Background: Light-harvesting complex II (LHCII) displays different spectroscopic properties in thylakoid membranes and in detergents.
Results: Circular dichroism and fluorescence lifetimes disclose structural effects of protein-protein, lipid-protein, and detergent interactions.
Conclusion: The native state of complexes is perturbed by detergents and best retained in lipid:LHCII assemblies.
Significance: The lipid environment is important for the proper function of LHCII, the most abundant of membrane proteins.
Keywords: Circular Dichroism (CD), Conformational Change, Fluorescence, Membrane Protein, Photosynthetic Pigment, Protein Aggregation, Detergent Solubilization, Non-photochemical Quenching
Abstract
Extraction of plant light-harvesting complex II (LHCII) from the native thylakoid membrane or from aggregates by the use of surfactants brings about significant changes in the excitonic circular dichroism (CD) spectrum and fluorescence quantum yield. To elucidate the cause of these changes, e.g. trimer-trimer contacts or surfactant-induced structural perturbations, we compared the CD spectra and fluorescence kinetics of LHCII aggregates, artificial and native LHCII-lipid membranes, and LHCII solubilized in different detergents or trapped in polymer gel. By this means we were able to identify CD spectral changes specific to LHCII-LHCII interactions, at (−)-437 and (+)-484 nm, and changes specific to the interaction with the detergent n-dodecyl-β-maltoside (β-DM) or membrane lipids, at (+)-447 and (−)-494 nm. The latter change is attributed to the conformational change of the LHCII-bound carotenoid neoxanthin, by analyzing the CD spectra of neoxanthin-deficient plant thylakoid membranes. The neoxanthin-specific band at (−)-494 nm was not pronounced in LHCII in detergent-free gels or solubilized in the α isomer of DM but was present when LHCII was reconstituted in membranes composed of phosphatidylcholine or plant thylakoid lipids, indicating that the conformation of neoxanthin is sensitive to the molecular environment. Neither the aggregation-specific CD bands, nor the surfactant-specific bands were positively associated with the onset of fluorescence quenching, which could be triggered without invoking such spectral changes. Significant quenching was not active in reconstituted LHCII proteoliposomes, whereas a high degree of energetic connectivity, depending on the lipid:protein ratio, in these membranes allows for efficient light harvesting.
Introduction
Light-harvesting complex II (LHCII),2 the principal photosynthetic antenna of plants, plays a key role in capturing and utilizing sunlight, in the macroorganization of the thylakoid membrane (1–3), stabilizing the bilayer and facilitating membrane stacking to form grana (4–9), and in protection against photodamage by dissipating excess absorbed light energy (non-photochemical quenching) (10, 11). The function of LHCII is regulated by interactions with its molecular surroundings. For example, non-photochemical quenching is controlled by lumenal pH, zeaxanthin, and the PsbS protein and possibly involves a change in LHCII-LHCII interactions (12). There is an ongoing debate whether, and to what extent and what nature of conformational changes are involved in these processes (13). It is generally agreed that activation of non-photochemical quenching is accompanied by structural reorganizations of thylakoid membrane that include rearrangement of LHCII (14–16) and possibly tight LHCII-LHCII interactions, or aggregation (12, 17). These effects are mimicked in vitro as, because of its hydrophobic surface, LHCII has a strong propensity to aggregate in the absence of surfactants, whereupon the fluorescence yield is substantially reduced (13, 18, 19), in contrast to the high yield when LHCII is solubilized in detergent micelles.
The aggregated and solubilized state of LHCII in vitro show clear differences in resonance Raman spectra (13, 20) signifying alterations in pigment conformation. Aggregates are also readily distinguished by the excitonic CD spectra (21, 22). The CD spectra report on small changes in the excitonic pigment-pigment interactions in the complexes (23), which are brought about by changes in the protein conformation or intermolecular interactions. When compared with the native thylakoid membranes, the CD spectra indicated that aggregated LHCII is structurally more similar to the native state despite the low fluorescence yield, and that detergent solubilization appeared to perturb the native structure (22). In light of this finding it becomes questionable whether LHCII in detergent micelles is a representative model for the native functional state of the complex. There can be several reasons for the witnessed CD changes, disrupted pigment-pigment interactions between complexes, changes in the conformation of the complexes due to the disrupted protein-protein or lipid-protein interactions, or changes directly induced by the detergent molecules. The question which of these possible effects is dominating the spectral changes has not been answered and their fingerprints have not been determined yet.
In this work we carefully investigated the exact CD changes occurring when LHCII is transferred from one molecular environment to another, namely from detergent micelles to the aggregated state, or reconstituted lipid membrane. We set out to separate the CD changes appearing due to LHCII-LHCII interactions from those brought about by artificial surfactants or lipids. To this end, we compared the effects of different detergents, embedded the complexes in lipid vesicles, and employed the technique used by Ilioaia et al. (24, 25) for trapping solubilized LHCII trimers in polymer gels, allowing us to exchange the surfactant while preventing aggregation. As a reference for the CD of LHCII in its native environment we used thylakoid membranes isolated from lincomycin-treated plants lacking photosystem core proteins. The CD spectra of LHCII proved highly sensitive to changes in the molecular environment. We identified specific CD bands signifying detergent-protein, protein-protein, and lipid-protein interactions. The CD bands attributed closely to LHCII-bound neoxanthin (Nx) were strongly altered in the detergent n-dodecyl-β-maltoside (β-DM) but did not respond to aggregation/disaggregation in the absence of detergent. Neither the aggregation-specific CD bands, nor the surfactant-specific bands were positively associated with the onset of fluorescence quenching as the CD spectra of native membranes had the signatures of LHCII aggregates but did not exhibit the strong quenching characteristic for aggregates. Furthermore, we evaluated the functional domain size of the aggregates and lipid membranes finding a high degree of connectivity (efficient energy transfer between complexes), higher with decreasing the relative amount of lipids.
EXPERIMENTAL PROCEDURES
Isolation of LHCII
LHCII was isolated and purified by sucrose density gradient ultracentrifugation (26) using pea (Pisum sativum) PSII-enriched membrane fragments as starting material, solubilized with 0.6% α-DM or β-DM. The LHCII trimer bands were concentrated with 30-kDa cutoff Amicon filters (Millipore) and stored at −80 °C until use. Aggregates were prepared by diluting the LHCII solution with 20 mm Tricine (pH 7.8) to 10–20 μg/ml of chlorophyll (Chl) content, then adding 80–100 mg/ml of absorbing beads (Bio-Beads SM-2, Bio-Rad) and stirring continuously for 2 h at room temperature to remove the detergent.
LHCII trimers were trapped in polyacrylamide gel to prevent protein aggregation, as described by Ilioaia et al. (24). Strips of 1.5-mm thickness, containing the detergent-LHCII solution and 6% acrylamide:bisacrylamide (30:1), were polymerized with 0.1% ammonium persulfate and 0.1% TEMED. To remove the detergent, the gel strips were incubated in 1000 volumes of 20 mm Tricine buffer (pH 7.8) for 2 h under vigorous shaking.
Reconstitution of LHCII Proteoliposomes
Reconstitution of LHCII:lipid membranes was carried out by following standard protocols (27–29). Large unilamellar lipid vesicles (liposomes) were prepared from egg phosphatidylcholine (PC), dimyristoylphosphatidylcholine (DMPC), or mixtures of plant thylakoid lipids (50.0% (w/v) monogalactosyldiacylglycerol, 31.0% digalactosyldiacylglycerol, 10.7% phosphatidylglycerol (PG), and 8.3% sulfoquinovosyldiacylglycerol. The lipids dissolved in chloroform:methanol were placed in a round-bottom flask and the solvent was dried in a rotary vacuum evaporator to form a thin film. The lipids were hydrated with 10 mm HEPES buffer (pH 7.6) containing 10 mm NaCl via agitating the solution. The hydrated lipids were extruded through a 100-nm pore membrane (Mini-Extruder, Avanti Polar Lipids) to form unilamellar vesicles. Detergent was added to the preformed liposomes (0.05% Triton X-100 for PC liposomes, 0.5% n-octyl-β-d-glucoside for DMPC, and 0.05% DM for thylakoid lipids). LHCII-detergent solution was added in drops to the liposome suspension to the desired lipid:protein ratio (reconstitution was successful in the range of 70–300:1). The detergent was then removed by repeated incubation with absorbent beads (Bio-Beads SM2, Bio-Rad). Protein aggregates not incorporated into the lipid were sedimented by centrifugation at 15,000 × g for 15 min and subsequent centrifugation at 40,000 × g for 40 min pelleted the proteoliposomes. Similar results were obtained when purifying the membranes by sucrose density gradient ultracentrifugation (140,000 × g for 16 h). At lipid:protein ratios of 100:1 or more, the amount of non-incorporated protein was negligible.
Isolation of Neoxanthin-deficient Thylakoid Membranes
Thylakoid membranes of Arabidopsis wild type (col-0) and the Nx-deficient mutant aba4 were prepared from fully expanded leaves by using the method described by Ruban et al. (30) with slight modifications.
Membranes from Lincomycin-treated Plants
Pea (P. sativum) seedlings were grown hydroponically in ¼ strength Knop's solution in darkness for 6 days and then exposed to normal daylight after adding 250 mg/liter of lincomycin to the growth medium. Thylakoid membranes were isolated from 2-week-old plants according to the protocol used in Lambrev et al. (22).
Pigment Analysis
The pigment composition of thylakoid membranes was determined by reversed-phase HPLC. Pigments were extracted with 100% acetone. Samples were centrifuged and the extracts were passed through a PTFE 0.2-μm pore size syringe filter. Pigments were separated using a 4.6 × 250-mm ReproSil-Pur Basic RP-18 column with 5 μm particle size (Dr. Maisch, Ammerbuch, Germany) and a Shimadzu LC-20 HPLC system. The acetone extract (20 μl) was injected to the column and the pigments were eluted by a linear gradient from acetonitrile, water, and triethylamine (9:1:0.01) to ethyl acetate. The gradient was run from 0 to 25 min at a flow rate of 1 ml/min.
CD Spectroscopy
CD spectra in the range of 350–750 nm were recorded at room temperature with a JASCO 815 spectropolarimeter. LHCII samples in solution were diluted in 20 mm Tricine buffer (pH 7.8) with 0.03% DM (for solubilized trimers) to an absorbance of 1 at the red maximum. Measurements were performed in a standard glass cell of 1-cm optical path length. Gel strips of 1.5-mm thickness were sandwiched between glass plates.
Freeze-fracture Transmission Electron Microscopy
For freeze-fracture experiments, droplets of 1–2 μl of concentrated LHCII proteoliposome suspension were pipetted onto a gold sample holder and frozen by plunging it immediately into partially solidified Freon for 20 s and stored in liquid nitrogen. Fracturing was performed at −100 °C in a freeze-fracture device (BAF 400D, Balzers AG, Liechtenstein). The fractured faces were etched for 30 s at −110 °C. The replicas, prepared by platinum-carbon shadowing, were cleaned with a water solution of surfactant and washed with distilled water. From pure water, the replicas were picked up on 200 mesh copper grids and examined in a Morgagni 268D (FEI, The Netherlands) transmission electron microscope.
Time-resolved Fluorescence
Fluorescence decays were recorded at room temperature with an effective time resolution of 5–10 ps by time-correlated single-photon counting using a FluoTime 200 instrument (PicoQuant, Germany). Excitation pulses of 6 ps duration, 20 MHz repetition rate, 633 nm wavelength were given by a WhiteLase Micro supercontinuum laser (Fianium, UK). Fluorescence emission was detected through a monochromator at wavelengths between 670 and 740 nm. Global lifetime analysis was done in MATLAB using homemade routines.
RESULTS
CD Spectra of Detergent-solubilized and Aggregated LHCII
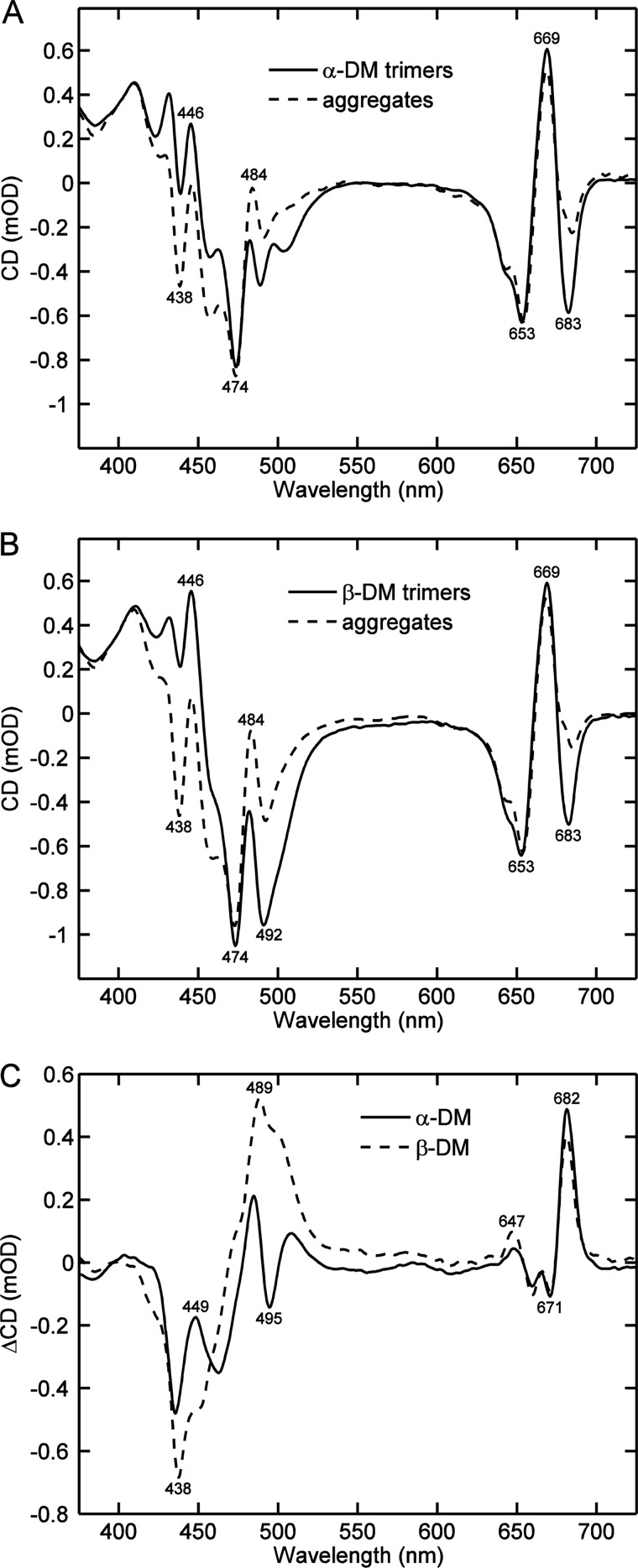
Removal of the detergent from a solution of LHCII invariably leads to aggregation of the highly hydrophobic protein complexes. The excitonic CD spectra of LHCII aggregates are markedly different from those of detergent-solubilized LHCII trimers. This has been well documented in the literature (21, 22) but the origin of the differences has not been clarified. For instance, whether the native conformation of the membrane-embedded protein is perturbed by the detergent or by aggregation. In the present work, to identify the effects of different detergents on the CD, we systematically reproduced these comparisons, whereas making the effort to optimize the experimental setup as much as possible to reveal the CD differences and avoid artifacts due to high pigment concentrations, light scattering, flattening, and baseline distortions in the aggregates. The CD spectra of LHCII trimers solubilized with α-DM and β-DM and aggregates obtained by extracting the detergent, as well as the aggregates minus trimers difference spectra, are shown in Fig. 1. The CD spectra of detergent-solubilized LHCII are well known and published in many works (21, 22, 26, 31, 32). In the red region, the spectra are characterized by the bands (−)-653, (+)-669, and (−)-683 nm, associated with the Qy exciton states of Chl b and a. In the blue region, the spectra have a more complex structure, because of the multitude of Chl and carotenoid transitions. The dominating peaks for LHCII in α-DM are (−)-439, (+)-446, (−)-474, and (+)-484. The spectrum of LHCII in β-DM micelles displays additionally a strong peak at (−)-492 nm, which is the most prominent difference between the two DM isomers.
FIGURE 1.
Typical CD spectra of LHCII aggregates and LHCII solubilized with DM. LHCII was isolated by the use of n-dodecyl-α-maltoside (A) or n-dodecyl-β-maltoside (B) and aggregated by extracting the detergent with absorbent beads (dashed lines). Panel C shows the CD difference spectra of aggregated minus solubilized LHCII. The CD spectra are normalized to the absorbance at 675 nm. The numbers on the graphs indicate the wavelength positions, in nanometers, of selected peaks.
Removal of the detergent, regardless of the isomer used, led to distinctive changes in the CD spectra, most notably, an increase of the (−)-438 and (+)-484 nm bands and a decrease of the (−)-683 nm band. Less prominent but highly reproducible changes of the shoulders at 460 and 647 nm were observed as well. The difference spectra are very similar to those previously reported (22). The sharp, well resolved CD difference bands (Fig. 1C) evidently signify specific changes in pigment-pigment excitonic coupling and cannot be attributed to light scattering. Although some scattering (flattening) effects are noticeable in the absorption spectra of aggregates, they depend on the aggregate size, whereas the CD changes are completely uncorrelated (data not shown).
CD Spectra of Gel-trapped LHCII
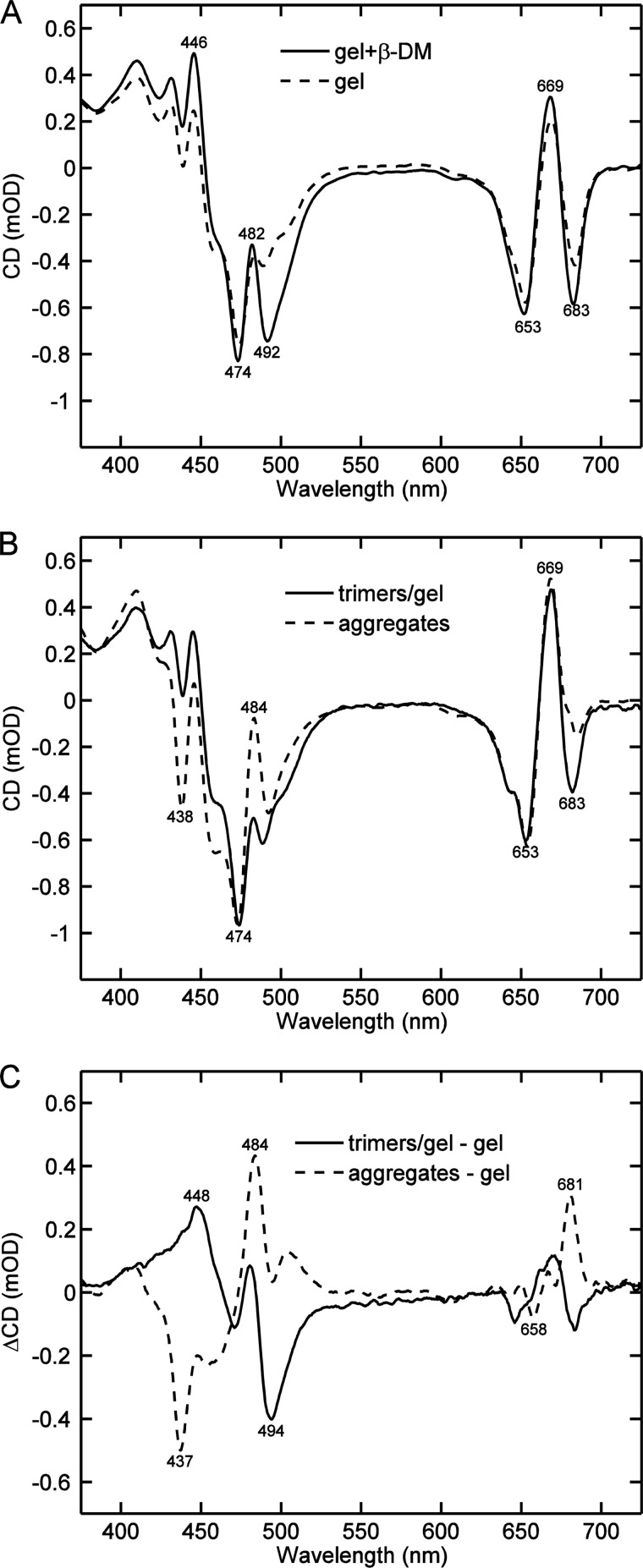
On the basis of the difference CD spectra of LHCII aggregates and solubilized trimers it is hard to know whether these changes are due to the loss of protein-protein interactions in the solubilized state or to changes in the pigment organization of the complexes brought about by the detergent environment. To separate these two effects, which we will refer to as “protein interactions” and “detergent interactions,” respectively, we recorded the CD spectra of LHCII fixed in polyacrylamide gel slabs. This system allows us to extract the detergent, while keeping the proteins from aggregating (24). By comparing the CD spectra of the LHCII gels after dialysis and after subsequent re-addition of the detergent (Fig. 2A), the effects of the detergent alone can be identified. It is evident that β-DM is responsible for the (−)-492 nm band and for a small enhancement of the (−)-683 nm band but it has no effect on the (−)-438 and (+)-482 nm bands. Enhancement of the latter two bands is clearly a signature of aggregation, or protein interactions, as can be seen in the comparison of aggregates in solution and trimers in a detergent-free gel (Fig. 2B). To make the comparison as accurate as possible, the CD spectrum of the dialyzed gel was corrected for distortions, such as pigment losses during the dialysis. Thus, the two difference spectra shown in Fig. 2C, aggregates − trimers in gel, and gel with β-DM − gel without β-DM, show separately the effects of aggregation and the detergent (β-DM). As an added check, the double difference spectrum (aggregates − trimers in gel, and gel + β-DM − dialyzed gel), which shows the combined effect of aggregation and detergent removal, completely reproduced the difference spectrum in Fig. 1C (not shown).
FIGURE 2.
Comparison of typical CD spectra of LHCII in the presence and absence of detergent. A, LHCII trimers trapped in polyacrylamide gel: the gel was dialyzed for 2 h to remove the detergent β-DM first (dashed line), then 0.1% β-DM was added to the dialyzed gel (solid line). B, LHCII trimers in dialyzed gel (solid line) and aggregates in detergent-free buffer solution (dashed line). All CD spectra are normalized to the absorbance at 675 nm. C, CD difference spectra of LHCII in gel with β-DM minus dialyzed gel (solid line) and aggregates minus dialyzed gel (dashed line).
Reconstituted LHCII:Lipid Membranes
Neither LHCII aggregates in aqueous solution, nor LHCII trimers in detergent micelles or gels can be regarded as proxies for the native state of LHCII, which is the thylakoid lipid membrane. To some extent LHCII aggregates resemble the thylakoid membrane (22), where LHCII interactions also take place; however, unspecific random contacts in the aggregates may disturb the structure and lipid-protein interactions in the native membrane cannot be neglected. To check the effects of lipids and to distinguish CD changes due to nonspecific protein-protein interactions from those due to protein-protein interactions in the membrane, LHCII was reconstituted into artificial lipid vesicles according to well established protocols (27, 28). We used PC (egg lecithin), DMPC, and a native thylakoid lipid mixture (monogalactosyldiacylglycerol, digalactosyldiacylglycerol, sulfoquinovosyldiacylglycerol, and phosphatidylglycerol).
The morphology of the reconstructed LHCII:PC proteoliposomes is revealed by electron micrographs obtained after the freeze-fracture procedure. Large nearly spherical vesicles appear typically in TEM images as it can be observed in Fig. 3. The size of vesicles containing LHCII complex is significantly larger than that of the extruded starting PC form. The fractured membrane surface is patchy, closely packed small entities with a characteristic size of about 20 nm are embedded, showing that LHCII is the constituent of the reconstructed membrane.
FIGURE 3.
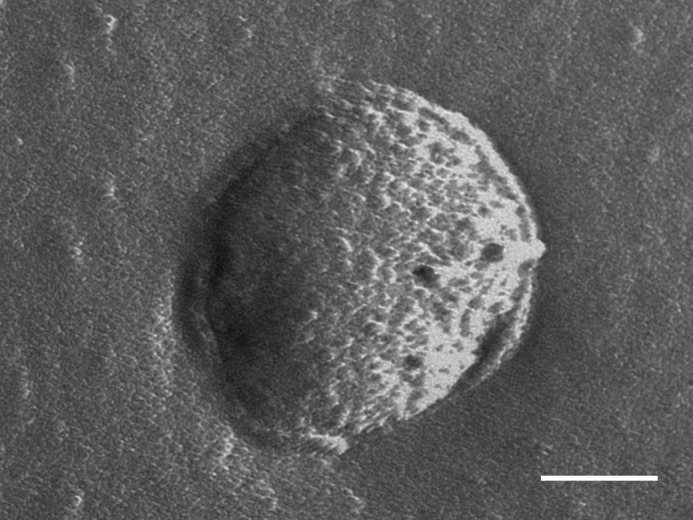
Representative freeze-fracture transmission electron microscopy image of LHCII-phosphatidylcholine membranes. The membranes were reconstituted at a lipid:protein ratio of 300:1 and purified by sucrose density gradient ultracentrifugation. The scale bar corresponds to 200 nm.
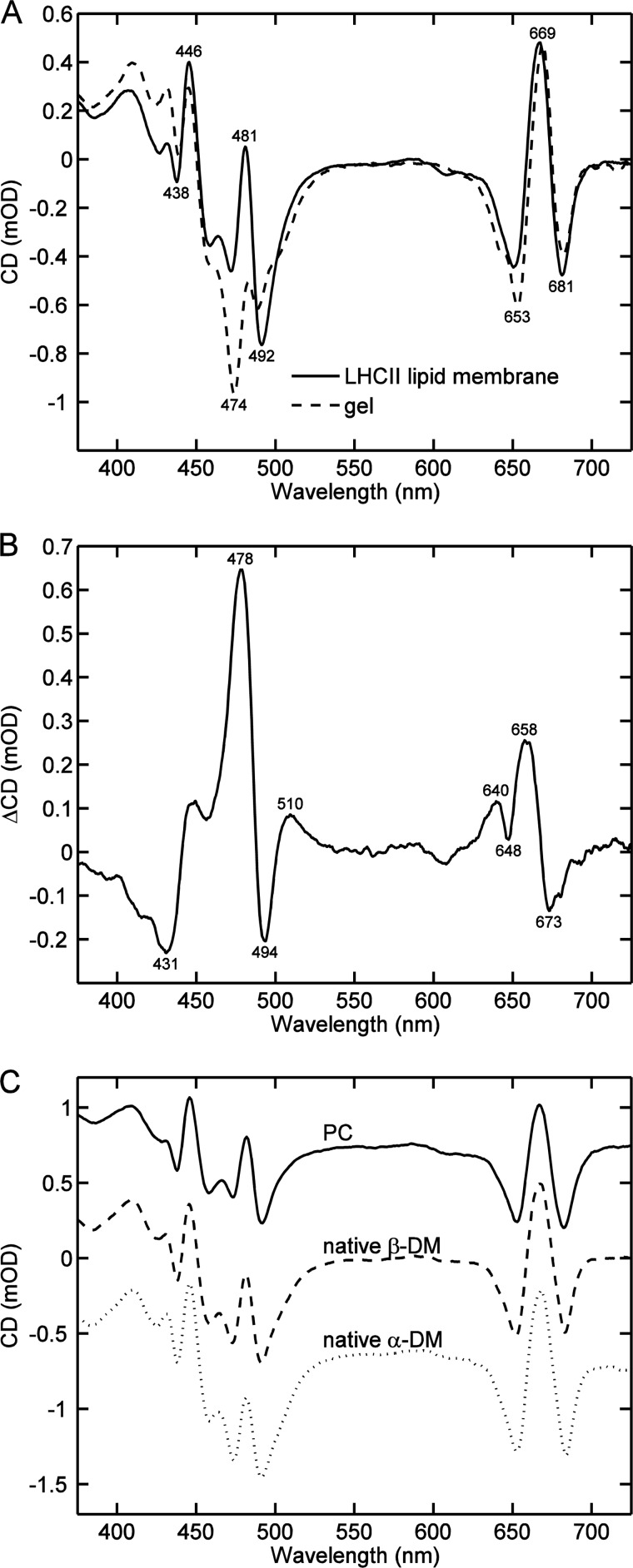
The CD spectra of LHCII proteoliposomes were not dissimilar to those already published (27, 28). A comparison of the CD spectrum of LHCII:DMPC membranes reconstituted at a lipid:protein ratio of 300:1 with LHCII in detergent-free gel (Fig. 4A) showed a decrease of the (−)-474 nm band and an increase of the (−)-492 nm band (Fig. 4B), yielding CD difference bands at 478 and 494 nm (Table 1). Interestingly, the strong (−)-492 nm band characteristic for β-DM-solubilized trimers is also present in all lipid membranes. It is also of note that the specific aggregation signatures at (+)-484 and (−)-437 nm were not observed at this lipid:protein ratio. The largest amplitude difference in the CD of membranes compared with trimers, at 478 nm (Fig. 4B), seems to have a different origin than the aggregate-specific band at 484 nm. Reconsitution with different lipids, including native thylakoid lipids, and with different intermediate detergents, resulted in essentially the same CD spectra (Fig. 4C).
FIGURE 4.
Comparison of typical CD spectra of LHCII reconstituted into lipid membranes and in detergent-free gel. A, LHCII:DMPC membrane (solid line) and LHCII trimers in dialyzed gel (dashed line). The CD spectra are normalized to the absorbance at 675 nm. B, CD difference spectrum (membrane minus dialyzed gel). C, typical CD spectra of LHCII lipid membranes, LHCII (Triton X-100):PC membranes (solid line), LHCII (β-DM):native thylakoid lipids (dashed line), and LHCII (α-DM):native thylakoid lipids (dotted line).
TABLE 1.
CD difference bands characteristic for LHCII in different environments compared to detergent-free gel
| Aggregates | α-DM | β-DM | Proteo-liposomes | LHCII-enriched thylakoid membranes |
|---|---|---|---|---|
| (−)-437 | (+)-446 | (+)-447 | (−)-431 | (−)-437 |
| (+)-484 | (−)-494 | (+)-478 | (+)-484 | |
| (+)-681 | (−)-494 | (+)-681 |
Thylakoid Membranes from Lincomycin-treated Plants
Native LHCII-enriched membranes were prepared by lincomycin treatment of plants, which blocks the synthesis of chloroplast-encoded proteins and as a result the membranes are essentially devoid of photosystem II core complexes. The HPLC profile showed that the amount of β-carotene, normally found in the core complexes, was reduced by 92% (Table 2) in membranes from lyncomicin-treated plants (expressed per mol of chlorophyll a). At the same time the relative amount of xanthophylls and chlorophyll b, found in the light-harvesting complexes only, was increased. These membranes could then serve as a proxy for the native state of LHCII (33), with the caveat that they contain not only the major LHCIIb but also monomeric LHCII and LHCI, which is evident from their 77 K fluorescence emission spectra (not shown).
TABLE 2.
Pigment composition of lincomycin-treated membrane relative to control
| Chl a | Chl b | β-Carotene | Lutein | Neoxanthin | Violaxanthin |
|---|---|---|---|---|---|
| 100% | 144% | 8% | 196% | 194% | 199% |
The CD spectra of LHCII-enriched thylakoid membranes, shown in Fig. 5A, had dominating peaks at (−)-437, (−)-474, (+)-484, and (+)-669, similar to those observed in the CD spectrum of LHCII aggregates. In fact, the spectra are virtually indistinguishable from LHCII macroaggregates (22). Solubilization of the thylakoid membranes with β-DM had the same effect on the CD spectra as with LHCII aggregates. The resulting CD difference spectrum (Fig. 5B) was almost identical to the difference spectrum corresponding to solubilization of LHCII aggregates with β-DM (Fig. 1B).
FIGURE 5.
Typical CD spectra of thylakoid membranes (native LHCII-enriched membranes) isolated from lincomycin-grown pea leaves. A, membranes resuspended in 20 mm Tricine buffer (solid line) and solubilized with 0.1% β-DM (dashed line). B, CD difference spectrum, unsolubilized minus solubilized.
Neoxanthin-deficient Thylakoid Membranes
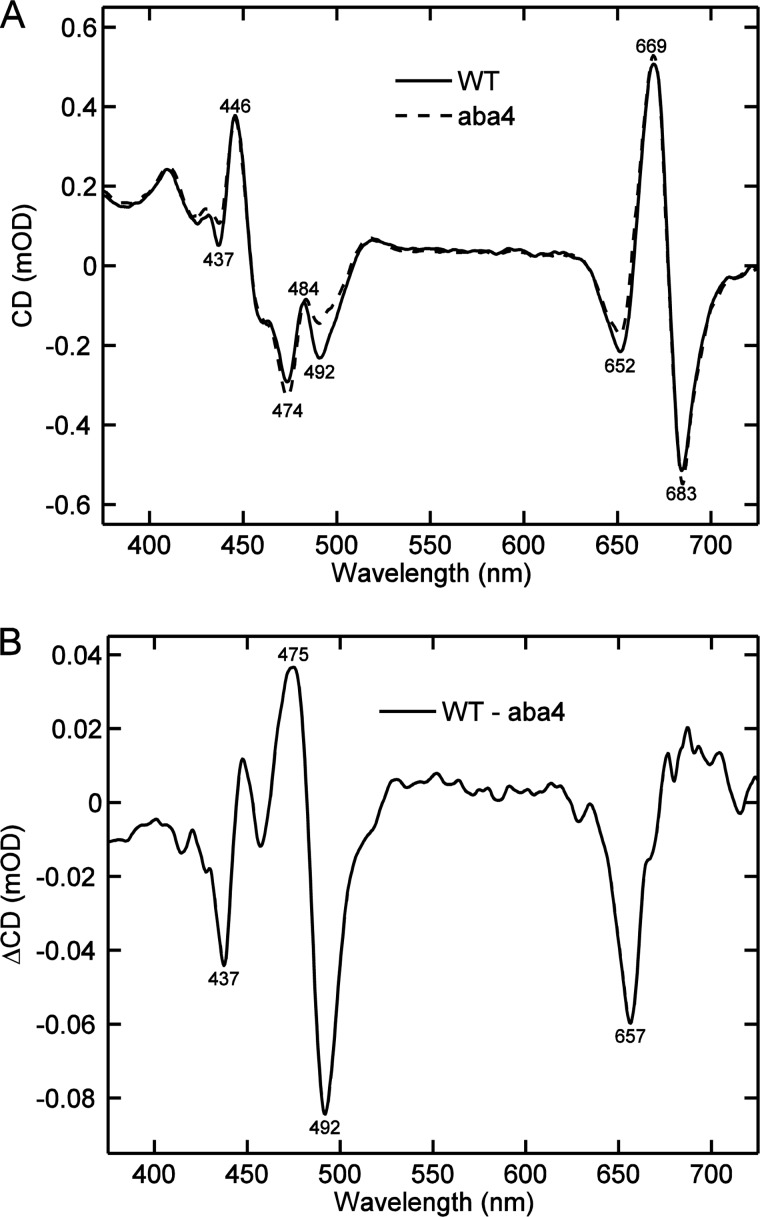
The strongest CD differences observed upon changing the LHCII environment are in the region of Chl b and xanthophyll absorption. Neoxanthin, located in the periphery of the trimeric complex, and in close interaction with Chl b, is most likely to be responsible for the CD changes in the blue region.
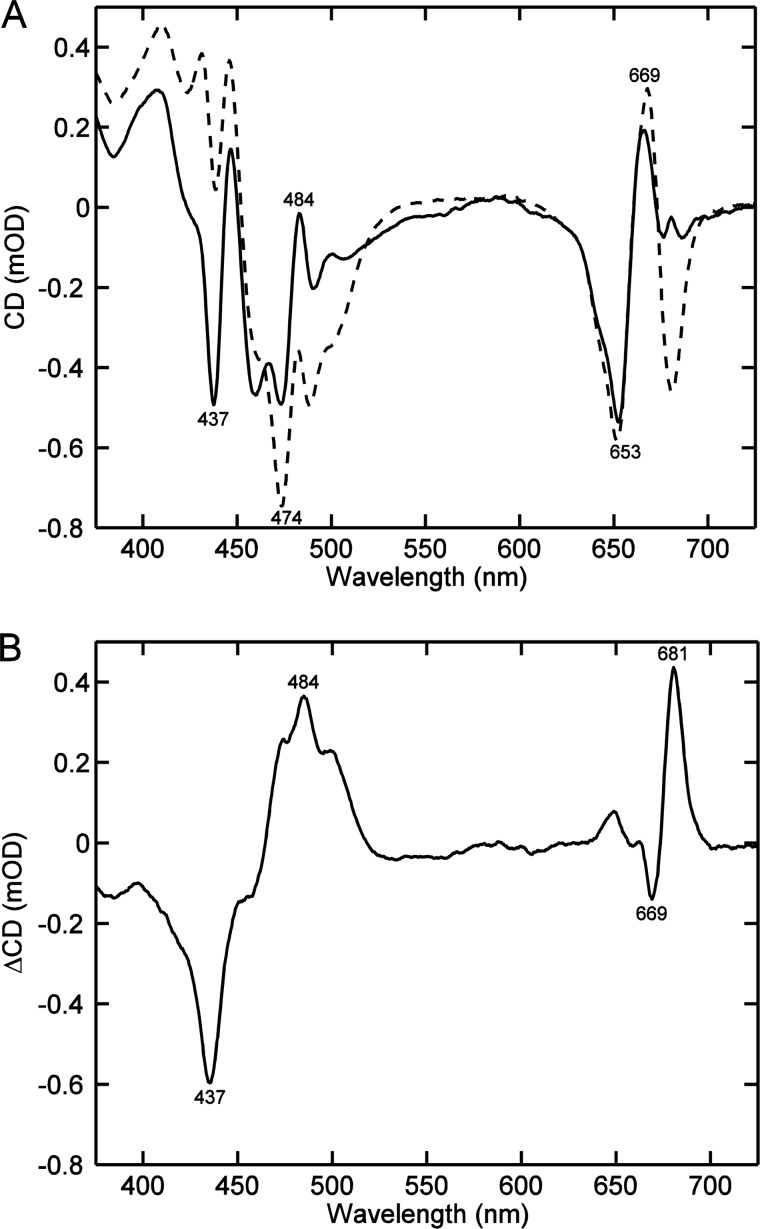
It has been known from Raman spectroscopy that the conformation of Nx changes upon aggregation of LHCII. To check the contribution of Nx to the CD spectrum of LHCII we compared the CD spectra of native thylakoid membranes isolated from wild type Arabidopsis thaliana and the Nx-deficient mutant aba4. The membranes were solubilized with β-DM (Fig. 6A). The most significant feature of the Nx-deficient CD spectrum of the membrane was the decrease of the amplitude at (−)-492 nm. A comparison of the absorption spectra of the thylakoid membranes also reveals missing absorption at 492 nm in the Nx-deficient mutant; the absence of Nx led to less pronounced but distinct changes at 437, 475, and 657 nm (data not shown). Virtually the same CD difference spectra were observed in the comparison of thylakoid membranes solubilized with α-DM (not shown). Reduction of the CD amplitude at (−)-492 nm and concomitant enhancement at (−)-475 nm was also observed in in vitro refolded LHCII lacking Nx (34, 35).
FIGURE 6.
Typical CD spectra of thylakoid membranes from A. thaliana, solubilized with 0.1% β-DM. A, wild type (solid line) and the neoxanthin-deficient mutant aba4 (dashed line). The CD spectra are normalized to the absorbance at 675 nm. B, CD difference spectrum of wild type minus aba4.
Fluorescence Lifetimes and Connectivity
It is well known that the fluorescence yield of LHCII drops dramatically in the absence of detergent (19, 36) in solution as well as in a polyacrylamide gel (24). In the lipid environment LHCII exhibits fluorescence yields that largely vary with the specific conditions (28). Fluorescence decays of the LHCII preparations used in this study were recorded by time-correlated single photon counting. Representative decays recorded at 680 nm and the calculated average lifetimes are shown in Fig. 7. The longest excited-state lifetime (3.6 ns) was observed in detergent micelles (β-DM or α-DM). The overall lifetime of the gels was mostly determined by the presence or absence of detergent. In detergent-free gels the lifetime was reduced 10-fold with decay kinetics similar to those reported by Rutkauskas et al. (37). The strongest quenching (20-fold) was observed in aggregates.
FIGURE 7.
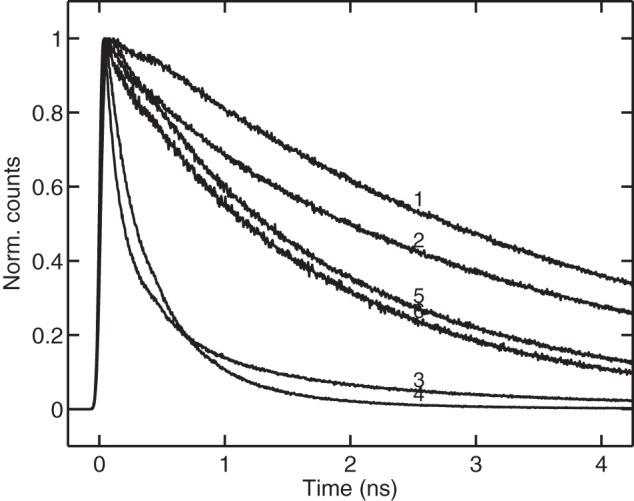
Fluorescence decay curves recorded by time-correlated single-photon counting from LHCII in different environments. 1, trimers in solution with β-DM (average lifetime, 3. 6 ns); 2, trimers in gel with β-DM (average lifetime, 2.5 ns); 3, DMPC membranes at lipid:protein ratio of 300:1 (average lifetime, 2.1 ns); 4, native thylakoid membranes isolated from lincomycin-treated pea (average lifetime, 1.6 ns); 5, LHCII trimers in gel without detergent (average lifetime, 0.4 ns); 6, aggregates in solution (average lifetime, 0.2 ns).
LHCII reconstituted in DMPC, PC, or native thylakoid lipid membranes exhibited average lifetimes between 1 and 2 ns, depending on the lipid:protein ratio. The average lifetime of thylakoid membranes isolated from lincomycin-treated pea plants was 1.6 ns. The average lifetime of detached intact leaves was 1.9 ns, consistent with the results reported by Belgio et al. (33) for Arabidopsis plants treated with lincomycin. In the cited work, a 100-ps lifetime component, presumably associated with the remaining Photosystem I cores, was omitted when calculating the average lifetime. Although we did observe a 140-ps lifetime component in our experiments, analysis of the decay-associated spectra (not shown) gave no indication that this component is related to Photosystem I. Incidentally, a 140-ps decay component of a similar amplitude and spectral shape was resolved in reconstituted proteoliposomes with thylakoid lipids.
It has been shown that aggregates of LHCII are energetically well interconnected and excitations can diffuse at long distances (38, 39). Here we evaluated the connectivity, or functional domain size (the average number of sites that excitations can diffuse to before being deactivated), in LHCII aggregates, in reconstituted lipid membranes, and native LHCII-enriched membranes. To this end, we measured the effective quenching rate constants of phenyl-p-benzoquine as in Ref. 39, following the assumption that it increases with the domain size. Briefly, the Stern-Volmer constant, KSV, was determined from the slope of the ratio of average lifetimes in the absence and presence of quencher, τ0/τ, plotted against the quencher concentration. According to the Stern-Volmer equation, the effective bimolecular quenching rate constant is kq = KSV/τ0. The effective quenching rate constants in aggregates, as reported previously (39), were on average, 30-fold higher than in solubilized trimers. For LHCII:DMPC proteoliposomes reconstituted at the lipid to protein ratio of 300 and 100, the relative domain size values were 10 and 27, respectively. Hence, the connectivity was dependent on the lipid:protein ratio, corroborating the results of Haferkamp et al. (40). The domain size values found for thylakoid membranes from lincomycin-treated plants were between 8 and 15 (average 9). Hence, reconstituted proteoliposomes exhibit effective energy transfer between adjacent LHCII trimers comparable with native LHCII-enriched membranes.
DISCUSSION
Isolation of LHCII from the native membrane or their aggregated state to detergent micelles and their solubilization in detergents leads to significant changes in the excitonic CD spectra (22). Moreover, the choice of surfactant can have a marked effect on different bands in the CD spectra as well as, in some cases, the fluorescence lifetime and thermal stability of the complexes (41). The CD changes may reflect any or all of the following: structural changes in the LHCII trimers that alter the interactions between chlorophylls and carotenoids; macrostructural changes (protein-protein interactions) that affect excitonic interactions between pigments belonging to different complexes; and intermolecular lipid-protein and detergent-protein interactions that change the immediate environment of pigments and cause spectral alterations. In this study we were able to separate the changes in excitonic interactions due to protein-protein interactions from those due to detergent-protein interactions. Table 1 summarizes our findings, reporting specific CD differences caused by LHCII aggregation (protein-protein interactions), α- and β-DM, and reconstitution into lipid membranes. The reported CD differences are relative to the CD of LHCII trimers in detergent free-gel. Evidently, the (−)-437, (+)-484, and (−)-681 nm difference bands can be attributed solely to protein-protein interactions in LHCII aggregates. In this respect it is intriguing that the same aggregation-specific bands were found in native LHCII-enriched membranes.
The two epimers of DM, when added to LHCII in dialyzed gels, alter the CD spectra in an identical manner, except for the difference band at 494 nm. The rest of the CD changes are of small magnitude and also appeared in the region of Chl b and carotenoid absorption, except for the change at 681 nm. It can be concluded that α-DM does not affect the LHCII structure to a large extent but β-DM brings about a specific and significant change, as do other detergents, such as Triton X-100. The differences in the two epimers with regards to solubilization of pigment-protein complexes are well documented but not entirely understood (42). It has been documented that pigment binding to LHCII is less stable in β-DM (26). Loss of pigments is not the reason for the CD differences reported here because of the reversibility of the changes in the gel. The differences can only be explained with conformational changes in the LHCII trimers induced by interaction with the detergent molecules. Interestingly, the same CD difference at (+)-448 and (−)-494 nm was also found in reconstituted lipid membranes, even with native thylakoid lipids. This is somewhat surprising because the negative band was neither observed in native thylakoid membranes (22) nor in LHCII-enriched membranes from lincomycin-treated plants. It can be concluded that the native conformation of the complex is not completely maintained in reconstituted proteoliposomes. One factor of possible significance is that in reconstituted membranes the complexes are inserted unspecifically with respect to their stromal/lumenal side3 (see also Ref. 43), possibly allowing irregular contacts between the protein complexes and thus additional pigment-pigment interactions. Another possibility is that the protein density and consequently interactions between complexes are different.
The CD signatures of the different molecular environments of LHCII reported here are quite specific and reproducible. The structural changes and affected pigment molecules, however, are not easy to identify, especially because the excitonic CD bands have contributions from many pigment interactions. The most striking differences are found in the blue spectral region and presumably involve interactions of carotenoids and chlorophylls. The conformation of Nx has been shown to change upon aggregation of LHCII (13, 44) and assumed to be responsible for the CD changes (24). However, based on the comparison of wild type and Nx-deficient thylakoid membranes (Fig. 6), as well as in vitro reconstituted LHCII (45), it seems more likely that Nx contributes to the changes at (−)-494 nm which are associated with the interaction of the detergent β-DM and lipids. This CD band was previously attributed to lutein (24) but our results do not support this assignment. Evidently, the Nx site is sensitive to β-DM and the conformation of this carotenoid is altered when the detergent (or lipid) is removed from the medium, which is also evident from resonance Raman spectra (25). However, aggregation itself does not appear to be associated with a change in Nx conformation. On the other hand, the change at (−)-437 nm, presumably reflecting Chl a interactions, is found both in aggregates and Nx-deficient membranes.
The light-harvesting antenna function of LHCII can only be fulfilled if the lifetime of the excited state is kept sufficiently long, i.e. in the absence of substantial fluorescence quenching. For this reason artificially aggregated LHCII can be regarded as a less suitable antenna model, because the fluorescence is strongly quenched. By comparing the lifetimes of LHCII in gels with and without detergent Ilioaia et al. (24) and Rutkauskas et al. (37) demonstrated that the quenching is not activated by aggregation itself and suggested that it is caused by a conformational change occurring upon removal of the detergent. If this is the case one may try to find a specific change in the excitonic CD that correlates with fluorescence quenching. The aggregation signatures at (−)-437 and (+)-484 nm are irrelevant because, on the one hand, quenching could be triggered in gel without aggregation and, on the other hand, thylakoid membranes from lincomycin-treated plants display all the aggregation CD signatures but no significant fluorescence quenching. The Nx-specific change at (−)-494 nm is also not likely related to quenching because the fluorescence lifetimes were unchanged when β-DM was replaced by α-DM. We can conclude that the structural changes affecting the CD signals in the spectral region of carotenoids are not the ones responsible for quenching.
It must be stressed that quenching is not a single on/off mechanism, there can be different pathways for nonradiative deactivation, triggered at different sites for different reasons that do not necessarily involve a change in excitonic interactions. The average lifetimes of LHCII:lipid membranes were consistently found between 1 and 2 ns, significantly shorter compared with detergent-solubilized LHCII trimers. It is worth noting that the “induced” quenching rate constant in lipid membranes (with reference to solubilized trimers) is only 0.2–0.7 ns−1, including native membranes from lincomycin-grown plants, whereas the quenching rate constant in detergent-free gel was 2.5 ns−1 and in aggregates 4–5 ns−1. Hence, it can be said that LHCII in the lipid membrane is relatively “unquenched,” although the excited-state lifetime is modulated compared with the 3.6 ns in detergent.
An important factor for the light-harvesting function of reconstituted antenna systems in vitro is the connectivity between antenna units allowing excitation energy to be delivered to the site of photochemical energy conversion. A characteristic of LHCII in the lipid membranes that may prove valuable is the long excited-state lifetime combined with large functional domain size. In this respect, the lipid membrane can be regarded as the most “native” in vitro environment for LHCII.
In conclusion, in this work we separated CD signatures of LHCII aggregation from detergent-induced perturbations. We were able to identify specific changes in excitonic interactions associated with aggregation of LHCII in detergent-free solutions or in protein-dense membranes, as well as a change in the excitonic interactions involving Nx, brought about by the interaction of LHCII with β-DM or lipids. It remains an open question which of these environments, if any, are able to stabilize the conformation of LHCII as found in the native thylakoid membrane, and how they relate to the high resolution crystal structures. Another important conclusion from our study is that the changes in chlorophyll-chlorophyll and chlorophyll-carotenoid interactions observed upon LHCII aggregation in vitro as well as in the native membranes were not correlated to fluorescence quenching. Native LHCII-lipid membranes support protein-protein (and consequent pigment-pigment) interactions found also in artificial aggregates without invoking the significant quenching specific to aggregates. Last, reconstituted LHCII:lipid membranes in our experiment showed an altered conformation of the complex in comparison with the native membrane (particularly with regard to the Nx domain) but nevertheless provide a useful in vitro model and platform for supporting a functional light-harvesting antenna that combines a high degree of energetic connectivity with minimal induced quenching.
This work was supported by Hungarian Scientific Research Fund Grants OTKA-PD 104530 and TÁMOP-4.2.2.A-11/1/KONV-2012–0060 and Hungarian National Innovation Office and A*STAR Singapore Grant TET_10-1-2011-0279.
C. Yang, personal communication.
- LHCII
- light-harvesting complex II
- α-DM
- n-dodecyl-α-maltoside
- β-DM
- n-dodecyl-β-maltoside
- DMPC
- 1,2-dimyristoyl-sn-glycero-3-phosphocholine
- Nx
- neoxanthin
- PC
- phosphatidylcholine
- TEMED
- N,N,N′,N′-tetramethylethylenediamine
- Chl
- chlorophyll
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine.
REFERENCES
- 1. Allen J. F., Forsberg J. (2001) Molecular recognition in thylakoid structure and function. Trends Plant Sci. 6, 317–326 [DOI] [PubMed] [Google Scholar]
- 2. Barzda V., Istokovics A., Simidjiev I., Garab G. (1996) Structural flexibility of chiral macroaggregates of light-harvesting chlorophyll a/b pigment-protein complexes. Light-induced reversible structural changes associated with energy dissipation. Biochemistry 35, 8981–8985 [DOI] [PubMed] [Google Scholar]
- 3. Aro E. M., Ohad I. (2003) Redox regulation of thylakoid protein phosphorylation. Antioxid. Redox Signal. 5, 55–67 [DOI] [PubMed] [Google Scholar]
- 4. Simidjiev I., Stoylova S., Amenitsch H., Jávorfi T., Mustárdy L., Laggner P., Holzenburg A., Garab G. (2000) Self-assembly of large, ordered lamellae from non-bilayer lipids and integral membrane proteins in vitro. Proc. Natl. Acad. Sci. U.S.A. 97, 1473–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garab G., Lohner K., Laggner P., Farkas T. (2000) Self-regulation of the lipid content of membranes by non-bilayer lipids: a hypothesis. Trends Plant Sci. 5, 489–494 [DOI] [PubMed] [Google Scholar]
- 6. Garab G., Mustárdy L. (1999) Role of LHCII-containing macrodomains in the structure, function and dynamics of grana. Aust. J. Plant Physiol. 26, 649–658 [Google Scholar]
- 7. Mustárdy L., Garab G. (2003) Granum revisited: a three-dimensional model, where things fall into place. Trends Plant Sci. 8, 117–122 [DOI] [PubMed] [Google Scholar]
- 8. Barber J. (1982) Influence of surface charges on thylakoid structure and function. Annu. Rev. Plant Physiol. 33, 261–295 [Google Scholar]
- 9. Mullet J. E., Arntzen C. J. (1980) Simulation of grana stacking in a model membrane system: mediation by a purified light-harvesting pigment-protein complex from chloroplasts. Biochim. Biophys. Acta 589, 100–117 [DOI] [PubMed] [Google Scholar]
- 10. Horton P., Ruban A. (2005) Molecular design of the photosystem II light-harvesting antenna: photosynthesis and photoprotection. J. Exp. Bot. 56, 365–373 [DOI] [PubMed] [Google Scholar]
- 11. Ruban A. V., Johnson M. P., Duffy C. D. (2012) The photoprotective molecular switch in the photosystem II antenna. Biochim. Biophys. Acta 1817, 167–181 [DOI] [PubMed] [Google Scholar]
- 12. Holzwarth A. R., Miloslavina Y., Nilkens M., Jahns P. (2009) Identification of two quenching sites active in the regulation of photosynthetic light-harvesting studied by time-resolved fluorescence. Chem. Phys. Lett. 483, 262–267 [Google Scholar]
- 13. Ruban A. V., Berera R., Ilioaia C., van Stokkum I. H., Kennis J. T., Pascal A. A., van Amerongen H., Robert B., Horton P., van Grondelle R. (2007) Identification of a mechanism of photoprotective energy dissipation in higher plants. Nature 450, 575–578 [DOI] [PubMed] [Google Scholar]
- 14. Johnson M. P., Zia A., Ruban A. V. (2012) Elevated ΔpH restores rapidly reversible photoprotective energy dissipation in Arabidopsis chloroplasts deficient in lutein and xanthophyll cycle activity. Planta 235, 193–204 [DOI] [PubMed] [Google Scholar]
- 15. Betterle N., Ballottari M., Zorzan S., de Bianchi S., Cazzaniga S., Dall'Osto L., Morosinotto T., Bassi R. (2009) Light-induced dissociation of an antenna hetero-oligomer is needed for non-photochemical quenching induction. J. Biol. Chem. 284, 15255–15266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kirchhoff H., Sharpe R. M., Herbstova M., Yarbrough R., Edwards G. E. (2013) Differential mobility of pigment-protein complexes in granal and agranal thylakoid membranes of C3 and C4 plants. Plant Physiol. 161, 497–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Horton P., Wentworth M., Ruban A. (2005) Control of the light harvesting function of chloroplast membranes: the LHCII-aggregation model for non-photochemical quenching. FEBS Lett. 579, 4201–4206 [DOI] [PubMed] [Google Scholar]
- 18. Ruban A. V., Rees D., Pascal A. A., Horton P. (1992) Mechanism of ΔpH-dependent dissipation of absorbed excitation energy by photosynthetic membranes: 2. The relationship between LHCII aggregation in vitro and qE in isolated thylakoids. Biochim. Biophys. Acta 1102, 39–44 [Google Scholar]
- 19. Miloslavina Y., Wehner A., Lambrev P. H., Wientjes E., Reus M., Garab G., Croce R., Holzwarth A. R. (2008) Far-red fluorescence: a direct spectroscopic marker for LHCII oligomers forming in non-photochemical quenching. FEBS Lett. 582, 3625–3631 [DOI] [PubMed] [Google Scholar]
- 20. Ruban A. V., Horton P., Robert B. (1995) Resonance Raman spectroscopy of the photosystem II light-harvesting complex of green plants: a comparison of trimeric and aggregated states. Biochemistry 34, 2333–2337 [DOI] [PubMed] [Google Scholar]
- 21. Ruban A. V., Calkoen F., Kwa S. L. S., van Grondelle R., Horton P., Dekker J. P. (1997) Characterisation of LHC II in the aggregated state by linear and circular dichroism spectroscopy. Biochim. Biophys. Acta 1321, 61–70 [Google Scholar]
- 22. Lambrev P. H., Várkonyi Z., Krumova S., Kovács L., Miloslavina Y., Holzwarth A. R., Garab G. (2007) Importance of trimer-trimer interactions for the native state of the plant light-harvesting complex II. Biochim. Biophys. Acta 1767, 847–853 [DOI] [PubMed] [Google Scholar]
- 23. Garab G., van Amerongen H. (2009) Linear dichroism and circular dichroism in photosynthesis research. Photosynth. Res. 101, 135–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ilioaia C., Johnson M. P., Horton P., Ruban A. V. (2008) Induction of efficient energy dissipation in the isolated light-harvesting complex of photosystem II in the absence of protein aggregation. J. Biol. Chem. 283, 29505–29512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ilioaia C., Johnson M. P., Liao P. N., Pascal A. A., van Grondelle R., Walla P. J., Ruban A. V., Robert B. (2011) Photoprotection in plants involves a change in lutein 1 binding domain in the major light-harvesting complex of Photosystem II. J. Biol. Chem. 286, 27247–27254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Caffarri S., Croce R., Breton J., Bassi R. (2001) The major antenna complex of photosystem II has a xanthophyll binding site not involved in light harvesting. J. Biol. Chem. 276, 35924–35933 [DOI] [PubMed] [Google Scholar]
- 27. Yang C., Boggasch S., Haase W., Paulsen H. (2006) Thermal stability of trimeric light-harvesting chlorophyll a/b complex (LHCIIb) in liposomes of thylakoid lipids. Biochim. Biophys. Acta 1757, 1642–1648 [DOI] [PubMed] [Google Scholar]
- 28. Moya I., Silvestri M., Vallon O., Cinque G., Bassi R. (2001) Time-resolved fluorescence analysis of the photosystem II antenna proteins in detergent micelles and liposomes. Biochemistry 40, 12552–12561 [DOI] [PubMed] [Google Scholar]
- 29. Zhou F., Liu S., Hu Z., Kuang T., Paulsen H., Yang C. (2009) Effect of monogalactosyldiacylglycerol on the interaction between photosystem II core complex and its antenna complexes in liposomes of thylakoid lipids. Photosynth. Res. 99, 185–193 [DOI] [PubMed] [Google Scholar]
- 30. Ruban A. V., Solovieva S., Lee P. J., Ilioaia C., Wentworth M., Ganeteg U., Klimmek F., Chow W. S., Anderson J. M., Jansson S., Horton P. (2006) Plasticity in the composition of the light harvesting antenna of higher plants preserves structural integrity and biological function. J. Biol. Chem. 281, 14981–14990 [DOI] [PubMed] [Google Scholar]
- 31. Nussberger S., Dekker J. P., Kühlbrandt W., van Bolhuis B. M., van Grondelle R., van Amerongen H. (1994) Spectroscopic characterization of three different monomeric forms of the main chlorophyll a/b binding protein from chloroplast membranes. Biochemistry 33, 14775–14783 [DOI] [PubMed] [Google Scholar]
- 32. Georgakopoulou S., van der Zwan G., Bassi R., van Grondelle R., van Amerongen H., Croce R. (2007) Understanding the changes in the circular dichroism of light harvesting complex II upon varying its pigment composition and organization. Biochemistry 46, 4745–4754 [DOI] [PubMed] [Google Scholar]
- 33. Belgio E., Johnson M. P., Jurić S., Ruban A. V. (2012) Higher plant photosystem II light-harvesting antenna, not the reaction center, determines the excited-state lifetime-both the maximum and the nonphotochemically quenched. Biophys. J. 102, 2761–2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hobe S., Trostmann I., Raunser S., Paulsen H. (2006) Assembly of the major light-harvesting chlorophyll-a/b complex: thermodynamics and kinetics of neoxanthin binding. J. Biol. Chem. 281, 25156–25166 [DOI] [PubMed] [Google Scholar]
- 35. Yang C., Lambrev P., Chen Z., Jávorfi T., Kiss A. Z., Paulsen H., Garab G. (2008) The negatively charged amino acids in the lumenal loop influence the pigment binding and conformation of the major light-harvesting chlorophyll a/b complex of photosystem II. Biochim. Biophys. Acta 1777, 1463–1470 [DOI] [PubMed] [Google Scholar]
- 36. Horton P., Ruban A. V., Rees D., Pascal A. A., Noctor G., Young A. J. (1991) Control of the light-harvesting function of chloroplast membranes by aggregation of the LHCII chlorophyll-protein complex. FEBS Lett. 292, 1–4 [DOI] [PubMed] [Google Scholar]
- 37. Rutkauskas D., Chmeliov J., Johnson M., Ruban A., Valkunas L. (2012) Exciton annihilation as a probe of the light-harvesting antenna transition into the photoprotective mode. Chem. Phys. 404, 123–128 [Google Scholar]
- 38. Barzda V., Garab G., Gulbinas V., Valkunas L. (1996) Evidence for long-range excitation energy migration in macroaggregates of the chlorophyll a/b light-harvesting antenna complexes. Biochim. Biophys. Acta 1273, 231–236 [Google Scholar]
- 39. Lambrev P. H., Schmitt F. J., Kussin S., Schoengen M., Várkonyi Z., Eichler H. J., Garab G., Renger G. (2011) Functional domain size in aggregates of light-harvesting complex II and thylakoid membranes. Biochim. Biophys. Acta 1807, 1022–1031 [DOI] [PubMed] [Google Scholar]
- 40. Haferkamp S., Haase W., Pascal A. A., van Amerongen H., Kirchhoff H. (2010) Efficient light harvesting by photosytem II requires an optimized protein packing density in grana thylakoids. J. Biol. Chem. 285, 17020–17028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Opačić M., Durand G., Bosco M., Polidori A., Popot J.-L. (2014) Amphipols and photosynthetic light-harvesting pigment-protein complexes. J. Membr. Biol. 247, 1031–1041 [DOI] [PubMed] [Google Scholar]
- 42. Pagliano C., Barera S., Chimirri F., Saracco G., Barber J. (2012) Comparison of the α and β isomeric forms of the detergent n-dodecyl-d-maltoside for solubilizing photosynthetic complexes from pea thylakoid membranes. Biochim. Biophys. Acta 1817, 1506–1515 [DOI] [PubMed] [Google Scholar]
- 43. Wilk L., Grunwald M., Liao P. N., Walla P. J., Kühlbrandt W. (2013) Direct interaction of the major light-harvesting complex II and PsbS in nonphotochemical quenching. Proc. Natl. Acad. Sci. U.S.A. 110, 5452–5456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ruban A. V., Pascal A. A., Robert B., Horton P. (2001) Configuration and dynamics of xanthophylls in light-harvesting antennae of higher plants: spectroscopic analysis of isolated light-harvesting complex of photosystem II and thylakoid membranes. J. Biol. Chem. 276, 24862–24870 [DOI] [PubMed] [Google Scholar]
- 45. Croce R., Weiss S., Bassi R. (1999) Carotenoid-binding sites of the major light-harvesting complex II of higher plants. J. Biol. Chem. 274, 29613–29623 [DOI] [PubMed] [Google Scholar]