Abstract
Background:
Rapid Ultrasound in Shock (RUSH) is a recently reported emergency ultrasound protocol designed to help clinicians better recognize distinctive shock etiologies in a short time. We tried to evaluate the accuracy of early RUSH protocol performed by emergency physicians to predict the shock type in critically ill patients.
Materials and Methods:
Our prospective study was approved by the ethics committee of trauma research center, Baqiyatallah University of Medical Science, Iran. We enrolled 52 patients with shock state in the emergency department from April 2013 to October 2013. We performed early bed-side sonographic examination for participants based on RUSH protocol. Patients received all needed standard therapeutic and diagnostic interventions without delay and were followed to document their final diagnosis. Agreement (Kappa index) of initial impression provided by RUSH with final diagnosis, and also sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of RUSH for diagnosis of each shock type were calculated.
Results:
Fifty-two patients were enrolled in our study. Kappa index was 0.7 (P value = 0.000), reflecting acceptable general agreement between initial impression and final diagnosis. For hypovolemic and obstructive shocks, the protocol had sensitivity of 100% but had lower PPV. For shocks with distributive or mixed etiology, RUSH showed PPV of 100% but had low sensitivity. For cardiogenic shocks, all reliability indices were above 90%.
Conclusion:
We highlight the role of RUSH examination in the hands of an emergency physician in making a rapid diagnosis of shock etiology, especially in ruling out obstructive, cardiogenic, and hypovolemic types.
Keywords: Accuracy, predictive value, rapid ultrasound in shock, shock, ultrasound
INTRODUCTION
The final outcome of patients in shock state is strongly dependent on the duration and severity of hypotension. In fact, there really exists an important time window for a clinician dealing with a patient in shock state, within which he can perform more specific treatments rather than just crystalloid infusion and monitoring of hemodynamic indices, and subsequently, anticipate better final outcome for the patient. Therefore, earlier identification of shock etiology has always been a great concern in emergency medicine. However, clinical evaluation to reveal the etiology of shock in the setting of a critical patient is usually problematic and time-consuming. Physical findings of different types of shock can easily overlap each other. For instance, dyspnea and elevated jugular venous pressure would be expected in diverse clinical settings such as pulmonary thromboembolism (PTE), cardiac tamponade, and depression of myocardium either due to myocardial infarction (MI) or sepsis.
Since ultrasound is becoming widely available in emergency departments (ED), many ultrasonographic protocols have been introduced to evaluate pulmonary or cardiac problems in emergent settings even replacing standard imaging modalities like supine chest X-ray or echocardiography.[1,2,3,4,5,6,7]
Specific goal-directed ultrasound examinations in early evaluation of critical patients evaluating the heart, abdomen, and venous system have been reported to be helpful in rapid diagnosis of non-traumatic etiology of hypotension.[8,9,10] Rapid Ultrasound in Shock (RUSH) is a recent emergency ultrasound protocol that integrates pulmonary evaluation with cardiac, abdominal, and venous examination.[11,12,13] Perara et al., proposed this protocol to help the clinician better and faster.[11,12]
However, the practical efficacy of performing RUSH examination in an emergency setting has not been completely evaluated yet.
Hence, in this study, we tried to evaluate the accuracy of early RUSH protocol performed by emergency physicians to predict the shock type in critically ill patients.
MATERIALS AND METHODS
This was a prospective study of diagnostic accuracy for RUSH protocol based on the STARD guideline. The study was performed in accordance with Declaration of Helsinki and approved by The Ethics Committee of Trauma Research Center, Baqiyatallah Hospital, Baqiyatallah University of Medical Science, Tehran, Iran.
Participants
All patients in shock state in the working shift of our emergency physician in ED were enrolled in this study from April 2013 to October 2013. Shock was defined as systolic blood pressure under 100 mmHg or a shock index (heart rate divided by systolic blood pressure in mmHg) greater than one. A clear cause of shock at patients’ arrival mandating prompt life-saving treatment, such as external bleeding or active gastrointestinal bleeding, was our exclusion criteria.
Initial clinical evaluation and immediate resuscitative interventions (intravenous (IV)-line, hydration, etc.) in ED according to standard medical protocols were accomplished for all these patients. Meanwhile, equipment for bed-side sonographic examination in ED was prepared without any delay or interruption in patients’ initial care. Then, sonographic examination based on RUSH protocol was performed concurrent with patient's resuscitative care. Again, a point to remember is that all necessary therapeutic or diagnostic investigations, including supine chest X-ray, computerized tomography (CT)-scan, echocardiography, or any other laboratory tests) were carried out without delay during their hospitalization.
Performing RUSH protocol and shock classification
A one-page checklist was designed to obtain information regarding three main components of RUSH examination. It involves evaluation of heart (to assess tamponade, ejection fraction, and strain of right ventricle), inferior vena cava (to estimate central venous pressure), thoracic and abdominal compartments (to assess pneumothorax, pulmonary edema, pleural effusion, and peritoneal free fluid), and large arteries or veins (to assess aortic dissection or aneurysm and deep vein thrombosis).
They are simplified as the pump, tank, and the pipes of a patient (see checklist, supplemental Digital Content 1).
We examined the patients in a supine to 30° upright position with two-dimensional grayscale bedside sonography. A Medison V10 ultrasound system (Medison, South Korea) with 2.5-5 MHz curvilinear and 5-12 linear probes was used. One investigator (a board-certified emergency physician) performed RUSH assessment for all patients using the views described by Perara et al.[11,12] He had a five-year experience in emergency ultrasonography and had performed more than 200 examinations per year. He had qualified a 20-hour workshop for emergency ultrasound including RUSH protocol and was familiar with the main components of RUSH examination. The clinician suggested the shock type of the patient based on RUSH protocol findings. The time interval between the patient's arrival and the time when the emergency physician reached the conclusion using RUSH protocol was considered as duration of examination for each patient.
As described in specialty textbooks, four classic subtypes were defined for shock: Hypovolemic, cardiogenic, distributive (including septic or neurogenic shock), and obstructive (due to pneumothorax, tamponade, or PTE).[14,15] We also considered a mixed type for patients that demonstrate combined features of different shock types, for instance, a patient demonstrating features of both sepsis and cardiogenic failure. Those whose etiology could not be identified were classified as “not defined”.
Documenting final diagnosis
We followed all patients to document their final diagnosis, which would be reached based on all investigations performed during their course of hospitalization. The final diagnosis was usually established by a second physician in charge (other than emergency physician) whom the patient was transferred to his service (internal medicine, cardiology, or surgery). They were all board-certified specialists with acceptable expertise in their fields of interest. We should declare that these physicians were not blind relative to the information obtained from ultrasonographic examination.
Statistical methods
We carried out statistical analysis using SPSS 18 software. We first investigated the general agreement between shock type determine by RUSH protocol and the type finally outlined for each patient. Furthermore, we calculated sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and Kappa index of RUSH protocol in diagnosis of each individual type of shock. A 0.95 confidence interval was considered as the level of significance in our analysis. To calculate these reliability indices, we excluded patients with final diagnosis of “not defined etiology”.
RESULTS
We enrolled 52 patients consisting of 28 men and 24 women with mean age of 51.6 years (age range, 36-69 years) in a time interval from April 2013 up to October 2013. Mean time duration of the examination (from patient's arrival till sonographic conclusion) was about 20 minutes (range, 10-25 minutes).
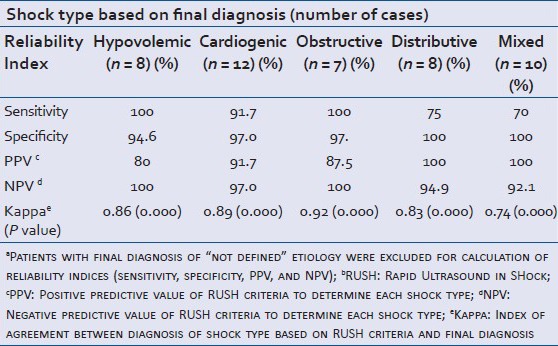
Table 1 shows the prevalence of different types of shock based on the final impression reached during hospitalization. The most frequent types of shock were cardiogenic shock (12 patients, 23.1%) and shock due to multiple etiologies (10 patients, 19.2%). Eight patients had hypovolemic, eight distributive, and seven obstructive type of shock. Seven cases (13.5%) died before we could clinically confirm the precise cause of the shock state and were classified as “not defined etiology”. In the early RUSH examination, undertaken for them, six of them were identified as mixed type and one as cardiogenic type. Kappa index for general agreement between shock type using RUSH protocol and final diagnosis was 0.70 (P value = 0.000), reflecting acceptable general agreement for this criteria. Table 2 shows sensitivity, specificity, PPV, NPV, and Kappa index of the protocol for individual types of shock.
Table 1.
Individual results and prevalence of each shock type based on final diagnosis
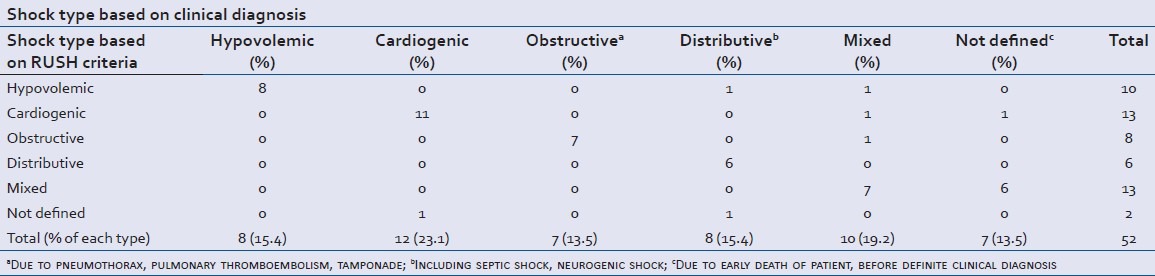
Table 2.
Reliability indicesa of RUSHb criteria in diagnosis of each shock type
Hypovolemic shock excellent sensitivity, good specificity
In hypovolemic patients, RUSH protocol showed 86% agreement with final diagnosis (P value < 0.001). We had eight cases finally diagnosed with hypovolemic shock. All were initially found by using RUSH protocol. Three were due to gastroenteritis, two had trauma, two due to diuretic overuse, and one had an aneurysm. For hypovolemic shock, the protocol had a high sensitivity and NPV (both 100%) but a lower specificity (94.6%) which resulted in substantial low PPV (80%). This means that using this protocol, one out of five patients diagnosed with hypovolemic shock may have had other causes. We misdiagnosed two patients as having hypovolemic shock; one was determined to have multiple etiologies and one had distributive shock (secondary to urinary tract infection and sepsis).
Cardiogenic shock: Good sensitivity and specificity
RUSH showed 89% agreement (P value < 0.001) with final diagnosis. We correctly diagnosed 11 of our 12 patients with cardiogenic shock representing 91.7% sensitivity (one had myocardial infarction (MI), one with digital toxicity, two had paroxysmal supraventricular tachycardia (PSVT), and seven with decompensated heart failure). The cardiac etiology of the other one with heart failure could not be clearly outlined using RUSH. Ejection fraction was assumed good for him and thus, his shock was labeled as “not defined”. We had also two patients who were diagnosed with cardiogenic shock; one of them was proven to have multiple etiologies and the other one died before definite diagnosis could be made. The specificity, PPV, and NPV of RUSH protocol for diagnosis of this shock type were 97.0%, 91.7%, and 97.0%, respectively.
Obstructive shock: Excellent sensitivity, and good specificity
The criteria had largest agreement with final diagnosis (92%, P value < 0.001) in this group of patients. Among our seven patients with obstructive shock, the examiner successfully diagnosed two patients with massive PTE, three with cardiac tamponade, and two of three patients with pneumothorax as the cause using RUSH protocol.
Pneumothorax in one patient could not be diagnosed with RUSH protocol; however, the patient was correctly defined to have an obstructive type of shock. Sensitivity and NPV were both 100%; specificity and PPV were 97.4% and 87.5%, respectively. We had one patient initially considered as obstructive shock was proved to have multiple etiologies for their shock state.
Distributive shock: Excellent specificity but low sensitivity
The protocol had an acceptable agreement with final diagnosis in these patients as well (83%, P value < 0.001). We had eight patients finally diagnosed with distributive shock; seven had sepsis (five with pneumonia, one tuberculosis, and one urinary tract infection, UTI) and one had neurogenic cause. During initial RUSH examination, the examiner outlined six of them as distributive shock. One was erroneously labeled as hypovolemic and one as “not defined” type. It was shown that the specificity of the protocol to diagnose distributive shock and its PPV were 100% but the sensitivity was considerably lower (75%) compared to other types.
Mixed etiology shock: Excellent specificity but low sensitivity
This protocol had lowest agreement (74%, P value < 0.001) with final diagnosis when patients had mixed etiologies as the cause for their shock state. We had 10 patients finally diagnosed as having multiple causes for their shock. Seven were correctly diagnosed using RUSH criteria (sensitivity of 70%), while three were initially misdiagnosed as hypovolemic, cardiogenic, and obstructive types of shock. The protocol had specificity and PPV of 100% and NPV of 92.1% in this group.
DISCUSSION
Our study clearly delineates that in the setting of patients in shock state and under the care of an emergency physician with expertise in emergency ultrasonography, initial impression provided by performing RUSH protocol early after admission was notably congruent with the final clinical diagnosis reached at the course of hospitalization (Kappa index = 0.70). Subsequently, we would be able to initiate appropriate goal directed therapies earlier (about 20 minutes after patients’ arrival) with much more confidence by performing this multi-organ-focused ultrasonographic examination. Volpicelli et al., have reported the same index of agreement (Kappa index = 0.71) between shock type diagnosed based on a similar protocol and final clinical diagnosis of patients.[16]
Obstructive, cardiogenic, and hypovolemic shocks
The most interesting finding was the valuable role of RUSH protocol to outline Obstructive causes of shock (sensitivity = 100%; specificity = 97%). We noted considerably high agreement (92%) with final diagnosis for this shock type. It seems that the examiners are good at finding signs of right ventricular strain in focused ultrasonography, after which they can direct their examination toward finding the possible underlying cause (pneumothorax, tamponade, or massive PTE).[2,6,10] There was only one case with obstructive shock due to pneumothorax (albeit later found in CT scan but not in supine chest X-ray); the pneumothorax itself was not diagnosed in the initial RUSH examination; however, the examiner could correctly outline that the shock state of the patient had obstructive features in the sonographic study.
The second highest agreement of this protocol was for diagnosis of patients with cardiogenic shock (89%), which was the most frequent cause of shock (23.1%) in our study. These criteria had quite good indices for diagnosis of shocks with cardiac etiology, especially to rule out cardiogenic state (NPV = 97%).
We found acceptable efficacy for RUSH protocol to define hypovolemic shock type (86% agreement, 100% sensitivity, and 94.6% specificity). The point here is that there are more straightforward life-saving therapeutic interventions for these three types of shock (obstructive, cardiogenic, and hypovolemic) compared to the other complicated types of shock (septic distributive and mixed). Fortunately, early RUSH examination enables us to rule out these critical states in the shortest possible time (all with NPV above 97%). As a result, the earlier goal-directed measures could be administered to improve their outcome.
Distributive shock
We found low sensitivity (75%) for RUSH protocol to detect distributive cause of shock state compared to other types. We believe that the main issue here is related to septic shock. From the pathophysiological point of view, a transition occurs in the body from systemic inflammatory response syndrome (SIRS) to severe sepsis and septic shock, and accordingly indices of circulation change accordingly in a dynamic manner.[17,18] Thus, a patient with septic shock may demonstrate a myriad of complex findings in a RUSH examination and does not demonstrate the straight forward findings of a distributive shock. As a result, the clinician cannot correctly regard the definite ongoing process. Thus, we miscategorized two patients with distributive shock, one in hypovolemic and one in “not defined” group. It is believed that the main benefit in the management of patients with septic shock arises from early identification of those at high risk for cardiovascular collapse.[19,20,21] Thus, we believe that in a patient clinically suspected to have septic shock, it would be more helpful to change our strategy and perform multiple sessions of RUSH examination instead of a single early examination. In this way, we would be able to monitor the response of cardiovascular system to resuscitative treatments.
In addition, when there is more than one underlying mechanism for shock (mixed type), the protocol showed the least sensitivity (only 70%) and had the lowest agreement (74%). Therefore, in patients clinically suspected to have distributive component or mixed etiology, we should interpret findings of early RUSH examination with more caution.
There exist certain considerations that need to be emphasized. The first issue is that in practice, we should not expect early RUSH protocol just to provide the patient's exact final diagnosis. Instead, the main role of this protocol should be to elucidate the most probable diagnosis among all potential etiologies and rule out certain life-threatening diagnosis in the initial precious time interval. In addition, we should bear in mind that findings on this goal directed ultrasonography often require further interpretation to define whether they are significant. In this way, the involved clinician could be the best candidate to use this protocol. For instance, in a hypotensive patient with cardiac dysfunction in ultrasonography, it is the role of clinician to decide whether low blood pressure is the result of a primary major cardiac process (MI or heart failure) or another ongoing process (such as sepsis or hypovolemia as a result of gastroenteritis).[9] We found similar scenarios in two of our patients who had underlying heart failure. Using RUSH examination, the exact cause of their shock was defined as hypovolemia (due to gastroenteritis) in one, and sepsis (due to pneumonia) in the other. In fact, this protocol enabled our clinician to plan his therapeutic strategies more efficiently.
Study limitations
There is an important consideration for using any type of ultrasonic protocol, which is the amount of required expertise to reach the desired evaluation (here, to outline the shock type of a critical patient). This study is an early report of applying RUSH protocol in practice and estimating its accuracy. In this study, only one emergency physician performed this examination, which means our results are affected by his personal experience and skills. We also had limited number of patients in each distinctive group of shock etiology, which made us interpret subgroup results with more caution.
CONCLUSION
We highlight the role of early RUSH examination in the care of an emergency physician to make a rapid and acceptably accurate diagnosis of the shock type in a hypotensive patient, especially to rule out obstructive (due to pneumothorax, tamponade, or PTE), cardiogenic, and hypovolemic shock types. This would guide the physician to begin a more specific life-saving resuscitative intervention earlier and more confidently. However, in our experience, RUSH protocol showed some limitations, particularly in its role in the diagnosis of distributive (specifically septic) or mixed types of shock. Further studies with multiple physicians performing this protocol and larger sample sizes are necessary to better outline these shortcomings.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Hendrickson RG, Dean AJ, Costantino TG. A novel use of ultrasound inpulseless electrical activity: The diagnosis of an acute abdominal aorticaneurysm rupture. J Emerg Med. 2001;21:141–4. doi: 10.1016/s0736-4679(01)00362-6. [DOI] [PubMed] [Google Scholar]
- 2.Plummer D, Heegaard W, Dries D, Reardon R, Pippert G, Frascone RJ. Ultrasound in HEMS: Its role in differentiating shock states. Air Med J. 2003;22:33–6. doi: 10.1067/mmj.2003.23. [DOI] [PubMed] [Google Scholar]
- 3.Hernandez C, Shuler K, Hannan H, Sonyika C, Likourezos A, Marshall J. C.A.U.S.E.: Cardiac arrest ultrasound exam--a better approach to managing patients in primary non-arrhythmogenic cardiac arrest. Resuscitation. 2008;76:198–206. doi: 10.1016/j.resuscitation.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 4.Lichtenstein D, Karakitsos D. Integrating lung ultrasound in thehemodynamic evaluation of acute circulatory failure (the fluid administration limited by lung sonography protocol) J Crit Care. 2012;27:533. doi: 10.1016/j.jcrc.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Volpicelli G, Mussa A, Garofalo G, Cardinale L, Casoli G, Perotto F, et al. Bedside lung ultrasound in the assessment of alveolar-interstitial syndrome. Am J Emerg Med. 2006;24:689–96. doi: 10.1016/j.ajem.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Volpicelli G. Sonographic diagnosis of pneumothorax. Intensive Care Med. 2011;37:224–32. doi: 10.1007/s00134-010-2079-y. [DOI] [PubMed] [Google Scholar]
- 7.Volpicelli G, Mussa A, Frascisco MF. Sonographic diagnosis of pulmonary embolism with cardiac arrest without major dilation of the right ventricle or direct sign of lower limb venous thrombosis. J Clin Ultrasound. 2012;40:529–33. doi: 10.1002/jcu.20860. [DOI] [PubMed] [Google Scholar]
- 8.Jones AE, Tayal VS, Sullivan DM, Kline JA. Randomized, controlled trial of immediate versus delayed goal-directed ultrasound to identify the cause of nontraumatic hypotension in emergency department patients. Crit Care Med. 2004;32:1703–8. doi: 10.1097/01.ccm.0000133017.34137.82. [DOI] [PubMed] [Google Scholar]
- 9.Rose JS, Bair AE, Mandavia D, Kinser DJ. The UHP ultrasound protocol: Anovel ultrasound approach to the empiric evaluation of the undifferentiated hypotensive patient. Am J Emerg Med. 2001;19:299–302. doi: 10.1053/ajem.2001.24481. [DOI] [PubMed] [Google Scholar]
- 10.Atkinson PR, McAuley DJ, Kendall RJ, Abeyakoon O, Reid CG, Connolly J, et al. Abdominal and cardiac evaluation with sonography in shock (ACES): An approach by emergency physicians for the use of ultrasound in patients with undifferentiated hypotension. Emerg Med J. 2009;26:87–91. doi: 10.1136/emj.2007.056242. [DOI] [PubMed] [Google Scholar]
- 11.Perera P, Mailhot T, Riley D, Mandavia D. The RUSH exam: Rapid ultrasound in shock in the evaluation of critically ill patient. Emerg Med Clin North Am. 2010;28:29–56. doi: 10.1016/j.emc.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Perera P, Mailhot T, Riley D, Mandavia D. The RUSH Exam 2012: Rapid ultrasound in shock in the evaluation of critically ill patient. Ultrasound Clin. 2012;7:255–78. doi: 10.1016/j.emc.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Seif D, Perera P, Mailhot T, Riley D, Mandavia D. Bedside Ultrasound in resuscitation and the rapid ultrasound in shock protocol. Crit Care Res Pract 2012. 2012 doi: 10.1155/2012/503254. 503254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loscalzo J, Harrison TR. New York: McGraw-Hill Medical; 2013. Harrison's Pulmonary and Critical Care Medicine. [Google Scholar]
- 15.Tintinalli JE, Stapczynski JS. New York: McGraw- Hill; 2011. Tintinalli's Emergency Medicine: A Comprehensive Study Guide. [Google Scholar]
- 16.Volpicelli G, Lamorte A, Tullio M, Cardinale L, Giraudo M, Stefanone V, et al. Point-of-care multiorgan ultrasonography for the evaluation of undifferentiated hypotension in the emergency department. Intensive Care Med. 2013;39:1290–8. doi: 10.1007/s00134-013-2919-7. [DOI] [PubMed] [Google Scholar]
- 17.Opal SM, Cross AS. Clinical trials for severe sepsis: Past failures and future hopes. Infect Dis Clin North Am. 1999;13:285–97. doi: 10.1016/s0891-5520(05)70075-1. [DOI] [PubMed] [Google Scholar]
- 18.King EG, Bauza GJ, Mella JR, Remick DG. Pathophysiologic mechanisms in septic shock. Lab Invest. 2014;94:4–12. doi: 10.1038/labinvest.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early Goal-Directed Therapy Collaborative Group. Early goal directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 20.Napoli AM, Machan JT, Corl K, Forcoda A. The use of impedance cardiography in predicting mortality in emergency department patients with severe sepsis and septic shock. Acad Emerg Med. 2010;17:452–5. doi: 10.1111/j.1553-2712.2010.00705.x. [DOI] [PubMed] [Google Scholar]
- 21.Haydar SA, Moore ET, Higgins GL, 3rd, Irish CB, Owens WB, Strout TD. Effect of bedside ultrasonography on the certainty of physician clinical decision making for septic patients in the emergency department. Ann Emerg Med. 2012;60:346–58. doi: 10.1016/j.annemergmed.2012.01.006. [DOI] [PubMed] [Google Scholar]


