Abstract
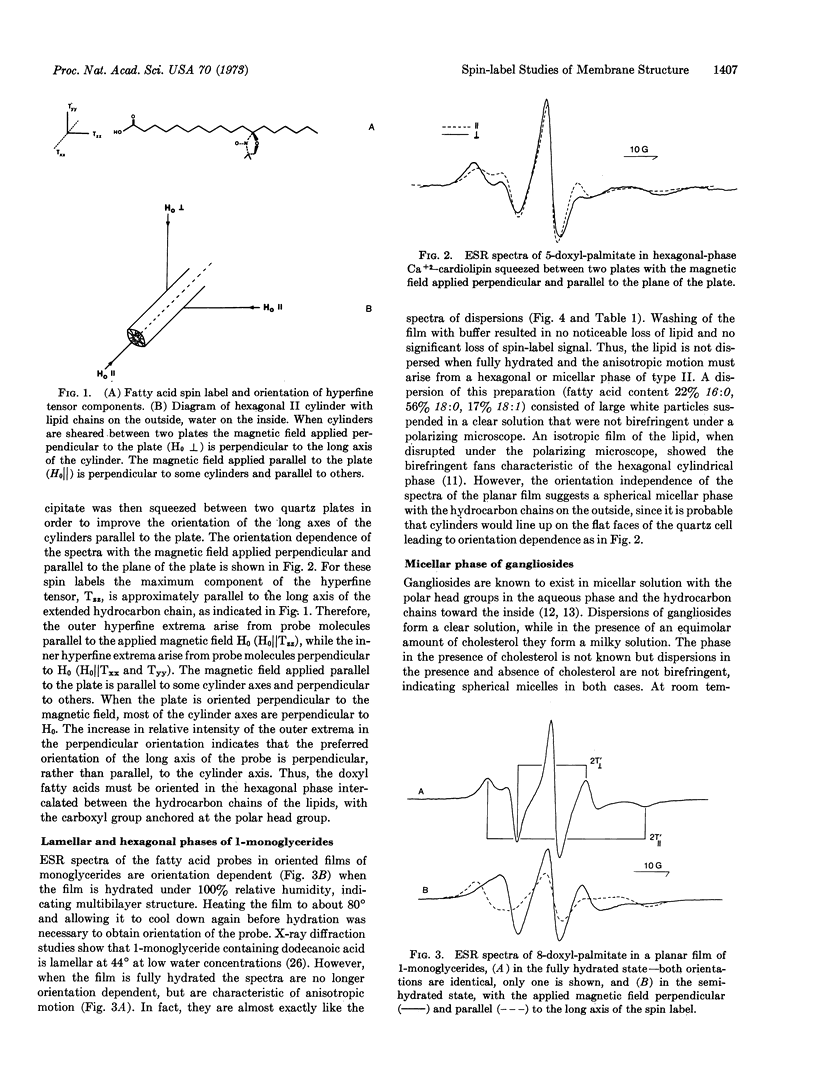
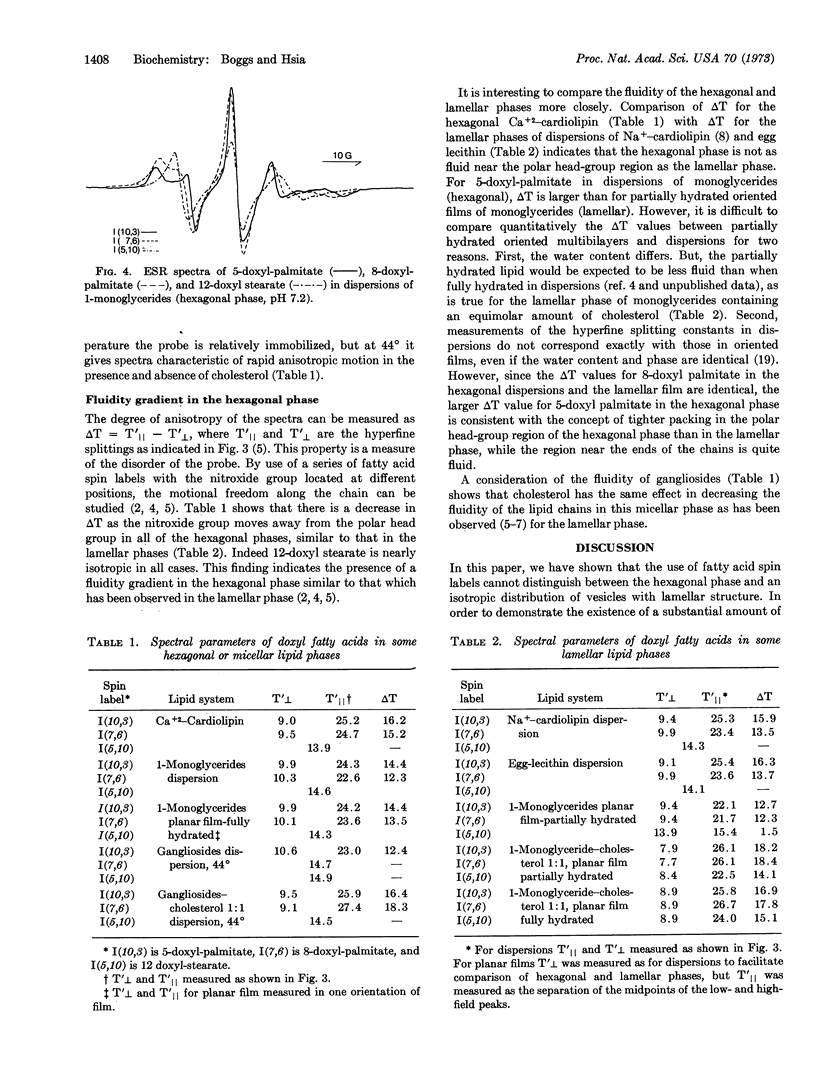
The acyl chain of spin-labeled fatty acids intercalates between the lipid hydrocarbon chains in hexagonal and micellar phases with the carboxyl group anchored at the lipid water interface. The spectra are characteristic of anisotropic motion and cannot be distinguished from the spectra of these probes in lamellar dispersions. In the hexagonal and micellar phases the molecular motion of the spin label increases as it is moved further away from the carboxyl group, similar to the behavior in the lamellar phase (Jost et al. (1971) J. Mol. Biol. 59, 77-98). The similarity in packing of the acyl chains in the hexagonal and lamellar phases suggests that localized regions of hexagonal phase are compatible with a bilayer matrix.
Keywords: membrane, cardiolipin, gangliosides, Ca++, phase transition
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boggs J. M., Hsia J. C. Effect of cholesterol and water on the rigidity and order of phosphatidylcholine bilayers. Biochim Biophys Acta. 1972 Dec 1;290(1):32–42. doi: 10.1016/0005-2736(72)90049-1. [DOI] [PubMed] [Google Scholar]
- Deamer D. W., Leonard R., Tardieu A., Branton D. Lamellar and hexagonal lipid phases visualized by freeze-etching. Biochim Biophys Acta. 1970;219(1):47–60. doi: 10.1016/0005-2736(70)90060-x. [DOI] [PubMed] [Google Scholar]
- Eletr S., Inesi G. Phospholipid orientation in sarcoplasmic membranes: spin-label ESR and proton MNR studies. Biochim Biophys Acta. 1972 Sep 1;282(1):174–179. doi: 10.1016/0005-2736(72)90321-5. [DOI] [PubMed] [Google Scholar]
- Esfahani M., Limbrick A. R., Knutton S., Oka T., Wakil S. J. The molecular organization of lipids in the membrane of Escherichia coli: phase transitions. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3180–3184. doi: 10.1073/pnas.68.12.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAMMACK D. B. PHYSICOCHEMICAL PROPERTIES OF OX-BRAIN GANGLIOSIDES. Biochem J. 1963 Aug;88:373–383. doi: 10.1042/bj0880373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulik-Krzywicki T., Shechter E., Vittorio Luzzati, Faure M. Interactions of proteins and lipids: structure and polymorphism of protein-lipid-water phases. Nature. 1969 Sep 13;223(5211):1116–1121. doi: 10.1038/2231116a0. [DOI] [PubMed] [Google Scholar]
- Hill M. W., Lester R. Mixtures of gangliosides and phosphatidylcholine in aqueous dispersions. Biochim Biophys Acta. 1972 Sep 1;282(1):18–30. doi: 10.1016/0005-2736(72)90307-0. [DOI] [PubMed] [Google Scholar]
- Hsia J. C., Chen W. L., Long R. A., Wong L. T., Kalow W. Existence of phospholipid bilayer structure in the inner membrane of mitochondria. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3412–3415. doi: 10.1073/pnas.69.11.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbell W. L., McConnell H. M. Molecular motion in spin-labeled phospholipids and membranes. J Am Chem Soc. 1971 Jan 27;93(2):314–326. doi: 10.1021/ja00731a005. [DOI] [PubMed] [Google Scholar]
- Hubbell W. L., McConnell H. M. Orientation and motion of amphiphilic spin labels in membranes. Proc Natl Acad Sci U S A. 1969 Sep;64(1):20–27. doi: 10.1073/pnas.64.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost P., Libertini L. J., Hebert V. C., Griffith O. H. Lipid spin labels in lecithin multilayers. A study of motion along fatty acid chains. J Mol Biol. 1971 Jul 14;59(1):77–98. doi: 10.1016/0022-2836(71)90414-1. [DOI] [PubMed] [Google Scholar]
- Libertini L. J., Waggoner A. S., Jost P. C., Griffith O. H. Orientation of lipid spin labels in lecithin multilayers. Proc Natl Acad Sci U S A. 1969 Sep;64(1):13–19. doi: 10.1073/pnas.64.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzati V., Reiss-Husson F., Rivas E., Gulik-Krzywicki T. Structure and polymorphism in lipid-water systems, and their possible biological implications. Ann N Y Acad Sci. 1966 Jul 14;137(2):409–413. doi: 10.1111/j.1749-6632.1966.tb50172.x. [DOI] [PubMed] [Google Scholar]
- McFarland B. G., McConnell H. M. Bent fatty acid chains in lecithin bilayers. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1274–1278. doi: 10.1073/pnas.68.6.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papahadjopoulos D., Miller N. Phospholipid model membranes. I. Structural characteristics of hydrated liquid crystals. Biochim Biophys Acta. 1967 Sep 9;135(4):624–638. doi: 10.1016/0005-2736(67)90094-6. [DOI] [PubMed] [Google Scholar]
- Phillips M. C., Hauser H., Paltauf F. The inter- and intra-molecular mixing of hydrocarbon chains in lecithin-water systems. Chem Phys Lipids. 1972 Mar;8(2):127–133. doi: 10.1016/0009-3084(72)90024-2. [DOI] [PubMed] [Google Scholar]
- Rand R. P., Sengupta S. Cardiolipin forms hexagonal structures with divalent cations. Biochim Biophys Acta. 1972 Feb 11;255(2):484–492. doi: 10.1016/0005-2736(72)90152-6. [DOI] [PubMed] [Google Scholar]
- Reiss-Husson F. Structure des phases liquide-cristallines de différents phospholipides, monoglycérides, sphingolipides, anhydres ou en présence d'eau. J Mol Biol. 1967 May 14;25(3):363–382. doi: 10.1016/0022-2836(67)90192-1. [DOI] [PubMed] [Google Scholar]
- Wilkins M. H., Blaurock A. E., Engelman D. M. Bilayer structure in membranes. Nat New Biol. 1971 Mar 17;230(11):72–76. doi: 10.1038/newbio230072a0. [DOI] [PubMed] [Google Scholar]


