Background: The Ca2+-induced Ca2+ release channel (mCrC) of Drosophila mitochondria is similar to the permeability transition pore (PTP).
Results: mCrC is modulated by PTP effectors and Drosophila F-ATPase forms 53-pS channels.
Conclusion: F-ATPase mediates Ca2+-induced Ca2+ release in Drosophila mitochondria.
Significance: Channel formation by F-ATPases has been conserved in evolution, but species-specific features exist that may underscore different roles in different organisms.
Keywords: calcium, Calcium Transport, Drosophila, Mitochondria, Permeability
Abstract
Mitochondria of Drosophila melanogaster undergo Ca2+-induced Ca2+ release through a putative channel (mCrC) that has several regulatory features of the permeability transition pore (PTP). The PTP is an inner membrane channel that forms from F-ATPase, possessing a conductance of 500 picosiemens (pS) in mammals and of 300 pS in yeast. In contrast to the PTP, the mCrC of Drosophila is not permeable to sucrose and appears to be selective for Ca2+ and H+. We show (i) that like the PTP, the mCrC is affected by the sense of rotation of F-ATPase, by Bz-423, and by Mg2+/ADP; (ii) that expression of human cyclophilin D in mitochondria of Drosophila S2R+ cells sensitizes the mCrC to Ca2+ but does not increase its apparent size; and (iii) that purified dimers of D. melanogaster F-ATPase reconstituted into lipid bilayers form 53-pS channels activated by Ca2+ and thiol oxidants and inhibited by Mg2+/γ-imino ATP. These findings indicate that the mCrC is the PTP of D. melanogaster and that the signature conductance of F-ATPase channels depends on unique structural features that may underscore specific roles in different species.
Introduction
Mitochondria of Drosophila melanogaster possess an array of Ca2+ transport pathways, i.e. the Ca2+ uniporter MCU, the Na+/Ca2+ exchanger NCLX, and a Na+-insensitive Ca2+ efflux system (1), that display the same features as those observed in mammalian mitochondria (2–5). An important difference, however, exists.
In mammalian mitochondria, Ca2+ and Pi induce opening of the permeability transition pore (PTP),2 a 500-pS channel that forms from the F-ATPase under conditions of oxidative stress (6). The PTP is permeable to sucrose, as is the pore of yeast mitochondria (7, 8) where Pi has an inhibitory effect and F-ATPase forms 300-pS channels (9). Also in D. melanogaster, Ca2+ opens a mitochondrial permeability pathway, resulting in depolarization and Ca2+ release (1); however, this Ca2+-induced Ca2+ release channel (mCrC, which like in yeast is inhibited rather than activated by Pi) is impermeable to sucrose, suggesting that its size may be considerably smaller than that of the PTP (1).
The recent demonstration that the PTP forms from the F-ATPase (6, 9) provides a new framework to analyze the nature of the mCrC of D. melanogaster. Here we have assessed whether F-ATPase dimers purified from Drosophila mitochondria possess channel activity. Our findings provide novel information on the channel function of F-ATPases, establish that the mCrC is the PTP of D. melanogaster, and shed new light on its possible role in regulation of Ca2+ homeostasis (10, 11).
EXPERIMENTAL PROCEDURES
Cell Cultures
Drosophila S2R+ cells (12, 13) were cultured in Schneider's insect medium (Life Technologies) supplemented with 10% heat-inactivated FBS (Life Technologies) and kept at 25 °C. Culture medium for the transfected S2R+pActCyPD-HA/pCoPuro cells was supplemented with 8 μg/ml puromycin.
Subcellular Fractionation
Cells were lysed in a medium containing 10 mm Tris-HCl, pH 6.7, 10 mm KCl, 150 μm MgCl2 supplemented with protease and phosphatase inhibitor cocktails (Sigma) for 30 min on ice, followed by passage through a 26-gauge × 0.5-inch syringe (Artsana). Sucrose was then added at a final concentration of 250 mm, and lysates were centrifuged three times at 2,200 × g for 10 min at 4 °C to remove nuclei and cell debris. Mitochondria were then sedimented at 8,200 × g for 10 min at 4 °C.
Cell Permeabilization
Cells were detached with a sterile cell scraper, centrifuged at 200 × g for 10 min, and washed twice with 130 mm KCl, 10 mm Mops-Tris, pH 7.4 (KCl medium) containing 10 μm EGTA-Tris. The resulting pellet was resuspended in KCl medium containing 150 μm digitonin and 1 mm EGTA-Tris and incubated for 20 min on ice (6 × 107 cells × ml−1). Cells were then diluted 1:5 in KCl medium containing 10 μm EGTA-Tris and centrifuged at 200 × g in a refrigerated centrifuge (4 °C) for 6 min. The final pellet was resuspended in KCl medium containing 10 μm EGTA-Tris at 4 × 108 cells × ml−1 and kept on ice.
Isolation of Mitochondria from Flies
Mitochondria were prepared from whole flies or 3rd instar larvae by differential centrifugation exactly as described (14).
Mitochondrial Membrane Potential and Ca2+ Retention Capacity
Mitochondrial membrane potential was measured using a Perkin-Elmer LS50B spectrofluorometer based on the fluorescence quenching of rhodamine 123 (15) at excitation and emission wavelengths of 503 and 523 nm, respectively, with the slit width set at 2.5 nm. Twenty million permeabilized S2R+ cells were added to the cuvette in a total volume of 2 ml. Further additions were as indicated in the figure legends. Extramitochondrial Ca2+ was measured based on Calcium Green 5N (Molecular Probes) fluorescence (15) at excitation and emission wavelengths of 485 and 538 nm, respectively, using a Fluoroskan Ascent FL plate reader (Thermo Scientific) with either 200 μg of isolated mitochondria or 2 × 106 permeabilized cells in a total volume of 200 μl/well. For measurements of Ca2+ retention capacity (CRC) and membrane potential during ATP synthesis at constant [ADP], the incubation medium contained 0.1 m glucose, 80 mm KCl, 10 mm Mops-Tris, pH 7.4, 5 mm succinate-Tris, 4 mm MgCl2, 1 mm Pi-Tris, 0.5 mm NADP+, 0.4 mm ADP-Tris, 50 μm P1,P5-di(adenosine-5′) pentaphosphate, 10 μm EGTA-Tris, 2 μm rotenone, 4 units/ml glucose-6-phosphate dehydrogenase, 3 units/ml hexokinase, and 0.5 μm Calcium Green 5N or 0.15 μm rhodamine 123. For measurements of CRC and membrane potential during ATP hydrolysis at constant [ATP], the incubation medium contained 0.1 m sucrose, 80 mm KCl, 10 mm Mops-Tris, pH 7.4, 4 mm MgCl2, 2 mm phosphocreatine, 1 mm Pi-Tris, 0.4 mm ATP-Tris, 10 μm EGTA-Tris, 2 μm rotenone, 1.5 units/ml creatine kinase, and 0.5 μm Calcium Green 5N or 0.15 μm rhodamine 123. For all other measurements, the composition of the assay medium is specified in the figure legends, and further additions were as indicated.
Mitochondrial Swelling Assay
Absorbance at 540 nm was monitored with a Multiskan Ex (Thermo Scientific) plate reader. Either 200 μg of isolated mitochondria or 2 × 106 permeabilized cells were added to each well in a total volume of 200 μl. Incubation media are specified in the figure legends.
Fly Stocks and Breeding Conditions
Flies were raised on standard cornmeal medium and were maintained at 23 °C, 70% relative humidity, on a 12-h light:12-h dark cycle. The fly strain used in all experiments was white1118 and was obtained from the Bloomington Stock Center.
Western Blotting
Cell or mitochondrial pellets were lysed in 150 mm NaCl, 20 mm Tris, pH 7.4, 5 mm EDTA-Tris, 10% glycerol, 1% Triton X-100, supplemented with protease and phosphatase inhibitor cocktails (Sigma), and kept on ice for 20 min. Suspensions were then centrifuged at 18,000 × g for 25 min at 4 °C, and the supernatants were solubilized in Laemmli gel sample buffer. For separation of F-ATPase dimers, the Laemmli buffer was directly added to the dimer bands eluted from a BN-PAGE gel. Samples were separated by 15% SDS-PAGE and transferred electrophoretically to nitrocellulose membranes using an SE400 vertical electrophoresis unit (Hoefer). Western blotting was performed in PBS containing 3% nonfat dry milk with polyclonal goat anti-actin (Santa Cruz Biotechnology), polyclonal rabbit anti-caspase-3 (Cell Signaling), monoclonal mouse anti-CyPD (Calbiochem), monoclonal mouse anti-HA (Sigma), monoclonal mouse anti-F-ATPase subunit β (Abcam), polyclonal rabbit anti-MCU (Sigma), or polyclonal rabbit anti-TOM20 (Santa Cruz Biotechnology) antibodies.
BN-PAGE
Pellets of mitochondria isolated from adult white1118 flies were suspended at 10 mg × ml−1 in 1× native PAGE sample buffer (Invitrogen) supplemented with protease inhibitor mixture (Sigma), solubilized with 2% (w/v) digitonin, and immediately centrifuged at 100,000 × g for 25 min at 4 °C. The supernatants were supplemented with native PAGE 5% G-250 sample additive (Invitrogen) and quickly loaded onto a blue native polyacrylamide 3–12% gradient gel (Invitrogen). After electrophoresis, gels were processed, and F-ATPase dimers were prepared for bilayer experiments exactly as described (9).
Immunofluorescence
One day before the experiments, stably transfected S2R+pActCyPD-HA cells were seeded on sterilized 13-mm round glass coverslips in a 24-well tissue culture plate at 2 × 105 cells/well in 0.5 ml of culture medium. On the day of the experiment, cells were washed once with PBS and incubated for 20 min at room temperature with 0.5 ml of serum-free Schneider's medium supplemented with 1 μg/ml CsH and 100 nm MitoTracker Red CMXRos (Molecular Probes). Cells were fixed with 4% paraformaldehyde for 20 min at room temperature, permeabilized with 50 mm NH4Cl in PBS + 0.1% Triton X-100 for 5 min at room temperature, and blocked with PBS containing 3% goat serum for 1 h at room temperature with PBS washes between each step. Monoclonal anti-HA (clone HA-7, Sigma) in PBS with 2% goat serum was added, and incubation was carried out overnight at 4 °C. On the next day, cells were washed with PBS, and the immunoreaction was revealed with FITC-conjugated anti-mouse IgG (Fab-specific, Sigma) in PBS with 2% goat serum for 45 min at room temperature. Coverslips were examined with an Olympus epifluorescence microscope at 60× magnification.
Cell Transfection, Plasmids, and Constructs
A construct of human cyclophilin (CyP) D cDNA carrying a N-terminal mitochondrial targeting sequence from Drosophila Hsp60 (a mitochondrial matrix protein) and a C-terminal HA tag was generated by PCR using total cDNA of the human osteosarcoma cell line HQB17 as template. Oligonucleotides were designed using the on-line tool Primer-BLAST (16). Primer sequences were 5′-ctggtaccatgttccgtttgccagtttcgcttgctcgctcctccattagccgccagttggccatgcgcggctatgccaaggatgtgtgcagcaagggctccggcgacccg-3′(forward) and 5′-ccgagctcttaagcgtaatctggaacatcgtatgggtagctcaactggccacagtctgtgatg-3′ (reverse). To generate a stable polyclonal cell population constitutively expressing human CyPD in Drosophila S2R+ cells, the construct was inserted into the pAct5c Drosophila expression vector under control of the Drosophila actin 5c promoter (a gift of M. F. Ceriani, Laboratorio de Genética del Comportamiento, Fundación Instituto Leloir and Instituto de Investigaciones Bioquímicas, Buenos Aires, Argentina) co-transfected with the selection vector pCoPuro (Addgene plasmid 17533), generated by Dr. Francis Castellino (17). Two million cells/well were plated in a 6-well tissue culture plate in 2 ml of culture medium/well. Cells were incubated for 5 h at room temperature and then transfected with the Effectene transfection reagent kit (Qiagen) with a ratio of selection vector (pCoPuro) to expression vector (pActCyPD-HA) of 1:20. After 3 days, the medium was replaced with culture medium containing 8 μg/ml puromycin for selection.
Electrophysiology
Planar lipid bilayer experiments were performed as described in Ref. 18. Briefly, bilayers of 150–200 picofarads capacitance were prepared using purified soybean asolectin. The standard experimental medium was 100 mm KCl, 10 mm Hepes, pH 7.4. All reported voltages refer to the cis chamber, zero being assigned to the trans (grounded) side. Currents are considered as positive when carried by cations flowing from the cis to the trans compartment. Freshly prepared F-ATPase dimers were added to the cis side. No current was observed when PTP activators were added to the membrane in the absence of F-ATPase dimers. The I-V curve was obtained in the range of −80 to +40 mV from three independent experiments and contained current values that were measured manually (n > 30 for each potential) using the PCLAMP8.0 program set. The conductance value thus obtained was 53.0 ± 7.2 pS.
Reagents and Statistics
All chemicals were of the highest purity commercially available. Reported results are typical of at least three replicates for each condition, and error bars refer to the S.E. p values were calculated with Student's t test.
RESULTS AND DISCUSSION
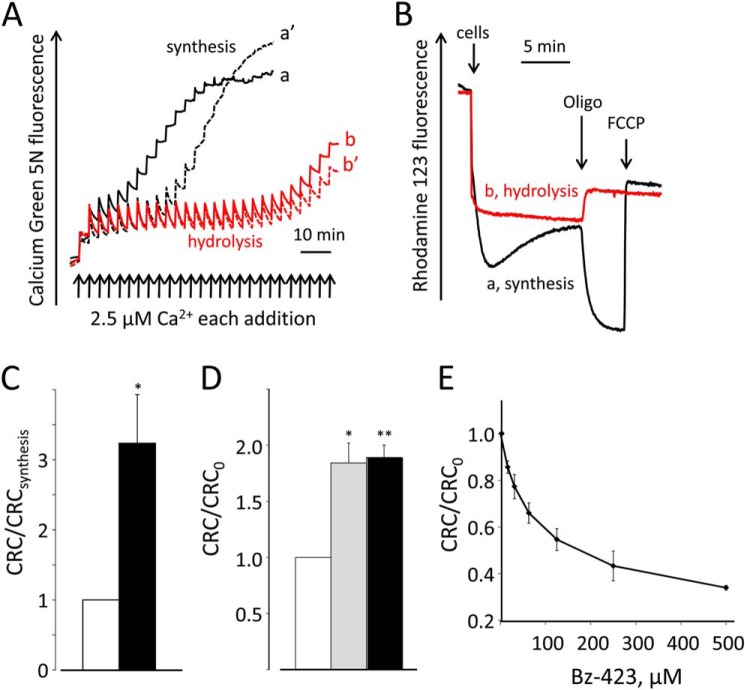
Drosophila S2R+ cells were permeabilized with digitonin and energized with succinate in the presence of ADP and Pi; an ATP-hydrolyzing system was present to maintain constant [ADP] and synchronize F-ATPases in the direction of ATP synthesis. A train of Ca2+ pulses was then added to determine the CRC, i.e. the threshold matrix Ca2+ load necessary to open the mCrC (1) (Fig. 1A, trace a). Next, we incubated permeabilized S2R+ cells in the absence of respiratory substrates, and then energized mitochondria with ATP in the presence of an ATP-regenerating system to maintain constant [ATP] and synchronize F-ATPases in the direction of ATP hydrolysis (Fig. 1A, trace b). The CRC was much larger in ATP-hydrolyzing than in ATP-synthesizing mitochondria (Fig. 1, compare traces a and b). Due to formation of glucose-6-P, the concentration of Pi decreases in the ATP synthesis experiments, and conversely, due to hydrolysis of P-creatine, it increases in the ATP-hydrolyzing protocols. However, in the presence of oligomycin, the CRC was only slightly higher than that observed during ATP synthesis (Fig. 1, trace a′), and in the presence of 5 mm Pi under conditions of ATP hydrolysis, the CRC was indistinguishable from that observed at 1 mm Pi (Fig. 1, trace b′). Thus, the difference between ATP-synthesizing and ATP-hydrolyzing mitochondria cannot be explained by differences in the Pi concentration. Note that the difference in CRC was also not due to different levels of the membrane potential, which was the same in ATP-synthesizing (Fig. 1B, trace a) and ATP-hydrolyzing mitochondria (Fig. 1B, trace b), and responded appropriately to oligomycin and uncoupler.
FIGURE 1.
Effect of ATPase catalysis and Bz-423 on the CRC of permeabilized S2R+ cells. A, digitonin-permeabilized S2R+ cells were incubated as detailed under “Experimental Procedures” either with succinate and ADP plus an ADP-regenerating system in the absence (trace a) or presence of 1 μg/ml oligomycin (trace a′) or with ATP plus an ATP-regenerating system with 1 mm (trace b) or 5 mm Pi (trace b′); extramitochondrial Ca2+ was monitored with 0.5 μm Calcium Green 5N, and the CRC was determined by the stepwise addition of 2.5 μm Ca2+ pulses (arrows). B, conditions for traces a and b were as described in the corresponding traces of panel A except that Calcium Green 5N was replaced with 0.15 μm rhodamine 123; where indicated, 2 × 107 permeabilized S2R+ cells, 1 μg/ml oligomycin (oligo), and 1 μm carbonylcyanide-p-trifluoromethoxyphenyl hydrazone (FCCP) were added. C, the amount of Ca2+ accumulated prior to onset of Ca2+-induced Ca2+ release was normalized to that obtained in ATP-synthesizing conditions (CRCsynthesis) in protocols identical to those of panel A; n = 6; *, p < 0.05. D, permeabilized S2R+ cells were incubated in 0.1 m sucrose, 80 mm KCl, 10 mm Mops-Tris, pH 7.4, 5 mm succinate-Tris, 4 mm MgCl2, 1 mm Pi-Tris, 10 μm EGTA-Tris, 2 μm rotenone, 1 μg/ml oligomycin, and 0.5 μm Calcium Green 5N, without further additions (open bar) or in the presence of 0.4 mm ADP (gray bar) or 0.4 mm ATP (closed bar). Data are normalized for the CRC determined in the absence of adenine nucleotides (CRC0); n = 3; *, p < 0.05, **, p < 0.005. E, digitonin-permeabilized S2R+ cells were incubated in 130 mm KCl, 10 mm Mops-Tris, 5 mm succinate-Tris, 1 mm Pi-Tris, 10 μm EGTA-Tris, 2 μm rotenone, and 0.5 μm Calcium Green 5N in the presence of the stated Bz-423 concentrations. Data are normalized for the CRC obtained in the absence of Bz-423 (CRC0). Error bars in C–E indicate S.E.
The threshold Ca2+ was increased 3-fold in ATP-hydrolyzing relative to ATP-synthesizing mitochondria (Fig. 1C), and the effect was not due to the different nucleotide as such because ADP and ATP displayed indistinguishable effects on the CRC when F-ATPase catalysis was blocked with oligomycin in the presence of Mg2+, and mitochondria were energized with succinate (Fig. 1D). We could also exclude participation of the endoplasmic reticulum because Ca2+ uptake was not observed in the presence of ATP plus oligomycin (results not shown; see Ref. 6). The intriguing effect of enzyme catalysis described in Fig. 1A is also observed for the mammalian PTP (6) and represents a first indication that the mCrC may originate from the F-ATPase as well. This effect could be due to the different conformations of F-ATPase during ATP synthesis and hydrolysis (see e.g. Ref. 19) and suggests that the sense of rotation of F-ATPase influences the mCrC of Drosophila mitochondria, possibly by modulating accessibility of Ca2+-sensitive site(s) that trigger channel opening (20).
The well characterized F-ATPase inhibitor Bz-423 binds the OSCP subunit of the enzyme in mammalian mitochondria (21) and inhibits both ATP synthesis and hydrolysis (22) while sensitizing the PTP to opening (6). Interestingly, the Ca2+ load necessary to trigger mCrC opening was decreased by Bz-423 in a concentration-dependent manner (Fig. 1E).
CyPD, a key regulator of the mammalian PTP, also binds the OSCP subunit of the F-ATPase, presumably at the same site as Bz-423, because the latter displaces CyPD from the enzyme complex (6). CyPD binding can be selectively inhibited by cyclosporin A (CsA) or by genetic ablation of CyPD, and both manipulations remarkably desensitize the PTP to Ca2+ in that its opening requires about twice the Ca2+ load necessary to open the PTP in untreated mitochondria (23). The Drosophila genome encodes 14 different CyPs (24). D. melanogaster CyP1 has an N-terminal sequence that according to Mitoprot (25) confers high probability of mitochondrial import, yet full sequence analysis led to the conclusion that no mitochondrial CyP is present in this species (24). Consistent with this conclusion, we could not detect mitochondrial localization of a CyP1-GFP fusion protein in S2R+ cells (results not shown).
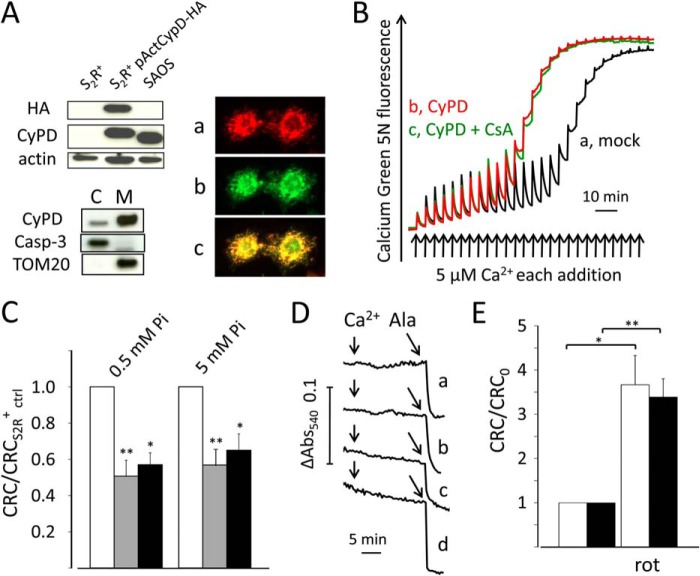
We prepared a cDNA construct with an N-terminal Drosophila mitochondrial targeting sequence followed by the human CyPD coding sequence and by a C-terminal HA tag. Transfected cells expressed the human CyPD construct, which was recognized by both the CyPD and the HA antibodies, with the increase of molecular weight expected of the HA tag (Fig. 2A, top left panel). The protein largely localized to mitochondria as judged (i) by Western blot analysis of cytosolic and mitochondrial fractions after subcellular fractionation (Fig. 2A, bottom left panel); and (ii) by colocalization with MitoTracker Red CMXRos in fixed cells immunostained with a mouse anti-HA antibody followed by a secondary fluoresceinated antibody against mouse IgG (Fig. 2A, right panel).
FIGURE 2.
Effect of CyPD expression and rotenone on permeabilized S2R+ cells and Drosophila mitochondria. A, top left, Western blot analysis with antibodies against CyPD, HA, and actin of total cell lysates from non-transfected S2R+ cells (S2R+), S2R+ cells stably transfected with pActCyPD-HA and pCoPuro vectors (S2R+ pActCyPD-HA), and human SAOS osteosarcoma cells (SAOS). Bottom left, Western blot analysis with antibodies against CyPD, caspase-3 (Casp-3), and TOM20 of cytosolic (C) and mitochondrial (M) fractions from S2R+pActCyPD-HA cells. Right, subcellular localization of CyPD in S2R+pActCyPD-HA cells determined after staining with MitoTracker Red CMXRos (panel a, red fluorescence) and an antibody against the HA tag followed by FITC-conjugated anti-mouse IgG (panel b, green fluorescence); the merged image (panel c) shows colocalization of the fluorescence signals. B, digitonin-permeabilized S2R+ cells transfected either with pActCyPD-HA expression vector and pCoPuro selection vector (trace b, CyPD) or with the selection vector alone (S2R+ ctrl, trace a, mock) were incubated in 250 mm sucrose, 10 mm Mops-Tris, pH 7.4, 5 mm glutamate-Tris, 2.5 mm malate-Tris, 0.5 mm Pi-Tris, 10 μm EGTA-Tris, and 0.5 μm Calcium Green 5N without further additions or in the presence of 0.8 μm CsA (trace c, CyPD + CsA). Where indicated, 5 μm Ca2+ pulses were added (arrows). C, CRC was measured in S2R+ ctrl cells (open bars) and in S2R+pActCyPD-HA cells in the presence or absence of 0.8 μm CsA (gray and closed bars, respectively). CRC was normalized to that obtained in non-transfected cells (CRCS2R+ ctrl); n = 7; *, p < 0.05, **, p < 0.005. D, permeabilized S2R+ ctrl cells (trace a) or S2R+pActCyPD-HA cells (trace b) were incubated as in panel B except that Calcium Green 5N was omitted; mitochondria isolated from white1118 Drosophila 3rd instar larvae (trace c), or adult flies (trace d) were incubated in 250 mm sucrose, 10 mm Mops-Tris, 5 mm succinate-Tris, 1 mm Pi-Tris, 10 μm EGTA-Tris, 2 μm rotenone, pH 7.4. The Ca2+ load necessary to open the mCrC was determined prior to each experiment, and the bolus Ca2+ addition was 150% of this amount, i.e. 127.5 (trace a), 82.5 (trace b), 18.8 (trace c) or 11.3 μm Ca2+ (trace d) followed by 10 μm alamethicin (Ala). Abs, absorbance. E, CRC of mitochondria from white1118 adult flies (open bars) or from 3rd instar larvae (closed bars) was determined in the absence or presence of 2 μm rotenone (rot), and values were normalized to those observed in the absence of rotenone (CRC0); n = 5; *, p < 0.05, **, p < 0.005. The incubation medium was as in panel D except that 0.5 μm Calcium Green 5N was added. Error bars in C and E indicate S.E.
Analysis of the CRC in cells co-transfected with the expression vector pActCyPD-HA and the pCoPuro selection vector as compared with cells containing only the selection vector showed that CyPD expression reduces the threshold Ca2+ load required to induce Ca2+ release (Fig. 2B, compare traces a and b). The effect of CyPD could not be counteracted by CsA (Fig. 2B, trace c), however, irrespective of the concentration of Pi (Fig. 2C), which in mammalian mitochondria favors CyPD binding to the F-ATPase, resulting in pore sensitization to Ca2+ (26, 27). The reason why CsA does not inhibit the mCrC after enforced expression of CyPD remains unclear, but we note (i) that D. melanogaster OSCP has a larger number of negative charges in the putative CyPD binding region, which could increase binding affinity; and (ii) that PTP inhibition does not depend on inhibition of CyPD enzymatic activity (28).
At variance from the PTP, the Drosophila mCrC is impermeable to sucrose (1). Of note, the size of the Drosophila mCrC was unaffected by expression of human CyPD because no Ca2+-induced swelling could be observed after the addition of enough Ca2+ to induce mCrC opening (not shown) irrespective of whether naive (Fig. 2D, trace a) or CyPD-expressing permeabilized cells (Fig. 2D, trace b) were used. S2R+ cells were derived from late embryonic stages (12). To assess whether the low exclusion size and inhibitory response to Pi of the mCrC are restricted to early developmental stages, or rather are defining properties of the Drosophila channel, we also measured the swelling response in mitochondria isolated from white1118 3rd instar larvae or adult flies. Neither mitochondrial preparation underwent Ca2+-induced swelling, which could instead be readily induced by the addition of the pore-forming peptide alamethicin (Fig. 2D, traces c and d).
In the absence of rotenone, succinate induces reverse electron flow through complex I and leads to generation of reactive oxygen species (29). Rotenone is a potent inhibitor of the mammalian PTP when succinate is used as the substrate (30), and inhibition of reverse electron flow at complex I provides a plausible mechanism for this inhibition because reactive oxygen species increase the probability of pore opening through thiol oxidation (31, 32). Remarkably, rotenone increased nearly 4-fold the CRC of Drosophila mitochondria from both larvae and adults (Fig. 2E). Thus, lack of swelling after mCrC opening and sensitivity to rotenone are defining features of Drosophila mitochondria irrespective of developmental stage.
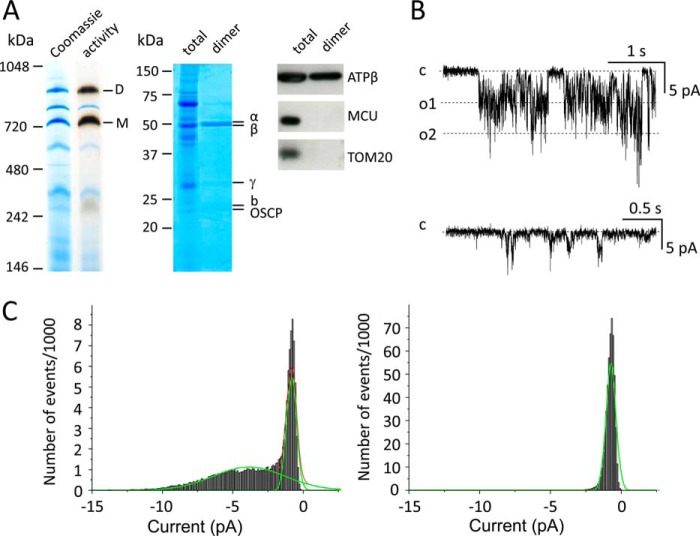
From these experiments, it appears extremely plausible that the mCrC is the PTP of Drosophila mitochondria despite its smaller exclusion size. Given that both the mammalian and yeast F-ATPase form channels (6, 9) whose electrophysiological features are indistinguishable from those of the mitochondrial megachannel (the electrophysiological equivalent of the PTP) (33–38), we next sought to test whether Drosophila F-ATPase forms channels, as well as the features of those channels. We purified mitochondria from adult white1118 flies, separated proteins by BN-PAGE after digitonin extraction, and identified F-ATPase monomers and dimers by in-gel activity staining (Fig. 3A, left lanes). SDS-PAGE analysis of excised ATPase dimers from the BN-PAGE revealed the typical subunit pattern of the enzyme complex after staining with Coomassie Colloidal Blue (Fig. 3A, middle lanes). Western blot analysis of the same preparation revealed a lack of contamination by inner membrane MCU and outer membrane TOM20 (Fig. 3A, right lanes).
FIGURE 3.
Ca2+-induced currents by F-ATPase dimers reconstituted in planar lipid bilayers. A, mitochondria from adult white1118 Drosophila flies were isolated, extracted with digitonin, and subjected to BN-PAGE as detailed under “Experimental Procedures.” Left lanes, F-ATPase dimers (D) and monomers (M) were identified by Coomassie Blue (Coomassie) and in-gel activity staining (activity). Central lanes, total mitochondrial extracts (total) or dimers excised from the BN-PAGE gels (dimer) were subjected to SDS-PAGE and stained with colloidal Coomassie Blue; α, β, γ, b, and OSCP refer to the position of the corresponding subunits of F-ATP synthase. Right lanes, Western blot analysis of total lysates (total) and F-ATPase dimers (dimer) for F-ATPase β subunit (ATPβ), MCU, and TOM20. F-ATPase dimers eluted from BN-PAGE (see left panel) were incorporated in planar lipid bilayers. B, top recording, representative current trace recorded at −100 mV (cis) (conductance = 53.0 ± 7.2 pS, established from the slope of I-V curves obtained from three independent experiments) following the addition of 2 mm Ca2+ plus 0.2 mm phenylarsine oxide, 0.125 mm Bz-423, and 10 μm copper-o-phenanthroline (added to the cis side) in a medium containing 100 mm KCl, 10 mm Hepes, pH 7.4. Bottom recording, effect of 0.8 mm Mg2+ (added to the cis side) and 0.1 mm γ imino ATP (added to both sides) on channel activity (−100 mV). C, amplitude histograms obtained from recordings in the presence of Ca2+, Bz-423, and oxidants (left panel) and after the addition of Mg2+/γ imino ATP (right panel). Gaussian fittings (green lines) were obtained using the Origin 6.1 Program Set.
The dimer preparation was eluted from the gel and incorporated into asolectin planar bilayers for determination of channel activity (6). The addition of Ca2+, Bz-423, phenylarsine oxide, and copper-o-phenanthroline induced opening of a channel with a prevalent single channel conductance of 53 pS (Fig. 3, B and C), which was consistently observed in different dimer preparations. The addition of γ-imino ATP and Mg2+ induced channel closure within a few seconds, as also demonstrated by the amplitude histograms obtained from current traces before and after the addition of the modulators (Fig. 3C). Further electrophysiological experiments confirmed that Drosophila F-ATPase dimers allow the passage of Ca2+ currents (results not shown). Thus, under conditions of oxidative stress and in the presence of Ca2+ and Bz-423, the F-ATPase of Drosophila forms channels whose conductance is 10-fold smaller than that of the mammalian PTP (6) and 6-fold smaller than that of yeast (9). These features are a perfect match to those defined for the mCrC in permeabilized S2R+ cells (1) and leave little doubt that the mCrC is the PTP of D. melanogaster. Thus, formation of channels that require Ca2+ and oxidation of critical thiol groups is a conserved feature of F-ATPases across species (6, 9, 20).
The finding that the Drosophila channel has a unit conductance of 53 pS heavily bears on the nature of the permeability pathway of the PTP. Jonas and co-workers (39) have recently proposed that the pore forms within F0 after Ca2+-dependent expulsion of F1, which would then be closed by the β subunit. F0 is formed by identical c-subunits arranged to form a barrel, the c-ring, whose rotation is tightly linked to the proton gradient. The number of c-subunits per F-ATPase monomer varies between species. In vertebrates and most invertebrates the c-ring has 8 c-subunits, whereas in prokaryotes, chloroplasts and fungi c-rings of 10–15 subunits have been observed (40). The N-terminal α-helix of members with c8-rings has 3 conserved Ala residues at positions 13, 19, and 23 (bovine numbering), which are essential to avoid side chain clashes (40), and the C-terminal α-helix has 1 conserved Lys residue at position 43, which is trimethylated in humans, pig, sheep and rabbit. This Lys appears to be the binding site for cardiolipin, which allows tight packing of c8-rings and is essential for enzyme activity and stability (41, 42). All these residues are conserved in Drosophila F-ATPase (40), which is consistently affected by cardiolipin loss (43). These structural features indicate that the Drosophila complex belongs to the c8-ring F-ATPase set. It is then difficult to see how a pore forming within c-rings of the same size could mediate formation of a channel with a conductance of 500 pS in mammals and of a mere 53 pS in Drosophila, and of 300 pS in yeast where the c-ring has 10 subunits (40). Our findings are therefore not consistent with the idea that PTP channel forms within the c-ring (39), whose actual conductance and Ca2+ dependence remain controversial (44–46); see also Refs. 47–49 for discussion.
Given that we readily observe channel formation with F-ATPase dimers but not monomers (6, 9), and that dimerization-resistant yeast strains display resistance to PTP opening (9), our working hypothesis is that the pathway for solute permeation forms between dimers (or higher order structures) (20). Intriguingly, cryoelectron tomography established that F-ATPase dimers from Drosophila flight muscle have a unique supramolecular structure, with two parallel rows at the high-curvature edge of cristae vesicles (43). It is possible that this supramolecular assembly prevents formation of high-conductance channels both in the native membrane and during electrophysiological experiments, where dimers could in principle be organized in higher order structures.
Depending on the open time, the mammalian PTP could be involved both in apoptosis induction and in Ca2+ homeostasis (50). Prolonged openings cause mitochondrial depolarization, osmotic swelling, outer mitochondrial membrane rupture, and release of apoptogenic proteins such as cytochrome c; transient openings, on the other hand, may be involved in physiological Ca2+ homeostasis and may protect mitochondria from Ca2+ overload (10, 11), as indicated by studies in isolated cardiomyocytes, CyPD knock-out mice, adult cortical neurons, and spinal chord mitochondria (51–54). It is tempting to speculate that the different PTP conductances observed in mammals, Drosophila, and yeast underscore different physiological functions. In particular, due to its smaller size, the Drosophila PTP could be involved in Ca2+ homeostasis rather than in apoptosis (1, 11), consistent with the finding that the mitochondrial pathway is not essential in most cases of Drosophila apoptosis (55–59). The discovery that the Drosophila PTP forms from F-ATPase will allow testing of this hypothesis with the powerful methods of Drosophila genetics.
This work was supported in part by AIRC Grants IG13392 (to P. B.) and IG11814 (to I. S.), PRIN Programs 20107Z8XBW (to P. B.) and 2010CSJX4F (to I. S.), National Institutes of Health-Public Health Service Grant 1R01GM069883 (to M. F. and P. B.), and the University of Padova Progetti Strategici di Ateneo Models of Mitochondrial Diseases (to P. B.).
- PTP
- permeability transition pore
- pS
- picosiemen(s)
- mCrC
- mitochondrial Ca2+ release channel
- BN-PAGE
- blue native polyacrylamide gel electrophoresis
- CyP
- cyclophilin
- CyPD
- cyclophilin D
- TOM20
- translocase of outer mitochondrial membranes of 20 kDa
- CsA
- cyclosporin A
- MCU
- mitochondrial Ca2+ uniporter
- CRC
- Ca2+ retention capacity
- OSCP
- oligomycin sensitivity conferral protein
- ctrl
- control.
REFERENCES
- 1. von Stockum S., Basso E., Petronilli V., Sabatelli P., Forte M. A., Bernardi P. (2011) Properties of Ca2+ transport in mitochondria of Drosophila melanogaster. J. Biol. Chem. 286, 41163–41170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baughman J. M., Perocchi F., Girgis H. S., Plovanich M., Belcher-Timme C. A., Sancak Y., Bao X. R., Strittmatter L., Goldberger O., Bogorad R. L., Koteliansky V., Mootha V. K. (2011) Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476, 341–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. De Stefani D., Raffaello A., Teardo E., Szabò I., Rizzuto R. (2011) A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476, 336–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Palty R., Silverman W. F., Hershfinkel M., Caporale T., Sensi S. L., Parnis J., Nolte C., Fishman D., Shoshan-Barmatz V., Herrmann S., Khananshvili D., Sekler I. (2010) NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc. Natl. Acad. Sci. U.S.A. 107, 436–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bernardi P., Azzone G. F. (1983) Regulation of Ca2+ efflux in rat liver mitochondria: role of membrane potential. Eur. J. Biochem. 134, 377–383 [DOI] [PubMed] [Google Scholar]
- 6. Giorgio V., von Stockum S., Antoniel M., Fabbro A., Fogolari F., Forte M., Glick G. D., Petronilli V., Zoratti M., Szabó I., Lippe G., Bernardi P. (2013) Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc. Natl. Acad. Sci. U.S.A. 110, 5887–5892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jung D. W., Bradshaw P. C., Pfeiffer D. R. (1997) Properties of a cyclosporin-insensitive permeability transition pore in yeast mitochondria. J. Biol. Chem. 272, 21104–21112 [DOI] [PubMed] [Google Scholar]
- 8. Bradshaw P. C., Pfeiffer D. R. (2013) Characterization of the respiration-induced yeast mitochondrial permeability transition pore. Yeast 30, 471–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carraro M., Giorgio V., Šileikytė J., Sartori G., Forte M., Lippe G., Zoratti M., Szabò I., Bernardi P. (2014) Channel formation by yeast F-ATP synthase and the role of dimerization in the mitochondrial permeability transition. J. Biol. Chem. 289, 15980–15985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bernardi P., Petronilli V. (1996) The permeability transition pore as a mitochondrial calcium release channel: a critical appraisal. J. Bioenerg. Biomembr. 28, 131–138 [DOI] [PubMed] [Google Scholar]
- 11. Bernardi P., von Stockum S. (2012) The permeability transition pore as a Ca2+ release channel: new answers to an old question. Cell Calcium 52, 22–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schneider I. (1972) Cell lines derived from late embryonic stages of Drosophila melanogaster. J. Embryol. Exp. Morphol. 27, 353–365 [PubMed] [Google Scholar]
- 13. Yanagawa S., Lee J. S., Ishimoto A. (1998) Identification and characterization of a novel line of Drosophila Schneider S2 cells that respond to Wingless signaling. J. Biol. Chem. 273, 32353–32359 [DOI] [PubMed] [Google Scholar]
- 14. Da-Rè C., von Stockum S., Biscontin A., Millino C., Cisotto P., Zordan M. A., Zeviani M., Bernardi P., De Pittà C., Costa R. (2014) Leigh syndrome in Drosophila melanogaster: morphological and biochemical characterization of Surf1 post-transcriptional silencing. J. Biol. Chem. 289, 29235–29246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fontaine E., Eriksson O., Ichas F., Bernardi P. (1998) Regulation of the permeability transition pore in skeletal muscle mitochondria: modulation by electron flow through the respiratory chain complex I. J. Biol. Chem. 273, 12662–12668 [DOI] [PubMed] [Google Scholar]
- 16. Ye J., Coulouris G., Zaretskaya I., Cutcutache I., Rozen S., Madden T. L. (2012) Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics 13, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iwaki T., Figuera M., Ploplis V. A., Castellino F. J. (2003) Rapid selection of Drosophila S2 cells with the puromycin resistance gene. BioTechniques 35, 482–484, 486 [DOI] [PubMed] [Google Scholar]
- 18. Szabò I., Soddemann M., Leanza L., Zoratti M., Gulbins E. (2011) Single-point mutations of a lysine residue change function of Bax and Bcl-xL expressed in Bax- and Bak-less mouse embryonic fibroblasts: novel insights into the molecular mechanisms of Bax-induced apoptosis. Cell Death Differ. 18, 427–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Buzhynskyy N., Sens P., Prima V., Sturgis J. N., Scheuring S. (2007) Rows of ATP synthase dimers in native mitochondrial inner membranes. Biophys. J. 93, 2870–2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bernardi P. (2013) The mitochondrial permeability transition pore: a mystery solved? Front. Physiol. 4, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Johnson K. M., Chen X., Boitano A., Swenson L., Opipari A. W., Jr., Glick G. D. (2005) Identification and validation of the mitochondrial F1F0-ATPase as the molecular target of the immunomodulatory benzodiazepine Bz-423. Chem. Biol. 12, 485–496 [DOI] [PubMed] [Google Scholar]
- 22. Stelzer A. C., Frazee R. W., Van Huis C., Cleary J., Opipari A. W., Jr., Glick G. D., Al-Hashimi H. M. (2010) NMR studies of an immunomodulatory benzodiazepine binding to its molecular target on the mitochondrial F1F0-ATPase. Biopolymers 93, 85–92 [DOI] [PubMed] [Google Scholar]
- 23. Basso E., Fante L., Fowlkes J., Petronilli V., Forte M. A., Bernardi P. (2005) Properties of the permeability transition pore in mitochondria devoid of cyclophilin D. J. Biol. Chem. 280, 18558–18561 [DOI] [PubMed] [Google Scholar]
- 24. Pemberton T. J., Kay J. E. (2005) Identification and comparative analysis of the peptidyl-prolyl cis/trans isomerase repertoires of H. sapiens, D. melanogaster, C. elegans, S. cerevisiae and Sz. pombe. Comp. Funct. Genomics 6, 277–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Claros M. G., Vincens P. (1996) Computational method to predict mitochondrially imported proteins and their targeting sequences. Eur. J. Biochem. 241, 779–786 [DOI] [PubMed] [Google Scholar]
- 26. Basso E., Petronilli V., Forte M. A., Bernardi P. (2008) Phosphate is essential for inhibition of the mitochondrial permeability transition pore by cyclosporin A and by cyclophilin D ablation. J. Biol. Chem. 283, 26307–26311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Giorgio V., Bisetto E., Soriano M. E., Dabbeni-Sala F., Basso E., Petronilli V., Forte M. A., Bernardi P., Lippe G. (2009) Cyclophilin D modulates mitochondrial F0F1-ATP synthase by interacting with the lateral stalk of the complex. J. Biol. Chem. 284, 33982–33988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Scorrano L., Nicolli A., Basso E., Petronilli V., Bernardi P. (1997) Two modes of activation of the permeability transition pore: the role of mitochondrial cyclophilin. Mol. Cell. Biochem. 174, 181–184 [PubMed] [Google Scholar]
- 29. Starkov A. A. (2008) The role of mitochondria in reactive oxygen species metabolism and signaling. Ann. N.Y. Acad. Sci. 1147, 37–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li B., Chauvin C., De Paulis D., De Oliveira F., Gharib A., Vial G., Lablanche S., Leverve X., Bernardi P., Ovize M., Fontaine E. (2012) Inhibition of complex I regulates the mitochondrial permeability transition through a phosphate-sensitive inhibitory site masked by cyclophilin D. Biochim. Biophys. Acta 1817, 1628–1634 [DOI] [PubMed] [Google Scholar]
- 31. Petronilli V., Costantini P., Scorrano L., Colonna R., Passamonti S., Bernardi P. (1994) The voltage sensor of the mitochondrial permeability transition pore is tuned by the oxidation-reduction state of vicinal thiols: increase of the gating potential by oxidants and its reversal by reducing agents. J. Biol. Chem. 269, 16638–16642 [PubMed] [Google Scholar]
- 32. Costantini P., Chernyak B. V., Petronilli V., Bernardi P. (1996) Modulation of the mitochondrial permeability transition pore by pyridine nucleotides and dithiol oxidation at two separate sites. J. Biol. Chem. 271, 6746–6751 [DOI] [PubMed] [Google Scholar]
- 33. Kinnally K. W., Campo M. L., Tedeschi H. (1989) Mitochondrial channel activity studied by patch-clamping mitoplasts. J. Bioenerg. Biomembr. 21, 497–506 [DOI] [PubMed] [Google Scholar]
- 34. Petronilli V., Szabò I., Zoratti M. (1989) The inner mitochondrial membrane contains ion-conducting channels similar to those found in bacteria. FEBS Lett. 259, 137–143 [DOI] [PubMed] [Google Scholar]
- 35. Szabó I., Zoratti M. (1991) The giant channel of the inner mitochondrial membrane is inhibited by cyclosporin A. J. Biol. Chem. 266, 3376–3379 [PubMed] [Google Scholar]
- 36. Szabó I., Bernardi P., Zoratti M. (1992) Modulation of the mitochondrial megachannel by divalent cations and protons. J. Biol. Chem. 267, 2940–2946 [PubMed] [Google Scholar]
- 37. Bernardi P., Vassanelli S., Veronese P., Colonna R., Szabó I., Zoratti M. (1992) Modulation of the mitochondrial permeability transition pore: effect of protons and divalent cations. J. Biol. Chem. 267, 2934–2939 [PubMed] [Google Scholar]
- 38. Szabó I., Zoratti M. (1992) The mitochondrial megachannel is the permeability transition pore. J. Bioenerg. Biomembr. 24, 111–117 [DOI] [PubMed] [Google Scholar]
- 39. Alavian K. N., Beutner G., Lazrove E., Sacchetti S., Park H. A., Licznerski P., Li H., Nabili P., Hockensmith K., Graham M., Porter G. A., Jr., Jonas E. A. (2014) An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc. Natl. Acad. Sci. U.S.A. 111, 10580–10585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Watt I. N., Montgomery M. G., Runswick M. J., Leslie A. G., Walker J. E. (2010) Bioenergetic cost of making an adenosine triphosphate molecule in animal mitochondria. Proc. Natl. Acad. Sci. U.S.A. 107, 16823–16827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Eble K. S., Coleman W. B., Hantgan R. R., Cunningham C. C. (1990) Tightly associated cardiolipin in the bovine heart mitochondrial ATP synthase as analyzed by 31P nuclear magnetic resonance spectroscopy. J. Biol. Chem. 265, 19434–19440 [PubMed] [Google Scholar]
- 42. Gonzalvez F., D'Aurelio M., Boutant M., Moustapha A., Puech J. P., Landes T., Arnauné-Pelloquin L., Vial G., Taleux N., Slomianny C., Wanders R. J., Houtkooper R. H., Bellenguer P., Møller I. M., Gottlieb E., Vaz F. M., Manfredi G., Petit P. X. (2013) Barth syndrome: cellular compensation of mitochondrial dysfunction and apoptosis inhibition due to changes in cardiolipin remodeling linked to tafazzin (TAZ) gene mutation. Biochim. Biophys. Acta 1832, 1194–1206 [DOI] [PubMed] [Google Scholar]
- 43. Acehan D., Malhotra A., Xu Y., Ren M., Stokes D. L., Schlame M. (2011) Cardiolipin affects the supramolecular organization of ATP synthase in mitochondria. Biophys. J. 100, 2184–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McGeoch J. E., Guidotti G. (1997) A 0.1–700 Hz current through a voltage-clamped pore: candidate protein for initiator of neural oscillations. Brain Res. 766, 188–194 [DOI] [PubMed] [Google Scholar]
- 45. McGeoch J. E., McGeoch M. W., Mao R., Guidotti G. (2000) Opposing actions of cGMP and calcium on the conductance of the F0 subunit c pore. Biochem. Biophys. Res. Commun. 274, 835–840 [DOI] [PubMed] [Google Scholar]
- 46. McGeoch J. E., Guidotti G. (2001) Batten disease and the control of the Fo subunit c pore by cGMP and calcium. Eur. J. Paediatr. Neurol. 5, Suppl. A, 147–150 [DOI] [PubMed] [Google Scholar]
- 47. Bernardi P., Di Lisa F. (2014) The mitochondrial permeability transition pore: molecular nature and role as a target in cardioprotection. J. Mol. Cell. Cardiol. 78C, 100–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chinopoulos C., Szabadkai G. (2014) What makes you can also break you, Part III: mitochondrial permeability transition pore formation by an uncoupling channel within the C-subunit ring of the F1FO ATP synthase? Front. Oncol. 4, 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rasola A., Bernardi P. (2014) The mitochondrial permeability transition pore and its adaptive responses in tumor cells. Cell Calcium 56, 437–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Petronilli V., Penzo D., Scorrano L., Bernardi P., Di Lisa F. (2001) The mitochondrial permeability transition, release of cytochrome c, and cell death: correlation with the duration of pore openings in situ. J. Biol. Chem. 276, 12030–12034 [DOI] [PubMed] [Google Scholar]
- 51. Altschuld R. A., Hohl C. M., Castillo L. C., Garleb A. A., Starling R. C., Brierley G. P. (1992) Cyclosporin inhibits mitochondrial calcium efflux in isolated adult rat ventricular cardiomyocytes. Am. J. Physiol. 262, H1699–H1704 [DOI] [PubMed] [Google Scholar]
- 52. Elrod J. W., Wong R., Mishra S., Vagnozzi R. J., Sakthievel B., Goonasekera S. A., Karch J., Gabel S., Farber J., Force T., Brown J. H., Murphy E., Molkentin J. D. (2010) Cyclophilin D controls mitochondrial pore-dependent Ca2+ exchange, metabolic flexibility, and propensity for heart failure in mice. J. Clin. Invest. 120, 3680–3687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Barsukova A., Komarov A., Hajnóczky G., Bernardi P., Bourdette D., Forte M. (2011) Activation of the mitochondrial permeability transition pore modulates Ca2+ responses to physiological stimuli in adult neurons. Eur. J. Neurosci. 33, 831–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Parone P. A., Da Cruz S., Han J. S., McAlonis-Downes M., Vetto A. P., Lee S. K., Tseng E., Cleveland D. W. (2013) Enhancing mitochondrial calcium buffering capacity reduces aggregation of misfolded SOD1 and motor neuron cell death without extending survival in mouse models of inherited amyotrophic lateral sclerosis. J. Neurosci. 33, 4657–4671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dorstyn L., Mills K., Lazebnik Y., Kumar S. (2004) The two cytochrome c species, DC3 and DC4, are not required for caspase activation and apoptosis in Drosophila cells. J. Cell Biol. 167, 405–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Means J. C., Muro I., Clem R. J. (2006) Lack of involvement of mitochondrial factors in caspase activation in a Drosophila cell-free system. Cell Death Differ. 13, 1222–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Oberst A., Bender C., Green D. R. (2008) Living with death: the evolution of the mitochondrial pathway of apoptosis in animals. Cell Death Differ. 15, 1139–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang C., Youle R. J. (2009) The role of mitochondria in apoptosis. Annu. Rev. Genet. 43, 95–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Xu D., Woodfield S. E., Lee T. V., Fan Y., Antonio C., Bergmann A. (2009) Genetic control of programmed cell death (apoptosis) in Drosophila. Fly (Austin) 3, 78–90 [DOI] [PMC free article] [PubMed] [Google Scholar]