Dietary restriction (DR) extends life span in many organisms, through unknown mechanisms that may or may not be evolutionarily conserved. Because different laboratories use different diets and techniques for implementing DR, the outcomes may not be strictly comparable. This complicates intra- and interspecific comparisons of the mechanisms of DR and is therefore central to the use of model organisms to research this topic. Drosophila melanogaster is an important model for the study of DR, but the nutritional content of its diet is typically poorly defined. We have compared fly diets composed of different yeasts for their effect on life span and fecundity. We found that only one diet was appropriate for DR experiments, indicating that much of the published work on fly ‘DR’ may have included adverse effects of food composition. We propose procedures to ensure that diets are suitable for the study of DR in Drosophila.
DIETARY restriction (DR) refers to a moderate reduction of food intake that leads to extension of life span beyond that of normal, healthy individuals. This intervention has principally been studied in rodents, but it also extends the life span of a wide range of organisms including the fruit fly, Drosophila melanogaster (1–7). Although extension of life span in response to DR is taxonomically widespread, it is unknown whether evolutionarily conserved mechanisms are at work or, instead, whether this is a case of evolutionary convergence (8). This issue is important, because upon its resolution depends the utility of the powerful invertebrate model organisms for understanding the mechanisms of the response to DR in mammals.
Considerable attention has been paid to the dietary components that are important for extension of life span by DR in rodents, where reduction of whole food intake can increase life span by approximately 40% (3). These studies have shown that altering the ratio of nutritional components, by reducing lipids, minerals, or vitamins in the diet, had no effect on rat life span, although reduction of the protein quantity or quality effected a relatively small increase (9–13). More recent work has shown that specific reduction of tryptophan (14) or methionine (15–17) can extend rodent life span to a similar magnitude as whole-food DR. On the one hand, these interventions with specific nutrients may reveal useful information about the mechanisms of whole-food DR; on the other hand, each intervention could operate through different molecular pathways to extend life span, thus revealing little or nothing about the mechanisms of whole-food DR (18). Similar debate exists over the potentially different mechanisms by which yeast replicative life span is increased when glucose is reduced from 2% to 0.05% (19) or from 2% to 0.5% (20,21). In Caenorhabditis elegans, several possible modes of life-span extension by food reduction exist as life span can be extended by dilution of the bacterial food source (22), complete removal of the bacterial food source (23,24), altering the strain of bacterium used in the worms’ diet (25,26), or using synthetic axenic media (27,28). To establish the mechanisms at work for any particular method of DR in any model organism, precise specification and, preferably, standardization of DR methods is desirable as a basis for intra- and interspecific comparisons.
DR is usually imposed in Drosophila by dilution of an agar-gelled food medium, which is always present in excess (29). In general, as food is diluted from a high concentration, life span increases to a peak at intermediate nutrient levels through DR, and then falls with further food dilution through starvation. It is generally assumed that the increase in life span with DR is a response to reduced nutrients. However, logically, it could just as well be a response to relief from a nonnutritional, toxic effect of the food (30). This is not an easy issue to address empirically, but some evidence can be drawn from parallel effects of diet on reproductive output, which can provide an independent indication of the effect of the diet on the organism’s nutritional status. In a manner similar to that for DR in worms and mice (22,31), a decrease in life span in response to increased nutrition should be accompanied by increased daily and lifetime fecundity (5,6). In contrast, increase in the concentration of a toxin would be expected to cause life span to decrease in parallel with a reduction or no increase in fecundity.
DR in Drosophila usually involves reduction of the yeast and sugar components of the diet (29), and yeast appears to account for the majority of the DR effect on life span (5,32). However, different laboratories use different sources of yeast and different concentrations of sugar, yeast, and agar for DR (5,6,33,34). Despite these differences, few laboratories have tested their diets to ensure that the effect of DR on lifespan in their experiments is a specific response to nutrition as evidenced by reduced reproductive output. To gauge the importance of these differences, and to establish a validated DR diet that should be reproducible between laboratories, we assembled a range of yeast-based diets and directly compared the life span and fecundity of flies in response to DR on each food type. Of the diets that we investigated, only one showed effects on survival and fecundity that is suitable for DR studies in Drosophila.
Methods
Fly Stocks, Maintenance and Handling Procedures
All experiments were performed with the wild type, outbred, laboratory strain Dahomey. The population is maintained in large population cages with overlapping generations on a 12-hour light/dark cycle at 25°C and 65% humidity.
Media
Rearing of flies and experiments were performed on standard sugar/yeast (SY) food (35). The arbitrary standard condition (1.0) is described in Table 1. In all cases, the food was prepared by adding the agar to water and bringing to a boil on a gas hob. At this point, the appropriate amounts of sugar and yeast (and cornmeal where indicated) were added with continuous stirring until the food was completely mixed. The food was then removed from the heat and allowed to cool to 65°C. At this point, preservatives were mixed in, and the food was dispensed. For the sugar range experiments, baker’s yeast (Table 1) was used. Media for the comparison of dietary yeasts were based on that in Table 1, with only the yeast component varied. For the water add-back experiment, a 1% agar solution was made (containing preservatives as for the SY media) and poured into individual 200-μL pipette tips. These tips were trimmed to a length that brought the agar solution close to the level of the food surface after being inserted into the food. A pipette tip filled with cotton wool, to prevent flies from crawling into the pipette tip and becoming trapped, was added to the control treatment.
Table 1. Recipe used to make food.
The values in this table describe the arbitrary reference condition (1.0) used in DR experiments and for rearing flies. Where indicated in the text, the yeast, sugar and agar concentrations were varied.
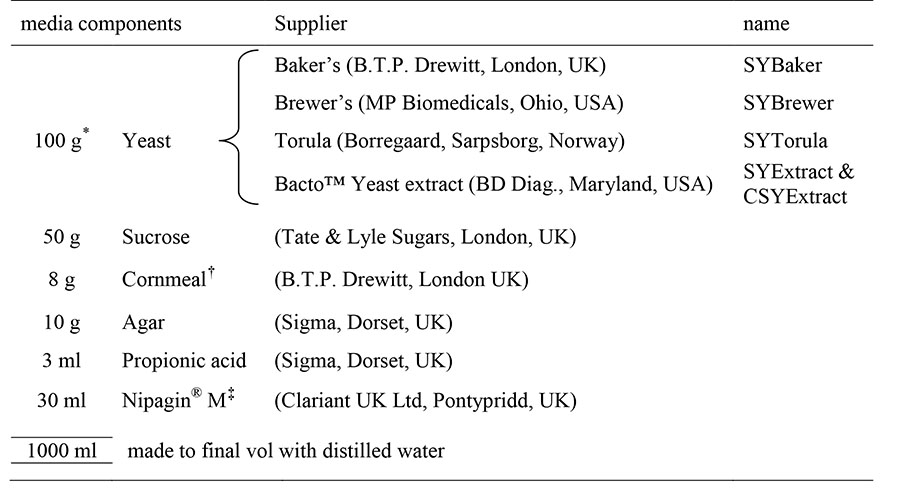
For yeast comparison experiments, the yeast concentration alone was varied from 10 g.l−1 (0.1) to 200 g.l−1 (2.0)
cornmeal (organic polenta) was used for the CSYExtract medium only
solution of 100 g.l−1 methyl 4-hydroxybenzoate in 95% ethanol
Life Span and Fecundity Assays
For life-span experiments, larvae were reared at standard density in 200-mL glass bottles containing 70 mL of 1.0 SY food (36). Flies emerged over 24 hours, were tipped into fresh bottles, and were allowed 48 hours to mate. Females were then separated from males under light CO2 anesthesia and randomly allocated to different food treatments at a density of 10 females per vial. Flies were transferred to fresh vials, and deaths were scored at least every 2 days. The yeast comparison experiment was performed in two batches, the first containing SYBaker’s, SYBrewer’s, and SYTorula, and the second containing SYBaker’s, SYBrewer’s, SYExtract, and CSYExtract. Due to the similarity between the two trials of SYBaker’s and SYBrewer’s (Supplementary Figure 1 and Supplementary Table 1), the data were combined. For each condition in each experiment, 100 flies were used.
For fecundity measurements, the same experimental flies as those used for life spans were kept in the same glass vials for between 18 and 24 hours; they were then transferred to fresh food. The eggs in the vacated vials were counted manually under a microscope. For the sugar concentration experiment, egg counts were performed on days 3, 7, 10, 14, and 21 of treatment. For the first yeast comparison experiment (SYBaker’s, SYBrewer’s, and SYTorula), eggs were counted on days 5, 9, 12, 16, 19, 23, 26, 30; for the second experiment (SYBaker’s, SYBrewer’s, SYExtract and CSYExtract), eggs were counted on days 4, 8, 11, 15, 18, 22, 25, and 29. Eggs were counted on days 3, 6, 10, 13, 17, 26, 31, and 38 for the water add-back experiment and on days 4, 11, 18, 25, 32, 46, and 60 for the agar concentration range experiment. As an index of lifetime fecundity, the sum of eggs laid during 24 hours on the days of counting by an average female was calculated. These sampling points cover the period of heaviest laying, and are therefore indicative of relative lifetime fecundity (6).
Data Analyses
Comparison of survivorship data was performed using the log-rank test implemented in Excel. Values of p from comparisons of fecundity data refer to the nonparametric Wilcoxon rank sum test performed in R, v2.2.1 (37). For the more complex comparisons of fecundity data illustrated in Figure 2, the nlme package in R was used (38), specifying a mixed model with yeast type, yeast concentration, and the quadratic function of concentration as fixed terms. Replicate vials were included as a random variable to compensate for multiple females per vial. To deal with the observed increasing variance with increasing fitted values (heteroscedasticity), we modeled the variance as a power function of the fitted values (such weighting of the variance structure improved the fit of the model, although it did not change the results). All factors and interactions were significant. Modeled versus actual data are shown in Supplementary Figure 2.
Figure 2.
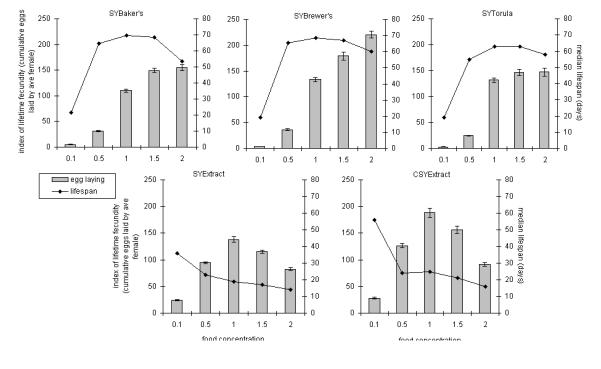
Effect of a range of concentrations of different commercially available yeasts on life span and fecundity. Five different yeast concentrations were prepared for each of five different sugar/yeast (SY) recipes. SYBaker’s, SYBrewer’s, and SYTorula each refer to food made with different, inactivated whole-yeast preparations, whereas SYExtract and CSYExtract refer to diets based on a water-soluble yeast extract. The nutritional components in each food type were sucrose and yeast or yeast extract and cornmeal (for CSYExtract only). Bars: index of lifetime fecundity ± standard error of the mean; connected black points: median life-span values. We specified a linear model to describe fecundity (Materials and Methods), which found all factors and interactions to be significant. The predicted values are plotted against observed values in Supplementary Figure 2. Each food concentration range was performed once, except for SYBaker’s and SYBrewer’s, which were performed twice.
Nutritional Analysis of Yeast
Chemical analysis of a sample of baker’s yeast was performed by Leatherhead Food International (Somerset, U.K.).
Results
High Levels of Dietary Sucrose Adversely Affect Fecundity With Little Effect on Life Span
Although it has been shown that the yeast component of an SY diet is critical for the response to DR in Drosophila, sucrose could also produce life-shortening effects similar to those of yeast if raised to sufficiently high concentrations [i.e., higher than those used previously (32)]. To test this, we looked at the effect of varying the sucrose concentration in the diet while keeping all other ingredients at a fixed level.
Interestingly, there was no requirement for dietary sucrose for maximum fecundity and, surprisingly, addition of sucrose at ≥ 100 g/L caused a decrease in female fecundity (p < .00002, Wilcoxon rank sum test), indicating that it had a detrimental effect on fly physiology and/or behavior. To ensure that nutrition, and therefore DR, is the key determinant of life span, fecundity should increase for increases in nutrition that cause life span to decrease. These data, therefore, show that sucrose concentrations > 50 g/L are not appropriate for DR studies. For optimum longevity, the flies required the level of dietary sucrose to be at least 50 g/L in an SY diet. This effect of sucrose is shown in Figure 1 as a small, but significant, increase in median life span when sucrose was added to a yeast-only diet (50 g/L vs 0 g/L; p < .00001, log-rank test). Raising the sucrose concentration further to 150 g/L caused no decrease in median life span in this experiment, but it has done so in other experiments that we have performed [data not shown and (32)]. As a result, further experiments reported herein used a fixed sucrose concentration of 50 g/L as this was neither detrimental to life span nor inhibitory to egg laying.
Figure 1.

Effect of dietary sucrose concentration on life span and fecundity of mated Drosophila females. Increasing concentrations of sucrose were added to a standard food background of 1.5 SYBaker’s (Table 1). Over the range of sucrose tested, very little change in life span was observed, whereas a significant decrease in fecundity was observed between 50 g/L and 100 g/L sucrose. Gray bars: index of lifetime fecundity (sum of the eggs laid by an average female on the days counted) ± standard error of the mean; connected black points: median life span. Representative data from one of two experiments are shown.
Varying the Quality of the Yeast Supply Produces a Range of Effects on Life Span and Fecundity
The above data and (32) show that DR in Drosophila is achieved solely by modulating the yeast component of the diet. We next compared a variety of different yeasts to determine their effects on life span and fecundity. These experiments included four sources of inactivated yeast: a baker’s yeast, a brewer’s yeast, a torula yeast, and a water-soluble extract of baker’s yeast. The first three of these yeasts are whole-cell lysates, whereas the fourth is a purified extract. Each of the yeasts was used over a range of concentrations from 10 g/L (labeled 0.1) to 200 g/L (labeled 2.0) while the other media constituents were held constant (Table 1).
Comparison of the three whole-yeast food types (SYBaker’s, SYBrewer’s, and SYTorula) showed a similar pattern for median life span, with a peak at 1.0 (100 g/L) and a decline as food concentration was changed above or below this point (top three graphs of Figure 2). SYBaker’s and SYBrewer’s yielded the longest life spans (69- and 70-day medians, respectively, on 1.0 food), whereas the longest life span on SYTorula (63-day median at 1.0) was significantly shorter (p < .0001 in both comparisons, log-rank test). For each of these three yeasts, lifetime fecundity increased with increasing food concentration to 1.5, above which there was no further increase for SYBaker’s and SYTorula, but there was for SYBrewer’s when the concentration was raised from 1.5 to 2.0 (Supplementary Figure 2). Furthermore, the level of egg laying on 2.0 SYBrewer’s was higher than the peak value for any of the other food types tested. Thus, the observed limit to egg laying on the other food types was not intrinsic to the physiology of the flies, but was restricted by some feature of the foods. In other experiments we have also raised the yeast concentration in SYBrewer’s medium to 300 g/L (3.0) and saw a further lifespan shortening from 2.0 (p < .05, log-rank test). However, this was not accompanied by a further increase in fecundity beyond the level in 2.0 (p = .53, Wilcoxon rank sum test; Supplementary Figure 3).
The flies responded differently to the yeast-extract–based media. The most obvious difference was that life span decreased for each addition of yeast extract to the medium. This was similar for CSYExtract and SYExtract (bottom two graphs of Figure 2), except for 0.1, at which level cornmeal addition resulted in a significantly longer life span (36 days on SYExtract vs 56 days on CSYExtract; p < .0001, log-rank test). Because the positive effect of cornflour on life span was only seen at the lowest concentration of yeast extract (0.1) and the longest life span on 0.1 SYExtract was low compared with all other treatments, the data are compatible with an argument that yeast extract caused dose-dependent toxicity. The pattern of lifetime fecundity was similar between SYExtract and CSYExtract, increasing with yeast extract addition to a maximum at 1.0, but decreasing at higher concentrations. Cornmeal addition augmented egg laying, which peaked in 1.0 CSYExtract at a level similar to that in 1.5 SYBrewer’s and higher than the maxima for the other three food types. In both the presence and absence of cornmeal, yeast extract was apparently more nutritionally dense than whole-yeast powders, because egg laying was greater on CSYExtract (up to 1.0) and SYExtract (up to 0.5) than on the whole-yeast diets at corresponding food concentrations. However, fecundity decreased for additions of yeast extract higher than 100 g/L (1.0). Thus, yeast extract at high concentrations is detrimental to fecundity in addition to negatively affecting life span.
Is DR in Drosophila a Nutritional Response?
In order to fulfill the requirements for DR, it is necessary that the longer-lived (restricted) animals are not simply less sick than those with higher nutritional intake. One indication of this comes from increased fecundity with increasing nutrients. However, if the food delivers both nutrients to benefit fecundity as well as a toxic effect that reduces life span, the phenotype would be indistinguishable from a true effect of DR (30). It is therefore important to try and distinguish directly between a toxin-based and a nutrient-based explanation for the life span–shortening effect of the high nutrient concentration
Increasing the food concentration could mimic a DR effect by increasing the hardness of the food. To test this possibility, we fixed the concentration of all food ingredients (at 2.0 SYBrewer’s) and varied the agar concentration on its own (Figure 3). For each increase in agar concentration, there was a trend toward a decrease in lifetime fecundity. However, this trend was only significant for the increase from 10 g/L to 15 g/L (p < .0005, Wilcoxon rank sum test). This reduction was accompanied by a significant increase in life span when the agar concentration was raised from 10 g/L to 15 g/L (p < .01, log-rank test) and a further, nonsignificant (p = .09, log-rank test) increase when agar was raised to 20 g/L. These data are consistent with agar controlling food availability in a nondetrimental way between 10 g/L and 20 g/L agar, and is therefore explicable as a DR effect. When the agar concentration was further increased to 25 g/L there was no change in median life span or lifetime fecundity, but maximum life span decreased from a median of 76 days to 71 days (Figure 3). This result argues that older flies do indeed differentially suffer if the food becomes sufficiently hard, but for agar concentrations < 20 g/L food hardness does not on its own cause the life-shortening (DR) effect seen in Figure 2. We also tested the effect of agar concentration for 1.0 SYBrewer’s and 3.0 SYBrewer’s (data not shown). Although qualitatively similar, this experiment also revealed an interaction with the yeast concentration, whereby flies were more sensitive to higher agar concentrations at higher yeast concentrations. This result indicates that the yeast content of the food can contribute to overall food hardness and adversely affect life span.
Figure 3.
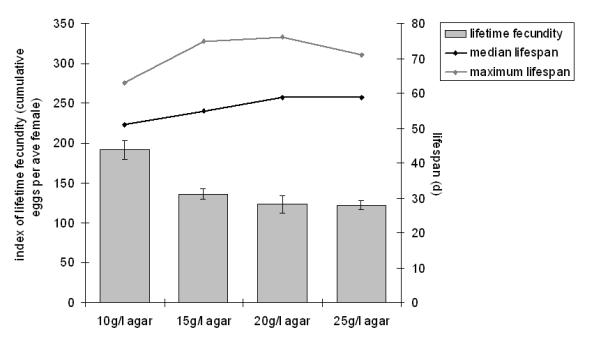
Effect of varying agar concentration on life span and fecundity of females on SYBrewer’s medium. The effect of food hardness on life span and fecundity was tested by altering the agar concentration while all other ingredients were held at fixed concentrations (Table 1). This medium contained Brewer’s yeast at 200 g/L (2.0 level) (agar concentration ranges were also tested at two other SYBrewer’s concentrations; data not shown). Bars: index of lifetime fecundity ± standard error of the mean; connected black points: median life span; connected gray points: maximum life span (median of the last 10% survivorship).
Another possible detrimental effect of high food concentrations concerns water availability, because the food is the only source of water. We therefore tested if water addition could overcome the adverse effects of high nutrition levels on life span. Figure 4 shows that addition of a fresh source of water to 1.0 and 2.0 SYBrewer’s could not rescue the life-shortening effect of high nutrient concentrations and had no effect on lifetime fecundity. Therefore, inability to access sufficient free water does not explain the life span–shortening effect accompanying high nutrient concentrations in the food.
Figure 4.
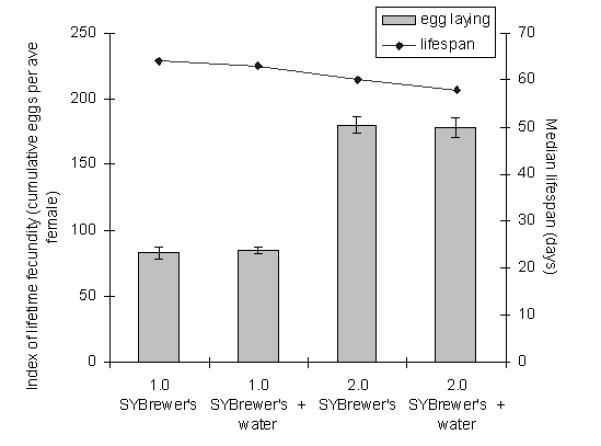
Effect of water addition on the dietary restriction (DR) response of flies on SYBrewer’s medium. Free access to water was provided in the form of 1% agar in a pipette tip inserted into the food. Bars: index of lifetime fecundity ± standard error of the mean; connected points: median life span. Experiment was performed twice.
Discussion
DR is a well-established intervention for extending fly life span. Indeed, the interaction among diet, life span, and fecundity has formed the basis for both practical and theoretical investigations into the possible trade-offs between these life-history traits (39). Here we have investigated DR more closely and found that, without careful attention to the food composition, studies that claim to be examining extended life span due to DR may simply be studying the rescue of normal life span from the effects of inappropriate food types that prematurely shorten life. It thus follows that any mechanistic conclusions drawn from such studies are likely to be obscured by the detrimental effects of the food and so would be inappropriate to address questions of how DR operates to preserve life span for Drosophila or other species.
Drosophila in the wild is thought to coconsume fruit material and microbes from fermenting and/or rotting fruit (40). In the laboratory, Drosophila can be maintained on a combination of sugar, yeast, and water (35). We found that addition of sugar > 50 g/L to the culture medium was detrimental for egg laying and that variations from 0 to 150 g/L had little effect on life span (Figure 1). These data indicate that Drosophila has a very low requirement for free sugar for maximal life span and fecundity, consistent with the finding that total sugar levels in rotting banana are no more than 20 mM (equivalent to 4.5 g/L sucrose) (41). Other experiments have shown that Drosophila modulate their feeding behavior only slightly, or not at all, when sucrose levels rise above 50 g/L (32,42,43). Thus, the dramatically lowered egg laying observed with high sugar is unlikely to be an effect of reduced feeding in response to the altered sucrose concentration, and instead probably reflects an adverse effect on physiology due to the presence of unnaturally high sugar levels. These data show that high sugar should be avoided in Drosophila DR experiments.
In contrast, increasing additions of one particular brewer’s yeast caused lifetime fecundity to continually increase over a concentration range that also decreased life span and so conformed to the expectations of a DR treatment. When recently changing our yeast supplier, we noted a shift in the concentration at which life span peaked from 65 g/L yeast [0.65 in (32)] to 100 g/L (1.0 shown here). Yeast quality is thus highly variable. Furthermore, high yeast concentrations that reduce life span are not always associated with increasing fecundity. This fact is at odds with the recognized effect of DR on fecundity in worms (22) and rodents (31), and is consistent with an explanation that the lifespan decrease on high food concentrations is not an effect of increased nutrition, but due to some detrimental effect of the yeast composition. This could be caused by either a direct effect of a specific toxic element whose increasing concentration reduces life span and perhaps also fecundity or an indirect effect of a nutritionally imbalanced diet that results in ill health.
Under the first explanation, one would expect a pattern of fecundity and life span similar to that seen for the flies fed increasing concentrations of yeast extract. In this situation, both nutrients and the toxin (e.g., a heavy metal) are delivered in the food. This situation results in increasing fecundity as nutrients increase and toxicity remains below a tolerable threshold (e.g., 1.0 in SYExtract and CSYExtract in Figure 2), beyond which fecundity is reduced. For this same concentration range, life span would be ever decreasing. This explanation is consistent with data for C. elegans grown on different types of bacteria. It is currently common practice to grow worms on Escherichia coli, which can support growth and reproduction and upon dilution elicit an apparent DR response (22). However, when the worms are grown on the soil bacterium Bacillus Subtilis, their life span is increased some 50% without changes in development time or reproductive output (26). Thus, any nutrient-dependent life-span shortening when increasing the concentrations of E. coli for worms or yeast extract for flies would be combined with the effects of food toxicity.
In contrast, nutritional imbalance would be expected to yield a life-history pattern like that for SYBaker’s and SYTorula, where the absence of a nutritional component imposes a limit on egg-laying capacity due to depletion from parental reserves. Previous data on the nutritional requirements of adult Drosophila showed that deficiencies for essential amino acids, chloride, phosphorous, or calcium reduced egg laying within 16 days, with little effect on the short-term viability of the adult (44). Thus a trace element shortfall may limit lifetime egg-laying capacity with little effect on immediate risk of death. An example of this phenotype is shown for flies on 1.5 and 2.0 SYBaker’s, which have the same level of lifetime fecundity but markedly different life spans (Figure 2). Because they both experience the same limitation to lifetime fecundity, the limitation in itself is not what causes shortened life span on 2.0. Rather, the increasing excess of other dietary components, and so nutrient imbalance, is the most likely explanation for the elevated mortality.
In an attempt to identify any such toxins or nutrient imbalances, we have compared the available nutritional data for each of the yeast types used (Supplementary Table 2). Unfortunately, these analyses have a limited scope because only standard nutritional constituents are measured; therefore, many potentially toxic compounds will be overlooked. It is possible, however, to compare nutrient ratios among yeasts. In this light it is notable that several vitamins are an order of magnitude lower in concentration in SYBaker’s than in SYBrewer’s. These vitamins include biotin, a deficiency of which is thought to shorten Drosophila life span (45). This could be tested by the addition of these vitamins to the food to see if they rescue fecundity and affect life span. As a note, it is possible that similarly subtle effects of food type belie unknown nutrient imbalances in DR experiments that have been performed in other model organisms. For example, rescue from a nutrient imbalance could explain the life-span extension found in rats when the dietary protein source casein was replaced with soy protein (12). Subtle differences in food affecting life span have also been demonstrated by experiments on mice and rats subjected to methionine restriction (15,17,46). Thus, diet optimization is also an important consideration for DR studies in rodents, in which food composition varies depending on the particular commercially available chow that is used.
Despite all these precautions to establish a diet suitable for Drosophila DR, it is still possible that the food could have a detrimental effect on life span unrelated to nutrition and with no adverse effect on lifetime fecundity, thus mimicking the DR effect. Because we use a food dilution method for DR, the hardness of the food and water availability are the most likely candidates to produce such an effect. Our experiments showed that neither could account for the life-span shortening seen when varying the yeast concentration. We did note, however, a detrimental effect on maximum life span when agar concentration was raised to an extremely high level (25 g/L, more than twice that used for our other experiments). This effect was exacerbated when the yeast concentration was also raised to 300 g/L, showing that food hardness can reduce Drosophila life span. This non-DR–based life-shortening effect of hard food is likely to have contributed heavily to the life-span shortening seen in studies when yeast and sugar are both raised to 300 g/L and agar to 20 g/L (34,47) (Supplementary Figure 3).
Based on the data presented above, we conclude that the brewer’s yeast is the most suitable of those that we tested for DR studies and it now forms the basis for our laboratory recipes. This change has the additional advantage of bringing the nutritional content of our fly diet in line with that of two other laboratories studying fly DR using the same yeast (Helfand and Pletcher laboratories, Scott Pletcher, personal communication, 2005 ). We are now in the process of extending this study by applying this DR regimen to male flies as well as a variety of commonly used laboratory strains of Drosophila. As there is an impact of genotype on the fly response to DR (48), it will be interesting to see if other laboratory strains (both inbred and outbred) exhibit a similar response to these foods. Modulations or even loss of the DR response in these lines may be informative about the mechanisms of DR.
In conclusion, this work highlights the need for validated diets used for DR as a step toward establishing some dietary uniformity in the DR community to allow direct comparison of different experiments with the same species and of different species. For flies, the dramatic variability in quality of yeasts from different suppliers, and presumably between seasons, points to the need for a defined synthetic medium that would avoid the potential problems of unwanted detrimental effects being introduced into Drosophila experiments from the yeast or its feedstock.
Supplementary Material
Supplementary Figure 1. Data for both trials run for SYBaker’s and SYBrewer’s. These are independently replicated data sets from nonoverlapping generations of flies.
Supplementary Figure 2. Model predictions versus actual egg-laying data reported in Figure 2. Model predictions are represented by the lines and actual data by symbols. All fixed terms (yeast type, concentration and the quadratic term for concentration) and interactions were significant.
Supplementary Figure 3. Effect of raising yeast concentration in SYBrewer’s above the range used for dietary restriction (DR). Yeast concentration was raised to 300 g/L, and life span and egg laying were monitored. Whereas life span showed a significant decline from that found at 2.0 SYBrewer’s, egg laying was not further increased, indicating that the flies did not experience a higher level of nutrition. Agar concentration was 15 g/L.
Acknowledgments
This work was supported by the Wellcome Trust Functional Genomic Analysis of Ageing Grant (MP and LP), the Medical Research Council (RW), Research into Ageing (RG), and the Biotechnology and Biological Science Research Council (LP and TB).
We also acknowledge the contribution by Will Mair in the early stages of this project and Bregje Wertheim for her assistance with statistical analyses.
REFERENCES
- 1.McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of lifespan and upon the ultimate body size. J Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- 2.Holehan AM, Merry BJ. The experimental manipulation of ageing by diet. Biol Rev Camb Philos Soc. 1986;61:329–368. doi: 10.1111/j.1469-185x.1986.tb00658.x. [DOI] [PubMed] [Google Scholar]
- 3.Weindruch R, Walford RL. The Retardation of Aging and Disease by Dietary Restriction. Thomas; Springfield, IL: 1988. [Google Scholar]
- 4.Masoro EJ, Shimokawa I, Yu BP. Retardation of the aging processes in rats by food restriction. Ann N Y Acad Sci. 1991;621:337–352. doi: 10.1111/j.1749-6632.1991.tb16990.x. [DOI] [PubMed] [Google Scholar]
- 5.Chippindale AK, Leroi AM, Kim SB, Rose MR. Phenotypic plasticity and selection in Drosophila life-history evolution. I. Nutrition and the cost of reproduction. J Evol Biol. 1993;6:171–193. [Google Scholar]
- 6.Chapman T, Partridge L. Female fitness in Drosophila melanogaster: an interaction between the effect of nutrition and of encounter rate with males. Proc R Soc Lond B Biol Sci. 1996;263:755–759. doi: 10.1098/rspb.1996.0113. [DOI] [PubMed] [Google Scholar]
- 7.Magwere T, Chapman T, Partridge L. Sex differences in the effect of dietary restriction on life span and mortality rates in female and male Drosophila melanogaster. J Gerontol A Biol Sci Med Sci. 2004;59:3–9. doi: 10.1093/gerona/59.1.b3. [DOI] [PubMed] [Google Scholar]
- 8.Partridge L, Brand MD. Special issue on dietary restriction. Dietary restriction, longevity and ageing--the current state of our knowledge and ignorance. Mech Ageing Dev. 2005;126:911–912. [Google Scholar]
- 9.Yu BP, Masoro EJ, Murata I, Bertrand HA, Lynd FT. Life span study of SPF Fischer 344 male rats fed ad libitum or restricted diets: longevity, growth, lean body mass and disease. J Gerontol. 1982;37:130–141. doi: 10.1093/geronj/37.2.130. [DOI] [PubMed] [Google Scholar]
- 10.Yu BP, Masoro EJ, McMahan CA. Nutritional influences on aging of Fischer 344 rats: I. Physical, metabolic, and longevity characteristics. J Gerontol. 1985;40:657–670. doi: 10.1093/geronj/40.6.657. [DOI] [PubMed] [Google Scholar]
- 11.Iwasaki K, Gleiser CA, Masoro EJ, McMahan CA, Seo EJ, Yu BP. Influence of the restriction of individual dietary components on longevity and age-related disease of Fischer rats: the fat component and the mineral component. J Gerontol. 1988;43:13–21. doi: 10.1093/geronj/43.1.b13. [DOI] [PubMed] [Google Scholar]
- 12.Iwasaki K, Gleiser CA, Masoro EJ, McMahan CA, Seo EJ, Yu BP. The influence of dietary protein source on longevity and age-related disease processes of Fischer rats. J Gerontol. 1988;43:5–12. doi: 10.1093/geronj/43.1.b5. [DOI] [PubMed] [Google Scholar]
- 13.Masoro EJ, Iwasaki K, Gleiser CA, McMahan CA, Seo EJ, Yu BP. Dietary modulation of the progression of nephropathy in aging rats: an evaluation of the importance of protein. Am J Clin Nutr. 1989;49:1217–1227. doi: 10.1093/ajcn/49.6.1217. [DOI] [PubMed] [Google Scholar]
- 14.De Marte ML, Enesco HE. Influence of low tryptophan diet on survival and organ growth in mice. Mech Ageing Dev. 1986;36:161–171. doi: 10.1016/0047-6374(86)90017-5. [DOI] [PubMed] [Google Scholar]
- 15.Orentreich N, Matias JR, DeFelice A, Zimmerman JA. Low methionine ingestion by rats extends life span. J Nutr. 1993;123:269–274. doi: 10.1093/jn/123.2.269. [DOI] [PubMed] [Google Scholar]
- 16.Richie JP, Jr, Leutzinger Y, Parthasarathy S, Malloy V, Orentreich N, Zimmerman JA. Methionine restriction increases blood glutathione and longevity in F344 rats. FASEB J. 1994;8:1302–1307. doi: 10.1096/fasebj.8.15.8001743. [DOI] [PubMed] [Google Scholar]
- 17.Zimmerman JA, Malloy V, Krajcik R, Orentreich N. Nutritional control of aging. Exp Gerontol. 2003;38:47–52. doi: 10.1016/s0531-5565(02)00149-3. [DOI] [PubMed] [Google Scholar]
- 18.Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Kaeberlein M, Hu D, Kerr EO, et al. Increased life span due to calorie restriction in respiratory-deficient yeast. PLoS Genet. 2005;1:e69. doi: 10.1371/journal.pgen.0010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin SJ, Kaeberlein M, Andalis AA, et al. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- 21.Lin SJ, Guarente L. Increased life span due to calorie restriction in respiratory-deficient yeast. PLoS Genet. 2006;2:e33. doi: 10.1371/journal.pgen.0020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klass MR. Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing life span. Mech Ageing Dev. 1977;6:413–429. doi: 10.1016/0047-6374(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 23.Kaeberlein TL, Smith ED, Tsuchiya M, et al. Lifespan extension in Caenorhabditis elegans by complete removal of food. Aging Cell. 2006;5:487–494. doi: 10.1111/j.1474-9726.2006.00238.x. [DOI] [PubMed] [Google Scholar]
- 24.Lee GD, Wilson MA, Zhu M, et al. Dietary deprivation extends lifespan in Caenorhabditis elegans. Aging Cell. 2006;5:515–524. doi: 10.1111/j.1474-9726.2006.00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garsin DA, Sifri CD, Mylonakis E, et al. A simple model host for identifying Gram-positive virulence factors. Proc Natl Acad Sci U S A. 2001;98:10892–10897. doi: 10.1073/pnas.191378698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garsin DA, Villanueva JM, Begun J, et al. Long-lived C. elegans daf-2 mutants are resistant to bacterial pathogens. Science. 2003;300:1921. doi: 10.1126/science.1080147. [DOI] [PubMed] [Google Scholar]
- 27.Vanfleteren JR, Braeckman BP. Mechanisms of life span determination in Caenorhabditis elegans. Neurobiol Aging. 1999;20:487–502. doi: 10.1016/s0197-4580(99)00087-1. [DOI] [PubMed] [Google Scholar]
- 28.Walker G, Houthoofd K, Vanfleteren JR, Gems D. Dietary restriction in C. elegans: from rate-of-living effects to nutrient sensing pathways. Mech Ageing Dev. 2005;126:929–937. doi: 10.1016/j.mad.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 29.Partridge L, Piper MD, Mair W. Dietary restriction in Drosophila. Mech Ageing Dev. 2005;126:938–950. doi: 10.1016/j.mad.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 30.Piper MD, Partridge L. Dietary restriction in Drosophila: delayed aging or experimental artefact? PLoS Genet. 2007;3:e57. doi: 10.1371/journal.pgen.0030057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holehan AM, Merry BJ. Lifetime breeding studies in fully fed and dietary restricted female CFY Sprague-Dawley rats. 1. Effect of age, housing conditions and diet on fecundity. Mech Ageing Dev. 1985;33:19–28. doi: 10.1016/0047-6374(85)90106-x. [DOI] [PubMed] [Google Scholar]
- 32.Mair W, Piper MD, Partridge L. Calories do not explain extension of lifespan by dietary restriction in Drosophila. PLoS Biol. 2005;7:e223. doi: 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bross TG, Rogina B, Helfand SL. Behavioral, physical, and demographic changes in Drosophila populations through dietary restriction. Aging Cell. 2005;4:309–317. doi: 10.1111/j.1474-9726.2005.00181.x. [DOI] [PubMed] [Google Scholar]
- 35.Ashburner M. Drosophila. A Laboratory Manual. Cold Spring Harbor Press; New York: 1989. Appendix N: food media; pp. 399–402. [Google Scholar]
- 36.Clancy DJ, Kennington WJ. A simple method to achieve consistent larval density in bottle cultures. Dros Inf Serv. 2001;84:168–169. [Google Scholar]
- 37.R Development Core Team . R: A language and environment for statistical computing (2.2.1) R Foundation for Statistical Computing; Vienna, Austria: 2005. [Google Scholar]
- 38.Pinheiro J, Bates D, DebRoy S, Sarkar D. nlme: Linear and non-linear mixed effects models. R package version 3.1-74 2006.
- 39.Barnes AI, Partridge L. Costing reproduction. Anim Behav. 2003;64:199–204. [Google Scholar]
- 40.Spieth HT. Courtship behaviour in Drosophila. Annu Rev Entomol. 1974;19:385–405. doi: 10.1146/annurev.en.19.010174.002125. [DOI] [PubMed] [Google Scholar]
- 41.Omura H, Honda K. Feeding responses of adult butterflies, Nymphalis xanthomelas, Kaniska canace and Vanessa indica, to components in tree sap and rotting fruits: synergistic effects of ethanol and acetic acid on sugar responsiveness. J Insect Physiol. 2003;49:1031–1038. doi: 10.1016/j.jinsphys.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 42.Edgecomb RS, Harth CE, Schneiderman AM. Regulation of feeding behavior in adult Drosophila melanogaster varies with feeding regimen and nutritional state. J Exp Biol. 1994;197:215–235. doi: 10.1242/jeb.197.1.215. [DOI] [PubMed] [Google Scholar]
- 43.Carvalho GB, Kapahi P, Benzer S. Compensatory ingestion upon dietary restriction in Drosophila melanogaster. Nat Methods. 2005;2:813–815. doi: 10.1038/nmeth798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sang JH, King RC. Nutritional requirements of axenically cultured Drosophila melanogaster adults. J Exp Biol. 1961;38:793–809. [Google Scholar]
- 45.Landenberger A, Kabil H, Harshman LG, Zempleni J. Biotin deficiency decreases life span and fertility but increases stress resistance in Drosophila melanogaster. J Nutr Biochem. 2004;15:591–600. doi: 10.1016/j.jnutbio.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 46.Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, Smith-Wheelock M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell. 2005;4:119–125. doi: 10.1111/j.1474-9726.2005.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng J, Mutcherson R, Helfand SL. Calorie restriction delays lipid oxidative damage in Drosophila melanogaster. Aging Cell. 2005;4:209–216. doi: 10.1111/j.1474-9726.2005.00159.x. [DOI] [PubMed] [Google Scholar]
- 48.Clancy DJ, Gems D, Hafen E, Leevers SJ, Partridge L. Dietary restriction in long-lived dwarf flies. Science. 2002;296:319. doi: 10.1126/science.1069366. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Data for both trials run for SYBaker’s and SYBrewer’s. These are independently replicated data sets from nonoverlapping generations of flies.
Supplementary Figure 2. Model predictions versus actual egg-laying data reported in Figure 2. Model predictions are represented by the lines and actual data by symbols. All fixed terms (yeast type, concentration and the quadratic term for concentration) and interactions were significant.
Supplementary Figure 3. Effect of raising yeast concentration in SYBrewer’s above the range used for dietary restriction (DR). Yeast concentration was raised to 300 g/L, and life span and egg laying were monitored. Whereas life span showed a significant decline from that found at 2.0 SYBrewer’s, egg laying was not further increased, indicating that the flies did not experience a higher level of nutrition. Agar concentration was 15 g/L.


