Abstract
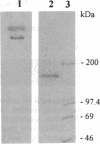
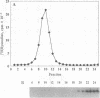
Membrane vesicles enriched in both ryanodine receptor and dihydropyridine receptor were obtained from rabbit skeletal muscle and solubilized with 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate. Analysis of the sedimentation behavior of the solubilized proteins showed the existence of a population of alpha 1 subunits of the dihydropyridine receptor which cosedimented with the ryanodine receptor. Solubilized proteins were immunoprecipitated with antibodies directed against either the ryanodine receptor or the alpha 1, alpha 2, or beta subunits of the dihydropyridine receptor. Immunoprecipitated proteins were identified by Western blot analysis and by specific labeling with [3H]ryanodine or [3H]PN200-110. Immunoprecipitation of the solubilized proteins with antibodies directed against the dihydropyridine receptor led to the coimmunoprecipitation of the ryanodine receptor. Conversely, immunoprecipitation with antibodies directed against the ryanodine receptor led to an immune complex containing both receptors, but these antibodies were unable to precipitate purified dihydropyridine receptor. These results demonstrate that ryanodine receptor and dihydropyridine receptor are present in the triad membrane preparation in a complex which may play an important role in excitation-contraction coupling.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlijanian M. K., Westenbroek R. E., Catterall W. A. Subunit structure and localization of dihydropyridine-sensitive calcium channels in mammalian brain, spinal cord, and retina. Neuron. 1990 Jun;4(6):819–832. doi: 10.1016/0896-6273(90)90135-3. [DOI] [PubMed] [Google Scholar]
- Ashley C. C., Mulligan I. P., Lea T. J. Ca2+ and activation mechanisms in skeletal muscle. Q Rev Biophys. 1991 Feb;24(1):1–73. doi: 10.1017/s0033583500003267. [DOI] [PubMed] [Google Scholar]
- Block B. A., Imagawa T., Campbell K. P., Franzini-Armstrong C. Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J Cell Biol. 1988 Dec;107(6 Pt 2):2587–2600. doi: 10.1083/jcb.107.6.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A. Excitation-contraction coupling in vertebrate skeletal muscle: a tale of two calcium channels. Cell. 1991 Mar 8;64(5):871–874. doi: 10.1016/0092-8674(91)90309-m. [DOI] [PubMed] [Google Scholar]
- Catterall W. A. Functional subunit structure of voltage-gated calcium channels. Science. 1991 Sep 27;253(5027):1499–1500. doi: 10.1126/science.1654596. [DOI] [PubMed] [Google Scholar]
- Chadwick C. C., Inui M., Fleischer S. Identification and purification of a transverse tubule coupling protein which binds to the ryanodine receptor of terminal cisternae at the triad junction in skeletal muscle. J Biol Chem. 1988 Aug 5;263(22):10872–10877. [PubMed] [Google Scholar]
- Chen S. R., Vaughan D. M., Airey J. A., Coronado R., MacLennan D. H. Functional expression of cDNA encoding the Ca2+ release channel (ryanodine receptor) of rabbit skeletal muscle sarcoplasmic reticulum in COS-1 cells. Biochemistry. 1993 Apr 13;32(14):3743–3753. doi: 10.1021/bi00065a029. [DOI] [PubMed] [Google Scholar]
- Corbett A. M., Bian J., Wade J. B., Schneider M. F. Depolarization-induced calcium release from isolated triads measured with impermeant fura-2. J Membr Biol. 1992 Jun;128(3):165–179. doi: 10.1007/BF00231810. [DOI] [PubMed] [Google Scholar]
- Curtis B. M., Catterall W. A. Solubilization of the calcium antagonist receptor from rat brain. J Biol Chem. 1983 Jun 25;258(12):7280–7283. [PubMed] [Google Scholar]
- De Jongh K. S., Merrick D. K., Catterall W. A. Subunits of purified calcium channels: a 212-kDa form of alpha 1 and partial amino acid sequence of a phosphorylation site of an independent beta subunit. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8585–8589. doi: 10.1073/pnas.86.21.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelice L. J. Molecular and biophysical view of the Ca channel: a hypothesis regarding oligomeric structure, channel clustering, and macroscopic current. J Membr Biol. 1993 May;133(3):191–202. doi: 10.1007/BF00232019. [DOI] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Fleischer S., Ogunbunmi E. M., Dixon M. C., Fleer E. A. Localization of Ca2+ release channels with ryanodine in junctional terminal cisternae of sarcoplasmic reticulum of fast skeletal muscle. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7256–7259. doi: 10.1073/pnas.82.21.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosset M., Jaimovich E., Delpont E., Lazdunski M. [3H]nitrendipine receptors in skeletal muscle. J Biol Chem. 1983 May 25;258(10):6086–6092. [PubMed] [Google Scholar]
- Galizzi J. P., Borsotto M., Barhanin J., Fosset M., Lazdunski M. Characterization and photoaffinity labeling of receptor sites for the Ca2+ channel inhibitors d-cis-diltiazem, (+/-)-bepridil, desmethoxyverapamil, and (+)-PN 200-110 in skeletal muscle transverse tubule membranes. J Biol Chem. 1986 Jan 25;261(3):1393–1397. [PubMed] [Google Scholar]
- Hawkes M. J., Díaz-Muñoz M., Hamilton S. L. A procedure for purification of the ryanodine receptor from skeletal muscle. Membr Biochem. 1989;8(3):133–145. doi: 10.3109/09687688909025827. [DOI] [PubMed] [Google Scholar]
- Horne W. A., Weiland G. A., Oswald R. E. Solubilization and hydrodynamic characterization of the dihydropyridine receptor from rat ventricular muscle. J Biol Chem. 1986 Mar 15;261(8):3588–3594. [PubMed] [Google Scholar]
- Ikemoto N., Antoniu B., Mészáros L. G. Rapid flow chemical quench studies of calcium release from isolated sarcoplasmic reticulum. J Biol Chem. 1985 Nov 15;260(26):14096–14100. [PubMed] [Google Scholar]
- Imagawa T., Smith J. S., Coronado R., Campbell K. P. Purified ryanodine receptor from skeletal muscle sarcoplasmic reticulum is the Ca2+-permeable pore of the calcium release channel. J Biol Chem. 1987 Dec 5;262(34):16636–16643. [PubMed] [Google Scholar]
- Jayaraman T., Brillantes A. M., Timerman A. P., Fleischer S., Erdjument-Bromage H., Tempst P., Marks A. R. FK506 binding protein associated with the calcium release channel (ryanodine receptor). J Biol Chem. 1992 May 15;267(14):9474–9477. [PubMed] [Google Scholar]
- Kim D. H., Ohnishi S. T., Ikemoto N. Kinetic studies of calcium release from sarcoplasmic reticulum in vitro. J Biol Chem. 1983 Aug 25;258(16):9662–9668. [PubMed] [Google Scholar]
- Kim K. C., Caswell A. H., Talvenheimo J. A., Brandt N. R. Isolation of a terminal cisterna protein which may link the dihydropyridine receptor to the junctional foot protein in skeletal muscle. Biochemistry. 1990 Oct 2;29(39):9281–9289. doi: 10.1021/bi00491a025. [DOI] [PubMed] [Google Scholar]
- Lai F. A., Erickson H. P., Rousseau E., Liu Q. Y., Meissner G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature. 1988 Jan 28;331(6154):315–319. doi: 10.1038/331315a0. [DOI] [PubMed] [Google Scholar]
- Lai F. A., Misra M., Xu L., Smith H. A., Meissner G. The ryanodine receptor-Ca2+ release channel complex of skeletal muscle sarcoplasmic reticulum. Evidence for a cooperatively coupled, negatively charged homotetramer. J Biol Chem. 1989 Oct 5;264(28):16776–16785. [PubMed] [Google Scholar]
- Lai Y., Seagar M. J., Takahashi M., Catterall W. A. Cyclic AMP-dependent phosphorylation of two size forms of alpha 1 subunits of L-type calcium channels in rat skeletal muscle cells. J Biol Chem. 1990 Dec 5;265(34):20839–20848. [PubMed] [Google Scholar]
- Leung A. T., Imagawa T., Campbell K. P. Structural characterization of the 1,4-dihydropyridine receptor of the voltage-dependent Ca2+ channel from rabbit skeletal muscle. Evidence for two distinct high molecular weight subunits. J Biol Chem. 1987 Jun 15;262(17):7943–7946. [PubMed] [Google Scholar]
- Meissner G., Darling E., Eveleth J. Kinetics of rapid Ca2+ release by sarcoplasmic reticulum. Effects of Ca2+, Mg2+, and adenine nucleotides. Biochemistry. 1986 Jan 14;25(1):236–244. doi: 10.1021/bi00349a033. [DOI] [PubMed] [Google Scholar]
- Mitchell R. D., Palade P., Fleischer S. Purification of morphologically intact triad structures from skeletal muscle. J Cell Biol. 1983 Apr;96(4):1008–1016. doi: 10.1083/jcb.96.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Reyes E., Kim H. S., Lacerda A. E., Horne W., Wei X. Y., Rampe D., Campbell K. P., Brown A. M., Birnbaumer L. Induction of calcium currents by the expression of the alpha 1-subunit of the dihydropyridine receptor from skeletal muscle. Nature. 1989 Jul 20;340(6230):233–236. doi: 10.1038/340233a0. [DOI] [PubMed] [Google Scholar]
- Pessah I. N., Francini A. O., Scales D. J., Waterhouse A. L., Casida J. E. Calcium-ryanodine receptor complex. Solubilization and partial characterization from skeletal muscle junctional sarcoplasmic reticulum vesicles. J Biol Chem. 1986 Jul 5;261(19):8643–8648. [PubMed] [Google Scholar]
- Rios E., Brum G. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature. 1987 Feb 19;325(6106):717–720. doi: 10.1038/325717a0. [DOI] [PubMed] [Google Scholar]
- Ruth P., Röhrkasten A., Biel M., Bosse E., Regulla S., Meyer H. E., Flockerzi V., Hofmann F. Primary structure of the beta subunit of the DHP-sensitive calcium channel from skeletal muscle. Science. 1989 Sep 8;245(4922):1115–1118. doi: 10.1126/science.2549640. [DOI] [PubMed] [Google Scholar]
- Ríos E., Ma J. J., González A. The mechanical hypothesis of excitation-contraction (EC) coupling in skeletal muscle. J Muscle Res Cell Motil. 1991 Apr;12(2):127–135. doi: 10.1007/BF01774031. [DOI] [PubMed] [Google Scholar]
- Schneider M. F., Chandler W. K. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973 Mar 23;242(5395):244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]
- Smith J. S., Coronado R., Meissner G. Sarcoplasmic reticulum contains adenine nucleotide-activated calcium channels. Nature. 1985 Aug 1;316(6027):446–449. doi: 10.1038/316446a0. [DOI] [PubMed] [Google Scholar]
- Striessnig J., Murphy B. J., Catterall W. A. Dihydropyridine receptor of L-type Ca2+ channels: identification of binding domains for [3H](+)-PN200-110 and [3H]azidopine within the alpha 1 subunit. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10769–10773. doi: 10.1073/pnas.88.23.10769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striessnig J., Scheffauer F., Mitterdorfer J., Schirmer M., Glossmann H. Identification of the benzothiazepine-binding polypeptide of skeletal muscle calcium channels with (+)-cis-azidodiltiazem and anti-ligand antibodies. J Biol Chem. 1990 Jan 5;265(1):363–370. [PubMed] [Google Scholar]
- Takahashi M., Seagar M. J., Jones J. F., Reber B. F., Catterall W. A. Subunit structure of dihydropyridine-sensitive calcium channels from skeletal muscle. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5478–5482. doi: 10.1073/pnas.84.15.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshima H., Nishimura S., Matsumoto T., Ishida H., Kangawa K., Minamino N., Matsuo H., Ueda M., Hanaoka M., Hirose T. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature. 1989 Jun 8;339(6224):439–445. doi: 10.1038/339439a0. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Adams B. A., Numa S., Beam K. G. Repeat I of the dihydropyridine receptor is critical in determining calcium channel activation kinetics. Nature. 1991 Aug 29;352(6338):800–803. doi: 10.1038/352800a0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenknecht T., Grassucci R., Frank J., Saito A., Inui M., Fleischer S. Three-dimensional architecture of the calcium channel/foot structure of sarcoplasmic reticulum. Nature. 1989 Mar 9;338(6211):167–170. doi: 10.1038/338167a0. [DOI] [PubMed] [Google Scholar]