Abstract
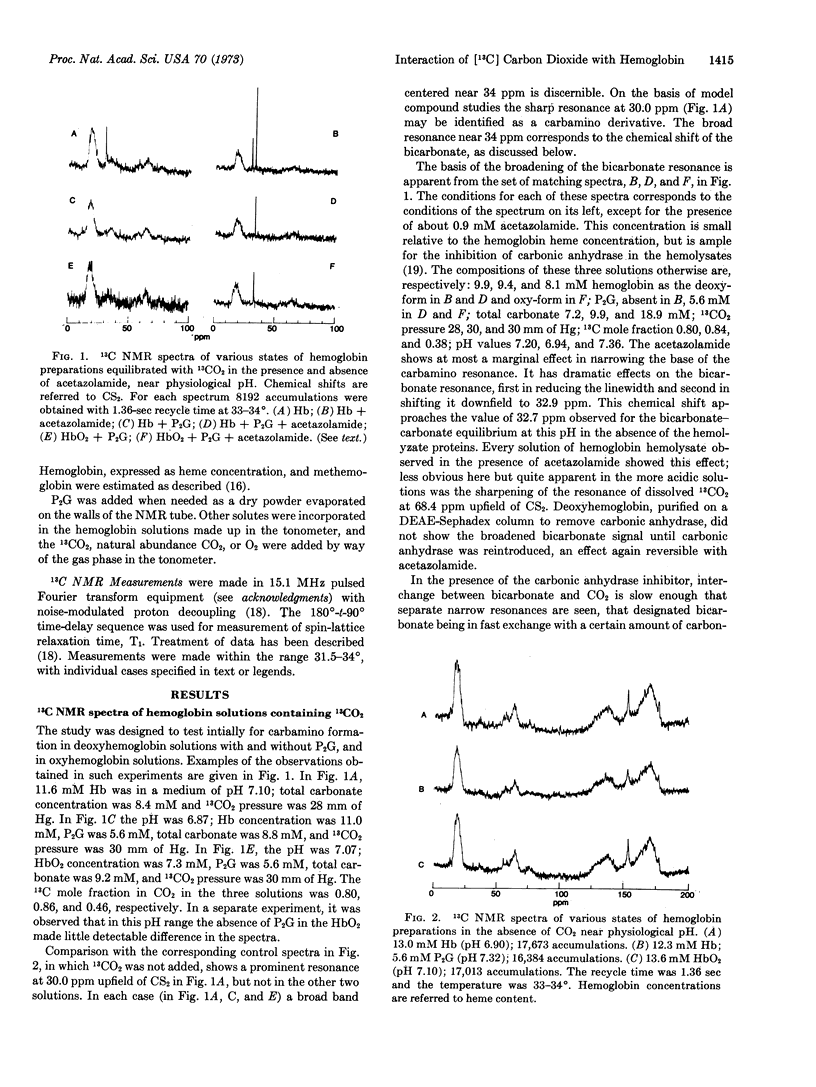
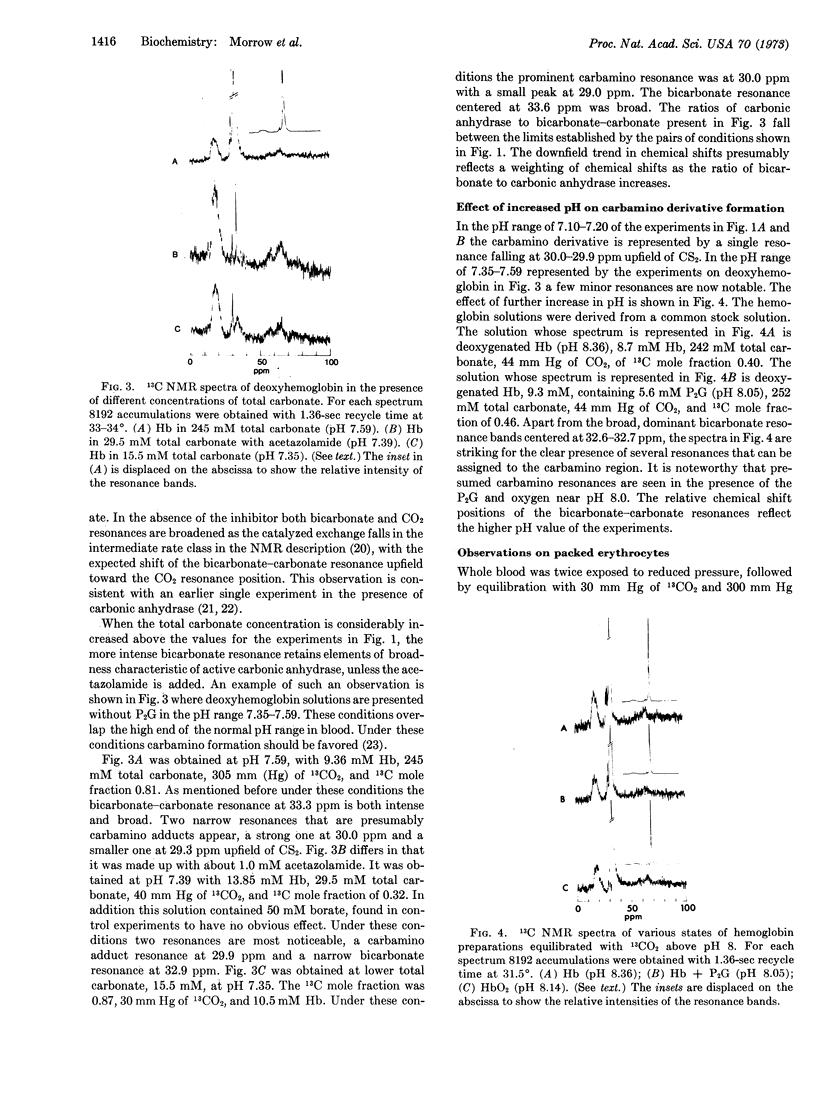
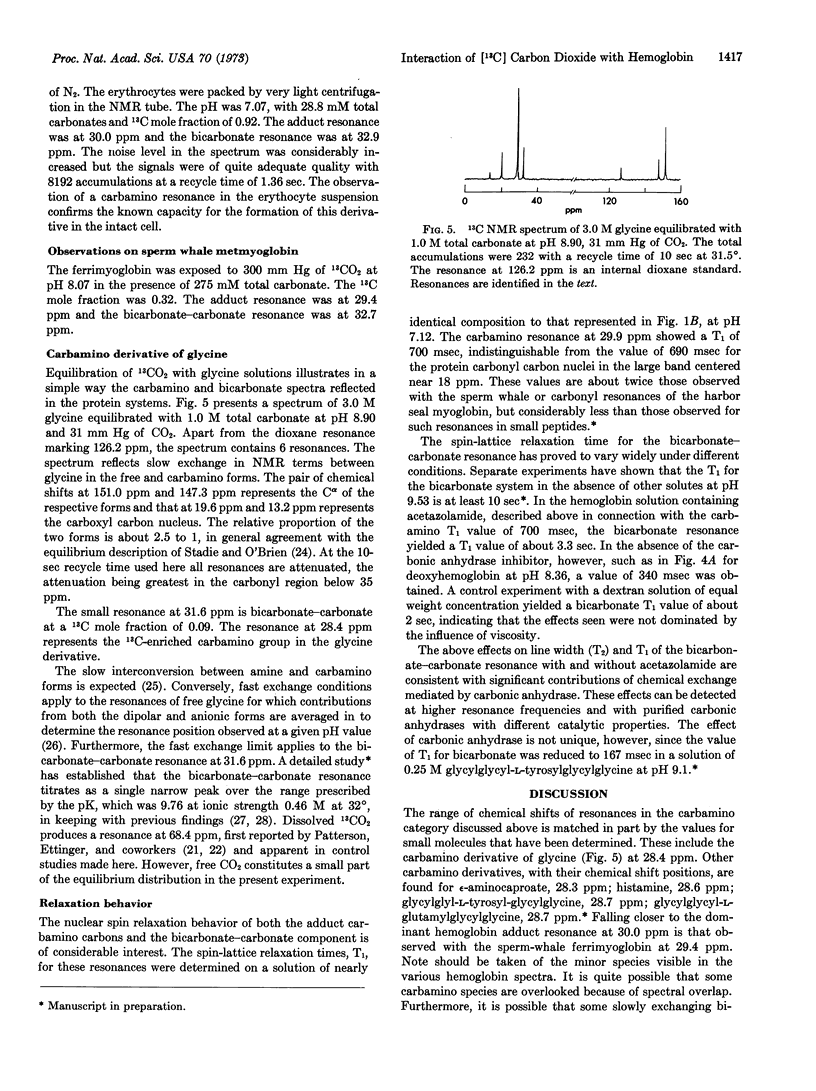
Formation of 13C-resonances attributable to carbamino derivatives has been observed in human erythrocyte hemolysate preparations equilibrated with 13CO2 at 33°. Carbamino formation is most marked in deoxyhemoglobin and at alkaline pH, and is very largely inhibited by the addition of 2,3-diphosphoglycerate or conversion to oxyhemoglobin. The prominent carbamino resonance at 30.0 ppm upfield of CS2 is visible in the spectrum of packed, deoxygenated erythrocytes equilibrated in 13CO2. This chemical shift falls close to that observed with sperm-whale myoglobin and within 2 ppm upfield of that seen with simple amino acids and peptides. The bicarbonate-carbonate resonance near 33 ppm is broad in the hemoglobin preparations, which always contain some carbonic anhydrase, but becomes narrow in the presence of the carbonic anhydrase inhibitor, acetazolamide. The nuclear magnetic resonance condition of intermediate exchange rate with dissolved CO2 (68.4 ppm) obtains in the absence of inhibitor. The process has marked consequences in reducing the spin-lattice relaxation time, T1, of the bicarbonate resonance by more than 10 times. The deoxyhemoglobin carbamino resonance has a T1 value of 700 msec, indistinguishable from that of the protein carbonyl resonance envelope.
Keywords: carbamino, NMR, carbonic anhydrase, relaxation
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENESCH R., MACDUFF G., BENESCH R. E. DETERMINATION OF OXYGEN EQUILIBRIA WITH A VERSATILE NEW TONOMETER. Anal Biochem. 1965 Apr;11:81–87. doi: 10.1016/0003-2697(65)90045-x. [DOI] [PubMed] [Google Scholar]
- Benesch R., Benesch R. E., Yu C. I. Reciprocal binding of oxygen and diphosphoglycerate by human hemoglobin. Proc Natl Acad Sci U S A. 1968 Feb;59(2):526–532. doi: 10.1073/pnas.59.2.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell P. R., Nagel R. L., Jaffe E. R. The effect of oxygen, carbon dioxide, pH and cyanate on the binding of 2,3-diphosphoglycerate to human hemoglobin. Biochem Biophys Res Commun. 1971 Sep 17;44(6):1504–1509. doi: 10.1016/s0006-291x(71)80256-5. [DOI] [PubMed] [Google Scholar]
- Forster R. E., Constantine H. P., Craw M. R., Rotman H. H., Klocke R. A. Reaction of CO2 with human hemoglobin solution. J Biol Chem. 1968 Jun 25;243(12):3317–3326. [PubMed] [Google Scholar]
- Glushko V., Lawson P. J., Gurd F. R. Conformational states of bovine pancreatic ribonuclease A observed by normal and partially relaxed carbon 13 nuclear magnetic resonance. J Biol Chem. 1972 May 25;247(10):3176–3185. [PubMed] [Google Scholar]
- Gurd F. R., Lawson P. J., Cochran D. W., Wenkert E. Carbon 13 nuclear magnetic resonance of peptides in the amino-terminal sequence of sperm whale myoglobin. J Biol Chem. 1971 Jun 10;246(11):3725–3730. [PubMed] [Google Scholar]
- Kilmartin J. V., Rossi-Bernardi L. The binding of carbon dioxide by horse haemoglobin. Biochem J. 1971 Aug;124(1):31–45. doi: 10.1042/bj1240031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matwiyoff N. A., Needham T. E. Carbon-13 NMR spectroscopy of red blood cell suspensions. Biochem Biophys Res Commun. 1972 Dec 4;49(5):1158–1164. doi: 10.1016/0006-291x(72)90590-6. [DOI] [PubMed] [Google Scholar]
- Moon R. B., Richards J. H. Conformation studies of various hemoglobins by natural-abundance 13 C NMR spectroscopy. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2193–2197. doi: 10.1073/pnas.69.8.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon R. B., Richards J. H. Nuclear magnetic resonance studies of 13 CO binding to various heme globins. J Am Chem Soc. 1972 Jul 12;94(14):5093–5095. doi: 10.1021/ja00769a058. [DOI] [PubMed] [Google Scholar]
- Nigen A. M., Keim P., Marshall R. C., Morrow J. S., Gurd F. R. Carbon 13 nuclear magnetic resonance spectroscopy of myoglobins and ribonuclease A carboxymethylated with enriched (2- 13 C)bromoacetate. J Biol Chem. 1972 Jun 25;247(12):4100–4102. [PubMed] [Google Scholar]
- Roughton F. J. Some recent work on the interactions of oxygen, carbon dioxide and haemoglobin. Biochem J. 1970 May;117(5):801–812. doi: 10.1042/bj1170801b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salhany J. M. Effect of carbon dioxide on human hemoglobin. Kinetic basis for the reduced oxygen affinity. J Biol Chem. 1972 Jun 25;247(12):3799–3801. [PubMed] [Google Scholar]



