] was purchased directly from ATCC (ATCC CRL1772) and was cultured in DMEM supplemented with 20% FBS and 2mM glutamine at 37°C/8%CO2. Stable cell lines were generated using the pIRESneo3 vector (Clontech). The pIRESneo3 vector was modified by subcloning a hemaglutinin (HA) epitope tag from the pCGN vector (Dr Tracey Wilson, WEHI, Australia) into the pIRESneo3 vector flanked by NheI and AgeI restriction sites (pIRESneo3-HA). The pIRES/FH-FRG1 construct was provided by Professor Rossella Tupler (University of Massachusetts Medical School, Worcester, MA), from which the FRG1 cDNA was PCR amplified and subcloned into the EcoR1 site of the modified pIRESneo3-HA vector. pIRESneo3-HA-FRG1 and pIRESneo3-HA (control) plasmids were transfected into C2C12 myoblasts using lipofectamine 2000 (Invitrogen) according to manufacturer’s instructions. Single, stably transfected C2C12 clones were isolated by sequential plating followed by selection in growth media containing 1 mg/ml G418 for up to 20 days, with selection media changed every 2–3 days. Several pIRESneo3-HA-FRG1 and pIRESneo3-HA clones were selected and amplified for further analysis. To confirm overexpression of HA-FRG1, cell lysates were prepared using Tris Saline pH 7.4 and 1% Triton X-100 and immunoblotted with HA (Covance, MMS-101R, 1:5000) and FRG1 (Abcam, 55024, 1:500)-specific antibodies. Lysates from C2C12 myoblasts transiently expressing HA-FRG1 (pCGN-FRG1) were used as a positive control.
RNA extraction and qRT-PCR analysis
Total RNA was prepared from 80% confluent proliferating or differentiating mouse myoblasts using the Isolate RNA Mini Kit (Bioline) and total RNA from muscle tissue prepared using the RNeasy Fibrous Mini Kit (Qiagen) as per manufacturer’s instructions. 50ng RNA was used for first-strand cDNA synthesis with Affinity Script QPCR cDNA Synthesis Kit (Stratagene). Quantitative RT-PCR (qRT-PCR) analysis was performed on the Corbett Rotorgene 3000 (Qiagen) using Brilliant II SYBR Green QPCR Mastermix (Stratagene) and Quantitect Primer Assay for human FRG1 (Qiagen, Hs_FRG1_1_SG), human FHL1 (Qiagen, HS_FHL1_1_SG), mouse Gapdh (Qiagen, Mm_Gapdh_3_SG), mouse Pax7 (Qiagen, Mm_Pax7_1_SG), mouse myod (forward 5’-GCCCGCGCTCCAACTGCTCTGAT- 3’, reverse 5’ -CCTACGGTGGTGCGCCCTCTGC- 3’) [50], mouse myogenin (forward 5’ –GGGCCCCTGGAAGAAAAG- 3’, reverse 5’—AGGAGGCGCTGTGGGAGT-3’) [50], mouse suv4–20h1 (forward 5’ TCGCAGTCGCTAAATTCCTT 3’, reverse 5’ CGACCAGTTGACACAAACTTAC 3’)[35] and mouse Eid3 (forward 5’ AGTTCCTGGTTTTGGCCTCT 3’, reverse 5’ TCGCAGTCGCTAAATTCCTT 3’) [35]. Relative mRNA for each amplicon was quantified after normalization against GAPDH using the comparative 2-∆∆Ct method [51]. For all samples the results are presented as the fold change over the baseline values from control samples (wild type muscle or vector control cells) as is the standard approach for the 2-∆∆Ct method. Each amplicon was analyzed in triplicate in a 72 well rotor in three independent experiments.
C2C12 differentiation and immunofluorescence staining
C2C12 cells stably transfected with HA-vector or HA-FRG1 were plated onto fibronectin-coated coverslips (5μg/ml; Sigma) at 1x105 cells per well (6-well dish for immunofluorescence) or onto fibronectin-coated 60mm dishes at 2.2x105 cells per dish (for Western blot analyses). To induce differentiation, 80% confluent cells were washed with PBS and switched to DMEM supplemented with 2% horse serum, 2mM glutamine, and maintained for 0–96 hours at 37°C/8%CO2. At 24 hour intervals post differentiation, cells were fixed in 4% formaldehyde for myosin heavy chain immunofluorescence analysis as described [52] and visualized using a Nikon C1 confocal inverted microscope, or lysates prepared for western blotting analysis. For Western blot analyses, Triton X-100–soluble lysates were prepared as described [52]. Protein concentration was measured using a DC protein assay reagent (Bio-Rad Laboratories), and 25μg lysates were separated by SDS-PAGE and immunoblotted with antibodies specific for myogenin [48] (Santa Cruz Biotechnology Cat# sc-12732, RRID:AB_627980, 1:500); MHC [53] (Developmental Studies Hybridoma Bank Cat# MF 20, RRID:AB_2147781, 1/500) and β-tubulin [54] (Life Technologies Cat# 480011, RRID:AB_10375603, 1:5000). Ponceau red staining of membranes was used to confirm equal protein loading. Western blot films were scanned and band signal intensities determined using MacBiophotonics ImageJ v1.43m software. Protein expression was corrected for protein loading by standardizing to the corresponding β-tubulin values (protein loading control) and expressed as the fold difference to control (0 time point) cells. Each experiment was performed in triplicate.
Mouse primary myoblasts
Primary myoblasts were derived from the hind limb muscles of mice post-natal day 1 and isolated using collagenase/dispase tissue digestion as described [55]. Primary myoblasts were cultured in growth media on 0.1% collagen (Sigma) coated plates at 37°C/8%CO2. The purity of all primary myoblast populations was confirmed by immunostaining for desmin. Myoblast cultures with >90% (range 89–95%) purity were used. For differentiation experiments, cells were plated at 5x104cell per well in 24 well plates coated with fibronectin (5μg/ml; Sigma). Differentiation was induced after 2 hours plating by washing with PBS and switching to DMEM supplemented with 5% horse serum (Invitrogen), 1% insulin-transferrin-selenium-A solution and penicillin/streptomycin. Cultures were differentiated for 48 and 96 hours.
Differentiated primary myoblast cultures were fixed and permeabilized in 4% formaldehyde/0.01% triton X-100 for immunofluorescence analysis and co-stained for MHC (to identify myotubes) and nuclei (DAPI) as described [52]. Myoblast differentiation was visualized using a Leica AF 6000 LX optical microscope and assessed by quantifying the nuclear fusion index (proportion of nuclei localized within MHC-positive myotubes. Each experiment was performed in triplicate. For all cell counts, a minimum of five—ten random fields were scanned for each slide to ensure that ≥ 100 cells were scored for each replicate. Cell counts were quantified using MacBiophotonics ImageJ v1.43m software (National Institutes of Health, USA).
Skeletal muscle lysates and Immunoblotting
The tibialis anterior, quadriceps, triceps and trapezius muscles were dissected, minced, and homogenized on ice for 5 × 30 s (Tissue Rupter, Qiagen) in 5 times the weight/volume of 1% NP-40, Tris-HCl buffer, pH 8, then extracted for 1 h at 4°C. Lysates were centrifuged at 1,000g for 5 minutes. Protein concentration was determined on the soluble fraction using a DC protein assay kit (Biorad), and 10μg of soluble protein was separated by SDS-PAGE and immunoblotted with antibodies specific for HA [48] (Covance Research Products Inc Cat# MMS-101R-500, RRID:AB_10063630, 1:5000), FRG1 (Abcam Cat# ab55024, RRID:AB_941653, 1:500), FHL1 [56] (Abcam Cat# ab23937, RRID:AB_732361, 1/1000), Eid3 [57] (Santa Cruz Biotechnology Cat# sc-167738, 1/500) and β-tubulin. Band signal intensities were determined as described above. Ponceau red staining of membranes was used to confirm equal protein loading.
Skeletal muscle histology
Muscles were snap frozen in isopentane cooled in liquid nitrogen. Serial 6μm—10μm transverse cryosections of muscle were fixed and stained with Harris Haematoxlin and Eosin (H&E) for morphological analysis, Masson’s Trichrome for fibrosis staining and Oil Red O for fat staining. All stains were performed using standard techniques. Sections stained with H&E and Masson’s Trichrome were mounted with a glass coverslip using DPX (Grail Scientific). Sections stained with Oil Red O were mounted with a glass coverslip and aquatex (aqueous mounting medium; Grail Scientific). All muscle histology was viewed using an Olympus AX70 Provis fitted with an Olympus DP70 color camera and captured using AnalySiS 5 software.
All quantitative analysis of muscle was performed using MacBiophotonics ImageJ v1.43m software. Prior to analysis, a spatial calibration was performed on Image J to set the image scale. For quantification of the area of fibrosis (Masson’s Trichrome) and fat deposition (Oil Red O), a minimum threshold was first determined from analysis of wild type muscle sections by performing an automated threshold to include only the fibrosis- and fat-stained areas within normal healthy muscle. This minimum threshold was maintained for analysis of FRG1 and FRG1/FHL1 muscle sections. The area occupied by fibrosis or fat deposition in muscle sections was determined from ten fibrosis- and fat-stained sections and expressed as the mean percentage area. Data represents the average of 4–6 mice per genotype for determination of fibrosis and 3–4 mice per genotype for determination of fat.
Muscle fiber diameter and centralized nuclei were analyzed using 8 μm transverse H&E stained muscle cryosections. Fiber Diameters were determined by measuring the minimal ferret’s diameter [58] using MacBiophotonics Image J. Between 500–1000 fibers were measured per muscle. Data represent the average of 10 sections from 3–5 mice per genotype. Histograms of fiber diameters were plotted and mean fiber diameter graphed and analyzed using GraphPad Prism 5 software. The total numbers of muscle fibers with centralized nuclei were counted from 500–1000 fibers per muscle. Data represent the mean from 3 wild type, 4 FRG1 and 4 FRG1/FHL1 mice.
Skeletal muscle immunostaining
Immunofluorescence staining for Pax7 [59] (Developmental Studies Hybridoma Bank Cat# pax7, RRID:AB_528428, 1/50), and DAPI was performed on 10μm transverse cryosections using the Vector M.O.M Immunodetection kit (Vector laboratories) for tissue sections as per manufacturer’s instructions. Co-staining for Pax7 and MyoD (Cell Signaling Technology, Cat# 13812S, 1/400) was achieved by double immunofluorescent labeling. Sections were first stained with Pax7 as described above, then, stained overnight with rabbit anti-MyoD, washed in PBS and incubated with goat anti-rabbit Alexa Fluor (1:400; Molecular probes) for 2 hours at room temperature. Sections were washed in PBS and coverslips mounted using fluoromount-G mounting media (Emgrid Australia, Cat# 17984–25). Images of Pax7+/MyoD-, Pax7+/MyoD+ and Pax7-/MyoD+ stained cells were captured using an Olympus AX70 Provis fitted with an Olympus DP70 color camera and AnalySiS 5 software. All analyses were manually performed using Image J. The total number of Pax7+/MyoD-, Pax7+/MyoD+or Pax7-/MyoD+ cells from transverse sections was determined from 4–5 consecutive fields at x200 magnification for each muscle section. Data presented as cell number per 100 myofibers.
For the determination of nuclei number per mm myofiber length [60], 10μm longitudinal cryosections from triceps muscle were fixed in 4% paraformaldehyde, washed in PBS and permeabilised/blocked (10% horse serum, 1% BSA and 0.1% triton X-100 in PBS) for 1 hour at room temperature. Sections were stained overnight with rabbit anti-dystrophin (Abcam Cat# ab15277, RRID:AB_301813, 1:400) [61], washed in PBS and incubated with goat anti-rabbit Alexa Fluor 594 and DAPI (1:100) for 1 hour at room temperature. Sections were washed in PBS and coverslips mounted using fluoromount mounting media.
Images of Dystrophin/DAPI stained longitudinal sections were captured using an Olympus BX-51 microscope using dotSlide Software. All analyses were manually performed using Imaging Software CellSens v1.6. The number of nuclei from longitudinal sections was determined from 20–40 myofibers [62] at x200 magnification for each muscle section. Data presented as the number of nuclei per mm of myofiber length. Data represent the mean ± SEM from 3–4 wild type, 3–4 FRG1 and 3–4 FRG1/FHL1 mice.
Analysis of muscle function
All procedures were approved by the Animal Experimentation Ethics Committee of The University of Melbourne (1112223) and conformed to the Guidelines for the Care and Use of Experimental Animals described by the National Health and Medical Research Council (Australia). Mice were housed in the Biological Research Facility at The University of Melbourne under a 12-hour light/dark cycle. In situ muscle function on mice was performed under sodium pentobarbitone anesthesia (Nembutal, 120 mg/kg), and all efforts were made to minimize suffering.
The methods for measuring contractile function of mouse tibialis anterior (TA) muscles in situ have been described in detail elsewhere [63]. Briefly, TA muscles were stimulated by supramaximal 0.2 ms square wave pulses of 300 ms duration, delivered via two wire electrodes adjacent to the peroneal nerve. Optimum muscle length (Lo) was determined from maximum isometric twitch force (Pt), and maximum isometric tetanic force (Po) was recorded from the plateau of a complete frequency-force relationship. Specific force (sPo) was determined by normalizing Po to muscle cross-sectional area (CSA) and expressed in kN/m2. The protocol for assessment of muscle fatigability was assessed using a standard fatigue protocol. Muscles were stimulated maximally once every 2 s for 4 minutes, with maximum force reported every minute. Muscles were trimmed of tendons and any adhering non-muscle tissue, blotted on filter paper and weighed on an analytical balance.
Statistical analysis
Data were graphed and analyzed using GraphPad Prism 5. Results were reported as mean ± SEM for the total number of observations, where n equals the number of mice or experiments used. Two tailed Student’s t tests and two-way ANOVA with Tukey’s multiple comparisons test were used to evaluate statistical significance and calculate P values with threshold values as described in the results or figure legends. P values of <0.05 were considered as statistically significant.
Results
C2C12 myoblasts over-expressing FRG1 exhibit a myoblast fusion defect
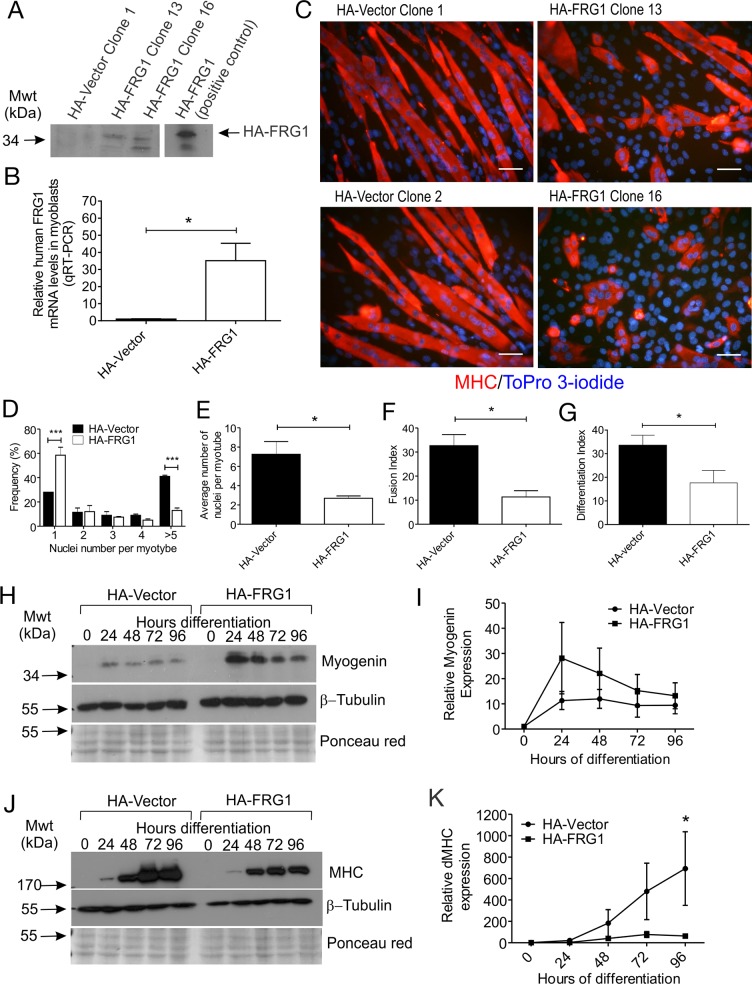
We selectively examined the effect of FRG1 overexpression on myogenesis by generating several C2C12 myoblast cell lines stably over-expressing HA-tagged FRG1. Immunoblot analysis using a HA-specific antibody confirmed expression of HA-FRG1 in two independent clones (Clones 13 and 16), which were selected for further analysis (Fig. 1A). HA-vector stably transfected cells were used as a control. QRT-PCR analysis demonstrated increased FRG1 mRNA in C2C12 myoblasts stably expressing HA-FRG1 relative to control HA-vector myoblasts (Fig. 1B). Myoblast fusion was evaluated by co-staining cultures for a myotube-specific marker myosin heavy chain (MHC), and ToPro 3-iodide to detect nuclei (as previously) [52]. Control myoblasts fused to form long, multinucleated MHC-positive myotubes that contained an average of 7 or more nuclei after 96 hours differentiation (Fig. 1C-E). In contrast, most C2C12 myoblasts over-expressing FRG1 remained as mononucleated myoblasts (Fig. 1C-D), or fused to form short rounded myotubes containing small numbers of nuclei (Fig. 1C-E). This myoblast fusion defect was observed for both HA-FRG1 clones. Analysis of all HA-vector clones versus all HA-FRG1 clones confirmed a 3-fold decrease in myoblast fusion with FRG1 overexpression (Fig. 1F) which was accompanied by an overall decrease in the proportion of MHC+ cells (Fig. 1G).
Fig 1. C2C12 myoblasts overexpressing FRG1 exhibit a fusion defect.
(A) Immunoblot analysis of FRG1 expression in C2C12 myoblasts expressing HA-vector or HA-FRG1. HA-tagged FRG1 was detected using a HA-specific antibody. Clones HA-FRG1 13 and HA-FRG1 16 were selected for further analysis. Positive control represents HA-FRG1 transfected COS1 cells. (B) qRT-PCR analysis of FRG1 mRNA levels in undifferentiated HA-FRG1 myoblasts relative to HA-vector control myoblasts. Data represent the mean +/- SEM from n = 3 independent experiments; *p<0.05. (C) Representative images of C2C12 myoblasts expressing either HA-vector control or HA-FRG1 as indicated, following 96 hours differentiation and stained with the differentiation marker MHC (red) and ToPro 3-iodide to detect nuclei (blue). (D-F) Several parameters were quantified to assess the efficiency of myoblast differentiation; (D) Frequency of MHC-positive myoblasts and myotubes containing 1, 2, 3, 4 or ≥5 nuclei. Data represent the mean ± SEM from n = 3 independent experiments;***p<0.0005 determined by two-way ANOVA with Tukey’s multiple comparisons test; (E) Average number of nuclei per myotube; (F) Fusion index; the percentage of total nuclei localized within MHC-positive myotubes; (G) Differentiation index; the percentage of total nuclei localized within MHC-positive cells (myocytes and myotubes); (H-I) Relative myogenin and (J-K) MHC expression in HA-vector versus HA-FRG1 expressing myoblasts during 0–96 hours differentiation. Immunoblotting for β-tubulin and staining membranes with ponceau red were used as a loading control. Myogenin and MHC expression were quantified using densitometry. Data for (B), (E-F), (I) and (K) represent the mean ± SEM from n = 3 independent experiments; *p< 0.05 determined by two-tailed Student’s T-test. Scale bars = 50μm.
To further define the myoblast fusion defect observed in FRG1-expressing myoblasts, expression of the myogenic regulatory transcription factor myogenin, which initiates differentiation and is typically induced within 24 hours following the induction of C2C12 myoblast differentiation [48], was examined. Expression of its downstream target MHC was also evaluated (Fig. 1H-K). Surprisingly, despite a myoblast fusion defect, a trend towards increased myogenin expression was observed in FRG1-expressing myoblasts undergoing differentiation, relative to control myoblasts (Fig. 1H-I). Myogenin mRNA is also increased in the muscles of FRG1-transgenic mice [4], an observation consistent with our study showing increased myogenin protein expression in cultured myoblasts overexpressing FRG1. Analysis of MHC expression revealed a significant reduction in FRG1-overexpressing myoblasts (Fig. 1J-K), consistent with our immunofluorescence experiments showing an overall decrease in the proportion of MHC+ cells in FRG1 cultures (Fig. 1G). Therefore our data suggests that FRG1 overexpression does not inhibit initiation of the differentiation program, as shown by the expression of myogenin but, rather impairs later events including MHC expression and myoblast fusion.
FHL1 reduces muscle wasting in the FRG1 mouse
Our in vitro data revealed that overexpression of FRG1 in C2C12 mouse myoblasts results in a fusion defect. Therefore, to provide proof of principle that FRG1 can impair myoblast fusion leading to muscular dystrophy, we investigated if co-expression of an agent that promotes myoblast fusion could rescue the dystrophic phenotype of FRG1 mice. We have previously reported that the LIM-only protein, FHL1, promotes myoblast fusion in vitro and skeletal muscle hypertrophy in vivo by enhancing NFAT transcriptional activity [48]. To determine if FHL1 expression can reduce muscle wasting in FRG1 mice, we crossed our skeletal muscle-specific FHL1-transgenic mice [48] with the dystrophic FRG1 mouse model [2]. A previous study has demonstrated that the effect of FRG1 overexpression on muscle is dose-dependent [2]. This was shown by generating FRG1-low, FRG1-med and FRG1-high transgenic mice, which express FRG1 at different levels. In the current study we use the FRG1-high line, which exhibits the most severe dystrophic phenotype.
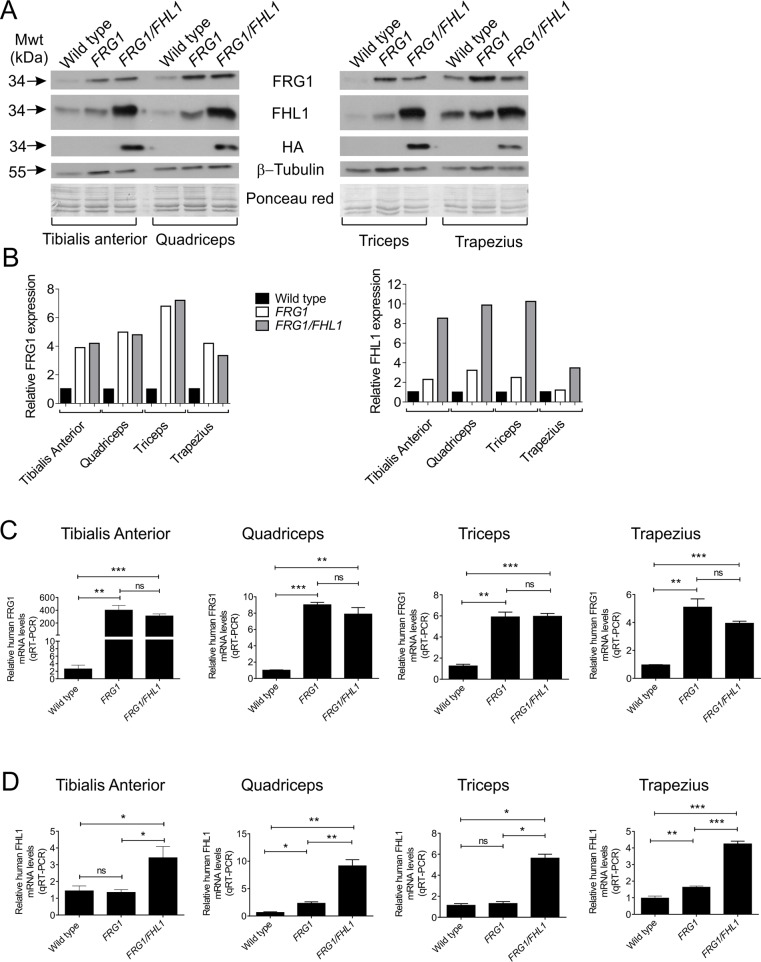
Different muscles in FRG1 mice are affected to varying extents and show pathological changes [2] in a distribution similar to FSHD [64]. Muscles affected in order of severity include the trapezius, vastus lateralis (quadriceps), triceps and the tibialis anterior [2]. Immunoblot analysis of these affected muscles confirmed increased FRG1 protein expression in FRG1 and FRG1/FHL1 mice relative to wild type littermates (Fig. 2A-B). The expression of transgenic HA-tagged FHL1 in FRG1/FHL1 mouse muscles was detected by immunoblotting with a HA-specific antibody. Increased FHL1 protein expression was further confirmed using a FHL1 antibody, which detects both the endogenous and transgene-derived FHL1, and revealed a modest 4–5 fold increase in FHL1 expression in FRG1/FHL1 muscle (Fig. 2B). QRT-PCR analysis using human-specific primers (to distinguish transgene-derived human transcripts from endogenous murine transcripts) confirmed a ∼5–9-fold increase in FRG1 mRNA across various muscles in FRG1 and FRG1/FHL1 mice (Fig. 2C). FHL1 mRNA was increased 4–10 fold in FRG1/FHL1 muscles (Fig. 2D).
Fig 2. Generation of FRG1 and FRG1/FHL1 mice.
(A) Immunoblot analysis of protein expression in the tibialis anterior, quadriceps, triceps and trapezius muscles from wild type, FRG1 and FRG1/FHL1 mice. HA-tagged FHL1 was detected using a HA-specific antibody; β-tubulin immunoblotting and ponceau red staining of membranes were used as loading controls. (B) Relative FRG1 and FHL1 expression levels in the tibialis anterior, quadriceps, triceps and trapezius muscles from wild type, FRG1 and FRG1/FHL1 mice. Protein expression was quantified using densitometry. Quantitative RT-PCR analysis of FRG1 (C) and FHL1 (D) mRNA in muscles from wild type, FRG1 and FRG1/FHL1 mice. Data represent the mean from n≥4 mice/genotype; *p<0.05, **p<0.005, ***p<0.001 determined by two-tailed student’s T-test.
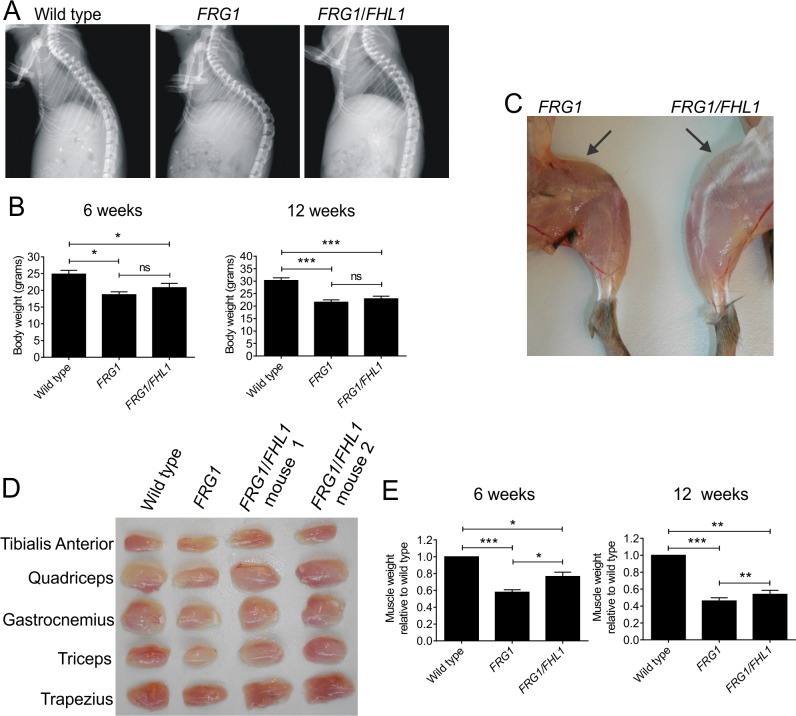
Dystrophy in FRG1 mice is characterized by progressive muscle wasting accompanied by spinal kyphosis (abnormal outward curvature of the spine), caused by muscle weakness [2]. X-ray images of representative 6-week-old FRG1 mice confirmed the presence of kyphosis which was absent from wild type mice (Fig. 3A). Expression of FHL1 was sufficient to reduce the dystrophic phenotype of FRG1 mice resulting in normal curvature of the spine, thus supporting the hypothesis that FHL1 expression can alleviate the reduced muscular support of the spine. Analysis of whole body weight revealed a significant reduction in FRG1 relative to wild type mice (Fig. 3B), an effect previously shown to be caused by reduced muscle mass and not due to reduced caloric intake [2]. A trend towards increased body weight was observed in FRG1/FHL1 mice relative to FRG1 mice aged 6 weeks (but not at 12 weeks), but this difference was not statistically significant (Fig. 3B). However, examination of various muscles from mice at 6 weeks of age revealed FHL1 overexpression was sufficient to increase muscle mass in FRG1 mice (Fig. 3C-E; S2 and S3 Tables). The weights of four affected muscle groups (tibialis anterior, quadriceps, triceps and trapezius) from FRG1 mice showed a 40% reduction in muscle weight relative to wild type mice at 6 weeks of age (Fig. 3E). Significantly, a 33% increase in muscle mass in FRG1/FHL1 mice was observed relative to FRG1 littermates (aged 6 weeks) (Fig. 3E and S2 Table), which was sustained, albeit at lower levels (19% increase), in adult FRG1/FHL1 mice aged 12 weeks (Fig. 3E and S3 Table). Therefore FHL1 promotes increased muscle mass in FRG1 mice. This does not translate to an overall significant increase in body weight in FRG1/FHL1 mice due to the small contribution of these muscles to overall body weight (∼1–2%). Collectively, this data provides evidence that we have achieved increased FHL1 expression in key affected muscles in the dystrophic FRG1 mouse, and demonstrated that FHL1 expression is sufficient to reduce the severity of the dystrophic FRG1 phenotype including amelioration of muscle wasting and spinal kyphosis.
Fig 3. FHL1 reduces muscle wasting in dystrophic FRG1 mice.
(A) Representative X-ray images of the spine of mice from the indicated genotypes. (B) Whole body weight in 6-week-old mice; wild-type (n = 10); FRG1 (n = 6); FRG1/FHL1 (n = 9); and 12-week-old mice; wild-type (n = 11); FRG1 (n = 12); FRG1/FHL1 (n = 14). (C) Representative image of skinned hind limbs from FRG1 and FRG1/FHL1 mice. Arrows point to the quadriceps muscle to show the difference in muscle mass between the FRG1 and FRG1/FHL1 mice. (D) Representative images of various muscles dissected from wild type, FRG1 and FRG1/FHL1 mice. (E) Relative muscle weight from 6-week-old mice and 12-week-old mice. Data represents an average of the combined weights from 4 muscle groups (n = 6–10 mice per genotype for the tibialis anterior, quadriceps, triceps and trapezius) and is expressed relative to wild type muscle weight. Data represent the mean ± SEM; *p< 0.05; **p<0.005; ***p<0.0005 determined by two-tailed Student’s T-test.
FHL1 improves muscle pathology in FRG1 mice
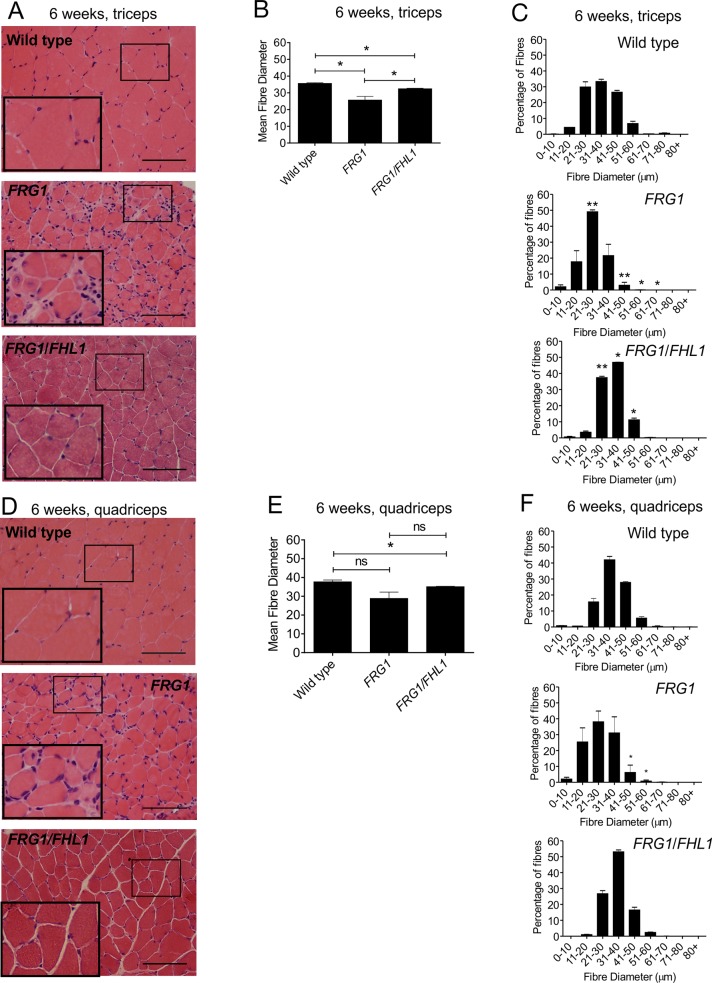
The dystrophic features reported in muscle from FRG1-transgenic mice include variation in muscle fiber size, centralized myonuclei and the presence of fibrosis [2] and were observed in H & E stained transverse muscle sections; (Fig. 4A and 4D, middle panels). We next examined specific features of the dystrophic phenotype by examining the quadriceps and triceps muscles, tissues representing high and intermediate degrees of muscle disease in the FRG1 mouse respectively [2]. These muscles, particularly the quadriceps, have been used in several previous studies to characterize the dystrophic phenotype of the FRG1 mouse including the examination of satellite cell and myoblast function [33]. Significantly, FHL1 expression in the triceps and quadriceps of 6-week-old FRG1 mice resulted in significantly improved pathology, with uniform tightly packed fibers and reduced fibrotic tissue (Fig. 4A and 4D, lower panels). Mean myofiber diameter was decreased in the FRG1 triceps and quadriceps compared to wild type (Fig. 4B and 4E) consistent with the presence of myofiber atrophy reported in this dystrophic model [2]. Myofiber atrophy was further examined by assessing the frequency of individual fiber diameters. In wild type mouse triceps and quadriceps muscles, fibers were 21–50μm in diameter, with some as large as 60–80 μm (Fig. 4C and 4F). FRG1 muscle showed a significant shift in myofiber size towards an increase in smaller atrophic fibers (11–30 μm) and a corresponding reduction in larger fibers > 30 μm (Fig. 4C and 4F). Importantly, FHL1 expression in FRG1 mouse triceps muscle resulted in a significant increase in both mean myofiber diameter (Fig. 4B) and also the frequency of larger myofibers > 30 μm (Fig 4C). FRG1/FHL1 muscle also exhibited a 3–4-fold reduction in the proportion of small atrophic fibers (>20 μm) (Fig. 4C). In 12-week-old adult FRG1/FHL1 mice the increase in myofiber size induced by FHL1 expression was sustained, although this was not as substantial as at 6 weeks (S1 Fig.). A previous study has examined the progressive course of muscle disease in the FRG1-transgenic mouse model between the ages of 3–14 weeks [33]. The dystrophic phenotype in the FRG1-transgenic mouse does not become apparent until 6-weeks of age and progressively worsens thereafter. Therefore, the improvement in mean fiber diameter observed at 6 weeks in the FRG1/FHL1 mice may not be as prominent at 12 weeks due to the progressive worsening of the dystrophic FRG1 phenotype, such that in older mice only a partial rescue was observed. Collectively these results show that FHL1 promotes increased myofiber size most significantly during the growth phase of juvenile FRG1 mice.
Fig 4. FHL1 improves muscle pathology in dystrophic FRG1 mice.
Representative images of transverse muscle sections from the triceps (A) or quadriceps (D) muscles of 6-week-old wild type, FRG1 and FRG1/FHL1 mice stained with H&E. Boxed region indicates area shown in high magnification image inset. Mean myofiber diameter from the triceps (B) and quadriceps (E) was measured for wild type, FRG1 and FRG1/FHL1 mice. Histograms showing the frequency of individual muscle fiber diameters from the triceps (C) or quadriceps (F) of wild type, FRG1 and FRG1/FHL1 mice. 500–1000 muscle fibers were measured per muscle for each mouse; Wild type (n = 3 mice), FRG1 (n = 4 mice) and FRG1/FHL1 (n = 4 mice). Data represent mean ± SEM; *p<0.05; **p<0.005 determined by two-tailed Student’s T-test. In (C) and (F), asterisks in FRG1 histograms indicate significant differences between FRG1 and wild type mice; Asterisks in FRG1/FHL1 histogram indicate significant differences between FRG1/FHL1 and FRG1 mice. Scale bars = 100μm.
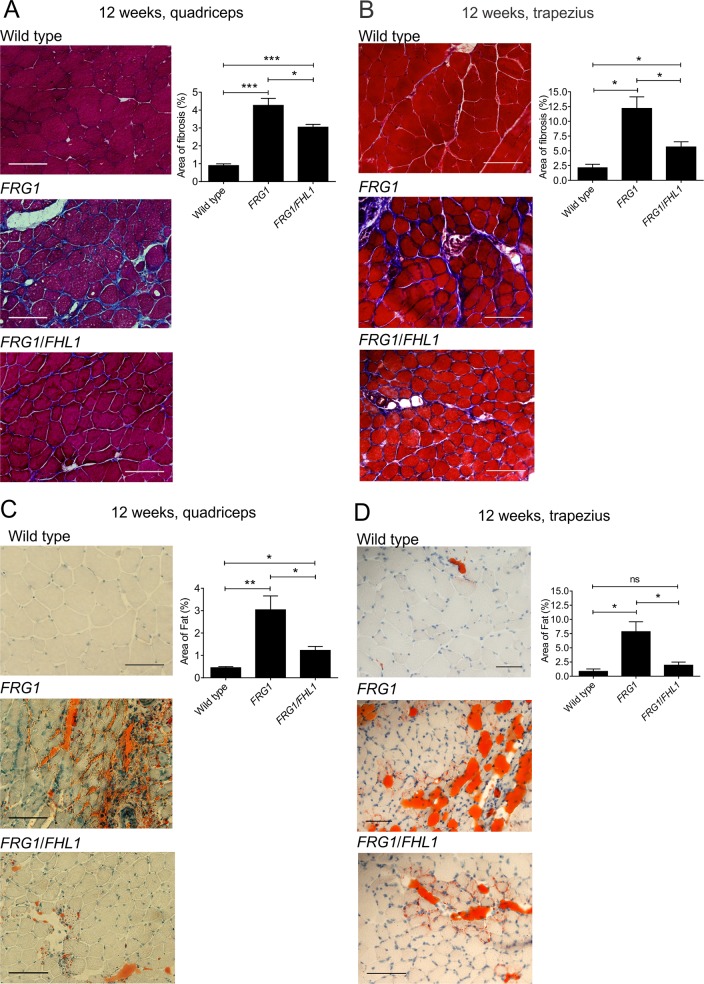
FHL1 reduces fibrosis in FRG1 mice
Dystrophic muscle is progressively replaced with fibro-fatty tissue as a consequence of the diminished capacity of satellite cells to repair damaged muscle. This may be caused by several factors including, replicative aging leading to satellite cell senescence, an unfavorable microenvironment for satellite cell mediated muscle repair, or due to the failure of muscle precursor cells (myoblasts) to differentiate efficiently [65]. The accumulation of fibrosis and fat in muscle was examined in two of the most severely affected muscles in FRG1-transgenic mice, the quadriceps and trapezius, which exhibit the greatest extent of fibrosis [2] and are therefore best suited to determine if FHL1 ameliorates this important disease feature. Masson’s trichrome staining of 12-week-old FRG1 muscle revealed extensive fibrosis between muscle fibers, occupying 4% of the total muscle area in quadriceps and 12.5% in the trapezius, and was largely absent in wild type muscle (Fig. 5A-B). Notably, expression of FHL1 in both quadriceps and trapezius FRG1 muscle resulted in a significant decrease in fibrosis, relative to FRG1 muscle in the absence of exogenous FHL1 (Fig. 5A-B). Oil Red O staining of fat revealed a ∼6-fold increase in fat deposition in FRG1 muscles relative to wild type and this was reduced in FRG1/FHL1 muscle (Fig. 5C-D). Therefore, FHL1 expression is sufficient to reduce the accumulation of fibro-fatty scar tissue, not only in the quadriceps of FRG1-transgenic mice, but also in the trapezius where fibrosis and fat deposition are prominent pathological features.
Fig 5. FHL1 reduces fibrosis and fat deposition in dystrophic FRG1 mice.
Representative images of transverse sections of (A) quadriceps and (B) trapezius muscle from 12-week-old wild type, FRG1 and FRG1/FHL1 mice stained with Masson’s trichrome to detect fibrosis within muscle. The percentage area of fibrosis staining in muscle was quantified from wild type (n = 4–6), FRG1 (n = 4–6) and FRG1/FHL1 (n = 5–6) mice. Representative images of transverse muscle sections from the (C) quadriceps and (D) trapezius muscle stained with Oil Red O to detect fat deposits within muscle. The percentage area of fat deposition in muscle was quantified in wild type (n = 3–4), FRG1 (n = 4) and FRG1/FHL1 (n = 4) mice. Data represent the mean ± SEM; *p<0.05; **p<0.005; ***p<0.0005 determined by two-tailed Student’s T-test. Scale bars = 100μm.
Analysis of muscle function in FRG1 versus FRG1/FHL1 mice
Analysis of several parameters was undertaken to compare muscle function in wild type, FRG1 and FRG1/FHL1 mice including, maximum force, specific (normalized) force, frequency force relationship and resistance to fatigue (n ≥ 5 mice per genotype). For all parameters measured, a marked decrease in muscle function in FRG1 mice was observed compared to wild type, however no improvement was observed in FRG1/FHL1 mice (S2 Fig.). This indicates that despite several lines of evidence showing FHL1 promotes an improvement in FRG1 muscle pathology, this was not sufficient to improve muscle function.
FHL1 does not alter satellite cell number or activation in FRG1 mice
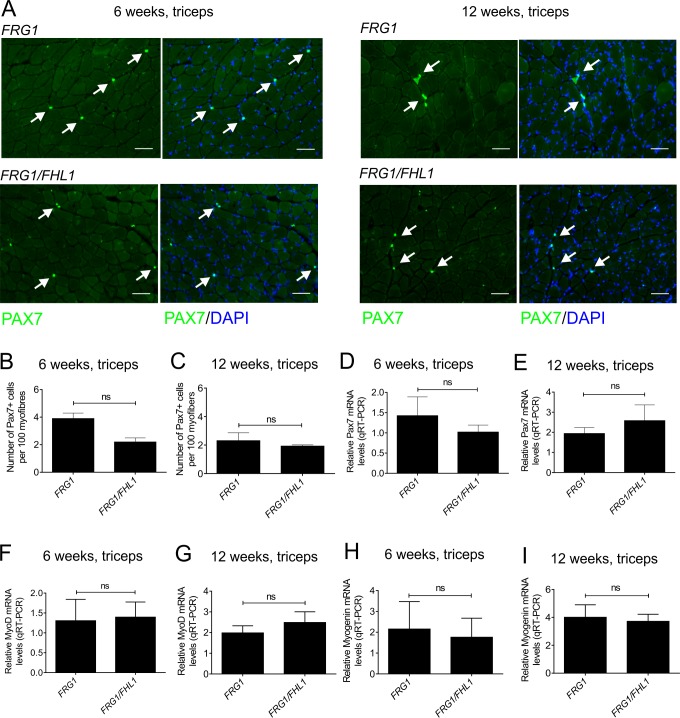
Previous studies have shown FRG1 mice exhibit decreased satellite cell proliferation and activation, which are thought to contribute to the dystrophic phenotype by restricting muscle growth and repair [33,66]. In contrast, we have shown muscle from FHL1-transgenic muscle exhibits increased numbers of satellite cells (in the absence of muscle damage) and features of enhanced myoblast fusion [48]. Therefore, we systematically examined the number of satellite cells, satellite cell activation, myoblast differentiation and fusion in FRG1 versus FRG1/FHL1 muscle to determine the mechanism(s) by which FHL1 reduces disease severity. Mice were examined at 6- and 12-weeks of age, representing both the early mild and late advanced stages of the dystrophic phenotype respectively in the FRG1 mice [33]. It should be noted that both the FRG1- [2] and FHL1-transgenic [48] mice were generated using the human skeletal actin (HSA)-promoter, which is active in differentiating myoblasts and mature muscle fibers, but not satellite cells [67,68]. Despite this, changes to the satellite cell population have been reported in both the FRG1 [33,66] and FHL1 [48] mouse models, via unknown mechanisms. Indeed it is widely accepted that extrinsic factors which are secreted from other sources including muscle fibers and resident non-muscle cells can influence satellite cell and myoblast function [69–71]. Therefore, a comparison of satellite cells and myoblasts in the FRG1 versus FRG1/FHL1 mouse models is a valid and necessary approach to understand how FHL1 expression can rescue the FRG1 dystrophic phenotype.
Satellite cell number was examined by immunostaining transverse muscle sections for the marker, Pax7 (paired-box gene 7) [72] and at both 6- and 12-weeks of age, no difference was observed between FRG1 versus FRG1/FHL1 muscle (Fig. 6 A-C triceps; S3 A–C Fig. quadriceps). This result was confirmed by qRT-PCR analysis of Pax7 mRNA (Fig. 6 D-E triceps; S3 D–E Fig. quadriceps). Satellite cell activation was examined by qRT-PCR analysis of MyoD mRNA [73], revealing no significant differences between FRG1 and FRG1/FHL1 muscle (Fig. 6 F-G triceps; Supplementary S3 F–G Fig., quadriceps). This was confirmed by Pax7/MyoD double immunofluorescent labeling of muscle sections, which revealed no difference in the Pax7+/MyoD+ or Pax7-/MyoD+ populations in FRG1 versus FRG1/FHL1 muscle (S4 Fig.; triceps muscle shown). Expression of myogenin, which marks the initiation of myoblast differentiation [48], was also unchanged between FRG1 and FRG1/FHL1 muscle (Fig. 6 H-I triceps; S3 H–I Fig. quadriceps). Collectively, this suggests that FHL1 does not enhance the activation of satellite cells or myoblast differentiation above that observed in dystrophic FRG1 muscle.
Fig 6. FHL1 does not alter satellite cell number or markers of satellite cell activation (MyoD) or differentiation (myogenin) in the triceps of FRG1 mice.
(A) Transverse muscle sections from the triceps of wild type, FRG1 and FRG1/FHL1 mice (aged 6- and 12-weeks) were co-stained with a satellite cell specific marker (pax7) and DAPI to detect nuclei. Arrows indicate pax7+ satellite cells. Boxed region indicates area shown in high magnification image inset. Scale bars = 100μm. The number of pax7+ satellite cells per 100 myofibers was counted for the triceps in mice aged (B) 6-weeks (FRG1 n = 3 and FRG1/FHL1 n = 3) and (C) 12-weeks (FRG1 n = 4 and FRG1/FHL1 n = 4). Quantitative RT-PCR analysis of pax7 (D- 6 weeks, E- 12 weeks) MyoD (F- 6 weeks, G- 12 weeks) and myogenin (H- 6 weeks, I- 12 weeks) mRNA in wild type, FRG1 and FRG1/FHL1 (n = 7 mice/genotype) triceps muscle. Data represent the mean ± SEM; ns not significant; *p<0.05; **p<0.001 determined by two-tailed Student’s T-test.
FHL1 increases myoblast fusion in FRG1 mice
The terminal phase of myoblast differentiation involves cell fusion to form multi-nucleated myotubes. A previous study has established that FRG1 mice have a myoblast fusion defect in skeletal muscle in vivo [33]. This was shown by examining young FRG1 mice aged 4 weeks, just prior to development of the dystrophic phenotype at 6 weeks, which exhibit a reduction in the number of nuclei per muscle fiber, compared to age-matched wild type mice. This indicates that in young pre-dystrophic FRG1 muscle, fewer myonuclei are incorporated into growing muscle fibers via myoblast fusion, causing a reduction in postnatal muscle growth.
After 6 weeks of age, the dystrophic phenotype develops in FRG1 mice and hence there is a requirement for muscle regeneration, which does not occur in the healthy muscle from wild type mice. Therefore after 6 weeks of age FRG1 mice exhibit an increase in the proportion of muscle fibers with centralized nuclei compared to wild type, in the latter there is no muscle disease and hence no requirement for muscle regeneration or increased myoblast fusion.
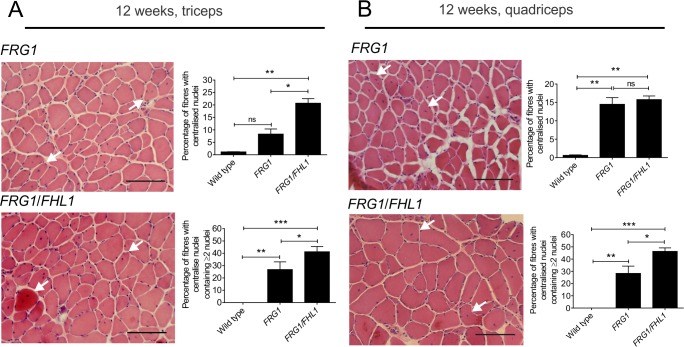
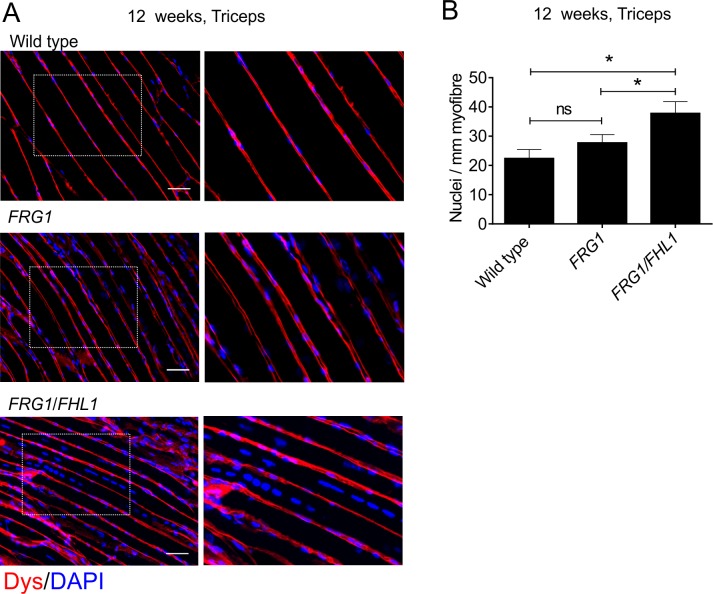
In previous work, we have shown that FHL1 enhances myoblast fusion in vitro [48]. Therefore, we examined if expression of FHL1 in FRG1/FHL1 mice was sufficient to enhance the level of myoblast fusion above that observed in FRG1 mice. Myoblast fusion was assessed in muscle in vivo by examining standard markers of this process; quantifying the proportion of fibers with centrally located nuclei and also the average number of myonuclei per muscle fiber [74]. First, we determined the proportion of total fibers containing centralized nuclei by analysis of H&E stained transverse sections of triceps and quadriceps muscles from adult 12-week-old mice. This analysis revealed FRG1/FHL1 muscles showed a significant increase in the proportion of myofibers with centralized nuclei relative to FRG1 mice in the triceps, but not quadriceps muscle (Fig. 7A-B). Next, we determined what subset of these fibers with centralized nuclei contained multiple centralized nuclei, as a further indicator of enhanced myoblast fusion [70,75]. This was increased in the FRG1/FHL1 triceps and quadriceps muscles compared to FRG1 muscle (Fig. 7A-B), further supporting the contention that FHL1 promotes increased nuclei addition to muscle fibers via enhanced myoblast fusion. We next quantified the number of nuclei per mm length of myofiber using longitudinal sections of the triceps, a dystrophic muscle in FRG1 mice [2]. The triceps long-head represents a single muscle group with all fibers running in parallel, allowing for accurate quantification of the number of myonuclei along the entire length of individual fibers.Analysis of longitudinal triceps muscle sections revealed the number of nuclei per mm of muscle fiber length was significantly increased in 12-week-old FRG1/FHL1 mice relative to both FRG1 and wild type mice (Fig. 8). Therefore, FHL1 enhances myoblast fusion during disease progression in FRG1 muscle.
Fig 7. FHL1 increases the proportion of muscle fibers with centralized nuclei in FRG1 mice.
Representative images of transverse sections of triceps (A) or quadriceps (B) muscles from 12-week-old FRG1 and FRG1/FHL1 mice stained with H&E. Arrows indicate myofibers with centralized nuclei, an indicator of myoblast fusion in vivo. Scale bars = 100μm. For both triceps and quadriceps muscles the percentage of muscle fibers with centralized nuclei was quantified for wild type (n = 3), FRG1 (n = 4) and FRG1/FHL1 (n = 4) mice. The subset of these fibers containing multiple centralized nuclei was further quantified at 12 weeks of age. Data represent the mean ± SEM; ns not significant, *p<0.05; **p<0.005; ***p<0.0005 determined by two-tailed Student’s T-test.
Fig 8. FHL1 enhances myoblast fusion in FRG1 mice.
(A) Representative images of longitudinal sections of triceps muscle from 12-week old wild type, FRG1 and FRG1/FHL1 mice co-stained for dystrophin to outline the muscle fiber membrane and DAPI to detect nuclei. Boxed region indicates area shown in high magnification image inset. Scale bars = 100μm. (B) The number of nuclei per mm of muscle fiber was counted as a measure of myoblast fusion at 12 weeks (wild type n = 3, FRG1 n = 4, FRG1/FHL1 n = 4). Data represent the mean ± SEM; ns not significant, *p<0.05determined by two-tailed Student’s T-test.
FHL1 does not alter expression of the methyltransferase Suv4–20h1 or differentiation inhibitor Eid3 in FRG1 mice
It was recently reported that the histone methyltransferase Suv4–20h1 is a gene-specific repressor required for myogenic differentiation [35]. FRG1 over-expression interferes with the repressive action of Suv4–20h1 leading to aberrant upregulation of the inhibitor, EP300 interacting inhibitor of differentiation 3 (Eid3), resulting in myoblast differentiation defects [35]. To determine if FHL1 enhances myoblast fusion through altering expression of Suv-20h1 or Eid3, we performed qRT-PCR analysis on triceps and quadriceps muscle from 6- and 12-week-old mice (S5 Fig.). We found no differences in Suv4–20h1 mRNA levels between wild type, FRG1 or FRG1/FHL1 mice, but observed a significant increase in Eid3 mRNA levels and protein expression in FRG1 muscle relative to wild type muscle (S4 E–I Fig.), as previously reported in FRG1 mice and FSHD muscle [35]. However, the levels of Eid3 remained elevated in FRG1/FHL1 muscle. Therefore FHL1 does not enhance myoblast fusion through alteration of the FRG1/Suv4–20h1/Eid3 pathway.
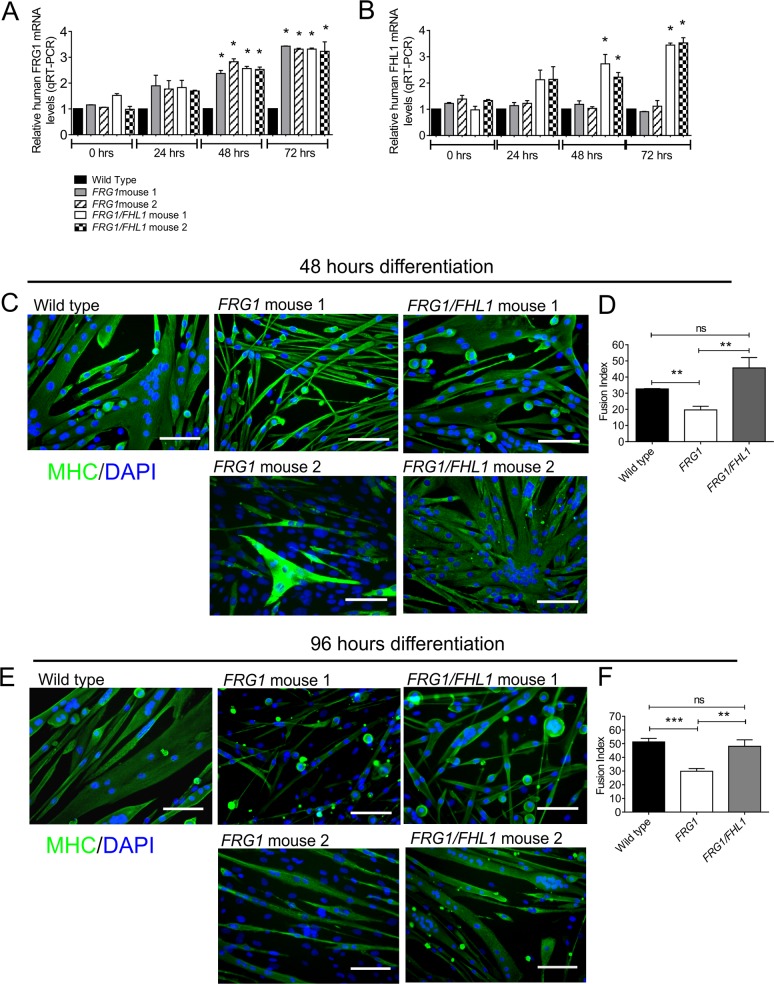
FRG1 myoblasts exhibit a myoblast fusion defect that is rescued in FRG1/FHL1 myoblasts
Primary myoblasts isolated from neonatal mice are routinely used for comparison of differentiation and fusion potential in healthy and disease models [76–78]. This approach has also been recently reported inFSHD disease mouse model, the DUX4 transgenic mouse [22]. Here, we compared the fusion of primary myoblasts isolated from wild type, FRG1 and FRG1/FHL1 mice. FRG1 and FHL1 are under control of the human skeletal muscle actin (HSA) promoter, which should only be active in mature muscle fibers or myoblasts post induction of differentiation [79,80]. In our study, qRT-PCR analysis using human-specific primers (to distinguish from endogenous murine transcripts) confirmed that both transgene-derived FRG1 and FHL1 mRNA were not induced in myoblast cultures until 48 hours differentiation consistent with the reported activation of the HSA promoter at this time point. We observed a 2–3-fold increase in FRG1 mRNA in all differentiating myoblast cultures from FRG1 and FRG1/FHL1 mice (Fig. 9A), and a 2–4-fold increase in FHL1 mRNA in differentiating myoblasts from FRG1/FHL1 mice (Fig. 9B).
Fig 9. FRG1 myoblasts exhibit a fusion defect that is rescued in FRG1/FHL1 myoblasts.
Quantitative RT-PCR analysis of human FRG1 (A) and human FHL1 (B) mRNA in primary mouse myoblasts isolated from wild type mice, and two FRG1 (1 and 2) and two FRG1/FHL1 (1 and 2) mice. Data represent the mean +/- SEM of n = 3 independent experiments and was standardized to GAPDH and expressed relative to control wild type myoblasts. *p<0.05. Representative images of primary myoblasts from wild type, FRG1 and FRG1/FHL1 mice following 48 hours (C) or 96 hours (E) differentiation. Cultures were stained with the differentiation marker MHC (green) and DAPI for detection of the fusion index; the percentage of total nuclei localized within MHC-positive myotubes after 48 hours (D) and 96 hours (F) differentiation. Data represent the mean ± SEM from n = 3 independent experiments; wild type (n = 1); FRG1 (n = 2); FRG1/FHL1 (n = 2); ns not significant, **p<0.005, ***p<0.0001 determined by two-tailed Students T-test. Scale bars = 100μm.
Here we established that the dystrophic pathology of FRG1 mice is rescued by FHL1 through enhancement of myoblast fusion. In a proof of principle experiment the differentiation of primary myoblasts isolated from FRG1 versus FRG1/FHL1 mice were compared. Myoblasts isolated from wild type mice fused to form long multinucleated MHC-positive myotubes, but many FRG1 myoblasts remained as mononucleated cells that did not fuse, however, occasional thin MHC-positive myotubes containing small numbers of nuclei formed (Fig. 9C and 9E). This defect in myoblast fusion was rescued in FRG1/FHL1 myoblasts, which formed long multinucleated MHC-positive myotubes (Fig. 9C and 9E) with a 2 fold-increase in the fusion index relative to FRG1 mouse-derived myoblasts (Fig. 9D and 9F), both at 48 hours and 96 hours differentiation. Therefore, myoblasts isolated from FRG1/FHL1 mice fused more efficiently than those from dystrophic FRG1 mice
Discussion
Here we investigated the selective involvement of FRG1 in regulating myoblast differentiation, revealing a significant myoblast fusion defect in C2C12 myoblasts overexpressing FRG1, and in primary myoblasts derived from FRG1-transgenic-mice. As such, we predicted in proof of principle experiments that agents which promote myoblast fusion may rescue the FRG1-transgenic muscle phenotype. Importantly, our study reveals that FHL1, a positive regulator of muscle hypertrophy and myoblast fusion, reduces muscle wasting and improves the muscular dystrophy phenotype observed in FRG1 mice by rescuing the FRG1-induced myoblast fusion defect. Critically, FHL1 is able to circumvent the myoblast fusion defects in FRG1 mice, despite not directly correcting an underlying molecular cause, the inhibitory FRG1/Suv4–20h1/Eid3 pathway.
FRG1 overexpression causes a myoblast fusion defect
Myoblast fusion is essential for skeletal muscle formation during development, post-natal muscle growth and for the regeneration of skeletal muscle. Increasing evidence supports the contention that defects in satellite cells and myoblasts contribute to disease pathogenesis in βconflicting and can vary with age and disease severity, and also differences have been noted between in vivo and in vitro analyses of satellite cell proliferation [33,66]. Satellite cell activation is also impaired in FRG1 muscle leading to impaired muscle growth and regeneration that precedes the development of the dystrophic phenotype [33]. Increased expression of FRG1 impairs the terminal differentiation of myoblasts [33] through interaction with the histone methytransferase Suv4–20h1 and de-repression of Eid3 [35]. In these recent reports, the differentiation defect of myoblasts overexpressing FRG1 was assessed by examining terminally differentiated myoblasts expressing the marker MHC and also by quantifying the nuclear fusion index (number of nuclei/MHC+ myotube). Our study has undertaken a more extensive characterization of myoblast differentiation in C2C12 myoblasts overexpressing FRG1 and as such has expanded upon the current knowledge of the effect of increased FRG1. This new data demonstrates that FRG1 overexpression does not impair the initiation of myoblast differentiation, as there was no decrease in the expression of the master regulator, myogenin [81]. However in myoblasts over-expressing FRG1, defects in the latter stages of differentiation, and a significant impairment of myoblast fusion was observed. This myoblast fusion defect was shown in C2C12 myoblasts overexpressing FRG1 and also primary myoblasts derived from FRG1-transgenic mice. Therefore our data, together with recent literature, firmly supports a model whereby defects in myoblast function underlie the pathogenesis of muscle disease in the FRG1-transgenic mouse. In our study we rigorously tested this hypothesis by attempting to rescue the dystrophic phenotype in FRG1 mice by enhancing muscle fusion via FHL1 expression.
FHL1 rescues the FRG1 muscular dystrophy phenotype
If myoblast fusion defects do play an important role in the pathogenesis of muscle disease, then it is reasonable to consider that circumventing this defect has potential as a therapeutic strategy. Ours is the first study to test the hypothesis that increasing myoblast fusion circumvents the dystrophic phenotype using the FRG1 mouse as a model. This was achieved by crossing the FRG1-transgenic mouse with a mouse model expressing increased levels of FHL1, a known activator of myoblast fusion [48]. FHL1-transgenic mice develop muscle hypertrophy, oassociated with increased muscle stem cells (satellite cells), increased muscle strength and protection from age-related muscle weakness [48]. Over-expression of FHL1 promotes secondary myoblast fusion, that is, the fusion of myoblasts with nascent myotubes [48]. FHL1 binds and co-activates the transcription factor NFATc1 (nuclear factor of activated T-cells) [48]. Activation of the calcineurin/NFAT pathway is essential for embryonic skeletal muscle development, hypertrophy, regeneration and protection against muscle atrophy [48,82–84]. Central to the role calcineurin/NFAT plays in regulating many of these processes is the activation of myoblast fusion by this pathway [48,85,86].
Notably, we demonstrated that FRG1 mice overexpressing FHL1 show significant improvement in the dystrophic phenotype, with reduced fibrosis and fat deposition associated with increased muscle mass and myofiber size relative to FRG1 mice. FHL1 did not alter satellite cell number, satellite cell activation or myogenic differentiation and therefore this suggests that FHL1 does not ameliorate the dystrophic phenotype in FRG1 mice through recovery of satellite cell function. However, we did observe an increase in the proportion of muscle fibers with one or more centralized nuclei and an increase in the number of nuclei along the length of individual muscle fiber in FRG1/FHL1 mice relative to FRG1, and both parameters are accepted indicators of enhanced myoblast fusion in vivo [74,87]. Critically, in a key proof of principle experiment, myoblasts isolated from FRG1/FHL1 mice exhibited rescue of the myoblast fusion defect that was observed in FRG1 mouse myoblasts. Therefore FHL1 ameliorates the dystrophic phenotype of FRG1 mice by enhancing myoblast fusion and thereby maintenance of muscle mass.
FHL1 does not improve muscle function in FRG1 mice
FHL1 promoted an improvement to the phenotype and muscle pathology in FRG1 mice via enhanced myoblast fusion. However despite this, FHL1 expression was not sufficient to recover muscle function in FRG1 mice. In human and mouse muscle FRG1 localizes to the contractile sarcomere [88] and one possible explanation for the lack of functional improvement in FRG1/FHL1 muscles is the recently reported role for FRG1 in regulating muscle contractility. FRG1-transgenic mice express an abnormal troponin T isoform in fast skeletal muscles due to aberrant splicing of the Tnnt3 mRNA [89]. As a result muscles from FRG1-transgenic mice exhibit a reduction in muscle strength and contractile properties due to an altered MyHC/actin ratio and reduced sensitivity to Ca2+. This decreased sensitivity of fast muscle fibers to Ca2+ is caused by expression of the abnormal troponin. FRG1 also binds actin and has a role in stabilizing actin filaments [5,6]. These results suggest that overexpression of FRG1 has two consequences in muscle, defects in myoblast fusion and impaired muscle contractility. In the current study we provide evidence that expression of FHL1 is sufficient to correct the myoblast fusion defect caused by FRG1 overexpression in FRG1-transgenic mice. However it is possible that an underlying defect in contractile proteins still exists in FRG1/FHL1 mice, which precludes functional improvement.
FHL1 alleviates myoblast fusion defects
There is an overt lack of effective treatments for many muscle diseases, including FSHD. The identification of myoblast differentiation and fusion defects in FSHD and also in an increasing number of other muscle diseases including Limb-girdle Muscular Dystrophy type 2B and Myotonic Dystrophy type 1 (DM1) [40,42,90], suggests that enhancing myoblast fusion may form part of a potential therapeutic strategy. There is a strong association between FHL1 expression/function and muscle health. The identification of multiple FHL1 mutations as causative for human muscle disease highlights the critical importance of FHL1 function for normal muscle homeostasis [44–47]. Myoblast fusion is regulated by the balance of FHL1 expression; increased FHL1 expression enhances myoblast fusion [48], whereas decreased FHL1 expression impairs myoblast [47,91]. The current study is the first to exploit this important regulatory function for FHL1 in controlling myoblast fusion in a muscular dystrophy model, revealing partial amelioration of the FSHD disease phenotype.
Supporting Information
Representative images of transverse muscle sections from the triceps (A) or quadriceps (D) muscles of 12-week-old wild type, FRG1 and FRG1/FHL1 mice stained with H&E. Boxed region indicates area shown in high magnification image inset. Mean myofiber diameter from the triceps (B) and quadriceps (E) was measured for wild type, FRG1 and FRG1/FHL1 mice. Histograms showing frequency of individual muscle fiber diameters from the triceps (C) or quadriceps (F) muscles of wild type, FRG1 and FRG1/FHL1 mice. 500–1000 muscle fibers were measured for per muscle for each mouse; Wild type (n = 3–4 mice), FRG1 (n = 4 mice) and FRG1/FHL1 (n = 5 mice) Data represent the mean ± SEM; *p<0.05; **p<0.005; ***p<0.0005 determined by two-tailed Student’s T-test. In (C) and (F), asterisks in FRG1 histograms indicate significant differences between FRG1 and wild type mice; Asterisks in FRG1/FHL1 histogram indicate significant differences between FRG1/FHL1 and FRG1 mice. Scale bars = 100μm.
(TIF)
Maximum force (A), specific (normalized) force (B), frequency force relationship (C), and resistance to fatigue expressed both as a percentage of initial force (D), and as raw force (E) of TA muscles from 8-week old wild type, FRG1 and FRG1/FHL1 mice, measured in situ. Data represent the mean ± SEM from n ≥ 5 mice per genotype; ns not significant; *p<0.05; ***p<0.0005 determined by two-tailed Student’s T-test.
(TIF)
(A) Transverse muscle sections from the quadriceps of FRG1 and FRG1/FHL1 mice (aged 6- and 12-weeks) co-stained with a satellite cell specific marker (pax7) and DAPI to detect nuclei. Arrows indicate pax7+ satellite cells. Boxed region indicates area shown in high magnification image inset. Scale bars = 100μm. The number of pax7+ satellite cells per 100 myofibers was counted for the quadriceps in mice aged (B) 6 weeks (FRG1 n = 3 and FRG1/FHL1 n = 3–4) and (C) 12 weeks (n = 4/genotype). Quantitative RT-PCR analysis of pax7 (D- 6 weeks, E- 12 weeks) MyoD (F- 6 weeks, G-12 weeks) and myogenin (H- 6 weeks, I- 12 weeks) mRNA in wild type, FRG1 and FRG1/FHL1 (n = 7 mice/genotype) quadriceps muscle. Data represent the mean ± SEM; ns not significant; *p<0.05; **p<0.001 determined by two-tailed Student’s T-test.
(TIF)
Transverse muscle sections from the triceps of FRG1 and FRG1/FHL1 mice aged (A) 6- and (B) 12-weeks, co-stained with a marker for quiescent satellite cells (Pax7) and activated satellite cells (MyoD). Green arrows indicate Pax7+/MyoD- cells; yellow arrows indicate Pax7+/MyoD+ cells; red arrows indicate Pax7-/MyoD+ cells. Boxed region indicates area shown in high magnification image at far right panel. Scale bars = 100μm. The number of Pax7+/MyoD+ cells per 100 myofibers from the triceps in mice aged (C) 6- and (D) 12-weeks; n = 3–4/genotype. The number of Pax7-/MyoD+ cells per 100 myofibers from the triceps muscle in mice aged (E) 6-and (F) 12-weeks; n = 3–4/genotype. Data represent the mean ± SEM and a Student’s T-test revealed no statistically significant difference (ns) between FRG1 and FRG1/FHL1 mice.
(TIF)
Quantitative RT-PCR analysis of Suv4–20h1 mRNA in triceps muscle at 6-weeks (A) and 12-weeks (B) and in quadriceps muscle at 6-weeks (C) and 12-weeks (D) from wild type, FRG1 and FRG1/FHL1 mice (n = 7/genotype). Quantitative RT-PCR analysis of Eid3 mRNA in triceps muscle at 6-weeks (E) and 12-weeks (F) and in quadriceps muscle at 6-weeks (G) and 12-weeks (H) from wild type, FRG1 and FRG1/FHL1 mice (n = 7/genotype). Data represent the mean ± SEM; ns not significant; *p<0.05 determined by two-tailed Student’s T-test. (C) Western blot of Eid3 protein expression in wild type, FRG1 and FRG1/FHL1 triceps muscle. Immunoblotting for β-tubulin or staining of membranes with ponceau red was used as a protein loading control.
(TIF)
(DOC)
(DOC)
(DOC)
(PDF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the FSHD Global Research Foundation Ltd Australian Research Grants (to C.A.M. and M.J.M.; http://www.fshdglobal.org/); and the National Institute of Health (NIAMS, RO1AR056129 to R.T; http://www.nih.gov/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Grewal PK, Todd LC, van der Maarel S, Frants RR, Hewitt JE (1998) FRG1, a gene in the FSH muscular dystrophy region on human chromosome 4q35, is highly conserved in vertebrates and invertebrates. Gene 216: 13–19. [DOI] [PubMed] [Google Scholar]
- 2. Gabellini D, D'Antona G, Moggio M, Prelle A, Zecca C, et al. (2006) Facioscapulohumeral muscular dystrophy in mice overexpressing FRG1. Nature 439: 973–977. [DOI] [PubMed] [Google Scholar]
- 3. van Koningsbruggen S, Straasheijm KR, Sterrenburg E, de Graaf N, Dauwerse HG, et al. (2007) FRG1P-mediated aggregation of proteins involved in pre-mRNA processing. Chromosoma 116: 53–64. [DOI] [PubMed] [Google Scholar]
- 4. Pistoni M, Shiue L, Cline MS, Bortolanza S, Neguembor MV, et al. (2013) Rbfox1 downregulation and altered calpain 3 splicing by FRG1 in a mouse model of Facioscapulohumeral muscular dystrophy (FSHD). Plos Genetics 9: e1003186 10.1371/journal.pgen.1003186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sun CY, van Koningsbruggen S, Long SW, Straasheijm K, Klooster R, et al. (2011) Facioscapulohumeral muscular dystrophy region gene 1 is a dynamic RNA-associated and actin-bundling protein. Journal of Molecular Biology 411: 397–416. 10.1016/j.jmb.2011.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu Q, Jones TI, Tang VW, Brieher WM, Jones PL (2010) Facioscapulohumeral muscular dystrophy region gene-1 (FRG-1) is an actin-bundling protein associated with muscle-attachment sites. Journal of Cell Science 123: 1116–1123. 10.1242/jcs.058958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hanel ML, Wuebbles RD, Jones PL (2009) Muscular dystrophy candidate gene FRG1 is critical for muscle development. Dev Dyn 238: 1502–1512. 10.1002/dvdy.21830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tawil R, van der Maarel SM, Tapscott SJ (2014) Facioscapulohumeral dystrophy: the path to consensus on pathophysiology. Skelet Muscle 4: 12 10.1186/2044-5040-4-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Padberg GW, Frants RR, Brouwer OF, Wijmenga C, Bakker E, et al. (1995) Facioscapulohumeral muscular dystrophy in the Dutch population. Muscle Nerve Suppl 2: S81–84. [PubMed] [Google Scholar]
- 10. Flanigan KM, Coffeen CM, Sexton L, Stauffer D, Brunner S, et al. (2001) Genetic characterization of a large, historically significant Utah kindred with facioscapulohumeral dystrophy. Neuromuscul Disord 11: 525–529. [DOI] [PubMed] [Google Scholar]
- 11. Mostacciuolo ML, Pastorello E, Vazza G, Miorin M, Angelini C, et al. (2009) Facioscapulohumeral muscular dystrophy: epidemiological and molecular study in a north-east Italian population sample. Clin Genet 75: 550–555. 10.1111/j.1399-0004.2009.01158.x [DOI] [PubMed] [Google Scholar]
- 12. Deenen JC, Arnts H, van der Maarel SM, Padberg GW, Verschuuren JJ, et al. (2014) Population-based incidence and prevalence of facioscapulohumeral dystrophy. Neurology 83: 1056–1059. 10.1212/WNL.0000000000000797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wijmenga C, Padberg GW, Moerer P, Wiegant J, Liem L, et al. (1991) Mapping of facioscapulohumeral muscular dystrophy gene to chromosome 4q35-qter by multipoint linkage analysis and in situ hybridization. Genomics 9: 570–575. [DOI] [PubMed] [Google Scholar]
- 14. Gabriels J, Beckers MC, Ding H, De Vriese A, Plaisance S, et al. (1999) Nucleotide sequence of the partially deleted D4Z4 locus in a patient with FSHD identifies a putative gene within each 3.3 kb element. Gene 236: 25–32. [DOI] [PubMed] [Google Scholar]
- 15. Lemmers RJ, van der Vliet PJ, Klooster R, Sacconi S, Camano P, et al. (2010) A unifying genetic model for facioscapulohumeral muscular dystrophy. Science 329: 1650–1653. 10.1126/science.1189044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dixit M, Ansseau E, Tassin A, Winokur S, Shi R, et al. (2007) DUX4, a candidate gene of facioscapulohumeral muscular dystrophy, encodes a transcriptional activator of PITX1. Proceedings of the National Academy of Sciences of the United States of America 104: 18157–18162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Geng LN, Yao ZZ, Snider L, Fong AP, Cech JN, et al. (2012) DUX4 Activates Germ line Genes, Retroelements, and Immune Mediators: Implications for Facioscapulohumeral Dystrophy. Developmental Cell 22: 38–51. 10.1016/j.devcel.2011.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Young JM, Whiddon JL, Yao Z, Kasinathan B, Snider L, et al. (2013) DUX4 binding to retroelements creates promoters that are active in FSHD muscle and testis. Plos Genetics 9: e1003947 10.1371/journal.pgen.1003947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pandey SN, Cabotage J, Shi R, Dixit M, Sutherland M, et al. (2012) Conditional over-expression of PITX1 causes skeletal muscle dystrophy in mice. Biol Open 1: 629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mitsuhashi H, Mitsuhashi S, Lynn-Jones T, Kawahara G, Kunkel LM (2013) Expression of DUX4 in zebrafish development recapitulates facioscapulohumeral muscular dystrophy. Human Molecular Genetics 22: 568–577. 10.1093/hmg/dds467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Krom YD, Thijssen PE, Young JM, den Hamer B, Balog J, et al. (2013) Intrinsic epigenetic regulation of the D4Z4 macrosatellite repeat in a transgenic mouse model for FSHD. Plos Genetics 9: e1003415 10.1371/journal.pgen.1003415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dandapat A, Bosnakovski D, Hartweck LM, Arpke RW, Baltgalvis KA, et al. (2014) Dominant Lethal Pathologies in Male Mice Engineered to Contain an X-Linked DUX4 Transgene. Cell Reports 8: 1484–1496. 10.1016/j.celrep.2014.07.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van Deutekom JC, Lemmers RJ, Grewal PK, van Geel M, Romberg S, et al. (1996) Identification of the first gene (FRG1) from the FSHD region on human chromosome 4q35. Human Molecular Genetics 5: 581–590. [DOI] [PubMed] [Google Scholar]
- 24. Tupler R, Berardinelli A, Barbierato L, Frants R, Hewitt JE, et al. (1996) Monosomy of distal 4q does not cause facioscapulohumeral muscular dystrophy. J Med Genet 33: 366–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferri G, Huichalaf CH, Caccia R, Gabellini D (2014) Direct interplay between two candidate genes in FSHD muscular dystrophy. Human Molecular Genetics: 1–11. [DOI] [PMC free article] [PubMed]
- 26. Cabianca DS, Casa V, Bodega B, Xynos A, Ginelli E, et al. (2012) A long ncRNA links copy number variation to a polycomb/trithorax epigenetic switch in FSHD muscular dystrophy. Cell 149: 819–831. 10.1016/j.cell.2012.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gabellini D, Green MR, Tupler R (2002) Inappropriate gene activation in FSHD: a repressor complex binds a chromosomal repeat deleted in dystrophic muscle. Cell 110: 339–348. [DOI] [PubMed] [Google Scholar]
- 28. Osborne RJ, Welle S, Venance SL, Thornton CA, Tawil R (2007) Expression profile of FSHD supports a link between retinal vasculopathy and muscular dystrophy. Neurology 68: 569–577. [DOI] [PubMed] [Google Scholar]
- 29. Klooster R, Straasheijm K, Shah B, Sowden J, Frants R, et al. (2009) Comprehensive expression analysis of FSHD candidate genes at the mRNA and protein level. European Journal of Human Genetics 17: 1615–1624. 10.1038/ejhg.2009.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Winokur ST, Chen YW, Masny PS, Martin JH, Ehmsen JT, et al. (2003) Expression profiling of FSHD muscle supports a defect in specific stages of myogenic differentiation. Human Molecular Genetics 12: 2895–2907. [DOI] [PubMed] [Google Scholar]
- 31. Jiang G, Yang F, van Overveld PG, Vedanarayanan V, van der Maarel S, et al. (2003) Testing the position-effect variegation hypothesis for facioscapulohumeral muscular dystrophy by analysis of histone modification and gene expression in subtelomeric 4q. Human Molecular Genetics 12: 2909–2921. [DOI] [PubMed] [Google Scholar]
- 32. Masny PS, Chan OYA, de Greef JC, Bengtsson U, Ehrlich M, et al. (2010) Analysis of allele-specific RNA transcription in FSHD by RNA-DNA FISH in single myonuclei. European Journal of Human Genetics 18: 448–456. 10.1038/ejhg.2009.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xynos A, Neguembor MV, Caccia R, Licastro D, Nonis A, et al. (2013) Overexpression of facioscapulohumeral muscular dystrophy region gene 1 causes primary defects in myogenic stem cells. Journal of Cell Science 126: 2236–2245. 10.1242/jcs.121533 [DOI] [PubMed] [Google Scholar]
- 34. Wuebbles RD, Hanel ML, Jones PL (2009) FSHD region gene 1 (FRG1) is crucial for angiogenesis linking FRG1 to facioscapulohumeral muscular dystrophy-associated vasculopathy. Disease Models & Mechanisms 2: 267–274. 10.1111/jvh.12392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Neguembor MV, Xynos A, Onorati MC, Caccia R, Bortolanza S, et al. (2013) FSHD muscular dystrophy region gene 1 binds Suv4–20h1 histone methyltransferase and impairs myogenesis. Journal of Molecular Cell Biology 5: 294–307. 10.1093/jmcb/mjt018 [DOI] [PubMed] [Google Scholar]
- 36. Celegato B, Capitanio D, Pescatori M, Romualdi C, Pacchioni B, et al. (2006) Parallel protein and transcript profiles of FSHD patient muscles correlate to the D4Z4 arrangement and reveal a common impairment of slow to fast fibre differentiation and a general deregulation of MyoD-dependent genes. Proteomics 6: 5303–5321. [DOI] [PubMed] [Google Scholar]
- 37. Cheli S, Francois S, Bodega B, Ferrari F, Tenedini E, et al. (2011) Expression profiling of FSHD-1 and FSHD-2 cells during myogenic differentiation evidences common and distinctive gene dysregulation patterns. Plos One 6: e20966 10.1371/journal.pone.0020966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tassin A, Leroy B, Laoudj-Chenivesse D, Wauters A, Vanderplanck C, et al. (2012) FSHD Myotubes with Different Phenotypes Exhibit Distinct Proteomes. Plos One 7: e51865 10.1371/journal.pone.0051865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tsumagari K, Chang SC, Lacey M, Baribault C, Chittur SV, et al. (2011) Gene expression during normal and FSHD myogenesis. Bmc Medical Genomics 4: 67–84. 10.1186/1755-8794-4-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Furling D, Lemieux D, Taneja K, Puymirat J (2001) Decreased levels of myotonic dystrophy protein kinase (DMPK) and delayed differentiation in human myotonic dystrophy myoblasts. Neuromuscular Disorders 11: 728–735. [DOI] [PubMed] [Google Scholar]
- 41. Timchenko NA, Iakova P, Cai ZJ, Smith JR, Timchenko LT (2001) Molecular basis for impaired muscle differentiation in myotonic dystrophy. Molecular and Cellular Biology 21: 6927–6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Storbeck CJ, Drmanic S, Daniel K, Waring JD, Jirik FR, et al. (2004) Inhibition of myogenesis in transgenic mice expressing the human DMPK 3 '-UTR. Human Molecular Genetics 13: 589–600. [DOI] [PubMed] [Google Scholar]
- 43. Brown S, McGrath MJ, Ooms LM, Gurung R, Maimone MM, et al. (1999) Characterization of two isoforms of the skeletal muscle LIM protein 1, SLIM1. Localization of SLIM1 at focal adhesions and the isoform slimmer in the nucleus of myoblasts and cytoplasm of myotubes suggests distinct roles in the cytoskeleton and in nuclear-cytoplasmic communication. Journal of Biological Chemistry 274: 27083–27091. [DOI] [PubMed] [Google Scholar]
- 44. Schessl J, Zou Y, McGrath MJ, Cowling BS, Maiti B, et al. (2008) Proteomic identification of FHL1 as the protein mutated in human reducing body myopathy. Journal of Clinical Investigation 118: 904–912. 10.1172/JCI34450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Quinzii CM, Vu TH, Min KC, Tanji K, Barral S, et al. (2008) X-linked dominant scapuloperoneal myopathy is due to a mutation in the gene encoding four-and-a-half-LIM protein 1. American Journal of Human Genetics 82: 208–213. 10.1016/j.ajhg.2007.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Windpassinger C, Schoser B, Straub V, Hochmeister S, Noor A, et al. (2008) An X-linked myopathy with postural muscle atrophy and generalized hypertrophy, termed XMPMA, is caused by mutations in FHL1. American Journal of Human Genetics 82: 88–99. 10.1016/j.ajhg.2007.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gueneau L, Bertrand AT, Jais JP, Salih MA, Stojkovic T, et al. (2009) Mutations of the FHL1 gene cause Emery-Dreifuss muscular dystrophy. American Journal of Human Genetics 85: 338–353. 10.1016/j.ajhg.2009.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cowling BS, McGrath MJ, Nguyen MA, Cottle DL, Kee AJ, et al. (2008) Identification of FHL1 as a regulator of skeletal muscle mass: implications for human myopathy. Journal of Cell Biology 183: 1033–1048. 10.1083/jcb.200804077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yaffe D, Saxel O (1977) Serial Passing and Differentiation of Myogenic Cells Isolated from Dystrophic Mouse Muscle. Nature 270: 725–727. [DOI] [PubMed] [Google Scholar]
- 50. Jorgensen LH, Jensen CH, Wewer UM, Schroder HD (2007) Transgenic overexpression of ADAM12 suppresses muscle regeneration and aggravates dystrophy in aged mdx mice. American Journal of Pathology 171: 1599–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3: 1101–1108. [DOI] [PubMed] [Google Scholar]
- 52. Cottle DL, McGrath MJ, Cowling BS, Coghill ID, Brown S, et al. (2007) FHL3 binds MyoD and negatively regulates myotube formation. Journal of Cell Science 120: 1423–1435. [DOI] [PubMed] [Google Scholar]
- 53. Kobayashi N, Homma S, Okada T, Masuda T, Sato N, et al. (2013) Elucidation of target muscle and detailed development of dorsal motor neurons in chick embryo spinal cord. J Comp Neurol 521: 2987–3002. 10.1002/cne.23326 [DOI] [PubMed] [Google Scholar]
- 54. Katsetos CD, Herman MM, Mork SJ (2003) Class III beta-tubulin in human development and cancer. Cell Motil Cytoskeleton 55: 77–96. [DOI] [PubMed] [Google Scholar]
- 55.Springer ML, Rando TA, Blau HM (2002) Gene delivery to muscle. Curr Protoc Hum Genet Chapter 13: Unit13 14. [DOI] [PubMed]
- 56. D'Arcy CE, Feeney SJ, McLean CA, Gehrig SM, Lynch GS, et al. (2014) Identification of FHL1 as a therapeutic target for Duchenne muscular dystrophy. Human Molecular Genetics 23: 618–636. 10.1093/hmg/ddt449 [DOI] [PubMed] [Google Scholar]
- 57. Bavner A, Matthews J, Sanyal S, Gustafsson JA, Treuter E (2005) EID3 is a novel EID family member and an inhibitor of CBP-dependent co-activation. Nucleic Acids Research 33: 3561–3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Briguet A, Courdier-Fruh I, Foster M, Meier T, Magyar JP (2004) Histological parameters for the quantitative assessment of muscular dystrophy in the mdx-mouse. Neuromuscul Disord 14: 675–682. [DOI] [PubMed] [Google Scholar]
- 59. Joven A, Morona R, Gonzalez A, Moreno N (2013) Spatiotemporal patterns of Pax3, Pax6, and Pax7 expression in the developing brain of a urodele amphibian, Pleurodeles waltl. J Comp Neurol 521: 3913–3953. 10.1002/cne.23385 [DOI] [PubMed] [Google Scholar]
- 60. Bruusgaard JC, Gundersen K (2008) In vivo time-lapse microscopy reveals no loss of murine myonuclei during weeks of muscle atrophy. Journal of Clinical Investigation 118: 1450–1457. 10.1172/JCI34022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kim JE, Yeo SI, Ryu HJ, Kim MJ, Kim DS, et al. (2010) Astroglial loss and edema formation in the rat piriform cortex and hippocampus following pilocarpine-induced status epilepticus. J Comp Neurol 518: 4612–4628. 10.1002/cne.22482 [DOI] [PubMed] [Google Scholar]
- 62. White RB, Bierinx AS, Gnocchi VF, Zammit PS (2010) Dynamics of muscle fibre growth during postnatal mouse development. Bmc Developmental Biology 10: 21 10.1186/1471-213X-10-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gehrig SM, van der Poel C, Sayer TA, Schertzer JD, Henstridge DC, et al. (2012) Hsp72 preserves muscle function and slows progression of severe muscular dystrophy. Nature 484: 394–398. 10.1038/nature10980 [DOI] [PubMed] [Google Scholar]
- 64. Wang CH, Leung M, Liang WC, Hsieh TJ, Chen TH, et al. (2012) Correlation between muscle involvement, phenotype and D4Z4 fragment size in facioscapulohumeral muscular dystrophy. Neuromuscul Disord 22: 331–338. 10.1016/j.nmd.2011.10.018 [DOI] [PubMed] [Google Scholar]
- 65. Wallace GQ, McNally EM (2009) Mechanisms of muscle degeneration, regeneration, and repair in the muscular dystrophies. Annu Rev Physiol 71: 37–57. 10.1146/annurev.physiol.010908.163216 [DOI] [PubMed] [Google Scholar]
- 66. Chen SC, Frett E, Marx J, Bosnakovski D, Reed X, et al. (2011) Decreased proliferation kinetics of mouse myoblasts overexpressing FRG1. Plos One 6: e19780 10.1371/journal.pone.0019780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Brennan KJ, Hardeman EC (1993) Quantitative analysis of the human alpha-skeletal actin gene in transgenic mice. Journal of Biological Chemistry 268: 719–725. [PubMed] [Google Scholar]
- 68. Miniou P, Tiziano D, Frugier T, Roblot N, Le Meur M, et al. (1999) Gene targeting restricted to mouse striated muscle lineage. Nucleic Acids Research 27: e27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Heredia JE, Mukundan L, Chen FM, Mueller AA, Deo RC, et al. (2013) Type 2 Innate Signals Stimulate Fibro/Adipogenic Progenitors to Facilitate Muscle Regeneration. Cell 153: 376–388. 10.1016/j.cell.2013.02.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Horsley V, Jansen KM, Mills ST, Pavlath GK (2003) IL-4 acts as a myoblast recruitment factor during mammalian muscle growth. Cell 113: 483–494. [DOI] [PubMed] [Google Scholar]
- 71. Tidball JG, Villalta SA (2010) Regulatory interactions between muscle and the immune system during muscle regeneration. Am J Physiol Regul Integr Comp Physiol 298: R1173–1187. 10.1152/ajpregu.00735.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Loh KC, Leong WI, Carlson ME, Oskouian B, Kumar A, et al. (2012) Sphingosine-1-phosphate enhances satellite cell activation in dystrophic muscles through a S1PR2/STAT3 signaling pathway. Plos One 7: e37218 10.1371/journal.pone.0037218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zammit PS, Partridge TA, Yablonka-Reuveni Z (2006) The skeletal muscle satellite cell: the stem cell that came in from the cold. Journal of Histochemistry & Cytochemistry 54: 1177–1191. [DOI] [PubMed] [Google Scholar]
- 74. Millay DP, O'Rourke JR, Sutherland LB, Bezprozvannaya S, Shelton JM, et al. (2013) Myomaker is a membrane activator of myoblast fusion and muscle formation. Nature 499: 301–305. 10.1038/nature12343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Minetti GC, Feige JN, Bombard F, Heier A, Morvan F, et al. (2014) Galphai2 signaling is required for skeletal muscle growth, regeneration, and satellite cell proliferation and differentiation. Molecular and Cellular Biology 34: 619–630. 10.1128/MCB.00957-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fu W, Asp P, Canter B, Dynlacht BD (2014) Primary cilia control hedgehog signaling during muscle differentiation and are deregulated in rhabdomyosarcoma. Proc Natl Acad Sci U S A 111: 9151–9156. 10.1073/pnas.1323265111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chatterjee S, Nam D, Guo B, Kim JM, Winnier GE, et al. (2013) Brain and muscle Arnt-like 1 is a key regulator of myogenesis. Journal of Cell Science 126: 2213–2224. 10.1242/jcs.120519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ge X, McFarlane C, Vajjala A, Lokireddy S, Ng ZH, et al. (2011) Smad3 signaling is required for satellite cell function and myogenic differentiation of myoblasts. Cell Res 21: 1591–1604. 10.1038/cr.2011.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Nicole S, Desforges B, Millet G, Lesbordes J, Cifuentes-Diaz C, et al. (2003) Intact satellite cells lead to remarkable protection against Smn gene defect in differentiated skeletal muscle. Journal of Cell Biology 161: 571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Quinn LS, Anderson BG, Plymate SR (2007) Muscle-specific overexpression of the type 1 IGF receptor results in myoblast-independent muscle hypertrophy via PI3K, and not calcineurin, signaling. Am J Physiol Endocrinol Metab 293: E1538–1551. [DOI] [PubMed] [Google Scholar]
- 81. Mastroyiannopoulos NP, Nicolaou P, Anayasa M, Uney JB, Phylactou LA (2012) Down-Regulation of Myogenin Can Reverse Terminal Muscle Cell Differentiation. Plos One 7 10.1371/journal.pone.0051204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sakuma K, Nishikawa J, Nakao R, Watanabe K, Totsuka T, et al. (2003) Calcineurin is a potent regulator for skeletal muscle regeneration by association with NFATc1 and GATA-2. Acta Neuropathologica 105: 271–280. [DOI] [PubMed] [Google Scholar]
- 83. Li X, Zhu L, Yang A, Lin J, Tang F, et al. (2011) Calcineurin-NFAT signaling critically regulates early lineage specification in mouse embryonic stem cells and embryos. Cell Stem Cell 8: 46–58. 10.1016/j.stem.2010.11.027 [DOI] [PubMed] [Google Scholar]
- 84. van Rooij E, Doevendans PA, de Theije CC, Babiker FA, Molkentin JD, et al. (2002) Requirement of nuclear factor of activated T-cells in calcineurin-mediated cardiomyocyte hypertrophy. Journal of Biological Chemistry 277: 48617–48626. [DOI] [PubMed] [Google Scholar]
- 85. Horsley V, Friday BB, Matteson S, Kegley KM, Gephart J, et al. (2001) Regulation of the growth of multinucleated muscle cells by an NFATC2-dependent pathway. Journal of Cell Biology 153: 329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Pavlath GK, Horsley V (2003) Cell fusion in skeletal muscle—central role of NFATC2 in regulating muscle cell size. Cell Cycle 2: 420–423. [PubMed] [Google Scholar]
- 87. Hochreiter-Hufford AE, Lee CS, Kinchen JM, Sokolowski JD, Arandjelovic S, et al. (2013) Phosphatidylserine receptor BAI1 and apoptotic cells as new promoters of myoblast fusion. Nature 497: 263–267. 10.1038/nature12135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hanel ML, Sun CYJ, Jones TI, Long SW, Zanotti S, et al. (2011) Facioscapulohumeral muscular dystrophy (FSHD) region gene 1 (FRG1) is a dynamic nuclear and sarcomeric protein. Differentiation 81: 107–118. 10.1016/j.diff.2010.09.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sancisi V, Germinario E, Esposito A, Morini E, Peron S, et al. (2014) Altered Tnnt3 characterizes selective weakness of fast fibers in mice overexpressing FSHD region gene 1 (FRG1). Am J Physiol Regul Integr Comp Physiol 306: R124–137. 10.1152/ajpregu.00379.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Cohen TV, Cohen JE, Partridge TA (2012) Myogenesis in dysferlin-deficient myoblasts is inhibited by an intrinsic inflammatory response. Neuromuscular Disorders 22: 648–658. 10.1016/j.nmd.2012.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wilding BR, McGrath MJ, Bonne G, Mitchell CA (2014) FHL1 mutants that cause clinically distinct human myopathies form protein aggregates and impair myoblast differentiation. Journal of Cell Science 127: 2269–2281. 10.1242/jcs.140905 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative images of transverse muscle sections from the triceps (A) or quadriceps (D) muscles of 12-week-old wild type, FRG1 and FRG1/FHL1 mice stained with H&E. Boxed region indicates area shown in high magnification image inset. Mean myofiber diameter from the triceps (B) and quadriceps (E) was measured for wild type, FRG1 and FRG1/FHL1 mice. Histograms showing frequency of individual muscle fiber diameters from the triceps (C) or quadriceps (F) muscles of wild type, FRG1 and FRG1/FHL1 mice. 500–1000 muscle fibers were measured for per muscle for each mouse; Wild type (n = 3–4 mice), FRG1 (n = 4 mice) and FRG1/FHL1 (n = 5 mice) Data represent the mean ± SEM; *p<0.05; **p<0.005; ***p<0.0005 determined by two-tailed Student’s T-test. In (C) and (F), asterisks in FRG1 histograms indicate significant differences between FRG1 and wild type mice; Asterisks in FRG1/FHL1 histogram indicate significant differences between FRG1/FHL1 and FRG1 mice. Scale bars = 100μm.
(TIF)
Maximum force (A), specific (normalized) force (B), frequency force relationship (C), and resistance to fatigue expressed both as a percentage of initial force (D), and as raw force (E) of TA muscles from 8-week old wild type, FRG1 and FRG1/FHL1 mice, measured in situ. Data represent the mean ± SEM from n ≥ 5 mice per genotype; ns not significant; *p<0.05; ***p<0.0005 determined by two-tailed Student’s T-test.
(TIF)
(A) Transverse muscle sections from the quadriceps of FRG1 and FRG1/FHL1 mice (aged 6- and 12-weeks) co-stained with a satellite cell specific marker (pax7) and DAPI to detect nuclei. Arrows indicate pax7+ satellite cells. Boxed region indicates area shown in high magnification image inset. Scale bars = 100μm. The number of pax7+ satellite cells per 100 myofibers was counted for the quadriceps in mice aged (B) 6 weeks (FRG1 n = 3 and FRG1/FHL1 n = 3–4) and (C) 12 weeks (n = 4/genotype). Quantitative RT-PCR analysis of pax7 (D- 6 weeks, E- 12 weeks) MyoD (F- 6 weeks, G-12 weeks) and myogenin (H- 6 weeks, I- 12 weeks) mRNA in wild type, FRG1 and FRG1/FHL1 (n = 7 mice/genotype) quadriceps muscle. Data represent the mean ± SEM; ns not significant; *p<0.05; **p<0.001 determined by two-tailed Student’s T-test.
(TIF)
Transverse muscle sections from the triceps of FRG1 and FRG1/FHL1 mice aged (A) 6- and (B) 12-weeks, co-stained with a marker for quiescent satellite cells (Pax7) and activated satellite cells (MyoD). Green arrows indicate Pax7+/MyoD- cells; yellow arrows indicate Pax7+/MyoD+ cells; red arrows indicate Pax7-/MyoD+ cells. Boxed region indicates area shown in high magnification image at far right panel. Scale bars = 100μm. The number of Pax7+/MyoD+ cells per 100 myofibers from the triceps in mice aged (C) 6- and (D) 12-weeks; n = 3–4/genotype. The number of Pax7-/MyoD+ cells per 100 myofibers from the triceps muscle in mice aged (E) 6-and (F) 12-weeks; n = 3–4/genotype. Data represent the mean ± SEM and a Student’s T-test revealed no statistically significant difference (ns) between FRG1 and FRG1/FHL1 mice.
(TIF)
Quantitative RT-PCR analysis of Suv4–20h1 mRNA in triceps muscle at 6-weeks (A) and 12-weeks (B) and in quadriceps muscle at 6-weeks (C) and 12-weeks (D) from wild type, FRG1 and FRG1/FHL1 mice (n = 7/genotype). Quantitative RT-PCR analysis of Eid3 mRNA in triceps muscle at 6-weeks (E) and 12-weeks (F) and in quadriceps muscle at 6-weeks (G) and 12-weeks (H) from wild type, FRG1 and FRG1/FHL1 mice (n = 7/genotype). Data represent the mean ± SEM; ns not significant; *p<0.05 determined by two-tailed Student’s T-test. (C) Western blot of Eid3 protein expression in wild type, FRG1 and FRG1/FHL1 triceps muscle. Immunoblotting for β-tubulin or staining of membranes with ponceau red was used as a protein loading control.
(TIF)
(DOC)
(DOC)
(DOC)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.