Background: STIM calcium sensors are key modulators of store-operated channels (SOCs).
Results: Changes in the ratio of active STIM2/STIM1 switch Imin channel regulation between store-operated and store-independent modes.
Conclusion: Endogenous SOCs are differently regulated by STIM1 and STIM2.
Significance: Cross-talk between STIM1 and STIM2 and their different roles in channel activation are indicative of an additional level of SOC regulation.
Keywords: Calcium, Calcium Channel, Endoplasmic Reticulum (ER), Patch Clamp, Stromal Interaction Molecule 1 (STIM1), Stromal Interaction Molecule 2 (STIM2), HEK293, Imin, SOC, STIM2, Single Channel
Abstract
The endoplasmic reticulum calcium sensors stromal interaction molecules 1 and 2 (STIM1 and STIM2) are key modulators of store-operated calcium entry. Both these sensors play a major role in physiological functions in normal tissue and in pathology, but available data on native STIM2-regulated plasma membrane channels are scarce. Only a few studies have recorded STIM2-induced CRAC (calcium release-activated calcium) currents. On the other hand, many cell types display store-operated currents different from CRAC. The STIM1 protein regulates not only CRAC but also transient receptor potential canonical (TRPC) channels, but it has remained unclear whether STIM2 is capable of regulating store-operated non-CRAC channels. Here we present for the first time experimental evidence for the existence of endogenous non-CRAC STIM2-regulated channels. As shown in single-channel patch clamp experiments on HEK293 cells, selective activation of native STIM2 proteins or STIM2 overexpression results in store-operated activation of Imin channels, whereas STIM1 activation blocks this process. Changes in the ratio between active STIM2 and STIM1 proteins can switch the regulation of Imin channels between store-operated and store-independent modes. We have previously characterized electrophysiological properties of different Ca2+ influx channels coexisting in HEK293 cells. The results of this study show that STIM1 and STIM2 differ in the ability to activate these store-operated channels; Imin channels are regulated by STIM2, TRPC3-containing INS channels are induced by STIM1, and TRPC1-composed Imax channels are activated by both STIM1 and STIM2. These new data about cross-talk between STIM1 and STIM2 and their different roles in store-operated channel activation are indicative of an additional level in the regulation of store-operated calcium entry pathways.
Introduction
Calcium is an intracellular messenger that regulates a broad spectrum of processes within the cell (1). Cells can rapidly increase cytosolic calcium in two ways: by calcium release from intracellular stores or due to calcium influx from extracellular space (2). These two pathways are tightly coupled in nonexcitable cells. Depletion of intracellular calcium stores leads to activation of plasma membrane store-operated calcium channels via their interaction with calcium sensor proteins named stromal interaction molecules (STIMs)3 (3, 4).
STIMs are single-pass transmembrane proteins containing an N-terminal EF-hand domain responsible for calcium store sensing (5). The cytosolic C-terminal fragment contains several signal and interaction domains, including that responsible for activation of CRAC (calcium release-activated calcium) channels (6–10). Mammals have two STIM homologs, STIM1 and STIM2 (11), which differ in their properties. When compared with STIM1, STIM2 has lower affinity to calcium and therefore is more sensitive to minor changes in the calcium store (12, 13), shows lower cooperativity in redistribution to puncta, and has higher affinity to plasma membrane phosphatidylinositol 4,5-bisphosphate (PIP2) and phosphatidylinositol 1,4,5-trisphosphate (PIP3) (14). Therefore, the physiological functions of STIM proteins are also different. STIM1 is the main activator of store-operated calcium entry, whereas STIM2 controls the basal calcium concentration and is responsible for prolonged calcium entry and response to low concentrations of calcium agonists (12, 15). STIM1 and STIM2 regulate different modes of intracellular calcium oscillations (16–18). STIM2 participates in the regulation of calcium signaling in different cell types such as B and T lymphocytes, dendrite cells, muscle cells, etc. (15, 19–22). Disruption of STIM2-mediated signaling contributes to a broad spectrum of diseases, including immune and autoimmune disorders, cancer, ischemia, and neurodegeneration (15, 23–32).
CRAC channels are the first described and best studied store-operated channels (33). However, it is only in a few cell types (mainly in the immune system) that store-operated calcium currents are mediated by CRAC channels alone. Most other cells contain store-operated calcium channels of different types (2, 34–37), and CRAC channels may even be absent (38). For example, we have revealed four different store-operated channel types in the A431 cell line (39, 40).
It has been shown that STIM1 can activate not only Orai-mediated CRAC channels but also canonical transient receptor potential (TRPC) channels (35, 36, 41–47) and other calcium channel types (48, 49). The CRAC-activating domain of STIM1 is necessary for the interaction with TRPC, whereas its C-terminal cationic lysine-rich region is necessary for TRPC activation (50–53). This TRPC-STIM1 cooperation plays a role in physiological functions such as fluid secretion in salivary glands (54), postnatal differentiation of human myoblasts (36), and disruption of the endothelial barrier (35). STIM2 protein has been less studied than STIM1. The exact mechanisms of molecular interactions between the STIM2 cytosolic terminal and Orai or TRPC channels remain unclear. It has been shown that STIM2 can activate overexpressed Orai1, -2, and -3 (12, 55) or native Orai1 and -2 channels (17, 18, 20), but it is still unknown whether STIM2 contributes to activation of other non-CRAC store-operated channels, although this question is highly relevant for research in cell physiology, pathophysiology, and disease treatment.
In this study, STIM2-operated channels were analyzed by the single-channel patch clamp technique. To discriminate between STIM1- and STIM2-operated channels, we used a combined knockdown/overexpression strategy and partial depletion of calcium stores for selective activation of STIM2. The electrophysiological properties of STIM2-regulated store-operated channels were characterized, which proved to differ from those of CRAC channels. It was also shown that the ratio between active STIM2 and STIM1 proteins could switch the regulation of Imin channels between store-operated and store-independent modes and that STIM1 and STIM2 proteins differ in the ability to activate various types of store-operated channels.
EXPERIMENTAL PROCEDURES
Cell Culture and Transfection
HEK293 cells (Cell Culture Collection, Institute of Cytology, Russian Academy of Sciences, St. Petersburg, Russia) were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin, and 2 mm glutamine. The cultures were grown in an incubator at 37 °C in a humidified 5% CO2 atmosphere. For patch clamp and Ca2+ imaging experiments, the cells were plated on coverslips and cultured for 1–3 days before use. In transfection experiments, the cells were harvested by trypsinization, plated in 35-mm tissue culture dishes, and grown for 20–24 h prior to transfection with the Lipofectamine reagent. For STIM knockdown, the cells were transiently cotransfected with a mixture of STIM shRNA plasmid (STIM1 shRNA sequence CTGAGCAGAGTCTGCATGACCTTCAGGAA from OriGene and STIM2 shRNA sequence CCGGGCTCAATTTCAGACACTCATTCTCGAGAATGAGTGTCTGAAATTGAGCTTTTTTG from Sigma) and GFP plasmid at a 3:1 molar ratio. In control experiments, nonspecific (luciferase-targeted) siRNA plasmid and GFP plasmid were used for cotransfection. Recordings were made 48–72 h after transfection. For STIM1 overexpression, the cells were transiently cotransfected with a mixture of human STIM1 and GFP plasmid at a 3:1 molar ratio. For STIM2 overexpression, the cells were transiently transfected with STIM2-YFP constructs in pcDNA3 expression vector. In control experiments GFP plasmid was used for transfection. After transfection, the cells were cultured on coverslips for 9–18 h, and GFP-positive (or YFP-positive) cells were selected for measurements.
Western Blotting
HEK293 cells grown in 5-cm dishes under the conditions described above were lysed in assay buffer (10 mm Tris-HCl, pH 7.5, with 150 mm NaCl, 1% Triton X-100, 1% Nonidet P-40, 2 mm EDTA, 0.2 mm phenylmethylsulfonyl fluoride, and protease inhibitor mixture), and the total protein extract (30–50 μg) was resolved by 8% SDS-PAGE (in mini gels). The proteins were transferred onto Immobilon P membrane (Millipore Inc.); treated with monoclonal antibody to STIM1 (BD Biosciences, 1:250), polyclonal antibodies to STIM2 (Alomone Labs, 1:200), or monoclonal antibody to α-tubulin (Sigma, 1:5000) and then with appropriate secondary antibody (Sigma); and developed with SuperSignal chemiluminescent substrate (Pierce). Anti-α-tubulin antibody was used to test for equal protein loading. Western blotting was repeated at least three times using independent cell lysates.
Ca2+ Imaging
HEK293 cells grown on glass coverslips were loaded with 5 μm Fura-2AM in the presence of 0.025% Pluronic for 40 min at room temperature and then illuminated by alternating 340- and 380-nm excitation light at 2 Hz. Emission fluorescence intensity was measured at 510 nm with an InCyt Basic I/P dual wavelength fluorescence imaging system (Intracellular Imaging Inc., Cincinnati, OH). The change in cytosolic Ca2+ concentration was estimated from the ratio of emission fluorescence intensities at 340- and 380-nm excitation wavelengths (the 340/380 ratio).
Electrophysiological Experiments
were performed at room temperature (22–24 °C) using an Axopatch 200B patch clamp amplifier (Axon Instruments) and PClamp software (Axon Instruments) for data acquisition and off-line data analysis.
Whole-cell Recordings
were made with 3–5 megaohms of fire-polished glass microelectrodes. The pipette solution contained (in mm): 145 NMDG aspartate, 10 Cs-EGTA (or 12 Cs-BAPTA), 10 Cs-HEPES (pH 7.3), 1.5 MgCl2, and 4.5 CaCl2 (pCa 7.0). Extracellular solution contained (in mm): 140 NMDG aspartate, 10 BaCl2, and 10 Cs-HEPES, pH 7.3. During recording, the currents were sampled at 5 kHz and filtered digitally at 500 Hz. The holding potential was −40 mV in all whole-cell experiments. Once every 5 s, the holding potential was shifted to −100 mV for 30 ms and a 170-ms voltage ramp to +70 mV was applied. The traces recorded before current activation were used as a template for leak subtraction. Whole-cell currents were normalized relative to the cell capacitance. Its mean value was 19 ± 4 picofarads (n = 15).
Single-channel Recordings
were made with 8–15 megaohms of SYLGARD-coated, fire-polished glass microelectrodes. The pipette solution contained 105 mm BaCl2 and 10 mm Tris-HCl (pH 7.3). In cell-attached experiments, the bath (control) solution contained (in mm): 140 KCl, 5 NaCl, 10 K-HEPES (pH 7.4), 1 MgCl2, and 2 CaCl2 to nullify the resting membrane potential. The thapsigargin (Tg) and UTP were applied by bath perfusion. The time required for complete solution exchange around the patch pipette was less than 1 s. The recordings were digitized at 5 kHz and filtered at 80–150 Hz for analysis and presentation. The amplitudes of single-channel currents were determined from the current traces and all-point amplitude histograms. The channel open probabilities (NPo) were determined by the following equation: NPo = (I)/i, where (I) is the mean channel current and i is the unitary current amplitude. The (I) was estimated from the time integrals of the currents above the baseline, and i was determined from the current traces and all-point amplitude histograms. The data were collected after channel activity reached steady state at −70 mV holding potential. Because channel activity was transient and fluctuated significantly, we used NPo collected during 30 s of maximal activity (NPomax30) as a standard parameter for comparing channel open probabilities between experiments.
Chemicals
HEPES, EGTA, NMDG, and Tg were from Sigma-Aldrich; UTP was from Calbiochem; and Fura-2AM and Pluronic were from Molecular Probes.
RESULTS
The Imin Channels Are Store-operated in HEK293 Cell Line with STIM1 Knockdown
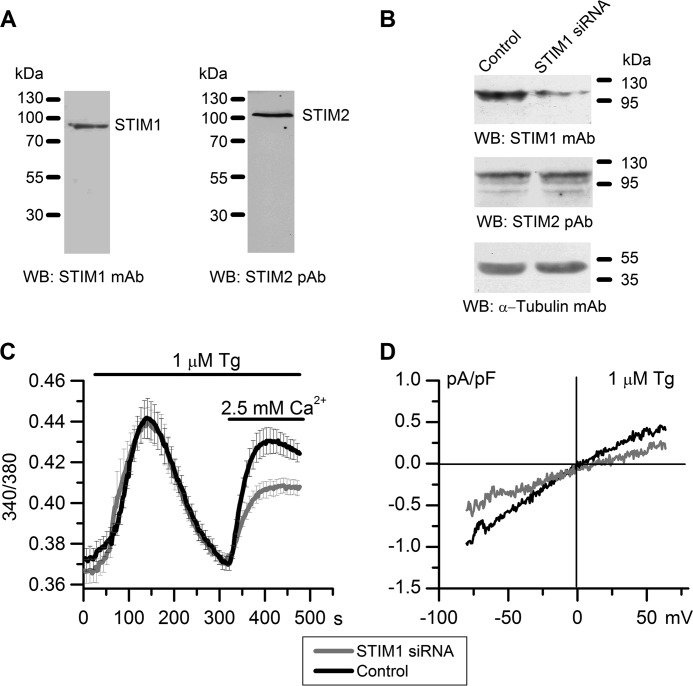
To evaluate the role of STIM2 calcium sensor in activating native store-operated channels, we used the HEK293 cell line. Electrophysiological properties of calcium channels in this cell line have been studied in detail, which makes it a good model for analyzing mechanisms of store-operated channel activation (56, 57). HEK293 cells express endogenous STIM1 and STIM2 proteins (11, 12, 58), as was confirmed in our Western blot experiments (Fig. 1A). To separate STIM2-regulated channels from STIM1-operated channels, STIM1 knockdown was performed by transient transfection of HEK293 cells with specific siRNA, and the results were verified by Western blotting, which also detected no effect on the STIM2 protein level (Figs. 1B and 3A).
FIGURE 1.
Suppression of STIM1 expression reduces store-operated calcium entry in HEK293 cells. A, endogenous expression of STIM1 and STIM2 proteins in HEK293 cells as analyzed by Western blotting (WB) with monoclonal antibodies to STIM1 and polyclonal antibodies to STIM2. B, Western blot analysis of HEK293 cells transiently transfected with nonspecific siRNA (Control) or siRNA against human STIM1 (STIM1 siRNA) using monoclonal antibodies to STIM1, polyclonal antibodies to STIM2, and α-tubulin (loading and specificity control). The results representative of at least three independent experiments are shown. C, calcium entry evoked by store depletion in STIM1 siRNA-transfected cells (gray line) was significantly reduced, when compared with the control (black line). Cytosolic Ca2+ levels in HEK293 cells transfected with STIM1 siRNA or control siRNA were monitored by ratiometric Fura-2 imaging. Calcium stores were depleted by incubation in Ca2+-free medium containing 0.2 mm EGTA and 1 μm Tg. Horizontal lines on the top indicate the time of treatment with 1 μm Tg and extracellular 2.5 mm Ca2+. Each trace represents an average of 14–15 experiments (mean ± S.E.), with calcium response from 10–20 cells recorded in each experiment. D, whole-cell recordings of store-operated currents in HEK293 cells transfected with STIM1 siRNA or control siRNA. Average current-voltage relationships for whole-cell currents evoked by passive depletion of calcium stores with 1 μm Tg in HEK293 cells transfected with STIM1 siRNA (gray line, n = 6) and control siRNA (black line, n = 9) are shown. The current-voltage relationships were measured when the currents reached a maximum. pF, picofarads.
FIGURE 3.
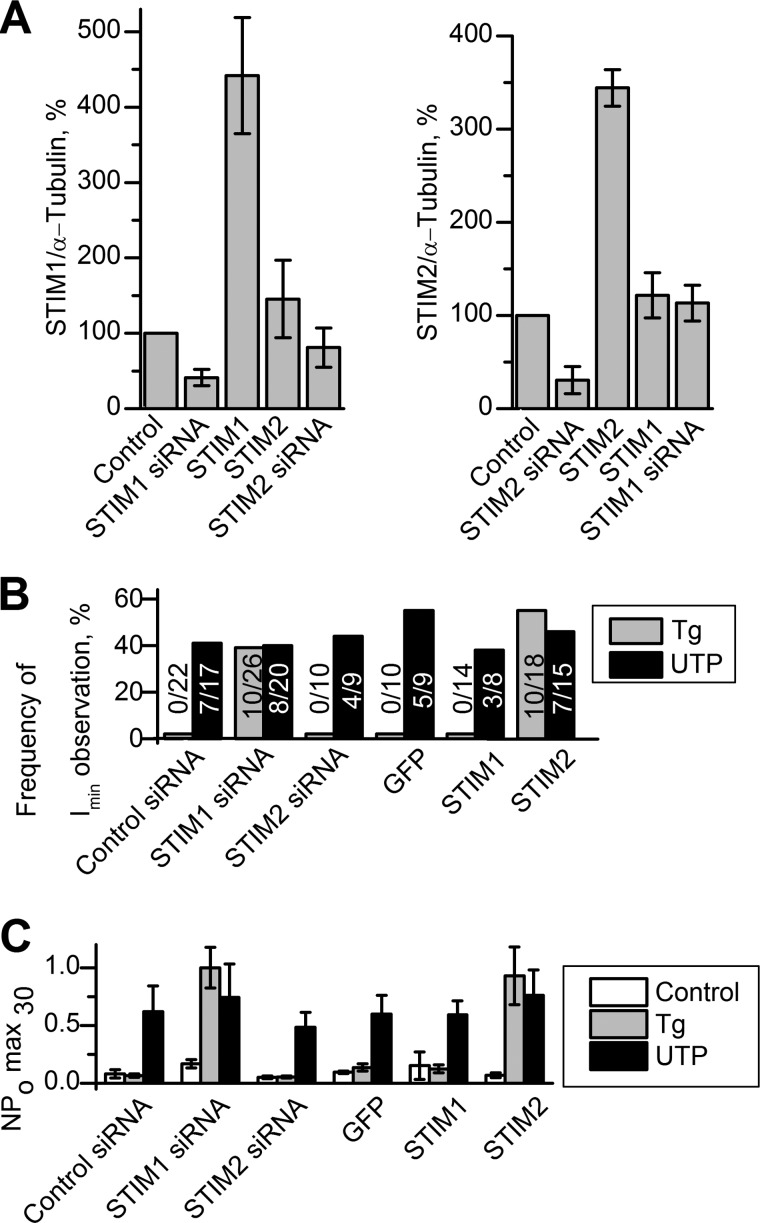
Activity of Imin channels in patches. A, quantitative summary data of STIM1 and STIM2 proteins levels obtained by Western blot analysis with antibodies to STIM proteins. The amount of STIM proteins was quantified by densitometry, normalized relative to the amount of α-tubulin, and plotted as a proportion of the control amount. The data are presented for control HEK293 cells (Control), HEK293 with STIM1 knockdown (STIM1 siRNA), HEK293 with STIM2 knockdown (STIM2 siRNA), HEK293 with STIM1 overexpression STIM1 (STIM1), and HEK293 with STIM2 overexpression (STIM2). Data represent an average of 3–6 experiments (mean ± S.E.) B, occurrence frequency of Imin channels in patches are plotted as a proportion (%) of positive experiments to the number of all experiments in the series. The activity of Imin channels was induced by bath application of 1 μm Tg (gray columns) and by subsequent addition of 100 μm UTP (black columns). The data are presented for HEK293 with control siRNA (Control siRNA), HEK293 with STIM1 knockdown (STIM1 siRNA), HEK293 with STIM2 knockdown (STIM2 siRNA), HEK293 with GFP overexpression (GFP), HEK293 with STIM1 overexpression STIM1 (STIM1), and HEK293 with STIM2 overexpression (STIM2). C, the summary plot of Imin open channel probability in cell-attached recordings before drug application (white columns), after bath application of 1 μm Tg (gray columns), after subsequent application of 100 μm UTP (black columns). The data are presented for control HEK293 cells (Control), HEK293 with STIM1 knockdown (STIM1 siRNA), HEK293 with STIM2 knockdown (STIM2 siRNA), HEK293 with STIM1 overexpression (STIM1), and HEK293 with STIM2 overexpression (STIM2). Imin activity is represented as NPomax30 (mean ± S.E.) (n = 5–8).
The involvement of STIM1 in the store-operated calcium influx was initially evaluated using the Ca2+ imaging method based on Fura-2 fluorescence. HEK293 cells transfected with anti-STIM1 or nonspecific (control) siRNA were incubated in Ca2+-free medium containing 1 μm sarcoendoplasmic reticulum Ca2+-ATPase (SERCA) pump inhibitor Tg to cause complete depletion of intracellular Ca2+ stores. Thereafter, the medium was supplemented with 2.5 mm Ca2+, and its influx via plasma membrane channels was monitored (Fig. 1C). The results showed that STIM1 knockdown resulted in reduction of Tg-stimulated Ca2+ influx to 60% of that in cells treated with control siRNA but had no effect on calcium release.
Experiments on measuring whole-cell currents at −80 mV holding potential showed that store-operated calcium entry stimulated by 1 μm Tg in STIM1 knockdown cells was only about 50% of that in cells transfected with control siRNA (Fig. 1D). These results are consistent with the concept that STIM1 is the main activator of store-operated calcium current (3, 4).
We then used single-channel analysis to evaluate the effects of STIM1 suppression on different types of calcium- and cation-selective channels expressed in HEK239 cells. In our previous studies, recordings in the cell-attached mode have shown that HEK293 cells contain three types of calcium channels, Imax, INS, and Imin, differing in protein composition, conductance, selectivity to divalent ions, open time, and number per cell (56, 57).
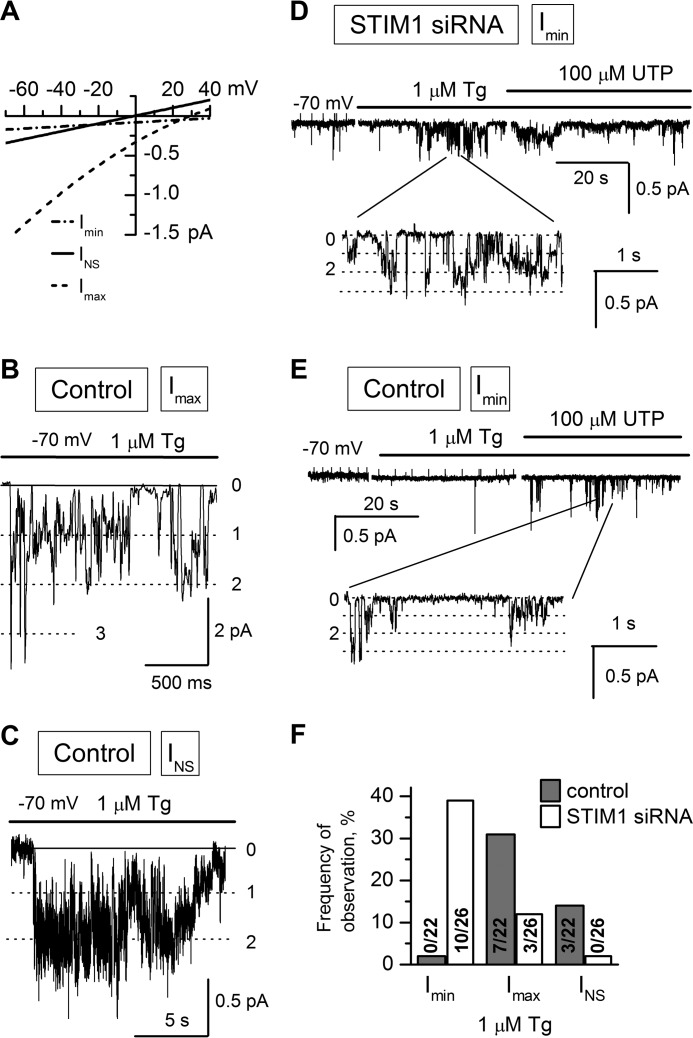
Store-operated Imax channels composed of TRPC1 proteins have medium selectivity and conductance of about 17 pS (57). Their activation was induced by calcium store depletion with 1 μm Tg. The occurrence of Imax channels in HEK293 cells decreased after STIM1 knockdown; they were detected in 3 out of 26 patches, when compared with 7 out of 22 patches in control cells (Fig. 2, A, B, and F; Table 1). The time lag of Imax activation after store depletion in STIM1 knockdown cells was prolonged to more than 90 s, whereas Imax channels in control cells were activated within less than 60 s after adding Tg.
FIGURE 2.
Activities of single calcium channels in HEK293 cells transfected with STIM1 siRNA or control siRNA. Recordings were made in the cell-attached patch clamp configuration at a membrane potential of −70 mV. In all experiments, the pipette solution contained 105 mm Ba2+ as a charge carrier. Representative fragments of the recordings are shown. A, average current-voltage relationships of Imin (dotted line solid line), INS (solid line), and Imax (dashed line) channels in control HEK293 cells. The fit to the data points yielded single-channel conductances of 1.2 pS for Imin, 5 pS for INS, and 17 pS for Imax. B and C, bath application of 1 μm Tg to cell-attached patches induced (B) Imax channel and (C) INS channel activity in HEK293 cells transfected with nonspecific siRNA (Control). D, Imin channel activity in STIM1 siRNA-transfected HEK293 cells (STIM1 siRNA) induced by bath application of 1 μm Tg and subsequent addition of 100 μm UTP. A fragment of the recording on an expanded time scale is shown at the bottom. E, the same as in D for control cells. F, occurrence frequencies of Imin, Imax, and INS channels in HEK293 and STIM1 siRNA cells. The cells were stimulated with Tg in cell-attached patches.
TABLE 1.
Occurrence frequency of Imax and INS channels in patches
| Cells | Activator | Imax channels | INS channels |
|---|---|---|---|
| % | % | ||
| HEK293 with control siRNA | 1 μm Tg | 31 | 14 |
| HEK293 with STIM1 knockdown | 1 μm Tg | 12 | 0 |
| HEK293 with GFP overexpression | 10 nm Tg | 16 | 0 |
| HEK293 with STIM2 overexpression | 10 nm Tg | 15 | 0 |
Store-operated TRPC3-containing INS channels are nonselective channels with a conductance of 5 pS (39, 56). No Tg-induced INS channel activity was revealed in STIM1 knockdown cells (n = 26). In control HEK293 cells, their activation after Tg application was observed in 14% of experiments (n = 22) (Fig. 2, A, C, and F; Table 1), in agreement with our previous data on the properties of INS channels in untreated HEK293 cells (56).
Imin channels, the third calcium channel type in HEK293 cells, have a single-channel conductance of 1.2 pS, show high selectivity to divalent cations (PBa/K = 20), and are activated by extracellular UTP or intracellular IP3, but not by Tg (56). Regardless of store depletion by UTP, intracellular IP3 at an increasing concentration is capable of activating Imin channels through conformational coupling with the IP3 receptor (IP3R) (59, 60). It is noteworthy that Imin channels in the A431 cell line display the basic properties of store-operated channels, such as activation upon calcium store depletion with Tg, BAPTA-AM, or N,N,N′,N″-tetrakis-(2-pyridylmethyl) ethylenediamine (TPEN) (61–63) and inhibition by store-operated entry blocker SKF95365 (59) or low concentrations of Gd3+. In our experiments with STIM1 knockdown HEK293 cells, application of 1 μm Tg induced Imin channel activity (n = 10/26) with NPomax30 raised from 0.08 ± 0.04 (n = 7) to 1.0 ± 0.18 (n = 7) (Figs. 2, A, D, and F, and 3C), whereas Imin channels in control HEK293 cells did not respond to this treatment (n = 0/22) (Figs. 2E and 3C). The presence of Imin channels in the patches was verified by adding UTP after Tg, which resulted in activation of Tg-insensitive Imin channels in control cells (n = 7/17) with NPomax30 equal to 0.62 ± 0.22 (n = 6) (Figs. 2E and 3, B and C) but had no significant effect on Imin activity in STIM1 knockdown cells, indicating that most of the Imin channels in the patch were already activated by Tg (Figs. 2D and 3, B and C).
Thus, STIM1 knockdown resulted in a decreased level of activation of store-operated INS and Imax channels in response to Tg treatment; moreover, previously Tg-independent Imin channels became sensitive to calcium store depletion with 1 μm Tg in STIM1 knockdown cells (Fig. 2). These results indicate that INS and Imax channels are probably STIM1-regulated, whereas Imin channels are not activated by STIM1 and appear to be regulated by another calcium sensor, namely, the STIM2 protein.
Store-operated Activity of Imin Channels Is Increased in STIM2-overexpressing HEK293 Cells
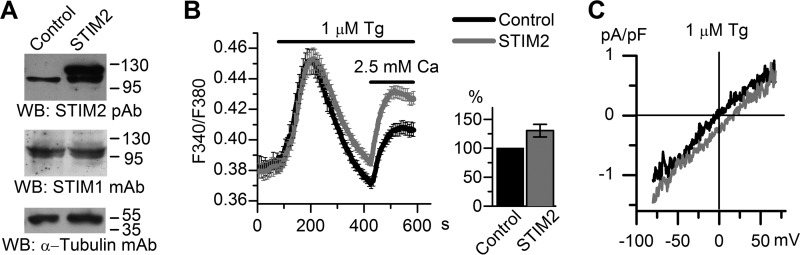
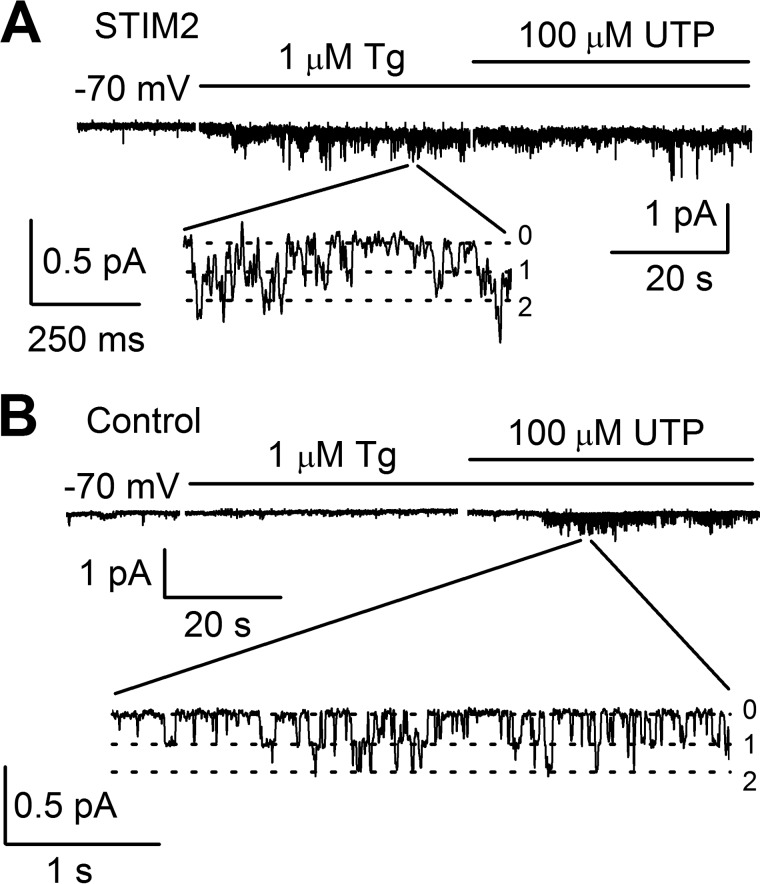
To test whether Imin channels are regulated by STIM2 proteins, we performed a series of experiments on STIM2 overexpression in HEK293 cells, using GFP-expressing cells as a control. As shown previously, STIM2 overexpression for more than 24 h could lead to inhibition of store-operated calcium entry (12, 58), with this inhibition being due to a certain cellular compensatory mechanism that does not manifest itself during the first 9 h (12). Therefore, the results of experiments were evaluated 9 h after transfection. STIM2 overexpression at this time point was confirmed by Western blot analysis, which also detected no effect on the STIM1 protein level (Figs. 3A and 4A). Intracellular calcium imaging with Fura-2 showed that Tg-induced calcium entry was slightly enhanced in STIM2-overexpressing cells, when compared with the control cells (Fig. 4B). The amplitude of whole-cell currents at −80 mV proved to be ∼20% higher in cells overexpressing Stim2 proteins than in control cells. The reversal potential of the whole-cell current-voltage relationship shifted to the positive potentials, indicating an increase in the activity of some store-operated channels selective to divalent ions (Fig. 4C). In 14 out of 20 single-channel experiments, 1 μm Tg proved to activate Imin channels in STIM2-overexpressing cells, with NPomax30 equal to 0.93 ± 0.25 (n = 8) remaining basically unchanged after subsequent treatment with 100 μm UTP (Figs. 3, B and C, and 5A). In contrast, Imin channels in control cells did not respond to Tg (n = 0/10) but were activated upon subsequent application of UTP (n = 5/9) with NPomax30 increased from 0.14 ± 0.03 (n = 8) to 0.60 ± 0.16 (n = 5) (Figs. 3, B and C, and 5B).
FIGURE 4.
Overexpression of exogenous STIM2 increases store-operated Ca2+ influx in HEK293 cells. A, control cells and cells transiently transfected with STIM2-encoding plasmid (STIM2) were analyzed by Western blotting (WB) with polyclonal antibodies to STIM2, monoclonal antibodies to STIM1, or monoclonal antibodies to α-tubulin (loading and specificity control). The results representative of at least three independent experiments are shown. B, left panel, calcium entry evoked by store depletion in STIM2-transfected cells (gray line, 49 cells) was increased, when compared with the control (black line, 43 cells). Cytosolic Ca2+ levels were monitored by ratiometric Fura-2 imaging. Calcium stores were depleted by incubation in Ca2+-free medium containing 0.2 mm EGTA and 1 μm Tg. Horizontal lines on the top indicate the time of treatment with 1 μm Tg and extracellular 2.5 mm Ca2+. Calcium entry was measured 9 h after transfection. Right panel, calcium entry in STIM2-overexpressing cells plotted as a proportion (%) of that in control cells. C, average current-voltage relationships of whole-cell currents evoked by passive Ca2+ stores depletion with 1 μm Tg in HEK293 cells overexpressing STIM2 (gray line, n = 7) and control (black line, n = 6). The current-voltage relationships were measured when the currents reached a maximum. pF, picofarads.
FIGURE 5.
Calcium store depletion with 1 μm Tg activates Imin channels in STIM2-overexpressing cells. A, Imin channel activity in STIM2-overexpressing cells (STIM2) was recorded in the cell-attached mode after bath application of 1 μm Tg and subsequent addition of 100 μm UTP. The holding potential was −70 mV. Expanded current traces are shown at the bottom. B, in control cells, calcium store depletion with 1 μm Tg did not activate Imin channels, whereas subsequent application of 100 μm UTP induced Imin activity.
Recordings in the cell-attached configuration showed that the induced activity of Imin channels was dependent on their activity in the control recording solution. To compare results between experiments, we calculated the ratio of channel open probability (NPo) after Tg or UTP treatment to that before treatment (ΔNPo = NPoafter/NPobefore), which reflected the amount of increase in the activity of Imin channels. Thus, it was found that the addition of 1 μm Tg to STIM2-overexpressing cells resulted in a 5.2-fold increase in Imin channel activity. A similar behavior of Imin channels in response to UTP treatment was observed in control cells, with their activity increasing 5.7-fold. This is evidence that all Imin channels that could be activated by agonist application in control cells were activated by store depletion with Tg in STIM2-overexpressing cells.
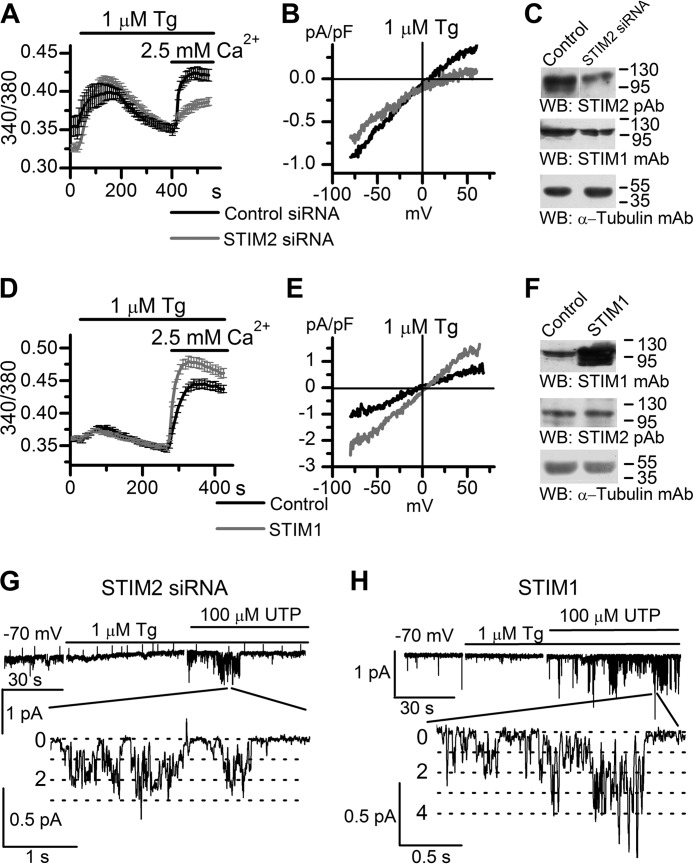
To further evaluate the role of STIM proteins in Imin activation, we performed experiments on cells with STIM2 knockdown or STIM1 overexpression (Figs. 3 and 6). Protein levels of STIM proteins were verified by Western blot (Figs. 3A and 6, C and F). Calcium imaging experiments demonstrated that Tg-induced calcium entry was down-regulated in cells with STIM2 knockdown and up-regulated in cells with STIM1 overexpression (Fig. 6, A and D). Similar results were obtained in whole-cell experiments, whereas STIM2 knockdown changed the shape of current-voltage relationships of whole-cell currents (Fig. 6, B and E). In single-channel experiments, we observed that in either case, Imin channels were not activated by calcium store depletion with 1 μm Tg, but subsequent addition of extracellular 100 μm UTP resulted in activation of these channels with NPomax30 up to 0.59 ± 0.13 (Figs. 3, B and C, and 6, G and H). On the whole, these data suggest that Imin channels can be regulated by STIM2 proteins upon calcium store depletion.
FIGURE 6.
Store depletion with 1 μm Tg does not activate Imin channels in cells with STIM2 knockdown (STIM2 siRNA) or STIM1-overexpressing cells (STIM1). A, calcium entry evoked by store depletion in STIM2 siRNA-transfected cells (gray line, 31 cells) and in control cells (black line, 16 cells). Cytosolic Ca2+ levels in HEK293 cells transfected with STIM2 siRNA or control siRNA were monitored by ratiometric Fura-2 imaging. Calcium stores were depleted by incubation in Ca2+-free medium containing 0.2 mm EGTA and 1 μm Tg. Horizontal lines on the top indicate the time of treatment with 1 μm Tg and extracellular 2.5 mm Ca2+. B, average current-voltage relationships of whole-cell currents evoked by passive depletion of calcium stores with 1 μm Tg in HEK293 cells transfected with STIM2 siRNA (gray line, n = 13) and control siRNA (black line, n = 10). The current-voltage relationships were measured when the currents reached a maximum. pF, picofarads. C, Western blot (WB) analysis of HEK293 cells transiently transfected with nonspecific siRNA (Control) or siRNA against human STIM2 (STIM2 siRNA) using polyclonal antibodies to STIM2, monoclonal antibodies to STIM1, and monoclonal antibodies to α-tubulin (loading and specificity control). The results representative of at least three independent experiments are shown. D, The same as in A for cells transfected with STIM1-encoding plasmid (STIM1). Calcium entry evoked by store depletion with 1 μm Tg in STIM1-transfected cells (gray line, 29 cells) and in control cells (black line, 33 cells) is shown. Cytosolic Ca2+ levels were monitored by ratiometric Fura-2 imaging. E, average current-voltage relationships of whole-cell currents evoked by passive depletion of calcium stores with 1 μm Tg in STIM1-transfected cells (gray line, n = 8) and control (black line, n = 6). The current-voltage relationships were measured when the currents reached a maximum. F, control cells and cells transiently transfected with STIM1-encoding plasmid (STIM1) were analyzed by Western blotting using monoclonal antibodies to STIM1, polyclonal antibodies to STIM2, and monoclonal antibodies to α-tubulin (loading and specificity control). G, in HEK293 cells transiently transfected with siRNA against human STIM2, bath application of 1 μm Tg did not induce Imin activity, whereas subsequent addition of 100 μm UTP activated Imin in the same patch. Imin currents are shown on compressed (top) and expanded (bottom) time scales. The holding potential was −70 mV. H, the same as in G for STIM1-overexpressing HEK293 cells.
Partial Calcium Store Depletion Activates Imin Channels in HEK293 Cells
The results described above were obtained in cells with STIM1 or STIM2 knockdown or overexpression. The next question was as to whether Imin channels could be regulated by STIM2 in native cells, without any manipulation with the STIM protein level. To answer this question, we made use of the difference in affinity to calcium between the STIM1 and STIM2 proteins (12, 13). STIM2 can be specifically activated by partial calcium store depletion, e.g. with low Tg concentrations (15, 17, 18), whereas STIM1 requires stronger depletion for its activation.
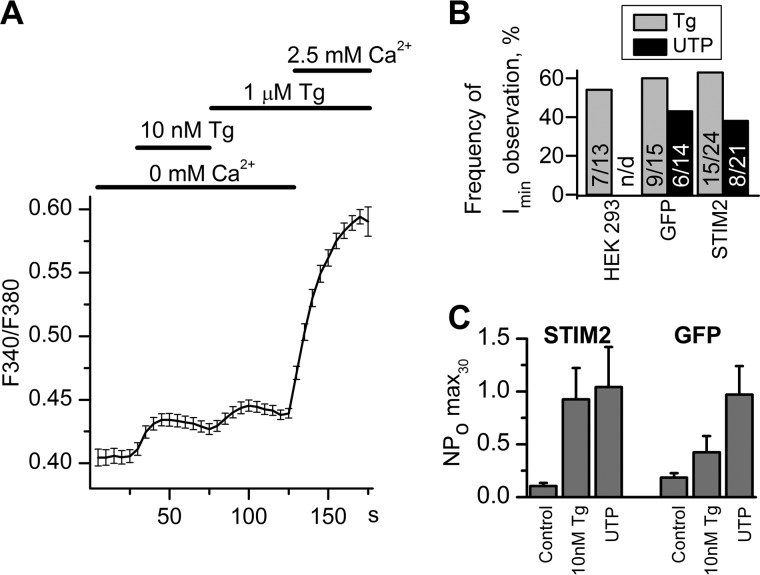
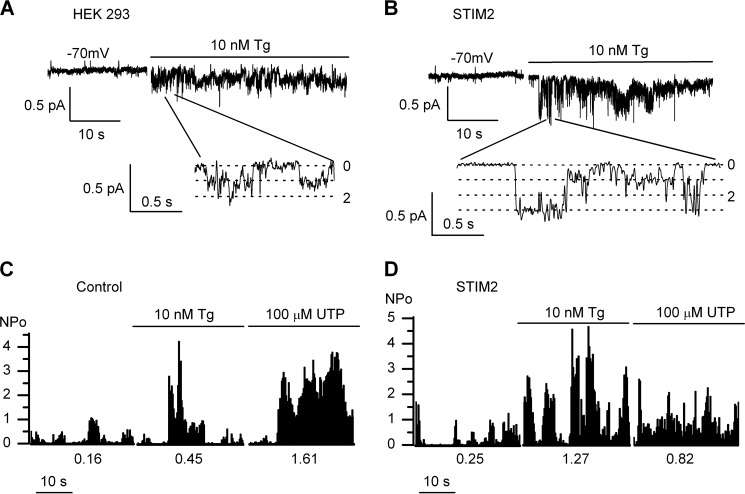
Calcium imaging experiments showed that 10 nm Tg induced only partial depletion of intracellular calcium store because an additional amount of calcium was released after subsequent treatment with 1 μm Tg (Fig. 7A). After extracellular application of 10 nm Tg (aimed at selective STIM2 activation), moderate activity of Imin channels was recorded in 54% of cell-attached patches (n = 7/13) (Figs. 7B and 8A), indicating that Imin channels could be regulated by endogenous STIM2 in native cells.
FIGURE 7.
Partial calcium store depletion with 10 nm Tg in HEK293 cells. A, cytosolic Ca2+ levels in HEK293 cells were monitored as 340/380 Fura-2 fluorescence ratio. Horizontal lines on the top indicate changes between 0 and 2.5 mm Ca2+ in extracellular solution and application of 10 nm Tg and 1 μm Tg. The Ca2+ release evoked by application of 10 nm Tg in Ca2+-free medium was followed by additional Ca2+ release upon stronger store depletion with 1 μm Tg. The data are presented as mean ± S.E. B, occurrence frequencies of Imin channels in patches are plotted as a proportion (%) of positive experiments to the number of all experiments in the series. Activity of Imin channels was induced by bath application of 10 nm Tg (gray columns) and by subsequent addition of 100 μm UTP (black columns). The data are presented for HEK293 (HEK293), HEK293 with GFP overexpression (GFP), and HEK293 with STIM2 overexpression (STIM2). C, the summary plot of Imin open channel probability in cell-attached recordings before drug application (Control), after bath application of 10 nm Tg (10 nm Tg), and after subsequent application of 100 μm UTP (UTP). The data are presented for HEK293 with GFP overexpression (GFP) and HEK293 with STIM2 overexpression (STIM2). Imin activity is represented as NPomax30 (mean ± S.E.) (n = 6–13).
FIGURE 8.
Partial store depletion activates Imin channels in HEK293 cells. A, application of 10 nm Tg to the bath solution activated endogenous Imin Ca2+ channels in cell-attached patches from native HEK293 cells. The unitary current amplitude at −70 mV membrane resting potential was −0.18 pA. Fragments of current recordings on an expanded time scale are shown at the bottom. B, the same as in A for STIM2-overexpressing HEK293 cells (STIM2). C, plot of Imin channel open probability (NPo) in the control cell-attached patch before and after bath application of 10 nm Tg and 100 μm UTP. The NPo was averaged over 500-ms intervals and plotted versus time. Mean NPomax30 was 0.16 in the control, 0.45 in the presence of 10 nm Tg, and 1.61 after subsequent application of 100 μm UTP (in the given experiment). The results representative of six experiments are shown. D, the same plot as in C for the experiment with STIM2-overexpressing HEK293 cells (STIM2). Mean NPomax30 was 0.25 in the control, 1.27 in the presence of 10 nm Tg, and 0.82 after subsequent application of 100 μm UTP (in the given experiment). The results representative of eight experiments are shown.
In the next experimental series, selective STIM2 activation by partial store depletion was performed in STIM2-overexpressing cells. After adding 10 nm Tg, strong activation of Imin channels in these cells was observed in 63% of experiments (n = 15/24). On average, NPomax30 was increased from 0.1 ± 0.03 (n = 10) to 0.93 ± 0.30 (n = 13) after 10 nm Tg treatment and was not changed significantly after subsequent treatment with 100 μm UTP (n = 8) (Figs. 7C and 8, B and D). In control cells, Imin channel NPomax30 increase was about 2-fold under the effect of 10 nm Tg (from 0.18 ± 0.04 (n = 9) to 0.42 ± 0.15 (n = 6), with an additional increase to 0.97 ± 0.27 (n = 6) being observed after subsequent addition of 100 μm UTP (Figs. 7C and 8C). These data indicate that the number of active channels (or NPo) correlates with the amount of active STIM2 proteins, which can be increased by means of their overexpression and/or selective activation.
Regulation of INS and Imax Channels by STIM Proteins
Although INS and Imax channels were found to be regulated by STIM1 protein (Fig. 2F), we were interested in evaluating the role of endogenous STIM2 protein in their activation. Partial calcium store depletion with 10 nm Tg failed to induce any INS channel activity (n = 33) (Table 1). Moreover, no activation of these channels was observed even after subsequent treatment with 100 μm UTP. Therefore, it appears that INS channels are activated by STIM1, but not by STIM2.
Application of 10 nm Tg to the cell-attached patch induced a moderate activity of Imax channel in only 16% of experiments (n = 3/19), with this proportion increasing to 31% in experiments with 1 μm Tg (Fig. 2, B and F; Table 1). In most experiments with partial calcium store depletion, the activity of Imax channels was induced with a delay of more than 3 min. We consider that both STIM1 and STIM2 proteins participate in Imax regulation, but STIM2 is a less potent activator.
DISCUSSION
Imin Channels Are STIM2-regulated Store-operated Channels
The results of this study show that endogenous Imin channels in HEK293 cells are regulated by STIM2 calcium sensors. In particular, this conclusion is based on the following facts. First, Imin channel activity in cell-attached patches was induced upon selective activation of endogenous STIM2 by partial calcium store depletion (Figs. 7B and 8A). Second, the overexpression of exogenous STIM2 (but not STIM1) resulted in an increased activity of Imin channels (Figs. 3, B and C, 5, and 6H). Moreover, these channels were found to be store-operated in STIM1 knockdown cells (Figs. 2, D and E, and 3, B and C). These data are not consistent with the accepted view that all store-operated channels are activated by STIM1. It is noteworthy, however, that STIM1 knockdown in our experiments led to a decrease in store-operated whole-cell currents (Fig. 1). This decrease may be explained by reduction of Imax and INS channel activity, with up-regulation of Imin channels being insufficient to rescue the current because of low conductance of these channels (Fig. 2, A and F).
Electrophysiological characteristics of Imin channels are described in our previous publications (39, 40, 56, 57, 59–64). These channels are similar to CRAC channels in properties such as high selectivity to divalent cations, low conductance, activation by calcium store depletion or increase in intracellular IP3, and inhibition by SKF95365 or Gd3+. As shown in several laboratories, CRAC channels are regulated by STIM1 or STIM2 proteins (17, 55). In contrast, Imin channels cannot be activated by STIM1 proteins alone, with STIM2 being necessary for their activation upon calcium store depletion. Thus, we have obtained the first evidence for the existence of endogenous STIM2-regulated non-CRAC store-operated channels.
Store Sensitivity of Imin Channels Depends on STIM2/STIM1 Ratio
Both STIM1 and STIM2 calcium sensors are essential for store-operated calcium entry (15) and can interact with each other in the cell (11). However, available data on the combined action of STIM1 and STIM2 in regulating store-operated calcium entry are scarce and controversial.
The first evidence for the role of cross-talk between STIM1 and STIM2 in activating store-operated calcium entry was obtained by Soboloff et al. (58), who found that the overexpression of exogenous STIM2 resulted in the inhibition of STIM1-induced store-operated calcium entry in HEK293, PC12, A7r5, and Jurkat T cells (58). Similar store-operated entry inhibition was obtained in experiments with intestinal epithelial cells (65), where the authors showed that changes in the level of polyamines altered the ratio of endogenous STIM1 to STIM2. Polyamine depletion enhanced the expression of endogenous STIM2, with consequent decrease in TRPC1-mediated store-operated calcium entry activated by STIM1 (65).
In contrast, data reported by other research groups are indicative of independent or synergistic action of STIM2 and STIM1 proteins (12, 15, 21). Thus, it has been shown that STIM2 overexpression results in activation of Orai1 channels in HeLa cells (12) and of overexpressed Orai1, -2, and -3 channels in HEK293 cells (55). Endogenous STIM2 proteins activate native Orai2 channels in dendritic cells (20) and also participate in calcium entry through native CRAC channels and in calcium oscillations in rat basophilic leukemia and HEK293 cells (17, 18). The expression of STIM2 has been proven to restore calcium entry in STIM1 knock-out mouse embryonic fibroblast cells (15).
An explanation for the inhibitory effect of STIM2 revealed by the Gill group (58) was provided by Brandman et al. (12). They demonstrated that STIM2 overexpression for 9 h enhanced store-operated calcium entry, but prolonged STIM2 overexpression (for 24 h) resulted in its inhibition, with the inhibitory effect being more distinct at a high level of STIM2. This is evidence for the existence of a slow-acting adaptive mechanism that down-regulates store-operated Ca2+ influx upon prolonged supramaximal STIM2 signaling (12).
Our data suggest that the sensitivity of Imin channels to calcium store depletion depends on the ratio between active STIM2 and STIM1 proteins. Activation of these channels in response to store depletion was observed in cells where the STIM2/STIM1 ratio shifted to the left as a result of STIM1 knockdown, STIM2 overexpression, or selective STIM2 activation by 10 nm Tg (Figs. 2, D–F, 3, 5, and 8A). A combination of these approaches evoked more robust Imin activation (Fig. 8). In contrast, calcium store depletion with 1 μm Tg failed to activate Imin channels in cells where the STIM2/STIM1 ratio was shifted to the right due to STIM2 knockdown or STIM1 overexpression (Figs. 3 and 6, G and H). In other words, changes in the relative amount of active STIM2 can switch the regulation of Imin channels between store-independent and store-operated modes.
The results of this study indicate that STIM1 activation blocks store-operated regulation of Imin, (12, 58, 65). In fact, calcium store depletion by 1 μm Tg combined with strong endogenous STIM1 activation (4, 12, 18) prevents Imin activation (Fig. 2E), whereas partial store depletion by 10 nm Tg and endogenous STIM2 activation (12, 15, 18) evoke Imin activation in native HEK293 cells (Fig. 8A). Therefore, STIM1 inhibitory effect is not associated with the long-term compensatory mechanism revealed in previous studies (12, 58). Similar results have recently been obtained in experiments with neurons; it has been shown that strong calcium store depletion with 2 μm Tg prevents the interaction between Orai1 and STIM2, whereas gradual reduction of intracellular calcium concentration by EGTA or BAPTA treatment promotes Orai1 interaction with STIM2 (66). Our data provide evidence that STIM1-STIM2 association or competition may be an additional mechanism modulating and regulating store-operated calcium entry (13, 58).
Different Store-operated Channel Types Are Differently Regulated by STIM1 and STIM2 Proteins
We have previously found that different calcium channels types coexist in A431 and HEK293 cells (39, 56). The results of this study show that these channels are differently regulated by STIM proteins (Fig. 3; Table 1).
According to single-channel patch clamp experiments, store-operated Imin channels in HEK293 cells are activated by STIM2 but not by STIM1 proteins (Fig. 3). TRPC3-containing INS channels (39) are activated by STIM1 but not by STIM2 (Table 1, Fig. 2C). This is in agreement with data that STIM2 has no effect on TRPC3-mediated calcium entry (58). Finally, Imax channels consisting of TRPC1 proteins are regulated by both STIM1 (51, 52) and STIM2 proteins (Table 1, Fig. 2B). However, our results show that Imax channel activation is slow when induced by STIM2. This is in agreement with data on activation of Orai channels by STIM proteins (55, 67), which show that STIM2 is a slower and weaker activator, when compared with STIM1.
Whole-cell data are in agreement with single-channel recordings. Overexpression or knockdown of STIM proteins changes the reversal potential and shape of whole-cell current-voltage relationship, indicating the different roles of STIM1 and STIM2 proteins in the regulation of various store-operated channels. Overexpression of STIM2 increases selectivity and amplitude of whole-cell currents due to the appearance of additional Tg-activated Imin channels. Based on the changes of the shape of current-voltage relationships, STIM2 knockdown seems to increase the activity of CRAC channels. Due to remarkable low single-channel CRAC conductance (2), we could not verify this supposition in single-channel experiments. This intriguing result requires further investigations.
As shown previously, different store-operated channels can coexist in the cell (2, 56), and different agonists cause activation of different STIM proteins (17). In this study, we have revealed that STIM1 and STIM2 proteins differ in potency for activating different store-operated channels (Fig. 3; Table 1) and therefore can exert additional regulatory control on store-operated calcium entry. Taken together, all these factors allow the cell to produce a broad spectrum of calcium signals and downstream responses. Further studies are needed to gain an insight into molecular mechanisms underlying differences in the activation response of individual store-operated channel types to STIM proteins.
This study was supported by the Russian Scientific Foundation, Project 14-14-00720 (to E. K. and A. S.); the program “Molecular and Cellular Biology” of the Russian Academy of Sciences (to G. N. M. and L. G.); the Scientific School Support Program, Project SS-1721.2014.4 (to G. N. M.); the Dynasty Foundation; and the Russian Foundation for Basic Research.
- STIM
- stromal interaction molecule
- CRAC
- calcium release-activated calcium
- TRPC
- canonical transient receptor potential
- IP3
- inositol 1,4,5-trisphosphate
- Tg
- thapsigargin
- NMDG
- N-methyl-d-glucamin
- BAPTA
- 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- pS
- picosiemens.
REFERENCES
- 1. Clapham D. E. (2007) Calcium signaling. Cell 131, 1047–1058 [DOI] [PubMed] [Google Scholar]
- 2. Parekh A. B., Putney J. W. (2005) Store-operated calcium channels. Physiol. Rev. 85, 757–810 [DOI] [PubMed] [Google Scholar]
- 3. Roos J., DiGregorio P. J., Yeromin A. V., Ohlsen K., Lioudyno M., Zhang S., Safrina O., Kozak J. A., Wagner S. L., Cahalan M. D., Veliçelebi G., Stauderman K. A. (2005) STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 169, 435–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liou J., Kim M. L., Heo W. D., Jones J. T., Myers J. W., Ferrell J. E., Jr., Meyer T. (2005) STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 15, 1235–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stathopulos P. B., Zheng L., Li G.-Y., Plevin M. J., Ikura M. (2008) Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry. Cell 135, 110–122 [DOI] [PubMed] [Google Scholar]
- 6. Yuan J. P., Zeng W., Dorwart M. R., Choi Y.-J., Worley P. F., Muallem S. (2009) SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat. Cell Biol. 11, 337–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Park C. Y., Hoover P. J., Mullins F. M., Bachhawat P., Covington E. D., Raunser S., Walz T., Garcia K. C., Dolmetsch R. E., Lewis R. S. (2009) STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell 136, 876–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Muik M., Fahrner M., Schindl R., Stathopulos P., Frischauf I., Derler I., Plenk P., Lackner B., Groschner K., Ikura M., Romanin C. (2011) STIM1 couples to ORAI1 via an intramolecular transition into an extended conformation. EMBO J. 30, 1678–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stathopulos P. B., Schindl R., Fahrner M., Zheng L., Gasmi-Seabrook G. M., Muik M., Romanin C., Ikura M. (2013) STIM1/Orai1 coiled-coil interplay in the regulation of store-operated calcium entry. Nat. Commun. 4, 2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou Y., Srinivasan P., Razavi S., Seymour S., Meraner P., Gudlur A., Stathopulos P. B., Ikura M., Rao A., Hogan P. G. (2013) Initial activation of STIM1, the regulator of store-operated calcium entry. Nat. Struct. Mol. Biol. 20, 973–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Williams R. T., Manji S. S., Parker N. J., Hancock M. S., Van Stekelenburg L., Eid J. P., Senior P. V., Kazenwadel J. S., Shandala T., Saint R., Smith P. J., Dziadek M. A. (2001) Identification and characterization of the STIM (stromal interaction molecule) gene family: coding for a novel class of transmembrane proteins. Biochem. J. 357, 673–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brandman O., Liou J., Park W. S., Meyer T. (2007) STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell 131, 1327–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zheng L., Stathopulos P. B., Li G.-Y., Ikura M. (2008) Biophysical characterization of the EF-hand and SAM domain containing Ca2+ sensory region of STIM1 and STIM2. Biochem. Biophys. Res. Commun. 369, 240–246 [DOI] [PubMed] [Google Scholar]
- 14. Ercan E., Momburg F., Engel U., Temmerman K., Nickel W., Seedorf M. (2009) A conserved, lipid-mediated sorting mechanism of yeast Ist2 and mammalian STIM proteins to the peripheral ER. Traffic 10, 1802–1818 [DOI] [PubMed] [Google Scholar]
- 15. Oh-Hora M., Yamashita M., Hogan P. G., Sharma S., Lamperti E., Chung W., Prakriya M., Feske S., Rao A. (2008) Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat. Immunol. 9, 432–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bird G. S., Hwang S.-Y., Smyth J. T., Fukushima M., Boyles R. R., Putney J. W., Jr. (2009) STIM1 is a calcium sensor specialized for digital signaling. Curr. Biol. 19, 1724–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kar P., Bakowski D., Di Capite J., Nelson C., Parekh A. B. (2012) Different agonists recruit different stromal interaction molecule proteins to support cytoplasmic Ca2+ oscillations and gene expression. Proc. Natl. Acad. Sci. U.S.A. 109, 6969–6974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thiel M., Lis A., Penner R. (2013) STIM2 drives Ca2+ oscillations through store-operated Ca2+ entry caused by mild store depletion. J. Physiol. 591, 1433–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matsumoto M., Fujii Y., Baba A., Hikida M., Kurosaki T., Baba Y. (2011) The calcium sensors STIM1 and STIM2 control B cell regulatory function through interleukin-10 production. Immunity 34, 703–714 [DOI] [PubMed] [Google Scholar]
- 20. Bandyopadhyay B. C., Pingle S. C., Ahern G. P. (2011) Store-operated Ca2+ signaling in dendritic cells occurs independently of STIM1. J. Leukoc. Biol. 89, 57–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Darbellay B., Arnaudeau S., Ceroni D., Bader C. R., Konig S., Bernheim L. (2010) Human muscle economy myoblast differentiation and excitation-contraction coupling use the same molecular partners, STIM1 and STIM2. J. Biol. Chem. 285, 22437–22447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gruszczynska-Biegala J., Pomorski P., Wisniewska M. B., Kuznicki J. (2011) Differential roles for STIM1 and STIM2 in store-operated calcium entry in rat neurons. PLoS ONE 6, e19285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schuhmann M. K., Stegner D., Berna-Erro A., Bittner S., Braun A., Kleinschnitz C., Stoll G., Wiendl H., Meuth S. G., Nieswandt B. (2010) Stromal interaction molecules 1 and 2 are key regulators of autoreactive T cell activation in murine autoimmune central nervous system inflammation. J. Immunol. 184, 1536–1542 [DOI] [PubMed] [Google Scholar]
- 24. Cheng K. T., Alevizos I., Liu X., Swaim W. D., Yin H., Feske S., Oh-hora M., Ambudkar I. S. (2012) STIM1 and STIM2 protein deficiency in T lymphocytes underlies development of the exocrine gland autoimmune disease, Sjogren's syndrome. Proc. Natl. Acad. Sci. U.S.A. 109, 14544–145449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McAndrew D., Grice D. M., Peters A. A., Davis F. M., Stewart T., Rice M., Smart C. E., Brown M. A., Kenny P. A., Roberts-Thomson S. J., Monteith G. R. (2011) ORAI1-mediated calcium influx in lactation and in breast cancer. Mol. Cancer Ther. 10, 448–460 [DOI] [PubMed] [Google Scholar]
- 26. Aytes A., Molleví D. G., Martinez-Iniesta M., Nadal M., Vidal A., Morales A., Salazar R., Capellà G., Villanueva A. (2012) Stromal interaction molecule 2 (STIM2) is frequently overexpressed in colorectal tumors and confers a tumor cell growth suppressor phenotype. Mol. Carcinog. 51, 746–753 [DOI] [PubMed] [Google Scholar]
- 27. Weidinger C., Shaw P. J., Feske S. (2013) STIM1 and STIM2-mediated Ca2+ influx regulates antitumour immunity by CD8+ T cells. EMBO Mol. Med. 5, 1311–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stanisz H., Saul S., Müller C. S. L., Kappl R., Niemeyer B. A., Vogt T., Hoth M., Roesch A., Bogeski I. (2014) Inverse regulation of melanoma growth and migration by Orai1/STIM2-dependent calcium entry. Pigment Cell Melanoma Res. 27, 442–453 [DOI] [PubMed] [Google Scholar]
- 29. Berna-Erro A., Braun A., Kraft R., Kleinschnitz C., Schuhmann M. K., Stegner D., Wultsch T., Eilers J., Meuth S. G., Stoll G., Nieswandt B. (2009) STIM2 regulates capacitive Ca2+ entry in neurons and plays a key role in hypoxic neuronal cell death. Sci. Signal. 2, ra67. [DOI] [PubMed] [Google Scholar]
- 30. Bojarski L., Pomorski P., Szybinska A., Drab M., Skibinska-Kijek A., Gruszczynska-Biegala J., Kuznicki J. (2009) Presenilin-dependent expression of STIM proteins and dysregulation of capacitative Ca2+ entry in familial Alzheimer's disease. Biochim. Biophys. Acta 1793, 1050–1057 [DOI] [PubMed] [Google Scholar]
- 31. Ryazantseva M., Skobeleva K., Kaznacheyeva E. (2013) Familial Alzheimer's disease-linked presenilin-1 mutation M146V affects store-operated calcium entry: Does gain look like loss? Biochimie 95, 1506–1509 [DOI] [PubMed] [Google Scholar]
- 32. Sun S., Zhang H., Liu J., Popugaeva E., Xu N.-J., Feske S., White C. L., 3rd, Bezprozvanny I. (2014) Reduced synaptic STIM2 expression and impaired store-operated calcium entry cause destabilization of mature spines in mutant presenilin mice. Neuron 82, 79–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hoth M., Penner R. (1993) Calcium release-activated calcium current in rat mast cells. J. Physiol. 465, 359–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zagranichnaya T. K., Wu X., Villereal M. L. (2005) Endogenous TRPC1, TRPC3, and TRPC7 proteins combine to form native store-operated channels in HEK-293 cells. J. Biol. Chem. 280, 29559–29569 [DOI] [PubMed] [Google Scholar]
- 35. Sundivakkam P. C., Freichel M., Singh V., Yuan J. P., Vogel S. M., Flockerzi V., Malik A. B., Tiruppathi C. (2012) The Ca2+ sensor stromal interaction molecule 1 (STIM1) is necessary and sufficient for the store-operated Ca2+ entry function of transient receptor potential canonical (TRPC) 1 and 4 channels in endothelial cells. Mol. Pharmacol. 81, 510–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Antigny F., Koenig S., Bernheim L., Frieden M. (2013) During post-natal human myogenesis, normal myotube size requires TRPC1- and TRPC4-mediated Ca2+ entry. J. Cell Sci. 126, 2525–2533 [DOI] [PubMed] [Google Scholar]
- 37. Saul S., Stanisz H., Backes C. S., Schwarz E. C., Hoth M. (2014) How ORAI and TRP channels interfere with each other: interaction models and examples from the immune system and the skin. Eur. J. Pharmacol. 739, 49–59 [DOI] [PubMed] [Google Scholar]
- 38. Hooper J. S., Hadley S. H., Mathews A., Taylor-Clark T. E. (2013) Store-operated calcium entry in vagal sensory nerves is independent of Orai channels. Brain Res. 1503, 7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kaznacheyeva E., Glushankova L., Bugaj V., Zimina O., Skopin A., Alexeenko V., Tsiokas L., Bezprozvanny I., Mozhayeva G. N. (2007) Suppression of TRPC3 leads to disappearance of store-operated channels and formation of a new type of store-independent channels in A431 cells. J. Biol. Chem. 282, 23655–23662 [DOI] [PubMed] [Google Scholar]
- 40. Shalygin A., Ryazantseva M., Glushankova L., Mozhayeva G. N., Bezprozvanny I., Kaznacheyeva E. (2010) Homer regulation of native plasma membrane calcium channels in A431 cells. Cell Calcium 48, 209–214 [DOI] [PubMed] [Google Scholar]
- 41. Horinouchi T., Higashi T., Higa T., Terada K., Mai Y., Aoyagi H., Hatate C., Nepal P., Horiguchi M., Harada T., Miwa S. (2012) Different binding property of STIM1 and its novel splice variant STIM1L to Orai1, TRPC3, and TRPC6 channels. Biochem. Biophys. Res. Commun. 428, 252–258 [DOI] [PubMed] [Google Scholar]
- 42. Selvaraj S., Sun Y., Watt J. A., Wang S., Lei S., Birnbaumer L., Singh B. B. (2012) Neurotoxin-induced ER stress in mouse dopaminergic neurons involves downregulation of TRPC1 and inhibition of AKT/mTOR signaling. J. Clin. Invest. 122, 1354–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jardin I., Gómez L. J., Salido G. M., Rosado J. A. (2009) Dynamic interaction of hTRPC6 with the Orai1-STIM1 complex or hTRPC3 mediates its role in capacitative or non-capacitative Ca2+ entry pathways. Biochem. J. 420, 267–276 [DOI] [PubMed] [Google Scholar]
- 44. Ong H. L., Cheng K. T., Liu X., Bandyopadhyay B. C., Paria B. C., Soboloff J., Pani B., Gwack Y., Srikanth S., Singh B. B., Gill D. L., Ambudkar I. S. (2007) Dynamic assembly of TRPC1-STIM1-Orai1 ternary complex is involved in store-operated calcium influx: evidence for similarities in store-operated and calcium release-activated calcium channel components. J. Biol. Chem. 282, 9105–9116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liao Y., Erxleben C., Abramowitz J., Flockerzi V., Zhu M. X., Armstrong D. L., Birnbaumer L. (2008) Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/Icrac channels. Proc. Natl. Acad. Sci. U.S.A. 105, 2895–2900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Alicia S., Angélica Z., Carlos S., Alfonso S., Vaca L. (2008) STIM1 converts TRPC1 from a receptor-operated to a store-operated channel: moving TRPC1 in and out of lipid rafts. Cell Calcium 44, 479–491 [DOI] [PubMed] [Google Scholar]
- 47. Ma H.-T., Peng Z., Hiragun T., Iwaki S., Gilfillan A. M., Beaven M. A. (2008) Canonical transient receptor potential 5 channel in conjunction with Orai1 and STIM1 allows Sr2+ entry, optimal influx of Ca2+, and degranulation in a rat mast cell line. J. Immunol. 180, 2233–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mignen O., Thompson J. L., Shuttleworth T. J. (2007) STIM1 regulates Ca2+ entry via arachidonate-regulated Ca2+-selective (ARC) channels without store depletion or translocation to the plasma membrane. J. Physiol. 579, 703–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang Y., Deng X., Mancarella S., Hendron E., Eguchi S., Soboloff J., Tang X. D., Gill D. L. (2010) The calcium store sensor, STIM1, reciprocally controls Orai and CaV1.2 channels. Science 330, 105–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Huang G. N., Zeng W., Kim J. Y., Yuan J. P., Han L., Muallem S., Worley P. F. (2006) STIM1 carboxyl-terminus activates native SOC, Icrac and TRPC1 channels. Nat. Cell Biol. 8, 1003–1010 [DOI] [PubMed] [Google Scholar]
- 51. Zeng W., Yuan J. P., Kim M. S., Choi Y. J., Huang G. N., Worley P. F., Muallem S. (2008) STIM1 gates TRPC channels, but not Orai1, by electrostatic interaction. Mol. Cell 32, 439–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cheng K. T., Liu X., Ong H. L., Swaim W., Ambudkar I. S. (2011) Local Ca2+ entry via Orai1 regulates plasma membrane recruitment of TRPC1 and controls cytosolic Ca2+ signals required for specific cell functions. PLoS Biol. 9, e1001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lee K. P., Choi S., Hong J. H., Ahuja M., Graham S., Ma R., So I., Shin D. M., Muallem S., Yuan J. (2014) Molecular determinants mediating gating of Transient Receptor Potential Canonical (TRPC) channels by stromal interaction molecule 1 (STIM1). J. Biol. Chem. 289, 6372–6382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pani B., Liu X., Bollimuntha S., Cheng K. T., Niesman I. R., Zheng C., Achen V. R., Patel H. H., Ambudkar I. S., Singh B. B. (2013) Impairment of TRPC1-STIM1 channel assembly and AQP5 translocation compromise agonist-stimulated fluid secretion in mice lacking caveolin1. J. Cell Sci. 126, 667–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Parvez S., Beck A., Peinelt C., Soboloff J., Lis A., Monteilh-Zoller M., Gill D. L., Fleig A., Penner R. (2008) STIM2 protein mediates distinct store-dependent and store-independent modes of CRAC channel activation. FASEB J. 22, 752–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bugaj V., Alexeenko V., Zubov A., Glushankova L., Nikolaev A., Wang Z., Kaznacheyeva E., Bezprozvanny I., Mozhayeva G. N. (2005) Functional properties of endogenous receptor- and store-operated calcium influx channels in HEK293 cells. J. Biol. Chem. 280, 16790–16797 [DOI] [PubMed] [Google Scholar]
- 57. Skopin A., Shalygin A., Vigont V., Zimina O., Glushankova L., Mozhayeva G. N., Kaznacheyeva E. (2013) TRPC1 protein forms only one type of native store-operated channels in HEK293 cells. Biochimie 95, 347–353 [DOI] [PubMed] [Google Scholar]
- 58. Soboloff J., Spassova M. A., Hewavitharana T., He L.-P., Xu W., Johnstone L. S., Dziadek M. A., Gill D. L. (2006) STIM2 is an inhibitor of STIM1-mediated store-operated Ca2+ entry. Curr. Biol. 16, 1465–1470 [DOI] [PubMed] [Google Scholar]
- 59. Zubov A. I., Kaznacheeva E. V., Nikolaev A. V., Alexeenko V. A., Kiselyov K., Muallem S., Mozhayeva G. N. (1999) Regulation of the miniature plasma membrane Ca2+ channel Imin by inositol 1,4,5-trisphosphate receptors. J. Biol. Chem. 274, 25983–25985 [DOI] [PubMed] [Google Scholar]
- 60. Kaznacheyeva E., Zubov A., Nikolaev A., Alexeenko V., Bezprozvanny I., Mozhayeva G. N. (2000) Plasma membrane calcium channels in human carcinoma A431 cells are functionally coupled to inositol 1,4,5-trisphosphate receptor-phosphatidylinositol 4,5-bisphosphate complexes. J. Biol. Chem. 275, 4561–4564 [DOI] [PubMed] [Google Scholar]
- 61. Kiselyov K. I., Semyonova S. B., Mamin A. G., Mozhayeva G. N. (1999) Miniature Ca2+ channels in excised plasma-membrane patches: activation by IP3. Pflugers Arch. 437, 305–314 [DOI] [PubMed] [Google Scholar]
- 62. Kaznacheyeva E., Zubov A., Gusev K., Bezprozvanny I., Mozhayeva G. N. (2001) Activation of calcium entry in human carcinoma A431 cells by store depletion and phospholipase C-dependent mechanisms converge on ICRAC-like calcium channels. Proc. Natl. Acad. Sci. U.S.A. 98, 148–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gusev K., Glouchankova L., Zubov A., Kaznacheyeva E., Wang Z., Bezprozvanny I., Mozhayeva G. N. (2003) The store-operated calcium entry pathways in human carcinoma A431 cells: functional properties and activation mechanisms. J. Gen. Physiol. 122, 81–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kiselyov K. I., Mamin A. G., Semyonova S. B., Mozhayeva G. N. (1997) Low-conductance high selective inositol (1,4,5)-triphosphate activated Ca2+ channels in plasma membrane of A431 carcinoma cells. FEBS Lett. 407, 309–312 [DOI] [PubMed] [Google Scholar]
- 65. Rao J. N., Rathor N., Zhuang R., Zou T., Liu L., Xiao L., Turner D. J., Wang J.-Y. (2012) Polyamines regulate intestinal epithelial restitution through TRPC1-mediated Ca2+ signaling by differentially modulating STIM1 and STIM2. Am. J. Physiol. Cell Physiol. 303, C308–C317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gruszczynska-Biegala J., Kuznicki J. (2013) Native STIM2 and ORAI1 proteins form a calcium-sensitive and thapsigargin-insensitive complex in cortical neurons. J. Neurochem. 126, 727–738 [DOI] [PubMed] [Google Scholar]
- 67. Zhou Y., Mancarella S., Wang Y., Yue C., Ritchie M., Gill D. L., Soboloff J. (2009) The short N-terminal domains of STIM1 and STIM2 control the activation kinetics of Orai1 channels. J. Biol. Chem. 284, 19164–19168 [DOI] [PMC free article] [PubMed] [Google Scholar]