Abstract
Borrelia turcica comprises the third major group of arthropod-transmitted borreliae and is phylogenetically divergent from other Borrelia groups. The novel group of Borrelia was initially isolated from Hyalomma aegyptium ticks in Turkey and it was recently found in blood and multiple organs of tortoises exported from Jordan to Japan. However, the ecology of these spirochetes and their development in ticks or the vertebrate hosts were not investigated in detail; our aims were to isolate the pathogen and to evaluate the possibility of transstadial transmission of Borrelia turcica by H. aegyptium ticks. Ticks were collected from Testudo graeca tortoises during the summer of 2013 from southeastern Romania. Engorged nymphs were successfully molted to the adult stage. Alive B. turcica was isolated from molted ticks by using Barbour-Stoenner-Kelly (BSK) II medium. Four pure cultures of spirochetes were obtained and analyzed by PCR and sequencing. Sequence analysis of glpQ, gyrB and flaB revealed 98%–100% similarities with B. turcica. H. aegyptium ticks collected from T. graeca tortoises were able to pass the infection with B. turcica via transstadial route, suggesting its vectorial capacity.
Introduction
Tick-borne infectious diseases are transmitted through the bite or ingestion of infected ticks [1]. Among the most widely distributed tick-borne bacterial pathogens are spirochetes of genus Borrelia [2, 3]. Genus Borrelia includes three main distinct phylogenetic lineages: Lyme borreliosis (LB) borreliae, relapsing fever (RF) borreliae and the recently described reptile-associated borreliae (REP) [4, 5]. The agents of LB are various species of the Borrelia burgdorferi sensu lato (s.l.) complex, all transmitted through the bite of hard ticks of genus Ixodes [6, 7]. RF Borrelia are transmitted by soft ticks, hard ticks or lice and have been reported primarily in northern US [8], Canada [9], Africa [10, 11] and Eurasia [12–16]. REP Borrelia isolates were described so far only from hard ticks or blood collected from tortoises.
In the last years, the epidemiologic role of reptiles has received increasing attention, mainly due to the international pet trade of animals originating from the wild [17]. Often, reptiles imported from the wild are harboring various tick species that facilitate the introduction of non-native tick-borne pathogens, thus posing a significant public health risk [17–19]. Various emerging and/or zoonotic pathogens have been isolated and characterized from reptiles or their associated ticks [20–25]. Borrelia turcica, a member of the REP group, was described from H. aegyptium ticks associated with Mediterranean tortoises in Turkey [21, 26]. B. turcica has been demonstrated to form a distinct monophyletic group and showed a relationship with both RF and LB groups. Based on the analysis of flaB and gyrB genes, respectively 16S rDNA sequences, B. turcica IST7 was distinct but branched off and clustered between the species of LB and RF Borrelia [21, 23, 26, 27]. Adeolu and Gupta (2014) grouped B. turcica spirochetes in a third distinct clade based on 16S rRNA gene [28]. REP Borrelia (Borrelia sp. tAG) spirochetes were also isolated from Amblyomma geoemydae ticks and they clustered with RF Borrelia based on another phylogenetic analysis [27].The natural cycle of tick-borne RF (TBRF) spirochetes involve a diversity of small mammals and their tick vectors [29]. They are a neglected cause of zoonotic diseases that result in significant illness and even death [30]. TBRF group of spirochetes currently includes ten already sequenced species of Borrelia [28], but many isolates are not yet characterized and further designations are expected.
Recently, H. aegyptium was shown to be an important carrier of various zoonotic agents: Theileria spp., Rickettsia spp. Ehrlichia spp., Coxiella spp., Anaplasma spp. and Crimean-Congo hemorrhagic fever virus [25, 31–35]. As nymphs and adults of H. aegyptium are found sporadically on humans they could play a role in the zoonotic transmission cycles of tick-borne pathogens [36–38]. Due to the low host specificity spectrum of hosts of H. aegyptium and its vectorial potential, these ticks could represent a threat to public health [37, 39, 40].
Considering the zoonotic potential of RF spirochetes, the increasing reptile trade and the common occurrence of reptile ticks on humans, studies aimed to elucidate the transmission patterns and vectorial competence of these ticks are needed. Except the tick-vertebrate cycle, the transstadial and transovarial transmission of Borrelia in ticks is also important for the maintenance of natural foci of pathogens [34]. Transstadial transmission of Borrelia spirochetes has been experimentally demonstrated for B. burgdorferi s.l. in various Ixodes species as well as in Dermacentor variabilis and Amblyomma americanum [41–43]. Additionally, transstadial transmission of REP Borrelia spp. (Borrelia sp. tAG) was demonstrated in A. geoemydae ticks [4, 27]. Nevertheless, there is no evidence about the possibility of transstadial transmission of B. turcica in ticks. The aim of the present study was to evaluate the possibility of transstadial passage of B. turcica in H. aegyptium ticks.
Methods
Collection of ticks and laboratory molting
In July 2013 six T. graeca tortoises (4 males, 2 females) were captured from Turcoaia locality in Tulcea County, Romania (45.09, 28.18). All of the tortoises were harboring ticks. Tortoises were brought to the laboratory of the University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca and kept for the duration of the natural tick feeding according to the national laws and ethical committee approvals. All ticks were allowed to feed until their full engorgement and spontaneous detachment. After detachment as well as after molting, all ticks were morphologically identified [44]. All detached nymphs were kept in the incubator at 21°C with 75% relative humidity and 12/12-hour light/dark photo period cycle at 7010 lux for four weeks to allow molting. The unengorged adults obtained as well as the still alive detached engorged adults were dissected for the isolation and collection of gut in order to allow the cultivation of spirochetes. After the tick detachment, all tortoises were released in their original habitat.
Tortoises blood sampling
Blood was collected from each tortoise upon their arrival to the laboratory from the ventral caudal vein. The blood samples were collected on EDTA and stored at 4°C until PCR examination.
Spirochete cultivation
Under a laminar flow cabinet adult ticks were dipped into 75% isopropyl alcohol for 5 min than air dried. Using forceps and scalpel blades, the midgut of each tick was dissected and inoculated individually into 7 ml of Barbour-Stoenner-Kelly (BSK) II medium (Sigma), incubated at 34°C and examined weekly for 2 months using dark-field microscopy (DFM) (Olympus BX53, magnification 200x). The BSK II medium contained 6% rabbit serum and was heat inactivated at 56°C 50 min prior to its use.
Detection and identification of Borrelia species using PCR
DNA extraction was performed individually from each tick, each blood sample and each culture. DNA extraction was performed using a commercial DNA extraction kit (DNeasy Blood & Tissue Kit, Qiagen), according to the manufacturer’s protocols. The DNA quantity and purity were assessed using spectrophotometer analyses (NanoDrop Technologies model ND-1000, Inc., Wilmington, DE, USA).
Borrelia species were identified based on the region of 5S-23S rRNA (rrf-rrl) intergenic spacer (IGS), respectively on flagellin gene (flaB), glycerophosphodiester phosphodiesterase (glpQ) and DNA gyrase B subunit (gyrB) genes (Table 1). The PCR reaction was carried out in Bio-Rad T1000 Thermal Cycle and amplifications were performed according to previously described protocols [26, 45, 46]. Two additional set of primers were designed on the basis of the glpQ and gyrB gene sequences of B. turcica isolate IST7 (GeneBank accession no. AB529430, AB473535). The primer pairs (0.2 μM) were tested for optimal primer annealing temperature (ranging from 48 to 62) and were employed to detect with 100% spot matching two different B. turcica genes. The primers BTglpQF and BTglpqR (Table 1) were designed to target 910 bp long region of the glpQ gene of B. turcica and was amplified under the following conditions: 1 cycle of initial denaturation at 95°C 15 min followed by 40 cycles of denaturation at 94°C for 30 sec, annealing at 60°C for 1 min and extension at 70°C for 1 min with a final extension at 72°C for 10 min. To increase the sensitivity of the assay, BTgyrBF and BTgyrBR primers were used to detect DNA gyrB gene (1780 bp long region) of B. turcica. The amplification program comprised 15 min at 95°C followed by 40 cycles of denaturation at 94°C for 30 sec, annealing at 50°C for 1 min and extension at 70°C for 2 min with a final extension at 72°C for 15 min.
Table 1. Oligonucleotide primer pairs used.
| Primer name | Sequence (5’…3’) | References |
|---|---|---|
| Rrf | CTGCGAGITCGCGGGAGA | [45] |
| Rrl | TCCTAGGCATTCACCATA | [45] |
| BflaA | TCTGATGATGCTGCTGGTATGG | [26] |
| BflaD | AGGTTTTCAATAGCATACTC | [26] |
| gyrB 3′ | GGCTCTTGAAACAATAACAGACATCGC | [46] |
| gyrB 5′ | GGTTTATGAGTTATGTTGCTAGTAATATTCAAGTGC | [46] |
| gyrB 5′+3 | TTTATTGGTTTTAAGTCAAGTTGAATATGTC | [46] |
| BTgyrBF | GACCTGGTATGTATATTGGATCTG | In this study* |
| BTgyrBR | CTCTTCTAGGTTCAACATCATCTCCC | In this study* |
| glpQ-F | GGTATGCTTATTGGTCTTC | [54] |
| glpQ-R | TTGTATCCTCTTGTAATTG | [54] |
| BTglpQF | GCATTACCTCTAGTCATAGCTCATAGAGGTGC | In this study |
| BTglpQR | GCCTAATACTACACTAGGAAAATCTGTAAATACTCC | In this study |
(* primers used in sequence analysis)
PCR reaction was carried out in a final volume of 25 μl using Ssofast EvaGreen Supermix (Bio-Rad). For each extraction procedure and PCR reaction, negative controls were used to check for cross-contamination of samples. Aliquots of each PCR product were electrophoresed on 2% agarose gel (1xTAE, pH 8.0) stained with SYBR Safe DNA gel stain (Invitrogen). The PCR products were purified by using QIAquick PCR purification kit (Qiagen) and further analyzed by sequence analysis (Macrogen Europe, Amsterdam). Nucleotide sequences were compared with those available in GenBank using Basic Local Alignment Search Tool.
Phylogenetic analysis
Phylogenetic analysis was performed with Mega 6.06 software [47]. The evolutionary history was inferred using the Neighbor-Joining method [48]. The bootstrap consensus tree inferred from 1000 replicates is taken to represent the evolutionary history of the taxa analyzed. Branches corresponding to partitions reproduced in less than 50% bootstrap replicates are collapsed. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown above the branches [49]. The evolutionary distances were computed using the Maximum Composite Likelihood method and are in the units of the number of base substitutions per site [50].
Ethics Statement
The field study was carried out in Tulcea County, Romania (45.09, 28.18) based on research permits issued by the Research Authorization Department of the Danube Delta Biosphere Reserve Administration. The studies were not performed on private land, or on other location requiring specific permissions.
Veterinary conditions regarding protection of animals used in this research are complied according to national standards and legislation. The Research Bioethics Commission of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca (USAMV CN) committee reviewed and approved the document (Registration no. of approval of application: 10/2013). The Research Bioethics Commission of USAMV CN approved this study. This declaration certifies that the use of animals for research purpose within the experiment complies with the rules and regulations of the national (Law no. 206/2004 on good conduct in scientific research, technological development and innovation) and international (DIRECTIVE 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes) legislation. No other ethics approvals were required, no animals were killed during the sample collection and ivasive methods were not used on the captured tortises.
Results
Ticks
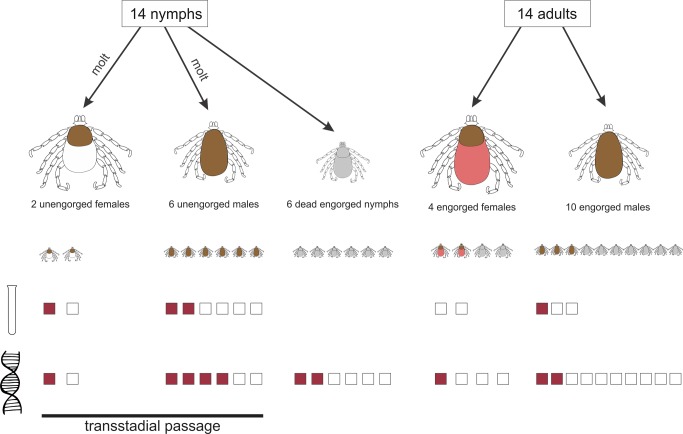
In total, 28 ticks were collected after full engorgement and spontaneous detachment from their tortoise hosts. After morphological examination, all were identified as H. aegyptium: 14 nymphs and 14 adults (4 females and 10 males). Out of the 14 fully engorged nymphs, 8 successfully molted after 3–4 weeks to the adult stage (resulting in 6 males and 2 females). Out of the 14 adults, only five (3 males and 2 females) were still found alive (Fig. 1).
Fig 1. H. aegyptium ticks used in this study together with the PCR and cultivation results.
Spirochetes presence in cultures from the midgut of ticks
Cultivation of the intestinal content of the 8 unengorged adults and 5 alive engorged adults produced cultures positive for helically shaped and very motile spirochetes in 4 cases after 28 days of cultivation. Three cultures originating from unengorged adults (2 males and 1 female) resulting after laboratory molting of nymphs were positive for spirochetes at DFM. One culture obtained from an engorged adult male midgut was also positive for spirochetes by DFM (Fig. 1).
Identification of spirochetes by PCR and sequencing
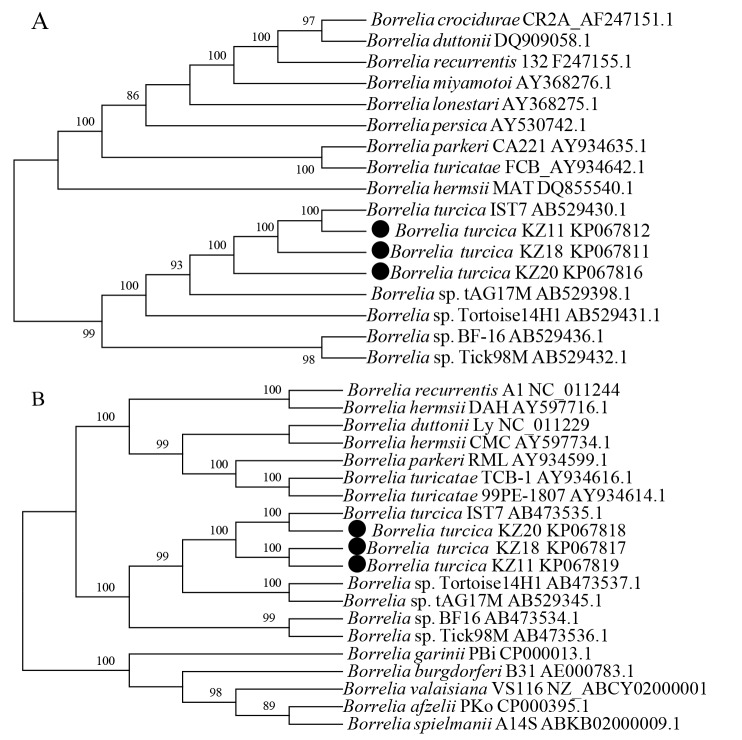
Ten H. aegyptium tick samples were positive for Borrelia using primers for flaB, gyrB and glpQ genes. All cultures were examined by PCR but only four (corresponding to those positive by DFM) were positive by using primers for flaB, gyrB and glpQ genes (Fig. 1). None of the 6 blood samples collected from T. graeca showed infection with Borrelia spirochetes by PCR, using the same primers. However, when using rrl/rrf primer pairs, a specific amplicon was not obtained by PCR of the IGS region. The PCR products were further analyzed by direct sequencing. Sequences were submitted to the GenBank sequence database (accession numbers: KP067811, KP067812, KP067816 for glpQ gene and KP067817, KP067818, KP067819 for gyrB gene). The gyrB and glpQ sequences showed 98%–100% similarities with reptile-associated B. turcica IST7. Phylogenetic analysis based on DNA sequences of glpQ and gyrB genes suggested that the sequences are distinct from LD, RF Borrelia and are similar with B. turcica IST7 and form together a monophyletic group (Fig. 2).
Fig 2. Phylogenetic analysis based on glpQ (A) and gyrB (B) genes of the genus Borrelia.
Discussion
H. aegyptium is generally found in the Western Palearctic and in central and southwest region of the Asian continent [51]. These ticks are associated with tortoises and certain micromammals (e.g. rodents, hedgehogs) as well as humans and might play an important role in the tick-borne pathogen transmission [39]. In Romania, T. graeca and Erinaceus roumanicus (E. roumanicus) were reported to be hosts for H. aegyptium [52]. Numerous studies have been published about the carrier or vectorial capacity of H. aegyptium [25, 32]. In our study, B. turcica was successfully isolated from unengorged adult ticks (obtained by laboratory molting of naturally engorged nymphs) gut. These findings suggest that the collected nymphs were infected with B. turcica and transstadial transmission occurred from nymph to adult stage. This proof represents an important prerequisite for the vectorial capacity of H. aegyptium for B. turcica. For the definitive proof that H. aegyptium is a competent vector for B. turcica, further investigations are needed to prove the ability of the larva to nymph transstadial passage, transovarial transmission and tick to vertebrate transmission. However, we were not able to obtain eggs from engorged female ticks; hence transovarial transmission of B. turcica couldn’t be evaluated.
As all blood samples collected from tortoises were negative for B. turcica, the infection of nymphs might have occurred during a previous blood meal on another host, via transovarial transmission or by co-feeding mechanisms on either host. The absence of B. turcica in the blood of tortoises from this study as well as in some previous assays by us (Z. Kalmár, unpublished) and the relatively high prevalence in ticks collected from the same tortoises (data from this study as well as [53]) indicate the possibility that the natural vertebrate hosts for B. turcica could be micromammalian hosts (e.g. rodents, hedgehogs) which are preferred by immature stages. Nevertheless, negative B. turcica PCR can be associated with low bacteremia. However, Takano et al. [23] reported the absence of infectivity of REP Borrelia for mice following subcutaneous inoculation. Moreover, B. turcica was successfully isolated from different tissues (heart, skin, blood, muscle, urinary bladder) of tick-free wild T. graeca exported from Jordan to Japan, but not from intraperitoneally experimentally inoculated Geochelone sulcata tortoises [23]. Our results and the literature data suggest the possibility of more complex vector-pathogen-reservoir host interactions which need to be investigated in more detail. Additionally, the pathogenicity and zoonotic potential of B. turcica are not known.
Data Availability
All sequences are available in GenBank sequence database (accession numbers: KP067811, KP067812, KP067816, KP067817, KP067818, KP067819).
Funding Statement
This paper was published under the frame of: 1. European Social Fund, Human Resources Development Operational Programme 2007-2013, project no. POSDRU/159/1.5/S/136893 (http://www.usamvcluj.ro/POSDRU136893/); 2. Grant IDEI PCE 236/2011 (http://www.geo-parasite.org/about.html); 3. The work of ZK, GD, DIM, AMI, DM, HS, VC, ADM was done under the frame of EurNegVec COST Action TD1303 (http://www.eurnegvec.org/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Orkun Ö, Karaer Z, Çakmak A, Nalbantoğlu S (2014) Identification of tick-borne pathogens in ticks feeding on humans in Turkey. PLoS Negl Trop Dis 8: e3067 10.1371/journal.pntd.0003067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rar VA, Fomenko NV, Dobrotvorsky AK, Livanova NN, Rudakova SA, et al. (2005) Tickborne pathogen detection, Western Siberia, Russia. Emerg Infect Dis 11: 1708–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dantas-Torres F, Chomel BB, Otranto D (2012) Ticks and tick-borne diseases: a One Health perspective. Trends Parasitol 28: 437–446. 10.1016/j.pt.2012.07.003 [DOI] [PubMed] [Google Scholar]
- 4. Takano A, Sugimori C, Fujita H, Kadosaka T, Taylor KR, et al. (2012) A novel relapsing fever Borrelia sp. infects the salivary glands of the molted hard tick, Amblyomma geoemydae . Ticks Tick Borne Dis 3: 259–261. 10.1016/j.ttbdis.2012.06.003 [DOI] [PubMed] [Google Scholar]
- 5. Franke J, Hildebrandt A, Dorn W (2013) Exploring gaps in our knowledge on Lyme borreliosis spirochaetes—updates on complex heterogeneity, ecology, and pathogenicity. Ticks Tick Borne Dis 4: 11–25. 10.1016/j.ttbdis.2012.06.007 [DOI] [PubMed] [Google Scholar]
- 6. Gern L, Humair PF (1998) Natural history of Borrelia burgdorferi sensu lato. Wien Klin Wochenschr 110: 856–858. [PubMed] [Google Scholar]
- 7. Stanek G, Reiter M (2011) The expanding Lyme Borrelia complex—clinical significance of genomic species? Clin Microbiol Infect 17: 487–493. 10.1111/j.1469-0691.2011.03492.x [DOI] [PubMed] [Google Scholar]
- 8. Barbour AG, Putteet-Driver AD, Bunikis J (2005) Horizontally acquired genes for purine salvage in Borrelia spp. causing relapsing fever. Infect Immun 73: 6165–6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Banerjee SN, Banerjee M, Fernando K, Burgdorfer W, Schwan TG (1998) Tick-borne relapsing fever in British Columbia, Canada: first isolation of Borrelia hermsii . J Clin Microbiol 36: 3505–3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Dam AP, van Gool T, Wetsteyn JC, Dankert J (1999) Tick-borne relapsing fever imported from West Africa: diagnosis by quantitative buffy coat analysis and in vitro culture of Borrelia crocidurae . J Clin Microbiol 37: 2027–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Elbir H, Raoult D, Drancourt M (2013) Relapsing fever borreliae in Africa. Am J Trop Med Hyg 89: 288–292. 10.4269/ajtmh.12-0691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Al-Gwaiz LA, Al-Mashhadani SA, Ayoola EA, Al-Khairy KS, Higgy KG, et al. (1995) Relapsing fever in Saudi Arabia: Report of two cases. Ann Saudi Med 15: 165–167. [DOI] [PubMed] [Google Scholar]
- 13. Dupont HT, La Scola B, Williams R, Raoult D (1997) A focus of tick-borne relapsing fever in southern Zaire. Clin Infect Dis 25: 139–144. [DOI] [PubMed] [Google Scholar]
- 14. Rizzoli A, Hauffe H, Carpi G, Vourc HG, Neteler M, et al. (2011) Lyme borreliosis in Europe. Euro Surveill 7: 16. [PubMed] [Google Scholar]
- 15. Richter D, Schlee DB, Matuschka FR (2003) Relapsing fever-like spirochetes infecting European vector tick of Lyme disease agent. Emerg Infect Dis 9: 697–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Masuzawa T (2004) Terrestrial distribution of the Lyme borreliosis agent Borrelia burgdorferi sensu lato in East Asia. Jpn J Infect Dis 57: 229–235. [PubMed] [Google Scholar]
- 17. Burridge MJ (2011) Invasive species In: Non-native and invasive ticks. Threats to human and animal health in the United States. University press of Florida; pp. 4–14. [Google Scholar]
- 18. Burridge MJ, Simmons LA, Allan SA (2000) Introduction of potential heart water vectors and other exotic ticks into Florida on imported reptiles. J Parasitol 86: 700–704. [DOI] [PubMed] [Google Scholar]
- 19. Burridge MJ, Simmons LA (2003) Exotic ticks introduced into the United States on imported reptiles from 1962 to 2001 and their potential roles in international dissemination of diseases. Vet Parasitol 113: 289–320. [DOI] [PubMed] [Google Scholar]
- 20. Paperna I, Kremer-Mecabell T, Finkelman S (2002) Hepatozoon kisrae sp. infecting the lizard Agama stellio is transmitted by the tick Hyalomma cf. aegyptium . Parasite 9: 17–27. [DOI] [PubMed] [Google Scholar]
- 21. Güner ES, Watanabe M, Hashimoto N, Kadosaka T, Kawamura Y, et al. (2004) Borrelia turcica sp. nov., isolated from the hard tick Hyalomma aegyptium in Turkey. Int J Syst Evol Microbiol 54: 1649–1652. [DOI] [PubMed] [Google Scholar]
- 22. Bitam I, Kernif T, Harrat Z, Parola P, Raoult D (2009) First detection of Rickettsia aeschlimannii in Hyalomma aegyptium from Algeria. Clin Microbiol Infec 2: 253–254. 10.1111/j.1469-0691.2008.02274.x [DOI] [PubMed] [Google Scholar]
- 23. Takano A, Goka K, Une Y, Shimada Y, Fujita H, et al. (2010) Isolation and characterization of a novel Borrelia group of tick-borne borreliae from imported reptiles and their associated ticks. Environ Microbiol 12: 134–146. 10.1111/j.1462-2920.2009.02054.x [DOI] [PubMed] [Google Scholar]
- 24. Harris DJ, Maia JP, Perera A (2011) Molecular characterization of Hepatozoon species in reptiles from the Seychelles. J Parasitol 97: 106–110. 10.1645/GE-2470.1 [DOI] [PubMed] [Google Scholar]
- 25. Paștiu AI, Matei IA, Mihalca AD, D’Amico G, Dumitrache MO, et al. (2012) Zoonotic pathogens associated with Hyalomma aegyptium in endangered tortoises: evidence for host-switching behaviour in ticks? Parasit Vectors 5:301 10.1186/1756-3305-5-301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Güner ES, Hashimoto N, Takada N, Kaneda K, Imai Y, et al. (2003) First isolation and characterization of Borrelia burgdorferi sensu lato strains from Ixodes ricinus ticks in Turkey. J Med Microbiol 52: 807–813. [DOI] [PubMed] [Google Scholar]
- 27. Takano A, Fujita H, Kadosaka T, Konnai S, Tajima T, Watanabe H, Ohnishi M, Kawabata H (2011) Characterization of reptile-associated Borrelia sp. in the vector tick, Amblyomma geoemydae, and its association with Lyme disease and relapsing fever Borrelia spp. Environ Microbiol Rep 3: 632–637. 10.1111/j.1758-2229.2011.00280.x [DOI] [PubMed] [Google Scholar]
- 28. Adeolu M, Gupta RS (2014) A phylogenomic and molecular marker based proposal for the division of the genus Borrelia into two genera: the emended genus Borrelia containing only the members of the relapsing fever Borrelia, and the genus Borreliella gen. nov. containing the members of the Lyme disease Borrelia (Borrelia burgdorferi sensu lato complex). Anton Leeuw 105: 1049–1072. [DOI] [PubMed] [Google Scholar]
- 29. Schwan TG, Anderson JM, Lopez JE, Fischer RJ, Raffel SJ, et al. (2012) Endemic foci of the tick-borne relapsing fever spirochete Borrelia crocidurae in Mali, West Africa, and the potentialfor human infection. PLoS Negl Trop Dis 6: e1924 10.1371/journal.pntd.0001924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cutler SJ (2006) Possibilities for relapsing fever reemergence. Emerg Infect Dis 12: 369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ray HN (1950) Hereditary transmission of Theileria annulata infection in the tick, Hyalomma aegyptium Neum. Trans R Soc Trop Med Hyg 44: 93–104. [DOI] [PubMed] [Google Scholar]
- 32. Kar S, Yılmazer N, Midilli K, Ergin S, Alp H, et al. (2011) Presence of the zoonotic Borrelia burgdorferi s.l. and Rickettsia spp. in the ticks from wild tortoises and hedgehogs. J Marmara Univ Inst Health Sci 1: 166–170. [Google Scholar]
- 33. Široký P, Mikulícek P, Jandzík D, Kami H, Mihalca AD, et al. (2009) Co-distribution pattern of a haemogregarine Hemolivia mauritanica (Apicomplexa: Haemogregarinidae) and its vector Hyalomma aegyptium(Metastigmata:Ixodidae). J Parasitol 95: 728–733. 10.1645/GE-1842.1 [DOI] [PubMed] [Google Scholar]
- 34. Široký P, Kubelová M, Modrý D, Erhart J, Literák I, et al. (2010) Tortoise tick Hyalomma aegyptium as long term carrier of Q fever agent Coxiella burnetii—evidence from experimental infection. Parasitol Res 107: 1515–1520. 10.1007/s00436-010-2037-1 [DOI] [PubMed] [Google Scholar]
- 35. Široký P, Bělohlávek T, Papoušek I, Jandzik D, Mikulíček P, et al. (2014) Hidden threat of tortoise ticks: high prevalence of Crimean-Congo haemorrhagic fever virus in ticks Hyalomma aegyptium in the Middle East. Parasit Vectors 11: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vatansever Z, Gargili A, Aysul NS, Sengoz G, Estrada-Peña A (2008) Ticks biting humans in the urban area of Istanbul. Parasitol Res 102: 551–553. [DOI] [PubMed] [Google Scholar]
- 37. Bursali A, Tekin S, Orhan M, Keskin A, Ozkan M (2010) Ixodid ticks (Acari: Ixodidae) infesting humans in Tokat Province of Turkey: species diversity and seasonalactivity. J Vector Ecol 35: 180–186. 10.1111/j.1948-7134.2010.00045.x [DOI] [PubMed] [Google Scholar]
- 38. Kar S, Dervis E, Akın A, Ergonul O, Gargili A (2013) Preferences of different tick species for human hosts in Turkey. Exp Appl Acarol 61: 349–355. 10.1007/s10493-013-9698-2 [DOI] [PubMed] [Google Scholar]
- 39. Apanaskevich DA: Host–parasite relationships of the genus Hyalomma Koch (Acari, Ixodidae) and their connection with microevolutionary processes. Parazitologiya 2004, 38:515–523. in Russian [PubMed] [Google Scholar]
- 40. Siroký P,Erhart J, Petrželková KJ, Kamler M. (2011). Life cycle of tortoise tick Hyalomma aegyptium under laboratory conditions. Exp Appl Acarol 54: 277–284. 10.1007/s10493-011-9442-8 [DOI] [PubMed] [Google Scholar]
- 41. Sanders FH, Oliver JH (1995) Evaluation of Ixodes scapularis, Amblyomma americanum, and Dermacentor variabilis (Acari:Ixodidae) from Georgia as vectors of a Florida strain of the Lyme disease spirochete, Borrelia burgdorferi . J Med Entomol 32: 402–406. [DOI] [PubMed] [Google Scholar]
- 42. Dolan MC, Maupin GO, Panella NA, Golde WT, Piesman J (1997) Vector competence of Ixodes scapularis , I. spinipalpis, and Dermacentor andersonii (Acari:Ixodidae) in transmitting Borrelia burgdorferi , the etiologic agent of Lyme disease. J Med Entomol 34: 128–135. [DOI] [PubMed] [Google Scholar]
- 43. Eisen L, Lane RS (2002) Vectors of Borrelia burgdorferi sensu lato In: Lyme borreliosis: Biology, Epidemiology and Control. Gray J., Kahl O., Lane R.S. and Stanek G. (eds.). Wallingford, Oxfordshire: CABI Publishing; pp: 102–115. [Google Scholar]
- 44. Feider Z (1965) Fauna RPR. Arachnida In romanian. Bucureşti: Academiei RPR, vol V, fasc 2. [Google Scholar]
- 45. Postic D, Assous MV, Grimont PA, Baranton G (1994) Diversity of Borrelia burgdorferi sensu lato evidenced by restriction fragment length polymorphism of rrf(5S)-rrl(23S) intergenic spacer amplicons. Int J Syst Bacteriol 44: 743–752. [DOI] [PubMed] [Google Scholar]
- 46. Schwan TG, Raffael JS, Schrumpf ME, Policastro PF, Rawlings JA, et al. (2005) Phylogenetic analysis of the spirochetes Borrelia parkeri and Borrliea turicatae and the potential for tick-borne relapsing fever in Florida. J Clin Microbiol 43: 3851–3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molec Biol Evol 30: 2725–2729. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Saitou N, Nei M (1987) The neighbor-joining method: A new method for reconstructing phylogenetic trees. Molec Biol Evol 4:406–425. [DOI] [PubMed] [Google Scholar]
- 49. Felsenstein J (1985) Confidence limits on phylogenies: An approach using the bootstrap. Evolution 39:783–791. [DOI] [PubMed] [Google Scholar]
- 50. Tamura K, Nei M, Kumar S (2004) Prospects for inferring very large phylogenies by using the neighbor-joining method. Proceedings of the National Academy of Sciences (USA) 101:11030–11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kolonin GV (1983) Mirovoe rasprostranenie iksodovykh kleshchey Rody Hyalomma, Aponomma, Amblyomma [World distribution of ixodid ticks. Genera Hyalomma, Aponomma, Amblyomma]. In Russian. Moskva, SSSR: Nauka. [Google Scholar]
- 52. Mihalca AD, Dumitrache MO, Magdaş C, Gherman CM, Domşa C, et al. (2012) Synopsis of the hard ticks (Acari: Ixodidae) of Romania with update on host associations and geographical distribution. Exp Appl Acarol 58: 183–206. 10.1007/s10493-012-9566-5 [DOI] [PubMed] [Google Scholar]
- 53. Kalmár Z, D’Amico G, Matei IA, Paștiu AI, Mărcuţan DI, et al. (2014) Borrelia turcica in Hyalomma aegyptium ticks in Romania. Parasit Vectors 7(Suppl 1): P6 25078468 [Google Scholar]
- 54. Bacon RM, Pilgard MA, Johnson BJ, Raffel SJ, Schwan TG (2004) Glycerophosphodiester phosphodiesterase gene (glpQ) of Borrelia lonestari identified as a target for differentiating Borrelia species associated with hard ticks (Acari:Ixodidae). J Clin Microbiol 42: 2326–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All sequences are available in GenBank sequence database (accession numbers: KP067811, KP067812, KP067816, KP067817, KP067818, KP067819).