Abstract
Sex differences in longevity and aging are seen throughout the animal kingdom. These are likely to result, in part, from sex differences in endocrinology. In the nematode C. elegans, males are the longer-lived sex. Here we explore the possibility that sex differences in insulin/IGF-1 and steroid endocrinology contribute to this sex difference in aging, studying C. elegans populations in liquid culture. We report that in hermaphrodite populations, mutational loss of the DAF-12 steroid receptor affected lifespan as in previous plate culture studies: mutant longevity is suppressed in a weak daf-2 insulin/IGF-1 receptor mutant, but enhanced in a stronger daf-2 mutant. However, in males mutation of daf-12 had little effect on aging in either weak or strong daf-2 mutants. Moreover, while mutation of daf-12 marginally reduced lifespan in daf-2(+) hermaphrodites, as in plate cultured populations, it did not in daf-2(+) males. These results could imply that in C. elegans, as in mammals, sex differences in steroid endocrinology contribute to sex differences in aging.
Keywords: C. elegans, aging, gender, steroid, insulin/IGF-1 signaling
INTRODUCTION
A salient feature of human aging is that women age slightly more slowly than men. In developed country this leads to a gender gap in mean lifespan of some 4 - 6 years1. Sex differences in aging are typical of animal species, but their biological basis remains poorly understood2. Many aspects of sexual dimorphism are specified by sex differences in endocrine function, and this may apply to aging too. For example, the shorter lifespan of men is thought to arise, in part, from the effects of testicular hormones (e.g. the steroid hormone testosterone) on behavior and on cardiovascular health1. While in many animal species, females are longer lived, in the nematode Caenorhabditis elegans the male is the longer lived sex, living some 20% longer than hermaphrodites3.
Several endocrine pathways are known to influence longevity and aging in C. elegans. Insulin/IGF-1 signaling (IIS) shortens lifespan4 and mutation of the daf-2 insulin/IGF-1 receptor gene can more than double adult lifespan5. Lifespan is also modulated by a bile acid-like steroid hormone which acts via the nuclear receptor DAF-126, 7. The interplay between insulin-like and steroid pathways in the control of aging is complex. In adult hermaphrodites, mutational inactivation of daf-12 slightly shortens lifespan in a daf-2(+) background, partially suppresses the longevity phenotype of weaker daf-2 mutants, but enhances that of more severe daf-2 mutants8, 9. Thus, the DAF-12-mediated steroid pathway can increase or decrease lifespan, depending on the genetic context.
In this study, we explore the possibility that steroid hormone biology in C. elegans might, like that of higher animals, be sexually dimorphic and contribute to sex differences in aging. To do this, we have directly compared the effect on lifespan in males and hermaphrodites of mutation of daf-12, either alone or in combination with mutations affecting daf-2. We report that the effects of daf-12 on aging differ substantially between the sexes.
EXPERIMENTAL METHODS
Nematodes were raised on NGM agar seeded with Escherichia coli OP50 bacteria, as previously described10. Measurement of lifespan in C. elegans males is complicated by male-male interactions which shorten lifespan, and frequent escape of males from agar plates3. Therefore, for this study, lifespan was measured in monoxenic liquid culture with E. coli OP50, with animals maintained individually in microtitre wells, as previously described11.
The three mutant alleles studied were as follows. daf-12(m20) results in a dauer defective phenotype, and has a stop codon near the 5′ end of the open reading frame, and therefore approximates to a null allele6. daf-2(m41) and daf-2(e1370) are both hypomorphic alleles resulting in constitutive formation of dauer larvae, and increased adult longevity. m41 is a less pleiotropic (class 1) allele with a missense mutation in the Cys-rich region of the receptor, while e1370 is a more pleiotropic (class 2) allele with a missense mutation in the kinase region of the receptor12, 13.
RESULTS AND DISCUSSION
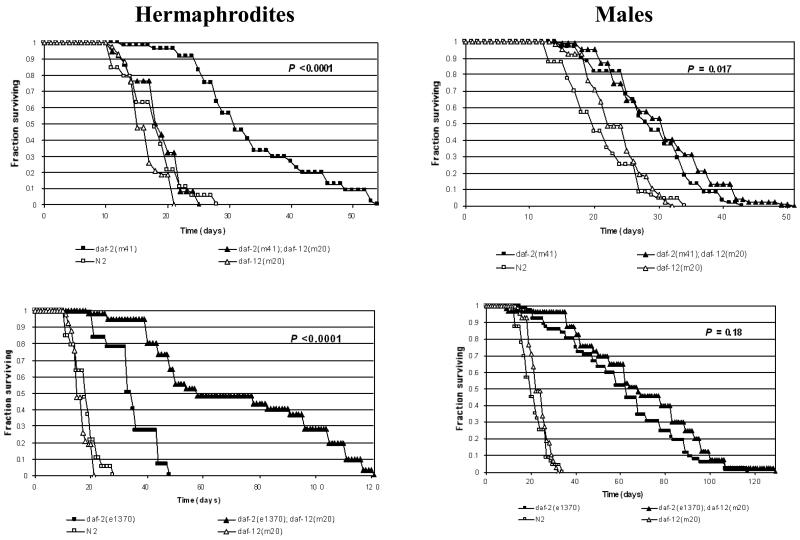
We compared the effects on aging in both sex of mutations of daf-12, alone or in combination with mutation of daf-2. In hermaphrodites, daf-12(m20) alone caused a marginal, non-significant reduction in lifespan (TABLE 1, FIGURE 1). This is consistent with earlier studies using agar plates, where daf-12 significantly shortened hermaphrodite lifespan8, 9. daf-12 also suppressed the increased longevity (Age phenotype) of the less pleiotropic (class 1) mutant daf-2(m41), but enhanced that of the more pleiotropic (class 2) mutant daf-2(e1370) (TABLE 1, FIGURE 1). These results also tally with earlier studies of plate cultured populations, where longevity of class 1 daf-2 alleles was suppressed and that of class 2 daf-2 alleles enhanced8,9. The only difference from earlier studies is that here daf-12 fully suppressed daf-2(m41) Age, whereas previously on partial suppression was seen. However, in a subsequent test of the effect of daf-12 on class 1 daf-2 lifespan in liquid culture we saw only partial suppression of Age (data not shown). Thus, the use of liquid culture rather than plate culture does not appear to markedly alter the effects on aging of mutation of daf-12 or daf-2, alone or in combination.
TABLE 1. Effect of daf-12 on lifespan in both sexes and in daf-2(+) and daf-2 mutant backgrounds.
| Genotype/gender | Median lifespan (days) ± 95% C.I. | % effect of daf-12 on median | Maximum lifespan (days) | % effect of daf-12 on maximum | N* | P † |
|---|---|---|---|---|---|---|
| + H1 | 17.7 (19.0, 16.5) | ---- | 23.0 | ---- | 165 (200) | ---- |
| + M2 | 19.8 (21.3, 18.3) | ---- | 29.0 | ---- | 135 (211) | ---- |
| daf-12(m20) H | 16.0 (17.5, 15.0) | −10 | 20.0 | −13 | 88 (151) | 0.56 |
| daf-12(m20) M | 23.5 (24.5, 22.1) | + 19 | 29.5 | +2 | 131 (152) | 0.07, 0.0001 |
| daf-2(m41) H | 31.8 (36.0, 28.8) | ---- | 51.5 | ---- | 44 (80) | ---- |
| daf-2(m41) M | 29.0 (32.0, 26.5) | ---- | 41.5 | ---- | 59 (80) | ---- |
| daf-2(m41); daf-12 H ‡ | 18.0 (20.0, 18.0) | −43 | 22.0 | −57 | 37 (80) | <0.0001 |
| daf-2(m41); daf-12 M | 30.0 (33.5, 28.0) | +3 | 44.0 | +6 | 87 (90) | 0.017, 0.74 |
| daf-2(e1370) H | 29.3 (32.0, 27.3) | ---- | 45.0 | ---- | 124 (332) | ---- |
| daf-2(e1370) M | 53.0 (56.0, 50.8) | ---- | 105.0 | ---- | 209 (262) | ---- |
| daf-2(e1370); daf-12 H ‡ | 54.0 (75.0, 43.5) | +84 | 113.5 | + 152 | 30 (72) | <0.0001 |
| daf-2(e1370); daf-12 M ‡ | 64.0 (83.0, 55.0) | +21 | 124.0 | + 18 | 40 (72) | 0.18 |
Hermaphrodite
Male
Senescent deaths (starting population)
Probability that survival curves of a strain with and without daf-12(m20) differ by random chance (log rank test). Multiple significance values represent results from trials performed at separate times.
Results from only one replicate, due to bacterial contamination in two other replicates. Trials performed at 22.5°C.
FIGURE 1.
Survival curves showing effect of daf-12(m20) on class 1 daf-2(m41) and class 2 daf-2(e1370) hermaphrodite and male survival (22.5°C). N2 (wild type) and daf-12(m20) are shown for comparison. P = probability that survival of daf-2(rf) and daf-2(rf); daf-12(m20) differ by random chance (log rank test).
In males, daf-12 did not shorten lifespan but instead produced a slight increase that was significant in one trial, and almost significant in another (FIGURE 1, TABLE 1). In other trials, we also saw a significant increase in male lifespan resulting from the null allele daf-12(rh61rh411) (data not shown). Thus, the DAF-12 nuclear receptor weakly promotes longevity in hermaphrodites but weakly antagonises it in males.
daf-2(m41) and daf-2(e1370) both increased lifespan in males in liquid culture (FIGURE 1, TABLE 1). daf-2(m41) suppressed the sex difference in longevity, something not seen in studies of another class 1 mutant, daf-2(m577), on plates3. By contrast, longevity was greatly increased in daf-2(e1370) males, as previously seen in plate cultured populations of this genotype3. Unexpectedly, addition of daf-12 had only minor effects on lifespan in daf-2 males (FIGURE 1, TABLE 1). daf-12 marginally increased daf-2 male lifespan, but the effects were very small compared to the effects seen in hermaphrodites. These slight increases reached statistical significance in one of two trials with daf-2(m41), but were not significant for daf-2(e1370).
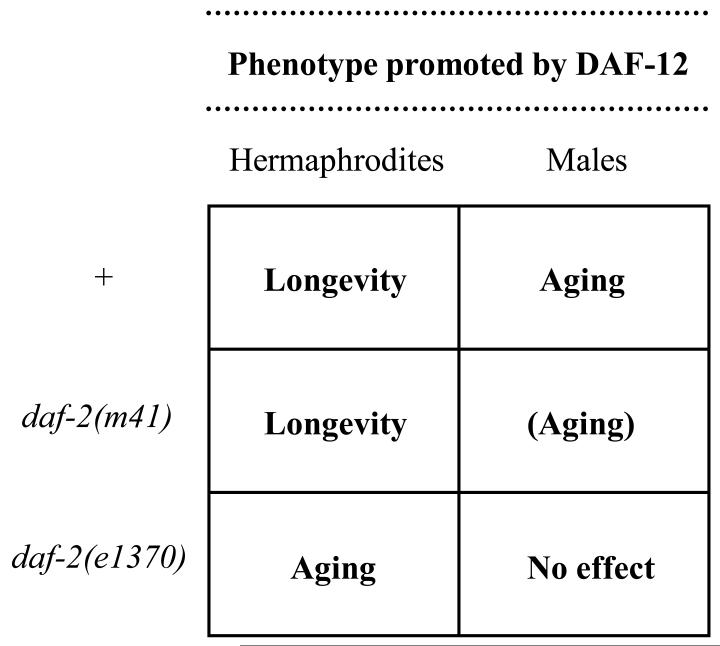
In summary, our results show that the effects of daf-12 on aging differ between the sexes in all three backgrounds tested: daf-2(+), daf-2(m41) and daf-2(e1370) (findings summarized in FIGURE 2). In a wild-type background, the DAF-12 receptor weakly promotes longevity in the hermaphrodite, but aging in the male. In a weak (class 1) daf-2 mutant background, DAF-12 promotes longevity in hermaphrodites, but marginally promotes aging in males. In a stronger (class 2) daf-2 mutant background, DAF-12 promotes longevity in hermaphrodites, but has little effect in males.
FIGURE 2.
Summary of the inferred effects of the DAF-12 steroid receptor on longevity and aging. In all three genetic contexts, the effects of mutation of daf-12 on lifespan differs between males and hermaphrodites.
These findings demonstrate sexual dimorphism in the effects on aging of the DAF-12 nuclear receptor. This could be determined in any of a number of ways, including sex differences in levels or tissue localization of DAF-12 protein levels, or of cofactors that interact with DAF-12, or in production or structure of the steroid ligand that controls DAF-12 activity. Whether nematodes are, like mammals, sexually dimorphic in terms of the steroid hormones that they secrete remains to be explored.
ACKNOWLEDGEMENTS
We thank Scott Pletcher for assistance with data analysis. This work was supported by the Biotechnology and Biological Science Research Council (UK) and the Wellcome Trust.
REFERENCES
- 1.Smith DWE. Human Longevity. Oxford University Press; New York, Oxford: 1993. Women live longer than men; pp. 78–98. [Google Scholar]
- 2.May R. Gender, immunity and the regulation of longevity. BioEssays. 2007 doi: 10.1002/bies.20614. In press. [DOI] [PubMed] [Google Scholar]
- 3.Gems D, Riddle DL. Genetic, behavioral and environmental determinants of male longevity in Caenorhabditis elegans. Genetics. 2000;154:1597–1610. doi: 10.1093/genetics/154.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–60. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Kenyon C, et al. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 6.Antebi A, et al. daf-12 encodes a nuclear receptor that regulates the dauer diapause and developmental age in C. elegans. Genes Dev. 2000;14:1512–1527. [PMC free article] [PubMed] [Google Scholar]
- 7.Gerisch B, et al. A bile acid-like steroid modulates Caenorhabditis elegans lifespan through nuclear receptor signaling. Proc. Natl. Acad. Sci. USA. 2007;104:5014–5019. doi: 10.1073/pnas.0700847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gems D, et al. Two pleiotropic classes of daf-2 mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans. Genetics. 1998;150:129–155. doi: 10.1093/genetics/150.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larsen PL, Albert PS, Riddle DL. Genes that regulate both development and longevity in Caenorhabditis elegans. Genetics. 1995;139:1567–1583. doi: 10.1093/genetics/139.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sulston J, Hodgkin J. Methods. In: Wood WB, editor. The nematode Caenorhabditis elegans. Cold Spring Harbor. N.Y.: 1988. pp. 587–606. [Google Scholar]
- 11.McCulloch D, Gems D. Evolution of male longevity advantage in nematodes. Aging Cell. 2003;2:165–173. doi: 10.1046/j.1474-9728.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 12.Kimura KD, et al. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- 13.Yu H, Larsen P. DAF-16-dependent and independent expression targets of DAF-2 insulin receptor-like pathway in Caenorhabditis elegans include FKBPs. J. Mol. Biol. 2001;314:1017–1028. doi: 10.1006/jmbi.2000.5210. [DOI] [PubMed] [Google Scholar]