Abstract
Cytoskeletal scaffolding complexes help organize specialized membrane domains with unique functions on the surface of cells. In this study, we define the scaffolding potential of the Schwann cell dystrophin glycoprotein complex (DGC) by establishing the presence of four syntrophin isoforms, (α1, β1, β2, and γ2), and one dystrobrevin isoform, (α–dystrobrevin-1), in the abaxonal membrane. Furthermore, we demonstrate the existence of two separate DGCs in Schwann cells that divide the abaxonal membrane into spatially distinct domains, the DRP2/periaxin rich plaques and the Cajal bands that contain Dp116, utrophin, α-dystrobrevin-1 and four syntrophin isoforms. Finally, we show that the two different DGCs can scaffold unique accessory molecules in distinct areas of the Schwann cell membrane. Specifically, the cholesterol transporter ABCA1, associates with the Dp116/syntrophin complex in Cajal bands and is excluded from the DRP2/periaxin rich plaques.
Introduction
The formation and maintenance of specialized membrane domains is critical for a myriad of cellular processes in virtually every cell type. Therefore, understanding the elements that divide the cell membrane into discrete functional domains is a fundamental aspect of many cellular processes. Cytoskeletal scaffolding networks contribute to the formation and maintenance of specialized membrane domains by anchoring networks of proteins that define the specific domain. The dystrophin glycoprotein complex (DGC) has emerged as one such cytoskeletal scaffold. The DGC organizes numerous membrane specializations, including the neuromuscular synapse (Bewick et al. 1992; Byers et al. 1991; Kramarcy and Sealock 2000; Luo et al. 2005; Newey et al. 2001a; Peters et al. 1994; Peters et al. 1998), the basolateral membrane of epithelial cells (Kachinsky et al. 1999), and astrocytic endfeet (Amiry-Moghaddam et al. 2004; Bragg et al. 2006).
The DGC’s ability to organize specialized membrane domains is primarily mediated by the syntrophins and dystrobrevins, (reviewed in (Albrecht and Froehner 2002). The syntrophins are a family of five modular adaptor proteins that tether kinases (Connors et al. 2004; Hogan et al. 2001; Lumeng et al. 1999), channels (Adams et al. 2001; Gee et al. 1998; Leonoudakis et al. 2004; Neely et al. 2001), transporters (Buechler et al. 2002; Munehira et al. 2004; Okuhira et al. 2005) and other proteins (Brenman et al. 1996; Newbell et al. 1997; Oak et al. 2001; Ort et al. 2000) to the DGC with their PDZ domains (Adams et al. 1993; Alessi et al. 2006; Newey et al. 2000). The dystrobrevins contribute to the DGC scaffold by doubling the number of syntrophins (Newey et al. 2000), and interacting with additional proteins (Albrecht and Froehner 2004; Benson et al. 2001; Ceccarini et al. 2007; Mizuno et al. 2001; Newey et al. 2001b).
Schwann cells express several DGC components, including dystrophin (Dp116), utrophin, DRP2, dystroglycan and the sarcoglycans (Byers et al. 1993; Fabbrizio et al. 1995; Imamura et al. 2000; Karpati et al. 1993; Yamada et al. 1994). Furthermore, the DGC helps stabilize the myelin sheath (Cai et al. 2007; Court et al. 2004; Saito et al. 2007; Saito et al. 2003; Sherman et al. 2001). While the dystroglycans and sarcoglycans are uniformly distributed on the Schwann cell plasma membrane, DRP2 aggregates with periaxin into discrete plaques where the myelin layers come in close proximity to the abaxonal membrane (Court et al. 2004). Elsewhere, the abaxonal membrane is separated from the myelin layers by strands of cytoplasm known as Cajal bands (Court et al. 2004), that presumably also contain a DGC. However, the distribution of Dp116, utrophin, dystrobrevin and the syntrophins on the Schwann cell plasma membrane are unknown.
Although the presence and importance of the DGC in Schwann cells is well established, little is known about the scaffolding potential of this complex in Schwann cells mediated by the syntrophins and dystrobrevins. In this study, we characterize the syntrophin and dystrobrevin isoforms in peripheral nerve, demonstrate the presence of two different DGC complexes with distinct distributions on the surface of myelin-forming Schwann cells, and show that the cholesterol transporter ABCA1 associates with syntrophin and Dp116 in Schwann cell Cajal bands.
Methods
Antibodies
Rabbit polyclonal antibodies previously generated and characterized in the Froehner lab include dystrophin (Kramarcy et al. 1994), utrophin (Kramarcy et al. 1994), α-dystrobrevin-1 (670) (Peters et al. 1998), α-dystrobrevin-2 (Peters et al. 1998), pan-dystrobrevin (433) (Peters et al. 1998), β-dystrobrevin (Peters et al. 1997b), α-syntrophin (Peters et al. 1997a), β1-syntrophin (Peters et al. 1997a), β2-syntrophin (Peters et al. 1994), γ1-syntrophin (289) and γ2-syntrophin (213) (Alessi et al. 2006). The mouse monoclonal antibody 1351 that recognizes the α and β syntrophins was characterized previously (Froehner et al. 1987). The rabbit anti DRP2 antibody (2164) was produced and characterized by the Brophy lab (Sherman et al. 2001). The rabbit anti-ABCA1 antibody was purchased from Affinity Bioreagents (Golden, CO). The monoclonal anti-dystrophin antibody Mandra1 was purchased from Sigma (St.Louis, MO).
Animals
Mice deficient in α-syntrophin, β2-syntrophin or both α- and β2-syntrophin were created in the Froehner lab and described previously(Adams et al. 2004; Adams et al. 2000). α-Dystrobrevin null mice (Grady et al. 1999) were a kind gift from Mark Grady and Joshua Sanes and maintained as a colony at the University of Washington. All animal experiments were performed with approval of the Institutional Animal Care and Use Committee at the University of Washington
Immunohistochemistry
C57 mice or Wistar rats were sacrificed and the sciatic nerves were rapidly removed, covered in OCT and frozen in liquid nitrogen cooled isopentane for cryosectioning. 10 μm frozen sections were fixed in either 2% paraformaldehyde for 10 min or 50% methanol/50% acetone for 1 min, rinsed with PBS, and blocked with 3% BSA, 1% fish gelatin and 0.05% tween-20 in PBS. The nerves were then labeled with primary antibody at a final concentration of 50 nM. Next, the nerves were washed in PBS and incubated with either, goat anti-rabbit IgG conjugated to Alexa 594 diluted 1:1000, or Donkey anti-mouse IgG conjugated to Alexa 488 diluted 1:1000 (MolecularProbes). After extensive washing in PBS, the slides were mounted and viewed using a Zeiss Axioskop2plus equipped with a Zeiss Axiocam and Axiovision software, a Leica TCS confocal microscope or a Zeiss Meta 510 confocal microscope maintained by the University of Washington Keck Imaging Center.
Tissue Homogenization and Immunoprecipitation
Whole tissue extracts were prepared by homogenizing nerve samples in 20 volumes of reducing sample buffer (125mM Tris pH 6.8, 4%SDS, 20% glycerol, 0.5% 2-mercapto ethanol) in a dounce homogenizer, and then boiling for 2 minutes. Insoluble material was removed by centrifuging at 10,000×g before separation of proteins by SDS-PAGE.
Dystrophin protein complexes were immunoprecipitated from mouse sciatic nerve using standard methods. Briefly, 10 sciatic nerves were homogenized in 1.5 ml of HB (10mM NaPO4, 150mM NaCl, 5mM EDTA, 1mM EGTA and 0.02% sodium azide) with 1% tritonX-100 and 0.5 μg/ml antipain, 50 ng/ml pepstatinA, 0.5 μg/ml benzamidine, 0.5 μg/ml leupeptin, and 0.5 μg/ml aproteinin. Homogenates were spun at 10,000 ×g for 15 min at 4°C to remove insoluble material and the supernatant was split into two separate tubes. Homogenates were then cleared with 20μl of protein G sepharose beads (Sigma) for 2hrs at 4°C. Immunoprecipitation was performed using 20μg of antibody or control IgG for 3hrs at 4°C. The immunocomplexes were isolated with protein G Sepharose beads and were washed twice in HB+1% tritonX-100, twice in HB+ 0.1% tritonX-100 and once with HB alone. The immune complexes were then boiled in reducing sample buffer, loaded onto 4-15% acrylamide gels (BioRad) and separated by SDS-PAGE.
Western blotting
Following SDS-PAGE, proteins were transferred to PVDF membranes (Millipore) using a miniprotean II tank transfer apparatus (Biorad) and then blocked in TBST+3%NFDM (define) overnight at 4° C. Antigen detection was performed by overlaying blots with the appropriate antibody diluted to a final concentration of 50nM. Blots were then washed and incubated with either goat anti-rabbit IgG or goat anti-mouse IgG secondary antibodies conjugated to horseradish peroxidase (Pierce) diluted 1:1,000. Bands were visualized by enhanced chemiluminescence (ECL) using super signal west pico or femto ECL substrate (Pierce) and an Alpha Innotech gel documentation system (Alpha Innotech Corp).
Results
Characterization of dystrobrevins and syntrophins in peripheral nerve
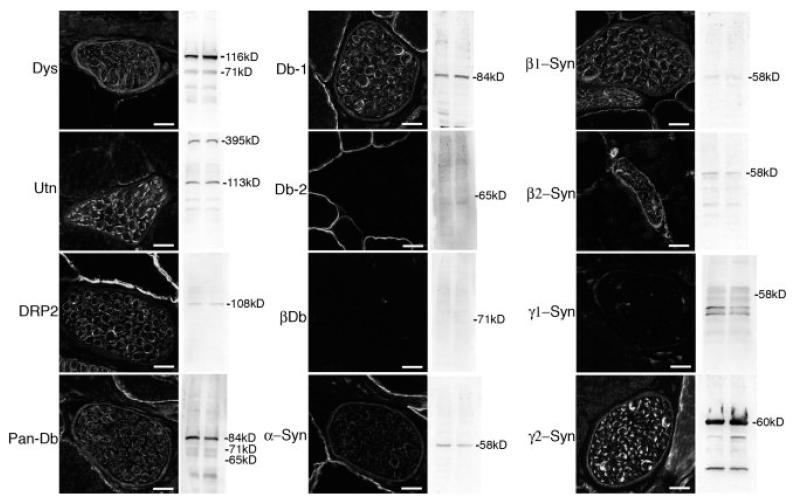
The presence and distribution of dystrophin, utrophin and DRP2 in peripheral nerve was confirmed (fig 1) as previously reported (Byers et al. 1993; Fabbrizio et al. 1995; Karpati et al. 1993). To identify the dystrobrevins and syntrophins that accompany the dystrophin complex in the perineurium and Schwann cell plasma membrane, we used isoform specific antibodies. α-Dystrobrevin-1 is the only dystrobrevin expressed in peripheral nerve, where it is found in both the perineurium and in Schwann cells (Fig. 1). In contrast, multiple syntrophin isoforms are present. These include α-, β1-, β2- and γ2-syntrophin, where γ2-syntrophin is qualitatively the most abundant isoform (Fig. 1). The α-, β2- and γ2-syntrophin isoforms are present in both the perineurium and in the Schwann cell plasma membrane, while β1-syntrophin is in the Schwann cells, but not the perineurium (fig.1).
Figure 1. Expression and localization of DGC proteins in peripheral nerve.
Immunofluorescence microscopy was used to determine the localization of dystrophin (Dys), utrophin (Utn), DRP2, α-dystrobrevin-1 (Db-1), α-dystrobrevin-2 (Db-2), β-dystrobrevin (βDb), α-, β1, β2, γ1, and γ2-syntrophin (Syn) in nerve branches running through skeletal muscle (transverse sections). Western blots using the corresponding antibody on duplicate samples of whole sciatic nerve homogenates are also shown. Scale bar is 12 μm.
Two different distributions of dystrophin family members in Schwann cells
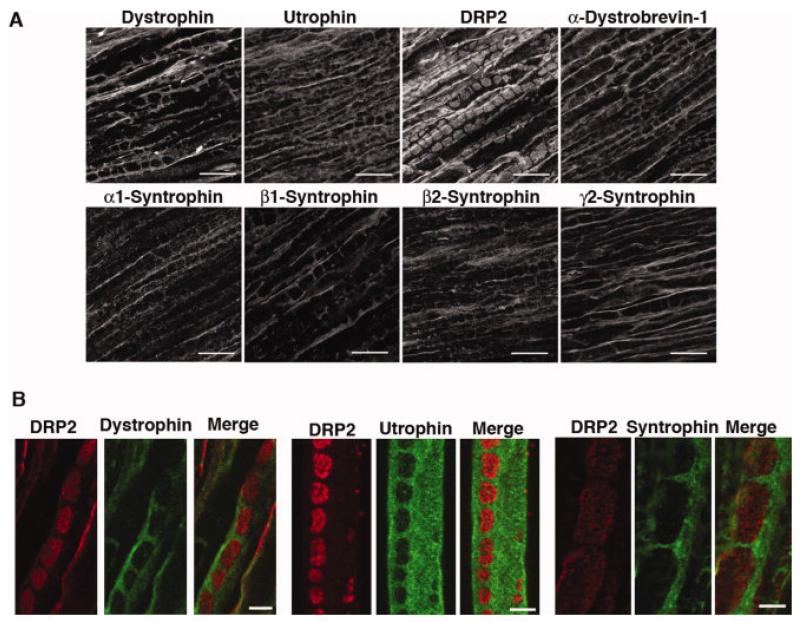
Analysis of the distribution of each member of the DGC along the length of the nerve revealed the presence two spatially distinct dystrophin complexes. As previously reported, DRP2 is located in discrete plaques on the Schwann cell plasma membrane (Sherman et al. 2001) (Fig. 2A). Dystrophin and utrophin are excluded from the DRP2 rich plaques, but are concentrated in the remainder of the abaxonal membrane - the Cajal bands (Fig. 2A). α-Dystrobrevin-1 and the syntrophins present in Schwann cells are exclusively in the Cajal bands, and thus are likely associated with dystrophin and/or utrophin on the membrane at these sites (Fig. 2A). Immunohistochemistry of sciatic nerves from C57, α-syntrophin null, β2-syntrophin null and α-dystrobrevin null mice, demonstrate the specificity of the syntrophin and dystrobrevin labeling in Schwann cells (Supplementary Figure). Confocal microscopy of longitudinal sections of sciatic nerve or teased quadriceps nerve double labeled for DRP2 and either dystrophin, utrophin, or syntrophin, demonstrate that their distributions are exclusive of one another (Fig. 2B).
Figure 2.
Localization of DGC proteins to Cajal bands and plaques and comparison of the distribution of DRP2 with dystrophin, utrophin, and α/β syntrophin in sciatic nerve. (A) Longitudinal sections of mouse sciatic nerve were stained with the indicated antibodies. Only DRP2 is localized to plaques. Dystrophin, utrophin, α-dystrobrevin-1, and α-, β1-, β2-, and γ2-syntrophin are all restricted to Cajal bands. Scale bar is 10 μm. (B) DRP2 (red) is localized to plaques while dystrophin, utrophin and α/β syntrophin (visualized with mAb 1351) (green) are restricted to Cajal bands. Right and left panels, rat sciatic nerve longitudinal sections. Middle panel, teased fiber. Scale bar is 5 μm.
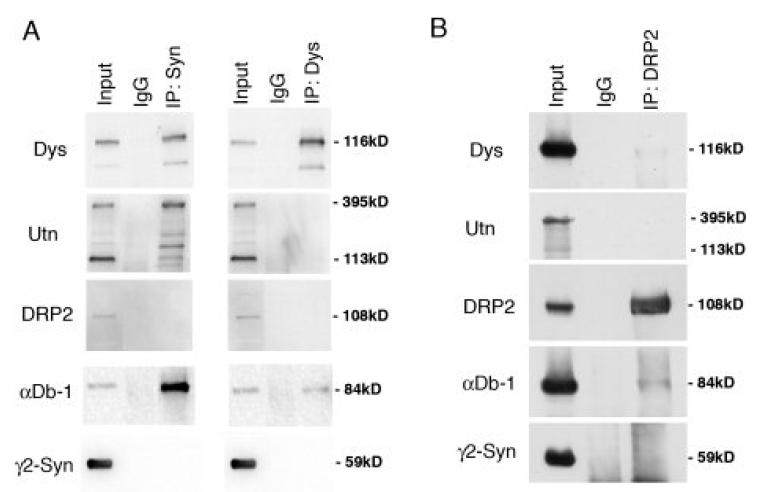
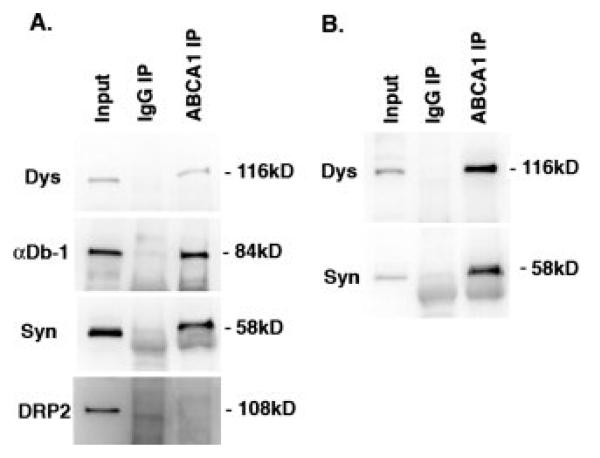
The presence of two distinct DGC complexes in the Schwann cell was confirmed by biochemical analysis. Immunoprecipitation of DGC complexes with anti-dystrophin, or anti-syntrophin, fail to bring down DRP2 (Fig. 3A). We also found that utrophin failed to coimmunoprecipitate with dystrophin. In the reverse experiment, immunoprecipitations with anti-DRP2 contained little to no dystrophin, utrophin, dystrobrevin, or syntrophin (Fig. 3B). Thus, by immunohistochemical and biochemical analysis, the Schwann cell plasma membrane contains two distinct dystrophin complexes. The first, originally characterized by Sherman et al. (2001), is composed of DRP2, dystroglycan and the PDZ domain containing protein, periaxin, and is concentrated into discrete plaques. The second is composed of the more traditional dystrophin complex members (Dp116, utrophin, α-dystrobrevin-1, α-, β1-, β2-, and γ2-syntrophin) and is present in Cajal bands.
Figure 3.
Coimmunoprecipitation of DGC proteins from mouse sciatic nerve. (A) Detergent extracts (Input) were subjected to immunoprecipitation (IP) with anti-α/β-syntrophin (Syn, mAb 1351), anti-dystrophin (Dys, mandra1), or control antibody (IgG); (B) immunoprecipation with anti-DRP2 or IgG. Immunoprecipitates were subjected to western blotting with the antibodies indicated. Dp116, α/β-syntrophin and α-Db-1 are coimmunoprecipitated. Neither DRP2 nor γ2-syntrophin are present in complexes with Dp116/syn/αDb-1 or with each other.
DGC scaffolding in Schwann cells
The presence of two distinct scaffolding complexes on the surface of Schwann cells suggests that the DGC organizes the abaxonal membrane into two distinct domains that have unique molecular compositions and functions. We used a collection of antibodies to known syntrophin binding proteins to determine if the DGC serves as a scaffold for any of these proteins in a specific pattern on the Schwann cell membrane. Our results reveal that the cholesterol transporter, ABCA1, previously shown to interact with the dystrophin complex through interactions with α-syntrophin (Munehira et al. 2004), β1-syntrophin (Okuhira et al. 2005), and β2-syntrophin (Buechler et al. 2002), is restricted to the Cajal bands in the Schwann cells of sciatic nerves (Fig. 6). Furthermore, analysis of protein complexes immunoprecipitated with anti-ABCA1 reveals the presence of α/β-syntrophin, α-dystrobrevin-1 and Dp116, but not DRP2 (Fig 5A). Attempts to identify the syntrophin isoform associated with ABCA1 by western blotting with isoform specific antibodies were foiled by the fact that multiple syntrophins associate with Dp116 and dystrobrevin, and thus are present in the complex. In an attempt to circumvent this problem, we performed immunoprecipitations using sciatic nerves from α–, β2-, and α/β2 double syntrophin null mice. The loss of α, β2 or both syntrophins from Schwann cells failed to disrupt the association of ABCA1 with the Dp116 complex. Thus, β1-syntrophin is likely the syntrophin interacting with ABCA1 in Schwann cells (Fig 5B). Alternatively, ABCA1 may be promiscuous, associating with more than one syntrophin isoform.
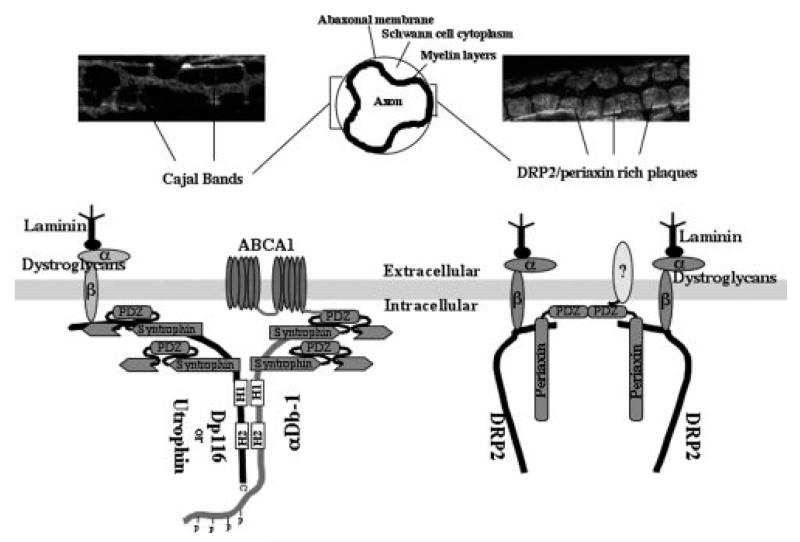
Figure 6.
A model of the dystrophin and DRP2 complexes and their division of the abaxonal membrane into Cajal bands and plaques.
Figure 5.
Coimmunoprecipitation of ABCA1 with the Dp116/syn/αDb-1 complex but not with DRP2. (A) Immunoprecipitations from C57 mouse sciatic nerve with ABCA1 were subjected to western blotting for Dp116 (Dys), αDb-1, α/β-syntrophins, and DRP2. (B) A similar experiment using sciatic nerve from the α/β2-syntrophin double null mice. Coimmunoprecipitation of the Dp116/syn/αDb-1 complex with anti-ABCA1 does not depend on the presence of α- or β2-syntrophin. DRP2 is not found in the ABCA1 complex. IgG IP, control.
Discussion
Our study defines the scaffolding potential of the DGC in the Schwann cell plasma membrane by identifying a single dystrobrevin isoform, and four syntrophin isoforms. Furthermore, we demonstrate that α-dystrobrevin-1 and all four syntrophins are excluded from the DRP2/periaxin rich plaques identified by Sherman et al (2001). Instead, the syntrophins and α-dystrobrevin-1 are concentrated in a separate DGC that contains Dp116 and utrophin. The two DGC complexes have distinct distributions. The DRP2 complex is clustered in large aggregates where the abaxonal membrane of the Schwann cell is in close apposition with the underlying myelin layers (Court et al. 2004). In contrast, the DGC that contains dystrobrevin and the syntrophins is located in the Cajal bands, where strands of cytoplasm tunnel between the abaxonal membrane and the layers of myelin to create a network of cytoplasm that radiates outward from the nucleus.
The presence of two spatially distinct DGC complexes on the Schwann cell membrane divides the surface into two domains organized by different cytoskeletal scaffolding networks that help dictate the protein composition, and therefore the function of each membrane domain. The syntrophin family localizes and stabilizes a variety of signaling molecules and channels in or near the membrane of many different cell types through interactions with their PDZ domains. Our findings demonstrate that all of the syntrophins that reside under the abaxonal Schwann cell membrane localize to Cajal bands, and that the DRP2 complex in Schwann cells does not contain any syntrophin isoforms. This result was surprising given that in vitro experiments demonstrate that DRP2 can associate with the γ-syntrophins, (Alessi et al. 2006) and that γ2-syntrophin is abundant in Schwann cells. However, the DRP2 DGC contains the PDZ protein, periaxin (Sherman et al. 2001), suggesting that this complex is capable of organizing a different set of signaling proteins and channels in the abaxonal membrane. A model summarizing our findings is shown in figure 6.
Although γ2-syntrophin localizes to the Cajal bands in Schwann cells, it fails to purify with either DGC by immunoprecipitation (Fig. 3). This is consistent with a previous study that showed γ2-syntrophin did not associate with dystrophin in vivo, even though in vitro pull down assays using GST fusion proteins demonstrated that γ2-syntrophin is capable of binding to one of the two syntrophin binding sites present on dystrophin, utrophin, DRP2, and both dystrobrevins (Alessi et al., 2006). Given the localization of γ2-syntrophin to Cajal bands (Fig. 2), it seems certain that it does not interact with DRP2 in Schwann cells, given that they are in different locations. However, γ2-syntrophin does not copurify with immunoprecipitated DGC complexes that are rich in Dp116, utrophin, and dystrobrevin. While these findings seem to contradict each other, there are several possible explanations. For example, γ2-syntrophin may have binding partners other than the DGC in Cajal bands. Also, the association of γ2-syntrophin with the DGC may be a low affinity interaction that is disrupted under the immunoprecipitation conditions used. Alternatively, the association of γ2-syntrophin with the DGC could be regulated. Thus, it is not clear if or how γ2-syntrophin contributes to the DGC scaffold in Cajal bands.
To determine if the Schwann cell DGC can serve as a scaffold for membrane associated proteins in the Cajal bands, we performed immunohistochemistry using antibodies to a variety of proteins known to bind syntrophin. Notably, most known syntrophin ligands, including nNOS (Brenman et al., 1996) and aquaporin-4 (Adams et al., 2001; Neely et al., 2001), were not on the Schwann cell membrane or in Cajal bands, (data not shown). Thus, our initial inspection suggests that the scaffolding function of the dystrophin complex in Schwann cells is quite different from that in skeletal muscle or astrocytes (Bragg et al., 2006). The cholesterol transporter, ABCA1, was found in Cajal bands by immunohistochemistry (Fig. 4), and its interaction with Dp116 and syntrophin, but not DRP2, was confirmed by immunoprecipitation (Fig. 5). These findings demonstrate the ability of the two separate DGCs in the Schwann cell plasma membrane to define distinct membrane domains with different functions.
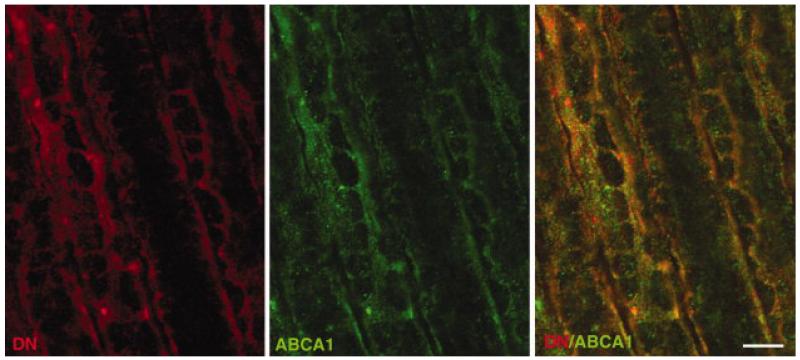
Figure 4.
Colocalization of ABCA1 and Dp116 in Cajal bands. Immunofluorescence labeling of rat sciatic nerve longitudinal sections shows significant, but not complete, colocalization of ABCA1 (green) and Dp116 (DN, Red). Scale bar is 5 μm.
Previous studies have described the interaction of ABCA1 with multiple syntrophin isoforms (Buechler et al., 2002; Munehira et al., 2004; Okuhira et al., 2005). Their interaction is dependent on the C-terminal tail of ABCA1, which has a sequence that binds class I PDZ domains, like those found in the syntrophins. Furthermore, the interaction of ABCA1 with α and β1-syntrophin decreases the turnover of ABCA1 (Munehira et al., 2004; Okuhira et al., 2005), and inhibition or over expression of β1-syntrophin modulates cholesterol efflux and ABCA1 cell surface expression in fibroblasts and macrophages (Okuhira et al., 2005).
ABCA1 plays a critical role in reverse cholesterol transport (Lawn et al., 1999), a process that removes excess cholesterol from peripheral tissues for transport to the liver and removal from the body. Mutations in ABCA1 are known to cause familial HDL deficiency (Bodzioch et al., 1999; Brooks-Wilson et al., 1999; McNeish et al., 2000; Orso et al., 2000; Rust et al., 1999) and Tangier disease. Patients with Tangier disease typically present with a wide range of peripheral nerve abnormalities that include relapsing peripheral neuropathy, deficits in temperature and pain sensation (Engel et al., 1967; Pollock et al., 1983) or syringomyelia-like neuropathy (Zuchner et al., 2003). Nerve biopsies reveal a range of myelination defects that reflect the severity of the patient’s neuropathy, and include nerves that are mostly normal with subtle signs of demyelination, to the complete loss of all myelinating Schwann cells and subsequent loss of nerve fibers (Pollock et al.,1983; Zuchner et al., 2003). Whether ABCA1 function and cholesterol metabolism are altered when the Schwann cell DGC is disrupted remains to be determined in future experiments.
Our studies in peripheral nerve have established the components of the DGC responsible for scaffolding proteins in the Schwann cell plasma membrane. In addition, we have demonstrated that two scaffolding networks divide the Schwann cell membrane into distinct domains, the DRP2 rich plaques and the Dp116 rich Cajal bands. These distinct distributions are likely to orchestrate the assembly of specialized membrane domains dedicated to different functions. As an example of this potential, we demonstrate that the syntrophin binding protein, ABCA1 interacts with the Dp116 based DGC and is localized to the Cajal bands, while being excluded from the DRP2 rich plaques. Presumably, more PDZ domain ligands are specifically targeted to membrane specializations by the syntrophins or periaxin. Their discovery will aid our understanding of the specific functions of these two specialized DGC complexes on the Schwann cell surface.
Supplementary Material
Acknowledgements
The authors thank the members of the Froehner lab for critical reading of the manuscript. This work was supported by a grant from the NIH to SCF (NS033145), and an NRSA to DEA (F32 NS10900). DLS and PJB thank the Wellcome Trust for their support.
References
- Adams ME, Butler MH, Dwyer TM, Peters MF, Murnane AA, Froehner SC. Two forms of mouse syntrophin, a 58 kd dystrophin-associated protein, differ in primary structure and tissue distribution. Neuron. 1993;11(3):531–40. doi: 10.1016/0896-6273(93)90157-m. [DOI] [PubMed] [Google Scholar]
- Adams ME, Kramarcy N, Fukuda T, Engel AG, Sealock R, Froehner SC. Structural abnormalities at neuromuscular synapses lacking multiple syntrophin isoforms. J Neurosci. 2004;24(46):10302–9. doi: 10.1523/JNEUROSCI.3408-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams ME, Kramarcy N, Krall SP, Rossi SG, Rotundo RL, Sealock R, Froehner SC. Absence of alpha-syntrophin leads to structurally aberrant neuromuscular synapses deficient in utrophin. J Cell Biol. 2000;150(6):1385–98. doi: 10.1083/jcb.150.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams ME, Mueller HA, Froehner SC. In vivo requirement of the alpha-syntrophin PDZ domain for the sarcolemmal localization of nNOS and aquaporin-4. J Cell Biol. 2001;155(1):113–22. doi: 10.1083/jcb.200106158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht DE, Froehner SC. Syntrophins and dystrobrevins: defining the dystrophin scaffold at synapses. Neurosignals. 2002;11(3):123–9. doi: 10.1159/000065053. [DOI] [PubMed] [Google Scholar]
- Albrecht DE, Froehner SC. DAMAGE, a novel alpha-dystrobrevin-associated MAGE protein in dystrophin complexes. J Biol Chem. 2004;279(8):7014–23. doi: 10.1074/jbc.M312205200. [DOI] [PubMed] [Google Scholar]
- Alessi A, Bragg AD, Percival JM, Yoo J, Albrecht DE, Froehner SC, Adams ME. gamma-Syntrophin scaffolding is spatially and functionally distinct from that of the alpha/beta syntrophins. Exp Cell Res. 2006;312(16):3084–95. doi: 10.1016/j.yexcr.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Amiry-Moghaddam M, Xue R, Haug FM, Neely JD, Bhardwaj A, Agre P, Adams ME, Froehner SC, Mori S, Ottersen OP. Alpha-syntrophin deletion removes the perivascular but not endothelial pool of aquaporin-4 at the blood-brain barrier and delays the development of brain edema in an experimental model of acute hyponatremia. Faseb J. 2004;18(3):542–4. doi: 10.1096/fj.03-0869fje. [DOI] [PubMed] [Google Scholar]
- Benson MA, Newey SE, Martin-Rendon E, Hawkes R, Blake DJ. Dysbindin, a novel coiled-coil-containing protein that interacts with the dystrobrevins in muscle and brain. J Biol Chem. 2001;276(26):24232–41. doi: 10.1074/jbc.M010418200. [DOI] [PubMed] [Google Scholar]
- Bewick GS, Nicholson LV, Young C, O’Donnell E, Slater CR. Different distributions of dystrophin and related proteins at nerve-muscle junctions. Neuroreport. 1992;3(10):857–60. doi: 10.1097/00001756-199210000-00009. [DOI] [PubMed] [Google Scholar]
- Bodzioch M, Orso E, Klucken J, Langmann T, Bottcher A, Diederich W, Drobnik W, Barlage S, Buchler C, Porsch-Ozcurumez M. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet. 1999;22(4):347–51. doi: 10.1038/11914. others. [DOI] [PubMed] [Google Scholar]
- Bragg AD, Amiry-Moghaddam M, Ottersen OP, Adams ME, Froehner SC. Assembly of a perivascular astrocyte protein scaffold at the mammalian blood-brain barrier is dependent on alpha-syntrophin. Glia. 2006;53(8):879–90. doi: 10.1002/glia.20347. [DOI] [PubMed] [Google Scholar]
- Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell. 1996;84(5):757–67. doi: 10.1016/s0092-8674(00)81053-3. others. [DOI] [PubMed] [Google Scholar]
- Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, van Dam M, Yu L, Brewer C, Collins JA, Molhuizen HO. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet. 1999;22(4):336–45. doi: 10.1038/11905. others. [DOI] [PubMed] [Google Scholar]
- Buechler C, Boettcher A, Bared SM, Probst MC, Schmitz G. The carboxyterminus of the ATP-binding cassette transporter A1 interacts with a beta2-syntrophin/utrophin complex. Biochem Biophys Res Commun. 2002;293(2):759–65. doi: 10.1016/S0006-291X(02)00303-0. [DOI] [PubMed] [Google Scholar]
- Byers TJ, Kunkel LM, Watkins SC. The subcellular distribution of dystrophin in mouse skeletal, cardiac, and smooth muscle. J Cell Biol. 1991;115(2):411–21. doi: 10.1083/jcb.115.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers TJ, Lidov HG, Kunkel LM. An alternative dystrophin transcript specific to peripheral nerve. Nat Genet. 1993;4(1):77–81. doi: 10.1038/ng0593-77. [DOI] [PubMed] [Google Scholar]
- Cai H, Erdman RA, Zweier L, Chen J, Shaw JHt, Baylor KA, Stecker MM, Carey DJ, Chan YM. The sarcoglycan complex in Schwann cells and its role in myelin stability. Exp Neurol. 2007;205(1):257–69. doi: 10.1016/j.expneurol.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Ceccarini M, Grasso M, Veroni C, Gambara G, Artegiani B, Macchia G, Ramoni C, Torreri P, Mallozzi C, Petrucci TC. Association of Dystrobrevin and Regulatory Subunit of Protein Kinase A: A New Role for Dystrobrevin as a Scaffold for Signaling Proteins. J Mol Biol. 2007 doi: 10.1016/j.jmb.2007.06.019. others. [DOI] [PubMed] [Google Scholar]
- Connors NC, Adams ME, Froehner SC, Kofuji P. The potassium channel Kir4.1 associates with the dystrophin-glycoprotein complex via alpha-syntrophin in glia. J Biol Chem. 2004;279(27):28387–92. doi: 10.1074/jbc.M402604200. [DOI] [PubMed] [Google Scholar]
- Court FA, Sherman DL, Pratt T, Garry EM, Ribchester RR, Cottrell DF, Fleetwood-Walker SM, Brophy PJ. Restricted growth of Schwann cells lacking Cajal bands slows conduction in myelinated nerves. Nature. 2004;431(7005):191–5. doi: 10.1038/nature02841. [DOI] [PubMed] [Google Scholar]
- Engel WK, Dorman JD, Levy RI, Fredrickson DS. Neuropathy in Tangier disease. Alpha-Lipoprotein deficiency manifesting as familial recurrent neuropathy and intestinal lipid storage. Arch Neurol. 1967;17(1):1–9. doi: 10.1001/archneur.1967.00470250005001. [DOI] [PubMed] [Google Scholar]
- Fabbrizio E, Latouche J, Rivier F, Hugon G, Mornet D. Re-evaluation of the distributions of dystrophin and utrophin in sciatic nerve. Biochem J. 1995;312(Pt 1):309–14. doi: 10.1042/bj3120309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehner SC, Murnane AA, Tobler M, Peng HB, Sealock R. A postsynaptic Mr 58,000 (58K) protein concentrated at acetylcholine receptor-rich sites in Torpedo electroplaques and skeletal muscle. J Cell Biol. 1987;104(6):1633–46. doi: 10.1083/jcb.104.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee SH, Madhavan R, Levinson SR, Caldwell JH, Sealock R, Froehner SC. Interaction of muscle and brain sodium channels with multiple members of the syntrophin family of dystrophin-associated proteins. J Neurosci. 1998;18(1):128–37. doi: 10.1523/JNEUROSCI.18-01-00128.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady RM, Grange RW, Lau KS, Maimone MM, Nichol MC, Stull JT, Sanes JR. Role for alpha-dystrobrevin in the pathogenesis of dystrophin-dependent muscular dystrophies. Nat Cell Biol. 1999;1(4):215–20. doi: 10.1038/12034. [DOI] [PubMed] [Google Scholar]
- Hogan A, Shepherd L, Chabot J, Quenneville S, Prescott SM, Topham MK, Gee SH. Interaction of gamma 1-syntrophin with diacylglycerol kinase-zeta. Regulation of nuclear localization by PDZ interactions. J Biol Chem. 2001;276(28):26526–33. doi: 10.1074/jbc.M104156200. [DOI] [PubMed] [Google Scholar]
- Imamura M, Araishi K, Noguchi S, Ozawa E. A sarcoglycan-dystroglycan complex anchors Dp116 and utrophin in the peripheral nervous system. Hum Mol Genet. 2000;9(20):3091–100. doi: 10.1093/hmg/9.20.3091. [DOI] [PubMed] [Google Scholar]
- Kachinsky AM, Froehner SC, Milgram SL. A PDZ-containing scaffold related to the dystrophin complex at the basolateral membrane of epithelial cells. J Cell Biol. 1999;145(2):391–402. doi: 10.1083/jcb.145.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpati G, Carpenter S, Morris GE, Davies KE, Guerin C, Holland P. Localization and quantitation of the chromosome 6-encoded dystrophin-related protein in normal and pathological human muscle. J Neuropathol Exp Neurol. 1993;52(2):119–28. doi: 10.1097/00005072-199303000-00004. [DOI] [PubMed] [Google Scholar]
- Kramarcy NR, Sealock R. Syntrophin isoforms at the neuromuscular junction: developmental time course and differential localization. Mol Cell Neurosci. 2000;15(3):262–74. doi: 10.1006/mcne.1999.0823. [DOI] [PubMed] [Google Scholar]
- Kramarcy NR, Vidal A, Froehner SC, Sealock R. Association of utrophin and multiple dystrophin short forms with the mammalian M(r) 58,000 dystrophin-associated protein (syntrophin) J Biol Chem. 1994;269(4):2870–6. [PubMed] [Google Scholar]
- Lawn RM, Wade DP, Garvin MR, Wang X, Schwartz K, Porter JG, Seilhamer JJ, Vaughan AM, Oram JF. The Tangier disease gene product ABC1 controls the cellular apolipoprotein-mediated lipid removal pathway. J Clin Invest. 1999;104(8):R25–31. doi: 10.1172/JCI8119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonoudakis D, Conti LR, Anderson S, Radeke CM, McGuire LM, Adams ME, Froehner SC, Yates JR, 3rd, Vandenberg CA. Protein trafficking and anchoring complexes revealed by proteomic analysis of inward rectifier potassium channel (Kir2.x)-associated proteins. J Biol Chem. 2004;279(21):22331–46. doi: 10.1074/jbc.M400285200. [DOI] [PubMed] [Google Scholar]
- Lumeng C, Phelps S, Crawford GE, Walden PD, Barald K, Chamberlain JS. Interactions between beta 2-syntrophin and a family of microtubule-associated serine/threonine kinases. Nat Neurosci. 1999;2(7):611–7. doi: 10.1038/10165. [DOI] [PubMed] [Google Scholar]
- Luo S, Chen Y, Lai KO, Arevalo JC, Froehner SC, Adams ME, Chao MV, Ip NY. {alpha}-Syntrophin regulates ARMS localization at the neuromuscular junction and enhances EphA4 signaling in an ARMS-dependent manner. J Cell Biol. 2005;169(5):813–24. doi: 10.1083/jcb.200412008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeish J, Aiello RJ, Guyot D, Turi T, Gabel C, Aldinger C, Hoppe KL, Roach ML, Royer LJ, de Wet J. High density lipoprotein deficiency and foam cell accumulation in mice with targeted disruption of ATP-binding cassette transporter-1. Proc Natl Acad Sci U S A. 2000;97(8):4245–50. doi: 10.1073/pnas.97.8.4245. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno Y, Thompson TG, Guyon JR, Lidov HG, Brosius M, Imamura M, Ozawa E, Watkins SC, Kunkel LM. Desmuslin, an intermediate filament protein that interacts with alpha -dystrobrevin and desmin. Proc Natl Acad Sci U S A. 2001;98(11):6156–61. doi: 10.1073/pnas.111153298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munehira Y, Ohnishi T, Kawamoto S, Furuya A, Shitara K, Imamura M, Yokota T, Takeda S, Amachi T, Matsuo M. Alpha1-syntrophin modulates turnover of ABCA1. J Biol Chem. 2004;279(15):15091–5. doi: 10.1074/jbc.M313436200. others. [DOI] [PubMed] [Google Scholar]
- Neely JD, Amiry-Moghaddam M, Ottersen OP, Froehner SC, Agre P, Adams ME. Syntrophin-dependent expression and localization of Aquaporin-4 water channel protein. Proc Natl Acad Sci U S A. 2001;98(24):14108–13. doi: 10.1073/pnas.241508198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbell BJ, Anderson JT, Jarrett HW. Ca2+-calmodulin binding to mouse alpha1 syntrophin: syntrophin is also a Ca2+-binding protein. Biochemistry. 1997;36(6):1295–305. doi: 10.1021/bi962452n. [DOI] [PubMed] [Google Scholar]
- Newey SE, Benson MA, Ponting CP, Davies KE, Blake DJ. Alternative splicing of dystrobrevin regulates the stoichiometry of syntrophin binding to the dystrophin protein complex. Curr Biol. 2000;10(20):1295–8. doi: 10.1016/s0960-9822(00)00760-0. [DOI] [PubMed] [Google Scholar]
- Newey SE, Gramolini AO, Wu J, Holzfeind P, Jasmin BJ, Davies KE, Blake DJ. A novel mechanism for modulating synaptic gene expression: differential localization of alpha-dystrobrevin transcripts in skeletal muscle. Mol Cell Neurosci. 2001a;17(1):127–40. doi: 10.1006/mcne.2000.0918. [DOI] [PubMed] [Google Scholar]
- Newey SE, Howman EV, Ponting CP, Benson MA, Nawrotzki R, Loh NY, Davies KE, Blake DJ. Syncoilin, a novel member of the intermediate filament superfamily that interacts with alpha-dystrobrevin in skeletal muscle. J Biol Chem. 2001b;276(9):6645–55. doi: 10.1074/jbc.M008305200. [DOI] [PubMed] [Google Scholar]
- Oak SA, Russo K, Petrucci TC, Jarrett HW. Mouse alpha1-syntrophin binding to Grb2: further evidence of a role for syntrophin in cell signaling. Biochemistry. 2001;40(37):11270–8. doi: 10.1021/bi010490n. [DOI] [PubMed] [Google Scholar]
- Okuhira K, Fitzgerald ML, Sarracino DA, Manning JJ, Bell SA, Goss JL, Freeman MW. Purification of ATP-binding cassette transporter A1 and associated binding proteins reveals the importance of beta1-syntrophin in cholesterol efflux. J Biol Chem. 2005;280(47):39653–64. doi: 10.1074/jbc.M510187200. [DOI] [PubMed] [Google Scholar]
- Orso E, Broccardo C, Kaminski WE, Bottcher A, Liebisch G, Drobnik W, Gotz A, Chambenoit O, Diederich W, Langmann T. Transport of lipids from golgi to plasma membrane is defective in tangier disease patients and Abc1-deficient mice. Nat Genet. 2000;24(2):192–6. doi: 10.1038/72869. others. [DOI] [PubMed] [Google Scholar]
- Ort T, Maksimova E, Dirkx R, Kachinsky AM, Berghs S, Froehner SC, Solimena M. The receptor tyrosine phosphatase-like protein ICA512 binds the PDZ domains of beta2-syntrophin and nNOS in pancreatic beta-cells. Eur J Cell Biol. 2000;79(9):621–30. doi: 10.1078/0171-9335-00095. [DOI] [PubMed] [Google Scholar]
- Peters MF, Adams ME, Froehner SC. Differential association of syntrophin pairs with the dystrophin complex. J Cell Biol. 1997a;138(1):81–93. doi: 10.1083/jcb.138.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters MF, Kramarcy NR, Sealock R, Froehner SC. beta 2-Syntrophin: localization at the neuromuscular junction in skeletal muscle. Neuroreport. 1994;5(13):1577–80. [PubMed] [Google Scholar]
- Peters MF, O’Brien KF, Sadoulet-Puccio HM, Kunkel LM, Adams ME, Froehner SC. beta-dystrobrevin, a new member of the dystrophin family. Identification, cloning, and protein associations. J Biol Chem. 1997b;272(50):31561–9. doi: 10.1074/jbc.272.50.31561. [DOI] [PubMed] [Google Scholar]
- Peters MF, Sadoulet-Puccio HM, Grady MR, Kramarcy NR, Kunkel LM, Sanes JR, Sealock R, Froehner SC. Differential membrane localization and intermolecular associations of alpha-dystrobrevin isoforms in skeletal muscle. J Cell Biol. 1998;142(5):1269–78. doi: 10.1083/jcb.142.5.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock M, Nukada H, Frith RW, Simcock JP, Allpress S. Peripheral neuropathy in Tangier disease. Brain. 1983;106(Pt 4):911–28. doi: 10.1093/brain/106.4.911. [DOI] [PubMed] [Google Scholar]
- Rust S, Rosier M, Funke H, Real J, Amoura Z, Piette JC, Deleuze JF, Brewer HB, Duverger N, Denefle P. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat Genet. 1999;22(4):352–5. doi: 10.1038/11921. others. [DOI] [PubMed] [Google Scholar]
- Saito F, Masaki T, Saito Y, Nakamura A, Takeda S, Shimizu T, Toda T, Matsumura K. Defective peripheral nerve myelination and neuromuscular junction formation in fukutin-deficient chimeric mice. J Neurochem. 2007;101(6):1712–22. doi: 10.1111/j.1471-4159.2007.04462.x. [DOI] [PubMed] [Google Scholar]
- Saito F, Moore SA, Barresi R, Henry MD, Messing A, Ross-Barta SE, Cohn RD, Williamson RA, Sluka KA, Sherman DL. Unique role of dystroglycan in peripheral nerve myelination, nodal structure, and sodium channel stabilization. Neuron. 2003;38(5):747–58. doi: 10.1016/s0896-6273(03)00301-5. others. [DOI] [PubMed] [Google Scholar]
- Sherman DL, Fabrizi C, Gillespie CS, Brophy PJ. Specific disruption of a schwann cell dystrophin-related protein complex in a demyelinating neuropathy. Neuron. 2001;30(3):677–87. doi: 10.1016/s0896-6273(01)00327-0. [DOI] [PubMed] [Google Scholar]
- Yamada H, Shimizu T, Tanaka T, Campbell KP, Matsumura K. Dystroglycan is a binding protein of laminin and merosin in peripheral nerve. FEBS Lett. 1994;352(1):49–53. doi: 10.1016/0014-5793(94)00917-1. [DOI] [PubMed] [Google Scholar]
- Zuchner S, Sperfeld AD, Senderek J, Sellhaus B, Hanemann CO, Schroder JM. A novel nonsense mutation in the ABC1 gene causes a severe syringomyelia-like phenotype of Tangier disease. Brain. 2003;126(Pt 4):920–7. doi: 10.1093/brain/awg074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.