Summary
The Lamina-associated polypeptide, Emerin, MAN1 - (LEM) domain defines a group of nuclear proteins, which bind chromatin through interaction of the LEM motif with the conserved DNA cross-linking protein, Barrier-to-Auto-Integration factor (BAF). Here, we describe a novel LEM protein, annotated in databases as “Ankyrin and LEM domain containing protein 1” (ANKLE1). We show that Ankle1 is conserved in metazoans and contains a unique C-terminal GIY-YIG motif that confers endonuclease activity in vitro and in vivo. In mammals, Ankle1 is predominantly expressed in hematopoietic tissues. While most characterized LEM proteins are components of the inner nuclear membrane, ectopic Ankle1 shuttles between cytoplasm and nucleus, and Ankle1 enriched in the nucleoplasm induces DNA cleavage and DNA damage response. This activity requires both the catalytic C-terminal GIY-YIG domain and the LEM motif, which binds chromatin via BAF. Hence, Ankle1 represents a novel LEM-protein with a GIY-YIG type endonuclease activity in higher eukaryotes.
Keywords: chromatin, DNA damage, GIY-YIG endonuclease, LEM-domain, nuclear envelope
Introduction
Polypeptides containing a Lamina-associated polypeptide, Emerin, MAN1 (LEM) domain (Lin et al., 2000) comprise a protein family with important and essential functions in nuclear architecture, mitosis, cell signaling and gene expression (Gruenbaum et al., 2005; Schirmer and Foisner, 2007; Wagner and Krohne, 2007; Anderson and Hetzer, 2008). The LEM domain is a structural motif of 45 amino acids that folds as two α-helices (Laguri et al., 2001) and binds to Barrier-to-Autointegration Factor (BAF) (Furukawa, 1999; Cai et al., 2001; Shumaker et al., 2001), an essential DNA cross-linking protein in metazoans (Umland et al., 2000; Zheng et al., 2000; Margalit et al., 2007).
The LEM protein family in mammals comprises the well characterized proteins Lamina-associated polypeptide 2 (LAP2), emerin and MAN1 (Schirmer and Foisner, 2007; Wagner and Krohne, 2007), the MAN1-related protein LEM2 (Brachner et al., 2005; Chen et al., 2006; Ulbert et al., 2006; Huber et al., 2009) and a partially characterized testis-specific protein, LEM domain-containing 1 (LEMD1, also LEM5) (Lee and Wilson, 2004; Yuki et al., 2004). Two of these proteins are conserved in Caenorhabditis elegans: emerin, and LEM2 (Lee et al., 2000; Gruenbaum et al., 2002; Liu et al., 2003). Most of the characterized LEM proteins are transmembrane proteins of the inner nuclear membrane where they interact with the nuclear lamina (Schirmer and Foisner, 2007; Wagner and Krohne, 2007). LEM protein - BAF complexes are involved in post-mitotic nuclear assembly and in chromatin organization in C. elegans (Liu et al., 2003; Margalit et al., 2005) and in mammalian cells (Haraguchi et al., 2001; Dechat et al., 2004; Shimi et al., 2004). In addition, several LEM domain proteins bind to and regulate transcription factors and signaling molecules such as β-catenin (Markiewicz et al., 2006), Smads (Lin et al., 2005; Pan et al., 2005; Jiang et al., 2008), germ-cell-less (gcl) (Nili et al., 2001; Holaska et al., 2003) and retinoblastoma protein (Markiewicz et al., 2002; Dorner et al., 2006). Mutations in genes encoding for emerin, MAN1 and LAP2α were linked to a number of human pathologies (Vlcek and Foisner, 2007; Wagner and Krohne, 2007; Worman and Bonne, 2007; Chi et al., 2009), reflecting their multiple and diverse functions.
In silico analyses of mammalian genomes identified novel genes predicted to be members of the LEM family, originally termed LEM3 and LEM4 (Lee et al., 2000; Lee and Wilson, 2004) and annotated in databases as “Ankyrin and LEM domain containing protein” (ANKLE) 1 and 2, respectively. In this study, we report the first biochemical and cell biological characterization of Ankle1 (formerly LEM3, ANKRD41 or FLJ39369), an evolutionary conserved non-membrane bound LEM protein that shuttles between nucleus and cytoplasm and is predominantly expressed in hematopoietic tissues and cells. Intriguingly, the Ankle1 C-terminus contains an enzymatically active GIY-YIG endonuclease domain, previously described as the characteristic feature of a subgroup of the homing endonuclease superfamily. Homing endonucleases are encoded within “selfish” group I introns or inteins. They catalyze their lateral transfer and integration into intron-less homologous alleles in the host genome (Stoddard, 2005; Edgell, 2009; Stoddard, 2011). A large number of these enzymes were identified in all three biological kingdoms - archae, bacteria and eukaryotes (Belfort and Roberts, 1997; Sokolowska et al., 2011). The GIY-YIG homing endonucleases that have been characterized in detail include I-TevI in T4 phage (Bell-Pedersen et al., 1991; Van Roey et al., 2002) and I-SceI in the yeast mitochondrial genome (Perrin et al., 1993; Moure et al., 2003). The GIY-YIG motif has also been found in enzymes other than homing endonucleases, such as UvrC, a protein involved in nucleotide excision repair in bacteria (Yoakum and Grossman, 1981; Truglio et al., 2005). Only few genes in higher eukaryotes are known to contain a GIY-YIG motif: the recently characterized human endonuclease SLX1 which is involved in structure-specific DNA repair (Fekairi et al., 2009; Svendsen et al., 2009), the transpositionally active Penelope-like elements in Drosophila virilis (Pyatkov et al., 2004; Evgen’ev and Arkhipova, 2005; Schostak et al., 2008) and the yet uncharacterized human gene encoding Ankle1 (Dunin-Horkawicz et al., 2006)
Results
Ankle1 is highly conserved in metazoans
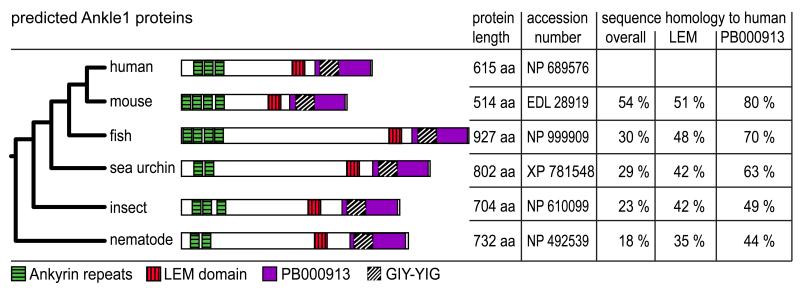
ANKLE1 (Ankle and LEM domain containing 1) – also termed ANKRD41 (Ankyrin repeat domain protein 41), FLJ39369 or LEM3 – was annotated in databases based on different computational screens for genes that contain either a LEM domain, Ankyrin repeats, or a GIY-YIG motif (Lee et al., 2000; Lee and Wilson, 2004; Dunin-Horkawicz et al., 2006). Assembly and alignment of Ankle1 sequences from numerous species obtained from the ENSEMBL and NCBI databases (Suppl. Fig. 1A) revealed a conserved domain organization of the protein: two to four (depending on the species) N-terminal Ankyrin repeats (Fig. 1, green boxes), a central putative LEM domain (red box), and a predicted C-terminal GIY-YIG motif (black hatched box) within a highly conserved C-terminal sequence stretch (violet box) termed “PB00913” in the Pfam database (www.pfam.sanger.ac.uk). This latter C-terminal domain including the GIY-YIG motif showed the highest homologies ranging from ~70% conserved residues among vertebrates to ~40% conservation between nematodes and mammals (Fig. 1 and Suppl. Fig. 1B). Unlike the domain architecture, the predicted molecular weights of Ankle1 vary considerably ranging from 58 kDa in mouse to 102 kDa in zebra fish (Fig. 1). Human Ankle1 (hAnkle1) has 615 residues and a predicted molecular weight of 65 kDa. The variability in size is particularly evident in the region between the Ankyrin repeats and the LEM-domain, indicating that the distance between these domains may be less important for its activity. In contrast, the spacing between the LEM-domain and the GIY-YIG motif was preserved and may thus be crucial for Ankle1’s function(s).
Fig. 1. Ankle1 is a novel LEM protein conserved among metazoans.
Comparison of the predicted domain organization of Ankle1 orthologs from various species representing major metazoan clades: Homo sapiens and Mus musculus (mammals), Danio rerio (vertebrates), Strongylocentrotus purpuratus (echinodermata), Drosophila melanogaster (arthropods) and Caenorhabditis elegans (nematodes). Ankyrin repeats are shown as green boxes, the LEM motif as red boxes, the C-terminal PB000913 sequence as violet box. The predicted GIY-YIG motif is marked as black-hatched box. Number of amino acids (aa) of full length proteins, database accession numbers, and sequence homologies of various domains to human Ankle1 are shown on the right.
Human Ankle1 is expressed in a tissue-restricted manner
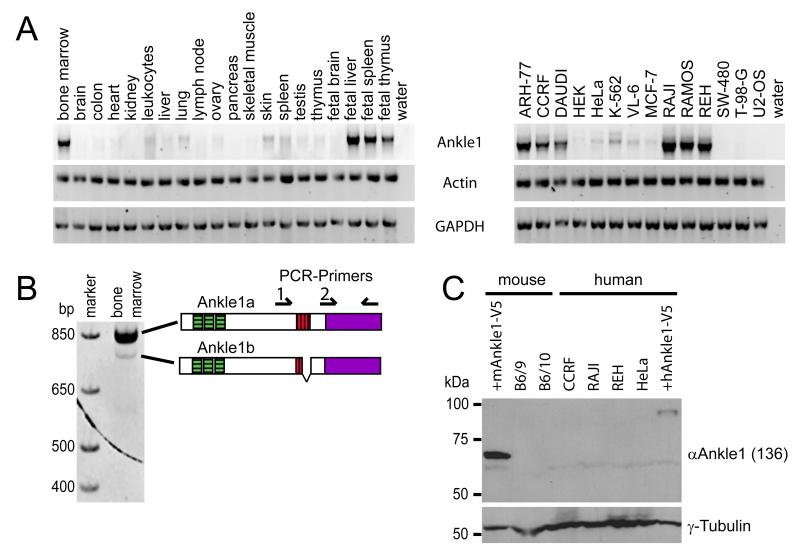
We analyzed the expression levels of Ankle1 in human tissues and cell lines by semiquantitative RT-PCR using primers for the 3′ region of Ankle1 cDNA. Analysis of mRNA samples from a collection of adult human tissues revealed predominant Ankle1 mRNA expression in bone marrow. Expression was also high in fetal liver, fetal spleen and fetal thymus, the primary organs of hematopoiesis during intrauterine development (Fig. 2A), suggesting that Ankle1 may be involved in hematopoiesis-specific processes. In addition, we observed expression in a panel of lymphoma- and leukemia-derived tumor cell lines (ARH77, CCRF, DAUDI, K562, RAJI, RAMOS, REH), while sarcoma- and carcinoma-derived lines (HACAT, HeLa, LSWW, MCF-7, SW-480, T-98-G, U2OS) expressed low or undetectable levels of Ankle1 mRNA (Fig. 2A). RT-PCR analysis of human bone marrow mRNA samples using a further upstream forward primer (Fig. 2B) identified a minor, slightly smaller isoform of Ankle1, originating from alternative splicing within exon 5 (see also Q8NAG6-1 in Uniprot database). Interestingly, the splice variant, which we termed Ankle1b, lacks half (aa 375-400) of the putative LEM domain (Fig. 2B).
Fig. 2. Ankle1 is predominantly expressed in human hematopoietic tissues and cell lines.
(A) Representative agarose gels showing semiquantitative RT-PCR products amplified from human tissue and cell line samples. Actin and GAPDH were used as a control for equal loading. (B) Resolution of human Ankle1 PCR products from human bone marrow on polyacrylamid gels revealed a low abundant smaller Ankle1 isoform. Position of PCR primers are indicated (primer 1 was used for Ankle1 splice variant identification; primer 2 for expression analyses). The identity of the isoform was verified sequencing. (C) Representative Western blot detecting Ankle1 protein in total lysates of HeLa cells expressing ectopic human or mouse Ankle1-V5. Tubulin was probed as a loading control.
Next, we tested Ankle1 protein expression, using rabbit polyclonal antibodies raised against the N-terminal recombinant mouse Ankle1 fragment (aa 95-260, antiserum ‘136′). Although the antibodies readily detected ectopically expressed Ankle1 in Western blots of HeLa cell lysates (Fig. 2C, hAnkle1-V5, mAnkle1-V5) as well as in immunofluorescence microscopy (see Fig. 4B), endogenous Ankle1 was not detectable in various human or mouse lymphoma cell lines which contain abundant Ankle1 mRNA levels (Fig. 2C). We obtained similar results with antibodies raised against a highly conserved peptide within the C-terminal region (aa 388-402) of mouse Ankle1 (data not shown). Thus, the antisera clearly detected ectopic Ankle1 protein in mammalian cells in both the unfolded and native state (Western blotting and immunofluorescence microscopy, respectively), but we could not detect endogenous Ankle1. Therefore, we assumed that endogenous Ankle1 protein levels may be strictly controlled and expressed at levels below the detection limit of our assays.
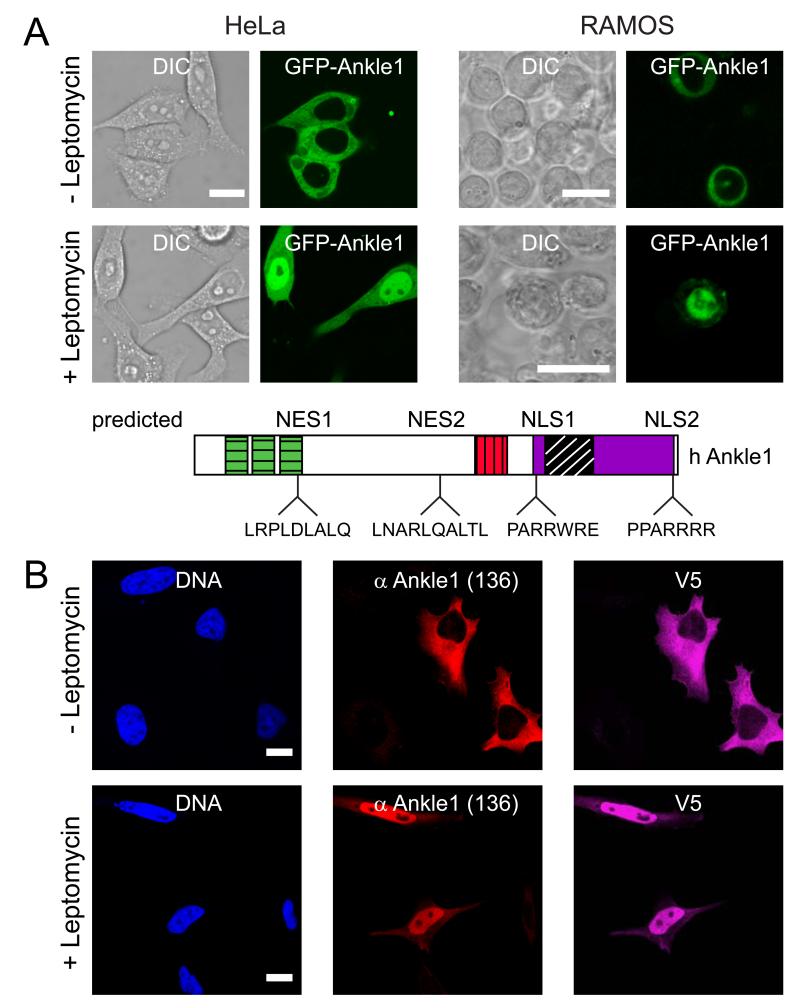
Fig. 4. Human Ankle1 shuttles between nucleus and cytoplasm.
(A) GFP-tagged Ankle1 was expressed in HeLa or RAMOS cells and imaged by live cell microscopy (GFP) and differential interference contrast (DIC) microscopy before (-) and after (+) treatment with Leptomycin for one hour. Nuclear export and nuclear localization signals identified by in silico prediction software are indicated in the cartoon (NES, NLS). (B) HeLa cells were transfected with hAnkle1-V5, and processed for immunofluorescence microscopy before and after three hours of Leptomycin treatment. Bars, 10 μm.
Ankle1 binds to BAF
A characteristic feature of LEM-proteins is their interaction with BAF via the LEM-motif. In order to test whether Ankle1 also binds BAF, we performed in vitro pull down assays using recombinant 6xhis-tagged BAF (BAF-his). Bacterial lysates containing BAF-his were mixed with E. coli cell lysates expressing recombinant full length Ankle1, an Ankle1 fragment, Ankle1ΔCT, which lacks the C-terminus including the predicted LEM domain, and, as a positive control, the N-terminal part of the LEM-protein emerin (EmerinΔTM), which was shown to bind BAF (Lee et al., 2001). BAF-his was precipitated using magnetic Ni particles and supernatant and pellet fractions were analyzed by Western blotting. BAF-his efficiently co-precipitated full-length Ankle1 and emerin, while Ankle1ΔCT remained in the unbound fraction (Fig. 3A), indicating that the C-terminal half of Ankle1 binds BAF.
Fig. 3. Co-precipitates of BAF and Ankle1 in vitro.
(A) BAF-6xHis was mixed with E.coli lysates containing recombinant Ankle1, Ankle1ΔCT or an N-terminal Emerin fragment EmerinΔTM and precipitated usining Ni-beads. BAF-bound proteins were detected by Western blotting using antiserum ‘136′ (Ankle1), India His-probe (BAF) and monoclonal antibody ‘MANEM5’ to emerin (gift of Glenn Morris). S, supernatant fraction (10% of input); P, precipitated fraction (50% of preciptated proteins). Molecular weights are indicated in kDa. The scheme shows the Ankle1 constructs used in the assays. (B) Recombinant 6xHis-V5 tagged Ankle1, Ankle1b or LAP2α were incubated with bacterial lysates containing untagged human BAF. His-tagged prey proteins were precipitated using Ni-beads and bound BAF was detected by Western blotting using antibodies to V5 tag and BAF. S, supernatant fraction (10% of input); P, precipitated fraction (50% of preciptated proteins). Note: BAF forms various homomeric complexes even under denaturating conditions (arrows indicating monomeric, dimeric and oligomeric states).
To test whether the LEM domain mediates Ankle1-BAF interaction we performed pull down assays with bacterially expressed full length 6xhis-V5-tagged Ankle1 (Ankle1-his-V5) and the corresponding Ankle1 splice variant lacking a part of its LEM domain (Ankle1b-his-V5). Precipitation of Ankle1-his-V5 with Ni beads brought down a significant fraction of recombinant, untagged BAF from bacterial cell lysates (Fig. 3B). Predominantly dimeric BAF was detected in the Ankle1 pellet fraction, while monomeric and oligomeric BAF mainly remained in the supernatant fraction. Similar results were obtained in the positive control using the LEM-protein LAP2α, which efficiently pelleted BAF dimers. Ankle1b-his-V5, in contrast, lacking a functional LEM domain precipitated only trace amounts of BAF, and very little dimeric BAF was detected in both the supernatant and pellet fractions. Overall, these findings show that Ankle1 interacts directly with BAF via its LEM-domain, and indicate that binding of BAF to the LEM domain may favor BAF dimerization.
Ankle1 shuttles between cytoplasm and nucleus
Since antisera failed to detect endogenous Ankle1 protein (see Fig. 2C) we expressed GFP-tagged human Ankle1 in a lymphoma and a carcinoma-derived cell line, expressing or lacking endogenous Ankle1 mRNA, respectively (see Fig. 1A). In both, lymphoma-derived RAMOS cells and in HeLa cells we observed predominant cytoplasmic localization of GFP-Ankle1 by fluorescence microscopy (Fig. 4A). Identical results were obtained using a C-terminal GFP tag (data not shown) or a small tag, such as V5 (Fig. 4B). Since in silico analyses of Ankle1 primary sequence predicted potential nuclear export and nuclear localization signals (NES and NLS, Fig. 4A, scheme), we hypothesized that Ankle1 shuttles between nucleus and cytoplasm. To test this hypothesis, we blocked Crm-dependent nuclear export using the drug Leptomycin B (Kudo et al., 1998). After one hour Leptomycin B treatment the majority of tagged Ankle1 accumulated in the nucleus in both HeLa and RAMOS cells (Fig. 4). Thus, Ankle1 is actively transported in and out of the nucleus and shows a predominant cytoplasmic localization at steady state.
Ankle1 has nuclease activity eliciting DNA damage signaling in the nucleus
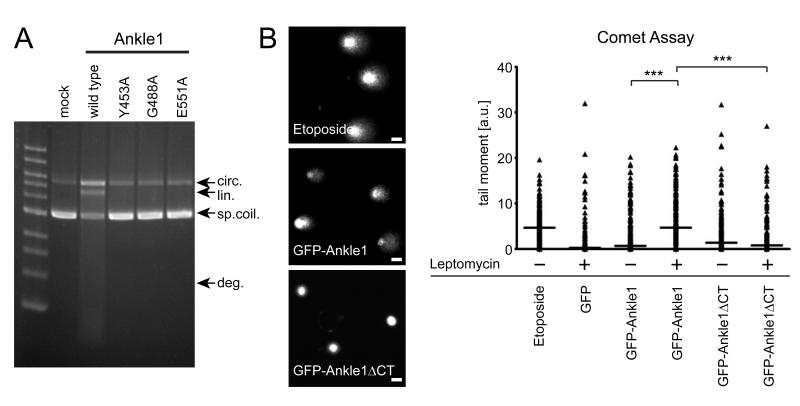
Given that Ankle1 contains a putative GIY-YIG type endonuclease domain, we performed a series of in vitro and in vivo assays to test for endonuclease activity. Recombinant Ankle1 purified from HEK cells cleaved supercoiled plasmid DNA into relaxed circular (nicked) and linearized DNA molecules (Fig. 5A), suggesting that Ankle1 possesses in vitro nuclease activity. Several conserved residues in the GIY-YIG motif (Y453, G488, E551 in human Ankle1, see Suppl. Fig. 3A) were previously identified to be crucial for the formation of the catalytic surface of the nuclease (Van Roey et al., 2002; Truglio et al., 2005; Lagerback and Carlson, 2008). Point mutations of either of these residues abrogated Ankle1’s in vitro DNA cleavage activity (Fig. 5A). Thus, we concluded that Ankle1 possesses intrinsic endonuclease activity mediated by its C-terminal canonical GIY-YIG motif.
Fig. 5. Ankle1 cleaves DNA in vitro and in vivo.
(A) Purified wildtype and mutated HAStrep-Ankle1 expressed in HEK cells was incubated with supercoiled pFastBac1 plasmid and DNA species were separated by agarose gel electrophoresis. Arrows indicate different intermediate steps of plasmid degradation (o.circ=open circle, lin=linear/nicked, sp.coil=supercoiled, deg=degraded). (B) Representative images of ethidiumbromide stained nuclei in a Comet assay. Bars indicate 10 μm. Comets were measured using the Comet Assay IV software (Perceptive Instruments, Haverhill, UK), quantified and visualized in Graphpad Prism. Median values are indicated as horizontal lines. *** indicate statistically significant differences in median values between populations (p<0.001). Comet formation (i.e. DNA fragmentation) was evaluated in single cells of three independent cell populations (n>50 each).
In order to test whether Ankle1 also cleaves DNA in cells in vivo, we performed COMET (i.e. single cell gel electrophoresis) assays, an established method to detect genomic DNA fragmentation in vivo at single cell level. Expression of GFP-Ankle1 in HeLa cells for 24 hours caused little DNA damage similar to the negative control, cells expressing GFP only (Fig. 5B). In contrast, treatment of cells with Leptomycin, causing GFP-Ankle1 accumulation in the nucleus, induced DNA cleavage to a similar extent as cells treated with the topoisomerase II inhibitor Etoposide, a drug known to induce massive DNA double strand breaks (Sullivan et al., 1986). Leptomycin treatment of cells expressing GFP or a truncated Ankle1 lacking the C-terminal GIY-YIG motif did not induce DNA breaks (Fig. 5B).
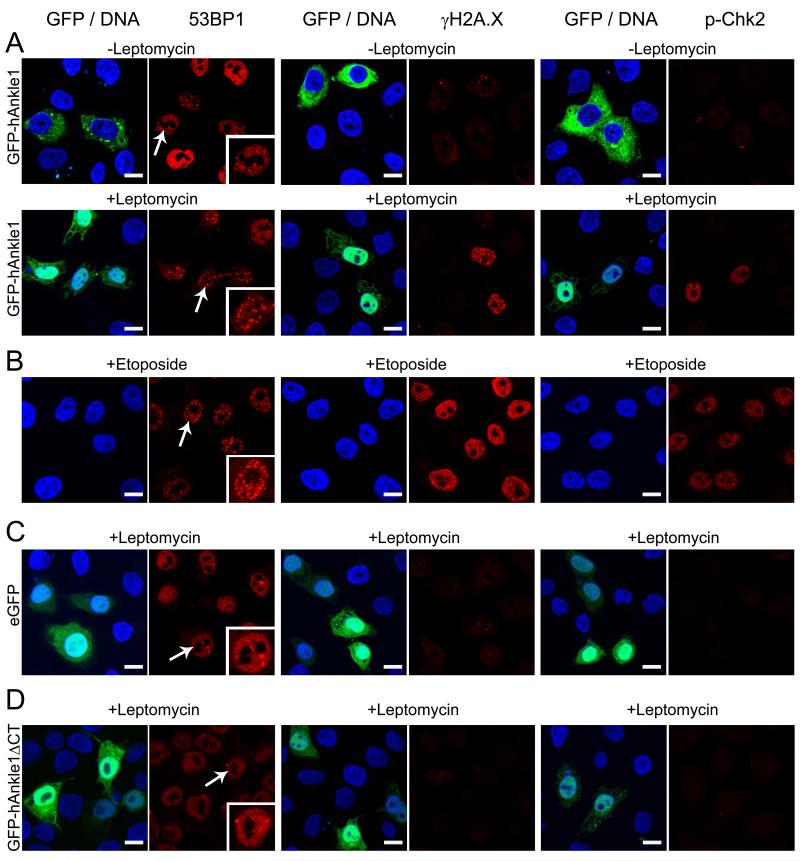
In order to find out whether Ankle1-mediated DNA cleavage activates DNA damage signaling, we analyzed the expression and localization of components of the DNA damage response pathway in GFP-Ankle1-expressing cells before and after Leptomycin treatment. In more than 90% of cells (n>100) nuclear accumulation of GFP-Ankle1 following Leptomycin treatment caused relocalization of 53BP1 to intranuclear foci (Schultz et al., 2000), phosphorylation of Histone 2A.X (γH2A.X) and activation of the downstream effector kinase Chk2 (pChk2) (Fig. 6A), similar as in Etoposide-treated cells (Fig. 6B). Identical results were obtained in cells expressing Ankle1-V5 (Suppl. Fig. 2). Leptomycin treatment of GFP expressing cells did not induce these markers (Fig. 6C), indicating that accumulation of Ankle1 in the nucleus, but not Leptomycin treatment and/or transfection, activated the DNA damage signaling pathway. Nuclear accumulation of a C-terminally truncated Ankle1 protein missing the GIY-YIG motif (GFP-Ankle1ΔCT) did not activate the DNA damage response pathway (Fig. 6D). These observations indicate that accumulation of Ankle1 in the nucleus causes DNA cleavage thereby activating the DNA damage response pathway.
Fig. 6. Accumulation of ectopic Ankle1 in the nucleus causes DNA damage.
HeLa cells were transiently transfected with GFP-Ankle1 (A), GFP (C) or GFP-Ankle1ΔCT (D) or incubated with Etoposide (B), and treated with Leptomycin as indicated. All samples were processed in parallel for confocal immunofluorescence microscopy. Fixed cells were stained for 53BP1, γH2A.X or phosphorylated Chk2 using specific antibodies (all red), DNA was stained with DAPI (blue), GFP and GFP-fusion proteins are shown in green. Bars indicate 10 μm; arrows, cells shown in the insets. Representative images out of >3 independent experiments.
The LEM domain of Ankle1 is required for DNA cleavage in vivo
Since defined DNA binding/targeting motifs described in homing endonucleases, such as zinc-finger domains, helix-turn-helix motifs and DNA minor groove-binding α-helical structures (Derbyshire et al., 1997; Kowalski et al., 1999; Liu et al., 2006; Carter et al., 2007), are not known in Ankle1, we hypothesized that Ankle1’s LEM motif might be involved in correct targeting of the protein. In order to test whether the LEM domain is involved in Ankle1-mediated DNA cleavage we measured γH2A.X staining intensity in mixed cultures of cells expressing either full length Ankle1 or the LEM-domain deficient variant Ankle1b. To correlate the extent of DNA damage (γH2A.X staining intensity) with Ankle1 expression levels in individual cells we also measured the intensity of the V5 staining. Unlike Ankle1b, full-length Ankle1 was expressed from a vector also expressing a GFP-marker, allowing us to discriminate between cells expressing full-length Ankle1 or Ankle1b in the mixed culture (Fig. 7). Immunofluorescence analyses of mixed cell populations were performed before and after Leptomycin treatment (Fig. 7). The fluorescence intensity of the V5-specific signal in the nucleus of Leptomycin treated cells was measured in GFP positive cells (i.e. cells expressing full length Ankle1-V5) and GFP-negative cells (expressing Ankle1b-V5) and plotted over the intensity of γH2A.X-specific fluorescence (Fig. 7, graph). DNA damage (measured via γH2A.X intensity) increased with increasing levels of full length Ankle1, while only low levels of γH2A.X were detectable in Ankle1b-expressing cells independent of the expression level (Fig. 7, graph). Thus, the presence of a functional LEM motif is required for the Ankle1-mediated activation of the DNA response pathway, suggesting that this domain might be involved in targeting Ankle1 to chromatin in vivo.
Fig. 7. The LEM domain in Ankle1 is required for inducing DNA damage response.
HeLa cells were transfected separately with constructs expressing Ankle1-V5 or the LEM domain-deficient Ankle1b-V5 and seeded as a mixed culture onto cover slips after 24 hours. The plasmid expressing Ankle1-V5 also expresses a CMV-driven GFP-Blasticidin marker gene (GFP-Bsd), the plasmid encoding Ankle1b-V5 contains a Blasticidin gene without GFP fusion (Bsd) (plasmid maps). After 48 hours cells were either treated with Leptomycin or ethanol for three hours, fixed, stained for V5 (red) and γH2A.X (yellow) and imaged on a confocal fluorescence microscope. DNA was stained with DAPI (blue). GFP-Blasticidin is shown in green. Cells marked with asterisks lack GFP signal and are thus expressing Ankle1b; cells expressing Ankle1 (expressing GFP) are marked with arrows. Relative fluorescence intensities of V5 and γH2A.X signals within the nucleus of untransfected, GFP-positive and GFP-negative transfected and Leptomycin-treated cells (n>30 each) were measured and plotted using Graphpad Prism software. Representative results out of three independent experiments are shown.
Discussion
In this study we performed biochemical and cell biological analyses of a novel human LEM protein, annotated as Ankle1 in databases. The LEM domain of Ankle1 interacts with BAF, confirming that Ankle1 is a bona fide LEM protein. Apart from its interaction with BAF, Ankle1 also showed unexpected novel and unique properties among the characterized LEM protein family members: (1) Ankle1 expression is largely restricted to hematopoietic tissues, while most other LEM proteins in mammals are widely expressed (Theodor et al., 1997; Ellis et al., 1998; Lin et al., 2000; Brachner et al., 2005). Only the LEM-domain-containing 1 gene (LEMD1, also LEM5) (Lee and Wilson, 2004) was reported to be expressed in a tissue-restricted manner (testis) so far (Yuki et al., 2004). (2) While all analyzed LEM proteins contain a LEM motif close to their nucleoplasmic N-terminus (Wagner and Krohne, 2007), Ankle1’s LEM domain is located in the middle of the polypeptide. (3) Ankle1 lacks transmembrane domains and shuttles in and out of the nucleus. Non-membrane bound localization among characterized LEM proteins has only been reported for two isoforms of the LAP2 gene, LAP2α (Dechat et al., 1998; Dechat et al., 2004) and LAP2ξ (Shaklai et al., 2008). All other studied LEM proteins contain one or two transmembrane domains and localize to the inner nuclear membrane. (4) The most intriguing and exceptional feature of Ankle1, however, is an enzymatically active GIY-YIG motif which was previously described in homing endonucleases and a few other proteins, including the bacterial nucleotide excision repair protein UvrC and the DNA structure specific repair enzyme SLX1 in humans (Perrin et al., 1993; Stoddard, 2005; Truglio et al., 2005; Dunin-Horkawicz et al., 2006; Edgell, 2009; Fekairi et al., 2009; Svendsen et al., 2009). The comparison of 3D models generated from the canonical GIY-YIG homing endonuclease I-TevI and from human, C. elegans and hydra Ankle1 revealed striking similarities (Suppl. Fig. 3), indicating a strong structural conservation between I-TevI and Ankle1 proteins despite low primary sequence identity. Thus, Ankle1 represents a novel member of the LEM protein family and is, along with SLX1, the only active GIY-YIG type endonuclease described in higher eukaryotes so far.
Besides the catalytically active GIY-YIG domain, homing endonucleases also contain DNA-binding motifs, such as zinc-finger, α-helix and helix-turn-helix domains that position the catalytic core at the DNA. It is tempting to speculate that Ankle1 is targeted to DNA through the interaction between its LEM domain and BAF. In addition a DNA-binding α-helix might be present within Ankle1’s C-terminus according to in silico modeling (Suppl. Fig. 3B), but so far we have no experimental evidence for additional DNA binding motifs in Ankle1. Our hypothesis that a Ankle1-BAF complex is involved in DNA cleavage is supported by the observation that the LEM-deficient splice isoform Ankle1b, did not induce DNA damage response.
What is the physiological role of Ankle1?
When forced to the nucleus by transient inhibition of nuclear export, Ankle1 caused DNA cleavage and induced DNA damage response. In all organisms, endonucleases are instrumental in diverse DNA repair pathways (Nishino and Morikawa, 2002; Marti and Fleck, 2004). Endonucleases are also essential for the processing of double strand breaks introduced during meiotic recombination (Borde, 2007; Mimitou and Symington, 2009) and for somatic genomic rearrangements during lymphocyte development in higher eukaryotes (Lieber et al., 2004; Rooney et al., 2004; Rivera-Munoz et al., 2007). Taking into account the predominant expression of human Ankle1 in hematopoietic tissues and in lymphoma-derived cell lines we postulate a role of Ankle1 in genomic rearrangement or associated DNA repair processes during the development of lymphocytes. In addition, Ankle1 may have a more general function in DNA repair pathways, which could explain its conservation in lower metazoan species. Consistent with this hypothesis, Dittrich et al. demonstrated that loss of function of the C. elegans ortholog of mammalian Ankle1, lem-3, causes a radiation hypersensitivity (Rad) phenotype (personal communication). Worms carrying an L to F point mutation in LEM-3 at position 659 (indicated in Supp. Fig. 3C) are hypersensitive to DNA damage caused by chemicals and irradiation. This leucine residue is invariably conserved in all identified Ankle1 proteins at the respective positions. Interestingly, this site resides within a predicted C-terminal α-helical structure (Suppl. Fig. 3B,C) and could thus be important for Ankle1-DNA coordination. Interestingly, human Ankle1 mutated at the respective residue (L590F) did not trigger the DNA damage response pathway upon accumulation in the nucleus (Supp. Fig. 4).
Altogether, Ankle1 is an evolutionary conserved GIY-YIG type endonuclease that is predicted to have functions in DNA damage repair pathways in lower metazoan organisms (Dittrich et al., personal communication), whereas it seems to have acquired more specialized functions during evolution in mammals, in particular in the human lymphocyte development.
Material and Methods
Cell culture and reagents
HeLa, HEK, SW-480, T-98-G and U2-OS cells were routinely cultivated in DMEM, ARH-77, CCRF, DAUDI, K-562, VL-6, RAJI, RAMOS and REH cells in RPMI, both supplemented with 10% fetal calf serum (Invitrogen, Carlsbad, USA), 100 U/mL Penicillin/Streptomycin and 2 mM L-Glutamine at 37°C in a humidified atmosphere containing 8,5% CO2 and 5% CO2 respectively. Transient transfections were performed using Nanofectin according to the manufacturer’s instructions (PAA, Pasching, Austria). Crm-dependent nuclear export was inhibited with 10 ng/ml Leptomycin B (Enzo Life Sciences, Lausen, Switzerland) in complete growth medium for 1 to 3 hours.
PCR analyses
Poly(A+) RNA purified from cell lines using the mRNA isolation kit (Roche, Mannheim, Germany), or purchased human tissue total RNA samples from Agilent Technologies (Santa Clara, USA) and Biochain (Hayward, USA) were reverse transcribed using the first-strand cDNA Synthesis Kit (Roche, Mannheim, Germany). Aliquots of the resulting products were used as templates for PCR-amplification of Ankle1, Actin and GAPDH using the Go-Taq PCR Master Mix (Promega, Germany) and the following primer pairs: Ankle1-forw(1) 5′-TGCCTGTGGGAGCACCAGACATC-3′, Ankle1-forw(2) 5′- GCCCTGCGGACGGGCTGTATTC-3′,, Ankle1-rev 5′-GCTCGCCTTCAGCCAGGAAGAC-3′, Actin-forw 5′-ATCTGGCACCACACCTTCTAC-3′, Actin-rev 5′-CAGCCAGGTCCAGACGCAGG-3′, GAPDH-forw 5′-CATCACCATCTTCCAGGAGCGA-3′ and GAPDH-rev 5′-CCTGCTTCACCACCTTCTTGAT-3′.
Antibodies
Mouse anti-V5 antibodies were purchased from Invitrogen, rabbit anti-Actin from Sigma Aldrich, rabbit anti-53BP1 and mouse anti-BAF both from Novus Biologicals (Littleton, USA), and rabbit anti-phosphorylated Chk2 (Thr68) from Cell Signaling Technology (Boston, USA). The MANEM5 anti-Emerin antibody was a kind gift from Glenn Morris (Manilal et al., 1996). Polyclonal rabbit anti-murine Ankle1 antibodies were raised against an N-terminal fragment encompassing aa 95-260, following the Austrian and European regulations on animal experimentation. Antibodies against Ankle1 were affinity purified from serum ‘136′ using a purified recombinant human Ankle1 fragment corresponding to mouse Ankle1 aa 95-260. His-tagged proteins were detected using the India His probe-HRP (Pierce, Rockford, USA).
Plasmids and cloning strategy
Human Ankle1 and the splice variant Ankle1b were amplified from bone marrow cDNA using the Platinum Pfx PCR Kit (Invitrogen, Carlsbad, USA). Oligos used were (all in 5′-3′ sequence direction): Ankle1-CACC-forw CACCGCTAGCATGTGCTCGGAGGCCCGCCTGG and Ankle1-woSTOP-rev GTATCTAGAGCCCCGGGCCTGGATGTC or Ankle1-STOP-rev TCAGCCCCGGGCCTGGATG. PCR products were cloned into pENTR/D-TOPO or pET102-TOPO by topoisomerase based cloning (Invitrogen). GFP-Ankle1 fusion constructs were created by PCR-amplification using primers Ankle1-SalI-forw 5′-AGCGTCGACATGTGCTCGGAGGCCCGCCTGG-3′ and Ankle1-STOP-rev. The PCR product was cut with SalI and ligated into SalI and SmaI sites of the peGFP-C1 vector (Clontech, Palo Alto, USA). The deletion construct hAnkle1ΔCT (aa 1-420) was constructed by cloning the PCR-generated fragment into pET102-TOPO and peGFP-C1 using the same cloning strategies as described above, using the respective forward primers and primer hAnkle1-1062-stop-rev 5′-CATTCTAGACCGGCTACAAGGGCCGACAG-3′. V5-tagged Ankle1 was generated by shuttling Ankle1 via LR-recombination reaction (Invitrogen) from pENTR plasmids into a Gateway®-compatible pTRACER plasmid (pTB) (Brachner et al., 2005) or pDEST-51 (Invitrogen). Quick-change site directed mutagenesis was performed according to the manufacturer’s protocol (Stratagene, Santa Clara, USA) by amplification of pEntry-hAnkle1 with primers containing the desired mutations. Introduced point mutations were verified by sequencing and Ankle1 mutants were shuttled into pTB or pDEST-51. The HA-Strep-Ankle1 plasmid was generated by cloning the PCR-amplified human Ankle1 cDNA into the pENTR11 vector (Invitrogen) followed by Gateway-mediated shuttling into the pTO_HA_StrepIII_GW_FRT vector (obtained from Matthias Gstaiger, ETH, Switzerland). PCR generated human BAF cDNA was cloned via topoisomerase reaction into pET102-D-Topo for bacterial expression with a C-terminal His-tag or with a downstream stop codon into pEntry-D-Topo. The pEntry-hBAF-stop was then used for Gateway cloning into the bacterial expression vector pDEST42.
Preparation of cell lysates, gel electrophoresis and immunoblotting
Cells were harvested, washed in cold PBS and resuspended in cold high-salt RIPA buffer (25 mM Tris-HCl pH 7.4, 500 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS, “Complete” protease inhibitor mix (Roche, Mannheim, Germany), 1 mM PMSF, 1 mM DTT). Following incubation on ice for 10 min, cell lysates were sonicated for 5 seconds and insoluble material was pelleted. Supernatants were mixed with Laemmli sample buffer, boiled for 3 minutes and resolved via polyacrylamid gel electrophoresis. Proteins were blotted onto nitrocellulose membranes and probed with antibodies. After incubation with HRP-conjugated secondary antibodies, the Supersignal West Pico chemiluminescent substrate and CL-XPosure films (Thermo Fisher Scientific, Rockford, IL, USA) were used to detect signals.
Pull down assay
Bacterial expression vectors containing hAnkle1a (pET102-hAnkle1a-stop or pET102-hAnkle1a-6xhis-V5), hAnkle1b (pET102-hAnkle1b-6xhis-V5), hAnkle1ΔCT (pET102-hAnkle1ΔCT-stop), hBAF (pET102-hBAF-6xhis or pDEST42-hBAF-stop), hLAP2α (pDEST42-hLAP2α-6xhis-V5) or EmerinΔTM (pET11c-EmerinΔTM, kindly provided by Kathy Wilson) were transformed into BL21-star (Invitrogen). After induction of recombinant protein expression with 1 mM IPTG for 3 hours at 37°C, bacteria were lysed in RIPA buffer (25 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS, “Complete” protease inhibitor mix, 1 mM PMSF, 1 mM DTT) and sonicated for 1 minute on ice. Unsoluble material was pelleted and 20 μL of soluble fractions containing hAnkle1-stop, hAnkle1ΔCT-stop or EmerinΔTM were mixed with 10 μg of human BAF-6xhis and incubated for 1 hour at 37°C in 100 μL pull down buffer (20 mM Tris pH7.4, 150 mM NaCl, 10 mM Imidazole, 0.5% NP40, 1 mM EDTA, protease inhibitor mix). BAF complexes were separated with magnetic Ni2+beads (Promega), washed 3 times with pull down buffer, eluted by incubation with HEPES elution buffer (100 mM HEPES pH7.4, 500 mM Imidazole, 1 mM DTT) for 10 minutes and analyzed by Western blotting. Pull down of untagged human BAF-stop was done accordingly: 20 μL of lysate containing BAF-stop were mixed with 20 μL of lysate of BL21 bacteria expressing hAnkle1a-6xhis-V5, hAnkle1b-6xhis-V5 or hLAP2α-6xhis-V5) in 100 μL pulldown buffer and incubated for 1 hour at 37°C. Complexes were precipitated with magnetic Ni particles, washed 3 times with pull down buffer and analyzed by Western Blotting.
Immunofluorescence
Cells were grown on poly-L-lysine-coated glass coverslips or seeded into ibidi-treat® microscopy slides for live cell imaging (see Fig. 4A) (Ibidi, Munich, Germany). Cells on coverslips were fixed in 4% paraformaldehyde / PBS for 10 minutes and permeabilized in PBS / 0.5% Triton X-100 for 5 minutes. Cells were blocked in PBS / 0.5% gelatine for 15 minutes, incubated with primary antibodies for 45 minutes, three times washed with PBS and re-probed with the appropriate secondary antibodies conjugated to either TexasRed or Cy-5 (Jackson Immuno Research, West-Grove, USA) for 45 minutes. After three washes with PBS, DNA was counterstained with 100 ng/mL DAPI (Sigma, Munich, Germany) for 5 minutes and samples were mounted in Mowiol (Fluka, Buchs, Switzerland). Images were taken using a confocal laser scanning microscope (LSM-Meta, Zeiss, Jena, Germany) using a Plan-Apochromat 63x oil immersion objective (NA=1.40). Live-cell imaging was performed on the same microscope with transfected cells seeded. Digital images were analyzed, adjusted for brightness and contrast and mounted using the LSM Image-Browser (Zeiss, Jena, Germany) and Adobe Illustrator (Adobe Systems Inc, San Jose, USA).
Computer-assisted analysis
Sequence alignments and database searches were performed by NCBI-BLAST (http://www.ncbi.nlm.nih.gov/blast/), GraphAlign (http://darwin.nmsu.edu/cgibin/graph_align.cgi) (Spalding and Lammers, 2004) and ClustalW2 (http://www.ebi.ac.uk/clustalw2/) (Thompson et al., 1994). Genomic analysis was done using the ENSEMBL Genome Browser (http://www.ensembl.org/) (Sanger Institute). Additional hAnkle1 orthologs were predicted using the GENSCAN software (http://genes.mit.edu/genscan.html) (Burge and Karlin, 1997). Protein motifs and pattern searches were performed using SMART (http://smart.embl-heidelberg.de/) (Schultz et al., 1998; Letunic et al., 2004) and PSORT-II (http://psort.nibb.ac.jp/form2.html). Transmembrane domains were calculated using the TMHMM 2.0 prediction software (http://www.cbs.dtu.dk/services/TMHMM/) (Krogh et al., 2001) and the DAS-TMfilter algorithm (Cserzo et al., 2004). Phylogenetic tree predictions were visualized using the Phylodendron and PhyloDraw software (Choi et al., 2000).
3D-modelling was performed employing the CPHmodels 3.0 Server (Nielsen et al., 2002) and visualized using the Discovery Visualizer Studio 2.5 (Accelrys Software Inc., San Diego, USA).
COMET assay
Alkaline single cell gel electrophoresis (COMET assays) were performed as described previously (Ehrlich et al., 2008). In brief, GFP positive HeLa cells were sorted by FACS, pelleted and embedded in a thin layer of low-melting agarose (Invitrogen) on microscopy slides. In order to prevent DNA damage caused by UV light, lysis and all subsequent steps were conducted under red light. After solidification of the agarose, slides were immersed in lysis buffer (2.5 M NaCl, 100 mM Na2EDTA, 10 mM Tris, 1% Triton X, 10% dimethyl sulfoxide, pH 10.0) at 4°C for at least 1 hour. DNA was unwound by incubation of slides in alkaline electrophoresis buffer (300 mM NaOH, 1 mM Na2EDTA, pH ≥ 12.5) for 20 min followed by electrophoresis for 20 minutes at 25 V and 300 mA. Then, the slides were neutralized by 2 times 10 minutes incubations in neutralization buffer (0.4 M Trizma base, pH 7.5) at 4°C. Slides were air dried and stained with ethidiumbromide. Formation of ‘comets’ was measured in randomly chosen nuclei (n>150) for each condition in three independent samples using the Comet Assay IV software (Perceptive Instruments, Haverhill, UK), quantified and visualized in Graphpad Prism.
Endonuclease assays with HA-Strep Ankle1
HEK293 cells transiently transfected with a vector expressing HA-Strep-Ankle1 were grown on 15 cm dishes to 80 % confluency. 24 hours post transfection, the cells were pelleted by centrifugation, washed in PBS, and snap-frozen. The pellets were lysed (20 mM HEPES-KOH pH 8.0, 200 mM NaCl, 1% Triton, 1 mM EDTA, 10% Glycerol and protease inhibitors) and incubated on ice for 20 minutes. The lysate was centrifuged and incubated with Streptactin beads (IBA biotechnology, Göttingen, Germany) for 2 hours at 4°C on a rotating wheel (300 rpm). Subsequently, the beads were washed three times with 1 ml lysis buffer and twice with 1 ml wash buffer (20 mM HEPES-KOH pH 7.5, 2 mM MnCl2, 0.05 mg/ml BSA and 50 mM KCl), each wash for 5 minutes at 4°C. HA-Strep Ankle1 was finally eluted in 100 μL of PBS containing 2.5 mM Desthiobiotin (IBA biotechnology) for 30 min at 4 °C on a shaker (600 rpm). Aliquots were immediately snap-frozen.
For the non-specific endonuclease assays, 80 ng of supercoiled pFastBac1 plasmid was incubated with wild-type Ankle1 or its variants in endonuclease buffer (20 mM HEPES-KOH pH 7.4, 2 mM MnCl2, 45 mM KCl, 50 μg/mL BSA). After incubation at 37°C for 1 hour, the reaction was terminated by the addition of 0.1% SDS, 14 mM EDTA and 0.1 mg/mL Proteinase K and incubation at 55°C for 15 minutes. 10% glycerol was added and the samples were separated on a 0.8% ethidium bromide agarose gel for 45 min at 80 V.
Supplementary Material
Acknowledgements
The authors would like to thank Chantal Rodgarkia-Dara, Christina Dittrich and Michael Hengartner for generous supply of materials as well as fruitful and pathbreaking discussions. We also thank Thomas Sauer, MFPL for assistance with FACS. We acknowledge grant support from the Austrian Science Research Fund (FWF P17871) to RF. JB was supported by grant I031-B from the University of Vienna.
References
- Anderson DJ, Hetzer MW. The life cycle of the metazoan nuclear envelope. Curr. Opin. Cell Biol. 2008;20:386–392. doi: 10.1016/j.ceb.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfort M, Roberts RJ. Homing endonucleases: keeping the house in order. Nucleic Acids Res. 1997;25:3379–3388. doi: 10.1093/nar/25.17.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell-Pedersen D, Quirk SM, Bryk M, Belfort M. I-TevI, the endonuclease encoded by the mobile td intron, recognizes binding and cleavage domains on its DNA target. Proc. Natl. Acad. Sci. USA. 1991;88:7719–7723. doi: 10.1073/pnas.88.17.7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borde V. The multiple roles of the Mre11 complex for meiotic recombination. Chromosome Res. 2007;15:551–563. doi: 10.1007/s10577-007-1147-9. [DOI] [PubMed] [Google Scholar]
- Brachner A, Reipert S, Foisner R, Gotzmann J. LEM2 is a novel MAN1-related inner nuclear membrane protein associated with A-type lamins. J. Cell Sci. 2005;118:5797–5810. doi: 10.1242/jcs.02701. [DOI] [PubMed] [Google Scholar]
- Burge C, Karlin S. Prediction of complete gene structures in human genomic DNA. J. Mol. Biol. 1997;268:78–94. doi: 10.1006/jmbi.1997.0951. [DOI] [PubMed] [Google Scholar]
- Cai M, Huang Y, Ghirlando R, Wilson KL, Craigie R, Clore GM. Solution structure of the constant region of nuclear envelope protein LAP2 reveals two LEM-domain structures: one binds BAF and the other binds DNA. EMBO J. 2001;20:4399–4407. doi: 10.1093/emboj/20.16.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JM, Friedrich NC, Kleinstiver B, Edgell DR. Strand-specific contacts and divalent metal ion regulate double-strand break formation by the GIY-YIG homing endonuclease I-BmoI. J. Mol. Biol. 2007;374:306–321. doi: 10.1016/j.jmb.2007.09.027. [DOI] [PubMed] [Google Scholar]
- Chen IH, Huber M, Guan T, Bubeck A, Gerace L. Nuclear envelope transmembrane proteins (NETs) that are up-regulated during myogenesis. BMC Cell Biol. 2006;7:38. doi: 10.1186/1471-2121-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi YH, Chen ZJ, Jeang KT. The nuclear envelopathies and human diseases. J. Biomed. Sci. 2009;16:96. doi: 10.1186/1423-0127-16-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Jung HY, Kim HS, Cho HG. PhyloDraw: a phylogenetic tree drawing system. Bioinformatics. 2000;16:1056–1058. doi: 10.1093/bioinformatics/16.11.1056. [DOI] [PubMed] [Google Scholar]
- Cserzo M, Eisenhaber F, Eisenhaber B, Simon I. TM or not TM: transmembrane protein prediction with low false positive rate using DAS-TMfilter. Bioinformatics. 2004;20:136–137. doi: 10.1093/bioinformatics/btg394. [DOI] [PubMed] [Google Scholar]
- Dechat T, Gotzmann J, Stockinger A, Harris CA, Talle MA, Siekierka JJ, Foisner R. Detergent-salt resistance of LAP2alpha in interphase nuclei and phosphorylation-dependent association with chromosomes early in nuclear assembly implies functions in nuclear structure dynamics. EMBO J. 1998;17:4887–4902. doi: 10.1093/emboj/17.16.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechat T, Gajewski A, Korbei B, Gerlich D, Daigle N, Haraguchi T, Furukawa K, Ellenberg J, Foisner R. LAP2alpha and BAF transiently localize to telomeres and specific regions on chromatin during nuclear assembly. J. Cell Sci. 2004;117:6117–6128. doi: 10.1242/jcs.01529. [DOI] [PubMed] [Google Scholar]
- Derbyshire V, Kowalski JC, Dansereau JT, Hauer CR, Belfort M. Two-domain structure of the td intron-encoded endonuclease I-TevI correlates with the two-domain configuration of the homing site. J. Mol. Biol. 1997;265:494–506. doi: 10.1006/jmbi.1996.0754. [DOI] [PubMed] [Google Scholar]
- Dorner D, Vlcek S, Foeger N, Gajewski A, Makolm C, Gotzmann J, Hutchison CJ, Foisner R. Lamina-associated polypeptide 2{alpha} regulates cell cycle progression and differentiation via the retinoblastoma-E2F pathway. J. Cell Biol. 2006;173:83–93. doi: 10.1083/jcb.200511149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunin-Horkawicz S, Feder M, Bujnicki JM. Phylogenomic analysis of the GIY-YIG nuclease superfamily. BMC Genomics. 2006;7:98. doi: 10.1186/1471-2164-7-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgell DR. Selfish DNA: homing endonucleases find a home. Curr. Biol. 2009;19:R115–117. doi: 10.1016/j.cub.2008.12.019. [DOI] [PubMed] [Google Scholar]
- Ehrlich VA, Nersesyan AK, Atefie K, Hoelzl C, Ferk F, Bichler J, Valic E, Schaffer A, Schulte-Hermann R, Fenech M, et al. Inhalative exposure to vanadium pentoxide causes DNA damage in workers: results of a multiple end point study. Environ. Health Perspect. 2008;116:1689–1693. doi: 10.1289/ehp.11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis JA, Craxton M, Yates JR, Kendrick-Jones J. Aberrant intracellular targeting and cell cycle-dependent phosphorylation of emerin contribute to the Emery-Dreifuss muscular dystrophy phenotype. J. Cell Sci. 1998;111:781–792. doi: 10.1242/jcs.111.6.781. [DOI] [PubMed] [Google Scholar]
- Evgen’ev MB, Arkhipova IR. Penelope-like elements--a new class of retroelements: distribution, function and possible evolutionary significance. Cytogenet. Genome Res. 2005;110:510–521. doi: 10.1159/000084984. [DOI] [PubMed] [Google Scholar]
- Fekairi S, Scaglione S, Chahwan C, Taylor ER, Tissier A, Coulon S, Dong MQ, Ruse C, Yates JR, 3rd, Russell P, et al. Human SLX4 is a Holliday junction resolvase subunit that binds multiple DNA repair/recombination endonucleases. Cell. 2009;138:78–89. doi: 10.1016/j.cell.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K. LAP2 binding protein 1 (L2BP1/BAF) is a candidate mediator of LAP2- chromatin interaction. J. Cell Sci. 1999;112:2485–2492. doi: 10.1242/jcs.112.15.2485. [DOI] [PubMed] [Google Scholar]
- Gruenbaum Y, Lee KK, Liu J, Cohen M, Wilson KL. The expression, lamin-dependent localization and RNAi depletion phenotype for emerin in C. elegans. J. Cell Sci. 2002;115:923–929. doi: 10.1242/jcs.115.5.923. [DOI] [PubMed] [Google Scholar]
- Gruenbaum Y, Margalit A, Goldman RD, Shumaker DK, Wilson KL. The nuclear lamina comes of age. Nat. Rev. Mol. Cell Biol. 2005;6:21–31. doi: 10.1038/nrm1550. [DOI] [PubMed] [Google Scholar]
- Haraguchi T, Koujin T, Segura-Totten M, Lee KK, Matsuoka Y, Yoneda Y, Wilson KL, Hiraoka Y. BAF is required for emerin assembly into the reforming nuclear envelope. J. Cell Sci. 2001;114:4575–4585. doi: 10.1242/jcs.114.24.4575. [DOI] [PubMed] [Google Scholar]
- Holaska JM, Lee KK, Kowalski AK, Wilson KL. Transcriptional Repressor Germ Cell-less (GCL) and Barrier to Autointegration Factor (BAF) Compete for Binding to Emerin in Vitro. J. Biol. Chem. 2003;278:6969–6975. doi: 10.1074/jbc.M208811200. [DOI] [PubMed] [Google Scholar]
- Huber MD, Guan T, Gerace L. Overlapping functions of nuclear envelope proteins NET25 (Lem2) and emerin in regulation of extracellular signal-regulated kinase signaling in myoblast differentiation. Mol. Cell. Biol. 2009;29:5718–5728. doi: 10.1128/MCB.00270-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Xia L, Chen D, Yang Y, Huang H, Yang L, Zhao Q, Shen L, Wang J. Otefin, a nuclear membrane protein, determines the fate of germline stem cells in Drosophila via interaction with Smad complexes. Dev. Cell. 2008;14:494–506. doi: 10.1016/j.devcel.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Kowalski JC, Belfort M, Stapleton MA, Holpert M, Dansereau JT, Pietrokovski S, Baxter SM, Derbyshire V. Configuration of the catalytic GIY-YIG domain of intron endonuclease I-TevI: coincidence of computational and molecular findings. Nucleic Acids Res. 1999;27:2115–2125. doi: 10.1093/nar/27.10.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- Kudo N, Wolff B, Sekimoto T, Schreiner EP, Yoneda Y, Yanagida M, Horinouchi S, Yoshida M. Leptomycin B inhibition of signal-mediated nuclear export by direct binding to CRM1. Exp. Cell Res. 1998;242:540–547. doi: 10.1006/excr.1998.4136. [DOI] [PubMed] [Google Scholar]
- Lagerback P, Carlson K. Amino acid residues in the GIY-YIG endonuclease II of phage T4 affecting sequence recognition and binding as well as catalysis. J. Bacteriol. 2008;190:5533–5544. doi: 10.1128/JB.00094-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laguri C, Gilquin B, Wolff N, Romi-Lebrun R, Courchay K, Callebaut I, Worman HJ, Zinn-Justin S. Structural characterization of the lem motif common to three human inner nuclear membrane proteins. Structure. 2001;9:503–511. doi: 10.1016/s0969-2126(01)00611-6. [DOI] [PubMed] [Google Scholar]
- Lee KK, Wilson KL. All in the family: evidence for four new LEM-domain proteins Lem2 (NET-25), Lem3, Lem4 and Lem5 in the human genome. Symp. Soc. Exp. Biol. 2004:329–339. [PubMed] [Google Scholar]
- Lee KK, Gruenbaum Y, Spann P, Liu J, Wilson KL. C. elegans nuclear envelope proteins emerin, MAN1, lamin, and nucleoporins reveal unique timing of nuclear envelope breakdown during mitosis. Mol. Biol. Cell. 2000;11:3089–3099. doi: 10.1091/mbc.11.9.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KK, Haraguchi T, Lee RS, Koujin T, Hiraoka Y, Wilson KL. Distinct functional domains in emerin bind lamin A and DNA-bridging protein BAF. J. Cell Sci. 2001;114:4567–4573. doi: 10.1242/jcs.114.24.4567. [DOI] [PubMed] [Google Scholar]
- Letunic I, Copley RR, Schmidt S, Ciccarelli FD, Doerks T, Schultz J, Ponting CP, Bork P. SMART 4.0: towards genomic data integration. Nucleic Acids Res. 2004;32:D142–144. doi: 10.1093/nar/gkh088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber MR, Ma Y, Pannicke U, Schwarz K. The mechanism of vertebrate nonhomologous DNA end joining and its role in V(D)J recombination. DNA Repair (Amst) 2004;3:817–826. doi: 10.1016/j.dnarep.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Lin F, Morrison JM, Wu W, Worman HJ. MAN1, an integral protein of the inner nuclear membrane, binds Smad2 and Smad3 and antagonizes transforming growth factor-beta signaling. Hum. Mol. Genet. 2005;14:437–445. doi: 10.1093/hmg/ddi040. [DOI] [PubMed] [Google Scholar]
- Lin F, Blake DL, Callebaut I, Skerjanc IS, Holmer L, McBurney MW, Paulin-Levasseur M, Worman HJ. MAN1, an inner nuclear membrane protein that shares the LEM domain with lamina-associated polypeptide 2 and emerin. J. Biol. Chem. 2000;275:4840–4847. doi: 10.1074/jbc.275.7.4840. [DOI] [PubMed] [Google Scholar]
- Liu J, Lee KK, Segura-Totten M, Neufeld E, Wilson KL, Gruenbaum Y. MAN1 and emerin have overlapping function(s) essential for chromosome segregation and cell division in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 2003;100:4598–4603. doi: 10.1073/pnas.0730821100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Derbyshire V, Belfort M, Edgell DR. Distance determination by GIY-YIG intron endonucleases: discrimination between repression and cleavage functions. Nucleic Acids Res. 2006;34:1755–1764. doi: 10.1093/nar/gkl079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manilal S, Nguyen TM, Sewry CA, Morris GE. The Emery-Dreifuss muscular dystrophy protein, emerin, is a nuclear membrane protein. Hum. Mol. Genet. 1996;5:801–808. doi: 10.1093/hmg/5.6.801. [DOI] [PubMed] [Google Scholar]
- Margalit A, Segura-Totten M, Gruenbaum Y, Wilson KL. Barrier-toautointegration factor is required to segregate and enclose chromosomes within the nuclear envelope and assemble the nuclear lamina. Proc. Natl. Acad. Sci.USA. 2005;102:3290–3295. doi: 10.1073/pnas.0408364102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margalit A, Brachner A, Gotzmann J, Foisner R, Gruenbaum Y. Barrier-to-autointegration factor--a BAFfling little protein. Trends Cell Biol. 2007;17:202–208. doi: 10.1016/j.tcb.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Markiewicz E, Dechat T, Foisner R, Quinlan RA, Hutchison CJ. Lamin A/C Binding Protein LAP2alpha Is Required for Nuclear Anchorage of Retinoblastoma Protein. Mol. Biol. Cell. 2002;13:4401–4413. doi: 10.1091/mbc.E02-07-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markiewicz E, Tilgner K, Barker N, van de Wetering M, Clevers H, Dorobek M, Hausmanowa-Petrusewicz I, Ramaekers FC, Broers JL, Blankesteijn WM, et al. The inner nuclear membrane protein emerin regulates beta-catenin activity by restricting its accumulation in the nucleus. EMBO J. 2006;25:3275–3285. doi: 10.1038/sj.emboj.7601230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti TM, Fleck O. DNA repair nucleases. Cell Mol Life Sci. 2004;61:336–354. doi: 10.1007/s00018-003-3223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimitou EP, Symington LS. Nucleases and helicases take center stage in homologous recombination. Trends Biochem. Sci. 2009;34:264–272. doi: 10.1016/j.tibs.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Moure CM, Gimble FS, Quiocho FA. The crystal structure of the gene targeting homing endonuclease I-SceI reveals the origins of its target site specificity. J. Mol. Biol. 2003;334:685–695. doi: 10.1016/j.jmb.2003.09.068. [DOI] [PubMed] [Google Scholar]
- Nielsen M, Lundegaard C, Lund O, Petersen T. CpHModels-3.0. Remote homology modeling using structure guided profile sequence alignment and double-sided baseline corrected scoring scheme. Abstract at the CASP8 conference 193.2002. [Google Scholar]
- Nili E, Cojocaru GS, Kalma Y, Ginsberg D, Copeland NG, Gilbert DJ, Jenkins NA, Berger R, Shaklai S, Amariglio N, et al. Nuclear membrane protein LAP2beta mediates transcriptional repression alone and together with its binding partner GCL (germ-cell-less) J. Cell Sci. 2001;114:3297–3307. doi: 10.1242/jcs.114.18.3297. [DOI] [PubMed] [Google Scholar]
- Nishino T, Morikawa K. Structure and function of nucleases in DNA repair: shape, grip and blade of the DNA scissors. Oncogene. 2002;21:9022–9032. doi: 10.1038/sj.onc.1206135. [DOI] [PubMed] [Google Scholar]
- Pan D, Estevez-Salmeron LD, Stroschein SL, Zhu X, He J, Zhou S, Luo K. The integral inner nuclear membrane protein MAN1 physically interacts with the R-Smad proteins to repress signaling by the transforming growth factor-{beta} superfamily of cytokines. J. Biol. Chem. 2005;280:15992–16001. doi: 10.1074/jbc.M411234200. [DOI] [PubMed] [Google Scholar]
- Perrin A, Buckle M, Dujon B. Asymmetrical recognition and activity of the I-SceI endonuclease on its site and on intron-exon junctions. EMBO J. 1993;12:2939–2947. doi: 10.1002/j.1460-2075.1993.tb05956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyatkov KI, Arkhipova IR, Malkova NV, Finnegan DJ, Evgen’ev MB. Reverse transcriptase and endonuclease activities encoded by Penelope-like retroelements. Proc. Natl. Acad. Sci. USA. 2004;101:14719–14724. doi: 10.1073/pnas.0406281101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Munoz P, Malivert L, Derdouch S, Azerrad C, Abramowski V, Revy P, Villartay JP. DNA repair and the immune system: From V(D)J recombination to aging lymphocytes. Eur. J. Immunol. 2007;37(Suppl 1):S71–82. doi: 10.1002/eji.200737396. [DOI] [PubMed] [Google Scholar]
- Rooney S, Chaudhuri J, Alt FW. The role of the non-homologous end-joining pathway in lymphocyte development. Immunol. Rev. 2004;200:115–131. doi: 10.1111/j.0105-2896.2004.00165.x. [DOI] [PubMed] [Google Scholar]
- Schirmer EC, Foisner R. Proteins that associate with lamins: Many faces, many functions. Exp. Cell Re.s. 2007;313:2167–2179. doi: 10.1016/j.yexcr.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Schostak N, Pyatkov K, Zelentsova E, Arkhipova I, Shagin D, Shagina I, Mudrik E, Blintsov A, Clark I, Finnegan DJ, et al. Molecular dissection of Penelope transposable element regulatory machinery. Nucleic Acids Res. 2008;36:2522–2529. doi: 10.1093/nar/gkm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J, Milpetz F, Bork P, Ponting CP. SMART, a simple modular architecture research tool: identification of signaling domains. Proc. Natl. Acad. Sci. USA. 1998;95:5857–5864. doi: 10.1073/pnas.95.11.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz LB, Chehab NH, Malikzay A, Halazonetis TD. p53 binding protein 1 (53BP1) is an early participant in the cellular response to DNA double-strand breaks. J. Cell Biol. 2000;151:1381–1390. doi: 10.1083/jcb.151.7.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaklai S, Somech R, Gal-Yam EN, Deshet-Unger N, Moshitch-Moshkovitz S, Hirschberg K, Amariglio N, Simon AJ, Rechavi G. LAP2zeta binds BAF and suppresses LAP2beta-mediated transcriptional repression. Eur. J. Cell Biol. 2008;87:267–278. doi: 10.1016/j.ejcb.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Shimi T, Koujin T, Segura-Totten M, Wilson KL, Haraguchi T, Hiraoka Y. Dynamic interaction between BAF and emerin revealed by FRAP, FLIP, and FRET analyses in living HeLa cells. J. Struct. Biol. 2004;147:31–41. doi: 10.1016/j.jsb.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Shumaker DK, Lee KK, Tanhehco YC, Craigie R, Wilson KL. LAP2 binds to BAF.DNA complexes: requirement for the LEM domain and modulation by variable regions. EMBO J. 2001;20:1754–1764. doi: 10.1093/emboj/20.7.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowska M, Czapinska H, Bochtler M. Hpy188I-DNA pre- and post-cleavage complexes--snapshots of the GIY-YIG nuclease mediated catalysis. Nucleic Acids Res. 2011;39:1554–1564. doi: 10.1093/nar/gkq821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding JB, Lammers PJ. BLAST Filter and GraphAlign: rule-based formation and analysis of sets of related DNA and protein sequences. Nucleic Acids Res. 2004;32:W26–32. doi: 10.1093/nar/gkh459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard BL. Homing endonuclease structure and function. Q. Rev. Biophys. 2005;38:49–95. doi: 10.1017/S0033583505004063. [DOI] [PubMed] [Google Scholar]
- Stoddard BL. Homing endonucleases: from microbial genetic invaders to reagents for targeted DNA modification. Structure. 2011;19:7–15. doi: 10.1016/j.str.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan DM, Glisson BS, Hodges PK, Smallwood-Kentro S, Ross WE. Proliferation dependence of topoisomerase II mediated drug action. Biochemistry. 1986;25:2248–2256. doi: 10.1021/bi00356a060. [DOI] [PubMed] [Google Scholar]
- Svendsen JM, Smogorzewska A, Sowa ME, O’Connell BC, Gygi SP, Elledge SJ, Harper JW. Mammalian BTBD12/SLX4 assembles a Holliday junction resolvase and is required for DNA repair. Cell. 2009;138:63–77. doi: 10.1016/j.cell.2009.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodor L, Shoham J, Berger R, Gokkel E, Trachtenbrot L, Simon AJ, Brok-Simon F, Nir U, Ilan E, Zevin-Sonkin D, et al. Ubiquitous expression of a cloned murine thymopoietin cDNA. Acta Haematol. 1997;97:153–163. doi: 10.1159/000203673. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truglio JJ, Rhau B, Croteau DL, Wang L, Skorvaga M, Karakas E, DellaVecchia MJ, Wang H, Van Houten B, Kisker C. Structural insights into the first incision reaction during nucleotide excision repair. EMBO J. 2005;24:885–894. doi: 10.1038/sj.emboj.7600568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbert S, Antonin W, Platani M, Mattaj IW. The inner nuclear membrane protein Lem2 is critical for normal nuclear envelope morphology. FEBS Lett. 2006;580:6435–6441. doi: 10.1016/j.febslet.2006.10.060. [DOI] [PubMed] [Google Scholar]
- Umland TC, Wei SQ, Craigie R, Davies DR. Structural basis of DNA bridging by barrier-to-autointegration factor. Biochemistry. 2000;39:9130–9138. doi: 10.1021/bi000572w. [DOI] [PubMed] [Google Scholar]
- Van Roey P, Meehan L, Kowalski JC, Belfort M, Derbyshire V. Catalytic domain structure and hypothesis for function of GIY-YIG intron endonuclease ITevI. Nat. Struct. Biol. 2002;9:806–811. doi: 10.1038/nsb853. [DOI] [PubMed] [Google Scholar]
- Vlcek S, Foisner R. Lamins and lamin-associated proteins in aging and disease. Curr. Opin. Cell Biol. 2007;19:298–304. doi: 10.1016/j.ceb.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Wagner N, Krohne G. LEM-Domain proteins: new insights into lamin-interacting proteins. Int. Rev. Cytol. 2007;261:1–46. doi: 10.1016/S0074-7696(07)61001-8. [DOI] [PubMed] [Google Scholar]
- Worman HJ, Bonne G. “Laminopathies”: a wide spectrum of human diseases. Exp. Cell Res. 2007;313:2121–2133. doi: 10.1016/j.yexcr.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoakum GH, Grossman L. Identification of E. coli uvrC protein. Nature. 1981;292:171–173. doi: 10.1038/292171a0. [DOI] [PubMed] [Google Scholar]
- Yuki D, Lin YM, Fujii Y, Nakamura Y, Furukawa Y. Isolation of LEM domain-containing 1, a novel testis-specific gene expressed in colorectal cancers. Oncol. Rep. 2004;12:275–280. [PubMed] [Google Scholar]
- Zheng R, Ghirlando R, Lee MS, Mizuuchi K, Krause M, Craigie R. Barrier-to-autointegration factor (BAF) bridges DNA in a discrete, higher-order nucleoprotein complex. Proc. Natl. Acad. Sci. USA. 2000;97:8997–9002. doi: 10.1073/pnas.150240197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.