Abstract
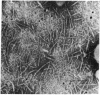
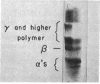
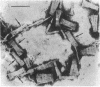
Insoluble, mature collagen fibers from bovine skin have been partially solubilized by mild, denaturing, but nonhydrolytic means. The soluble denatured collagen was fractionated by alcohol coacervation, and a fraction rich in high-molecular-weight α-chains was obtained. The heavy α-chains were isolated by carboxymethylcellulose chromatography. Renaturation, followed by measurements of optical rotation at 365 nm, showed that stable, in-register renaturation was more readily accomplished in mixtures of heavy α-chains than in α1-β11-chain mixtures. Renatured heavy α-chain preparations were precipitated in SLS form, negatively stained, and examined by electron microscopy. The SLS precipitates were compared with SLS segments from native soluble collagen and were found to match in band pattern and spacing along their entire length from the COOH-terminal region, except for an NH2-terminal extension of 170 ± 30 Å in the heavy α-chain SLS. The heavy α-chains correspond chromatographically with those previously reported to be intermediates in the conversion of procollagen to collagen, on the basis of their molecular weight and of labeling studies. The presence of NH2-terminal extensions, and their existence in mature insoluble collagen, suggest that these intermediates may have a special role in fibril formation.
Keywords: procollagen, biosynthetic precursors, fibril formation, renaturation, electron microscopy
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellamy G., Bornstein P. Evidence for procollagen, a biosynthetic precursors of collagen. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1138–1142. doi: 10.1073/pnas.68.6.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P., Ehrlich H. P., Wyke A. W. Procollagen: conversion of the precursor to collagen by a neutral protease. Science. 1972 Feb 4;175(4021):544–546. doi: 10.1126/science.175.4021.544. [DOI] [PubMed] [Google Scholar]
- Bornstein P., Von der Mark K., Wyke A. W., Ehrlich H. P., Monson J. M. Characterization of the pro- 1 chain of procollagen. J Biol Chem. 1972 May 10;247(9):2808–2813. [PubMed] [Google Scholar]
- Clark C. C., Veis A. A high-resolution acrylamide-gel electrophoresis technique for the analysis of collagen polymer components. Biochim Biophys Acta. 1968 Jan 22;154(1):175–182. doi: 10.1016/0005-2795(68)90269-9. [DOI] [PubMed] [Google Scholar]
- Clark C. C., Veis A. High molecular weight chains in acid-soluble collagen and their role in fibrillogenesis. Biochemistry. 1972 Feb 15;11(4):494–502. doi: 10.1021/bi00754a003. [DOI] [PubMed] [Google Scholar]
- Dehm P., Jimenez S. A., Olsen B. R., Prockop D. J. A transport form of collagen from embryonic tendon: electron microscopic demonstration of an NH 2 -terminal extension and evidence suggesting the presence of cystine in the molecule (chick embryo-tropocollagen-gel filtration). Proc Natl Acad Sci U S A. 1972 Jan;69(1):60–64. doi: 10.1073/pnas.69.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez S. A., Dehm P., Prockop D. J. Further evidence for a transport form of collagen. Its extrusion and extracellular conversion to tropocollagen in embryonic tendon. FEBS Lett. 1971 Oct 1;17(2):245–248. doi: 10.1016/0014-5793(71)80156-4. [DOI] [PubMed] [Google Scholar]
- Olsen B. R. Electron microscope studies on collagen. IV. Structure of vitrosin fibrils and interaction properties of vitrosin molecules. J Ultrastruct Res. 1965 Aug;13(1):172–191. doi: 10.1016/s0022-5320(65)80095-8. [DOI] [PubMed] [Google Scholar]
- PIEZ K. A., WEISS E., LEWIS M. S. The separation and characterization of the alpha- and beta-components of calf skin collagen. J Biol Chem. 1960 Jul;235:1987–1991. [PubMed] [Google Scholar]
- Veis A., Anesey J. R., Garvin J. E., Dimuzio M. T. High molecular weight collagen: a long-lived intermediate in the biogenesis of collagen fibrils. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1404–1411. doi: 10.1016/0006-291x(72)90869-8. [DOI] [PubMed] [Google Scholar]
- Veis A., Anesey J. Modes of intermolecular cross-linking in mature insoluble collagen. J Biol Chem. 1965 Oct;240(10):3899–3908. [PubMed] [Google Scholar]