Background: Interaction between the type 2 peroxisomal targeting signal and its receptor initiates import of the cargo protein.
Results: The binding of an additional co-receptor converts this cargo-receptor dimer into a highly stable import-competent complex.
Conclusion: The effective recognition of the signal requires the sequential binding of receptor and co-receptor.
Significance: This mechanism of sequential binding avoids the peroxisomal transport of cargo-free receptor.
Keywords: Conformational Change, Peroxisome, Protein Sorting, Protein Targeting, Receptor, PEX5, PEX7, PTS2, Mammalian Two-hybrid Assay, Trimeric Complex
Abstract
The destination of peroxisomal matrix proteins is encoded by short peptide sequences, which have been characterized as peroxisomal targeting signals (PTS) residing either at the C terminus (PTS1) or close to the N terminus (PTS2). PTS2-carrying proteins interact with their cognate receptor protein PEX7 that mediates their transport to peroxisomes by a concerted action with a co-receptor protein, which in mammals is the PTS1 receptor PEX5L. Using a modified version of the mammalian two-hybrid assay, we demonstrate that the interaction strength between cargo and PEX7 is drastically increased in the presence of the co-receptor PEX5L. In addition, cargo binding is a prerequisite for the interaction between PEX7 and PEX5L and ectopic overexpression of PTS2-carrying cargo protein drastically increases the formation of PEX7-PEX5L complexes in this assay. Consistently, we find that the peroxisomal transfer of PEX7 depends on cargo binding and that ectopic overexpression of cargo protein stimulates this process. Thus, the sequential formation of a highly stable trimeric complex involving cargo protein, PEX7 and PEX5L stabilizes cargo binding and is a prerequisite for PTS2-mediated peroxisomal import.
Introduction
Proteins compartmentalized within organelles reach their destination by the concerted action of a targeting signal and the corresponding receptor protein that recognizes such signals and mediates the initiation of protein transport (1). A variety of signal-receptor combinations have been investigated that govern the transport of newly synthesized proteins to mitochondria, the endoplasmic reticulum or peroxisomes (2–4). Soluble proteins destined for peroxisomes are folded before the import and, thus, this import system has a special status. Peroxisomes are single membrane-bound organelles that exert a variety of metabolic functions such as degradation of hydrogen peroxide and fatty acids (α- and β-oxidation) (5) and the synthesis of complex lipids (docosahexaenoic acid, ether phospholipids, such as plasmalogens and bile acids). Their importance is highlighted by the existence of severe inherited human disorders caused either by the absence of all peroxisomal functions (peroxisome biogenesis disorders) (6) or by single peroxisomal enzyme or transporter deficiencies (7). The import of peroxisomal matrix proteins is initiated by the recognition of a peroxisomal targeting signal (PTS)2 by cognate receptors. One type of signal (PTS1) is found at the extreme C terminus of the protein and is recognized by the receptor PEX5, whereas another type of signal (PTS2) is located in proximity to the N terminus of the protein and is recognized by the receptor PEX7. In fungi and metazoa the majority of peroxisomal matrix proteins is imported via the PTS1-dependent pathway, whereas in plants numerous proteins use the PTS2-dependent import pathway (8).
The PTS2-type peroxisomal targeting signal was originally described as a peptide with the consensus sequence (R/K)(L/V/I)X5(Q/H)(L/A) (9). We recently suggested a specification of this nomenclature that is derived from this original description, but labels the less defined residues as well (10). Thereby, the previously defined positions are indicated as S1 to S4, whereas the intermediate five less characterized positions are indicated from X1 to X5 resulting in S1S2X1X2X3X4X5S3S4, which will be used throughout the text. Frequency-based approaches predicted revised consensus sequences for PTS2 motifs: R-(L/V/I/Q)-X-X-(L/V/I/H)-(L/S/G/A)-X-(H/Q)-(L/A) for the most common PTS2 variants and (R/K)-(L/V/I/Q)-X-X-(L/V/I/H/Q)-(L/S/G/A/K)-X-(H/Q)-(L/A/F) comprising essentially all known possibilities (11). These reports observed a preference for hydrophobic residues at position X3 (8, 10, 11) and its relevance has been verified experimentally (10). Furthermore, we demonstrate that the PTS2 has to form an α-helical conformation, in which all important residues orient their side chains in the same direction and that a flexible linker domain connects the PTS2-helix with the core protein to expose the signal properly (10). The PTS2 receptor PEX7 belongs to the family of seven-bladed WD-40 repeat proteins, which share a characteristic shape (12). Patients harboring a mutation in PEX7 suffer from rhizomelic chondrodysplasia punctata type 1 (RCDP1) presenting with a variety of pathological manifestations such as shortening of the proximal limbs and aberrant calcification patterns of the cartilage (13). The structure of human PEX7 has been predicted independently several times (10, 14, 15), but only after the identification of an evolutionary conserved groove on top of the cone-shaped WD-40 structure, the binding site of the PTS2-helix, could be predicted and experimentally verified (10). In this model, two evolutionary conserved glutamate residues (Glu-113, Glu-200) are part of the binding groove and charge-inverting substitutions of these residues interfere with cargo binding, whereas a third similarly conserved glutamate (Glu-287) is proximal to the binding groove but its mutation retains cargo binding (10) (schematically presented in Fig. 1A). This model for the human PEX7-cargo interaction has recently been corroborated by resolution of the three-dimensional structure of the yeast ScPex7p bound to a PTS2-carrying cargo protein, which shows a virtually identical binding mode (16).
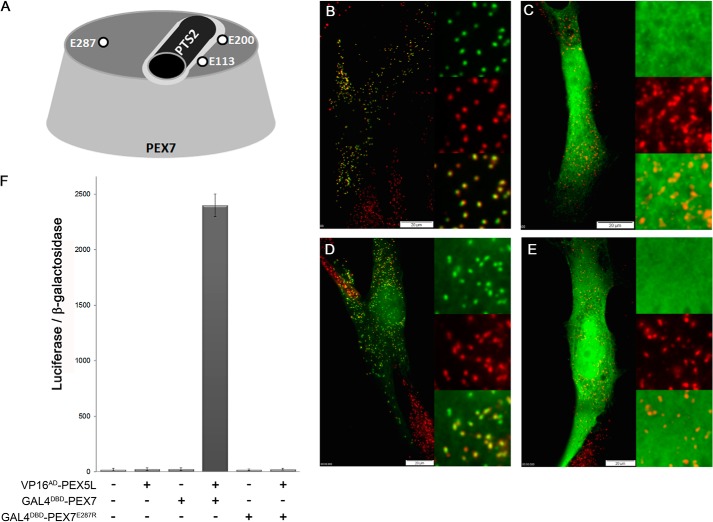
FIGURE 1.
Glutamate 287 of PEX7 is involved in binding to the co-receptor PEX5L. A, schematic representation of the top side of the PEX7-cone indicating three conserved glutamate residues and the PTS2-helix. B–E, human fibroblasts obtained from a healthy subject (B) or an RCDP1 patient (C–E) were co-transfected with the reporter protein PTS2thiolase-EGFP together with either the empty vector (B and C) or expression plasmids for native myc-PEX7 (D) or myc-PEX7E287R (E) and after about 40 h cells were processed for immunofluorescence microscopy. Autofluorescence of EGFP (green) and α-PMP70 antibody (red) were used for detection of the proteins. F, mammalian two-hybrid assay: COS7 cells were co-transfected with the expression plasmids for VP16AD-PEX5L and GAL4DBD-PEX7 or GAL4DBD-PEX7E287R together with the luciferase reporter plasmid pFR-Luc (GAL45xUAS-luciferase) and the β-galactosidase reporter plasmid (pCMV-β-Gal). The ratio of luciferase activity and β-galactosidase activity is indicated.
The transport of PEX7-cargo complexes to the peroxisomal surface is exerted by additional proteins summarized as co-receptors. In metazoan and plant species, the PTS1 receptor PEX5 acts as co-receptor (17–19), whereas in various yeast species the co-receptor function is performed either by one protein such as Pex20p in Yarrowia lipolytica (20) or a corresponding protein complex consisting of Pex18p and Pex21p in Saccharomyces cerevisiae (21). All these co-receptor proteins share a short, highly conserved domain, which is capable of PEX7 binding (22, 23). In mammalian PEX5 this domain is encoded by an independent exon that is facultatively omitted by alternative splicing giving rise to a long and short isoform of PEX5 (PEX5L and PEX5S), whereas in plants the short isoform occurs only in rice (24). Furthermore, all co-receptors harbor sequences for docking at the peroxisomal membrane and further integration (25, 26), but also for ubiquitination and recycling of the co-receptor·receptor complex from peroxisomes to the cytosol (27, 28). From this perspective, PEX7 exerts a bridging function that links various cargo proteins harboring a PTS2 signal with the co-receptor protein such as PEX5L that mediates transport and receptor recycling. Thus, PTS2-carrying proteins are imported by a piggyback-like mechanism that has been amply demonstrated in peroxisomal protein import (29–31), whereby PEX7 acts as platform to handle diverse proteins. The subsequent translocation of PTS2-carrying cargo proteins across the peroxisomal membrane is mechanistically still enigmatic, but most likely resembles the better understood import of PTS1-carrying proteins (32).
In the present work we use a modified application of the mammalian two-hybrid assay to reveal the counterwise stabilizing effect of cargo and co-receptor binding to PEX7 on the stability of the trimeric complex. Thereby, we provide evidence that in PTS2-mediated protein import the interaction between PEX7 and cargo protein is a prerequisite for co-receptor binding, which reciprocally stabilizes the receptor-cargo interaction and initiates transfer of this complex to peroxisomes.
EXPERIMENTAL PROCEDURES
Cell Culture and Immunofluorescence Microscopy
The green monkey kidney cell line COS7 (ATCC), and human fibroblasts from healthy patients (33) and fibroblasts from RCPD1 patients carrying mutations H39P/W206X have been previously described (14). Cells were cultivated in DMEM (COS7) or RPMI1640 (fibroblasts) supplemented with 10% fetal calf serum (FCS), 2 mm l-glutamine, 50 units/ml of penicillin, and 100 μg/ml of streptomycin (BioWhittaker). For transfection cells were incubated with Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions using Opti-MEM (Invitrogen) or electroporation. 32–48 h after transfection, cells were fixed for 15 min with 4% paraformaldehyde in phosphate-buffered saline (PBS). Cells were washed, permeabilized (5 min with 0.1% Triton X-100 in PBS), and blocked in blocking solution (PBS with 10% FCS and 5% bovine serum albumin (Roche Applied Science)). After incubation with primary antibodies from different species (rabbit, α-PMP70 (1:2000, ABR); mouse, α-EGFP (1:800, Roche Applied Science)), slides were washed with PBS several times and exposed to compatible secondary antibodies (Cy2- and Cy3-labeled goat α-rabbit IgG and goat α-mouse IgG, 1:300, Jackson ImmunoResearch). Finally, cells were mounted in PBS/glycerol (1:9) with 3% DABCO (Sigma). For microscopic analysis the invert microscope IX71 equipped with a CCD camera (CAM-XM10) and an appropriate filter set was used together with analysis and C-M-cell software (Olympus). During the analysis of subcellular distribution, cells that showed extremely high expression levels were avoided, because in these usually a cytosolic distribution of the reporter protein was observed, probably due to saturation of the PTS2-dependent import pathway.
Cloning of Plasmids
The expression plasmid pcDNA3.1_zeo-PEX5L was cloned by excising the ORF of PEX5L from pCD2 (PEX5L-IRES-EGFP-SKL, kindly provided by Marc Fransen, Leuven, Belgium) using restriction enzymes BglII/SalI and inserting this DNA fragment into pcDNA3.1_zeo that had been linearized using BamHI and XhoI. PTS2thiolase (RS1E)-EGFP was generated by digesting the reporter protein lacking a PTS2 signal (PTS2-tester) (10) with restriction enzymes PstI and EcoRI and inserting oligonucleotides 1674 and 1675.
pcDNA3.1-mRFP-PEX7
The plasmid myc-PEX7 (10) was digested with restriction enzymes EcoRI/NdeI and the ORF of mRFP-C1 together with the CMV-promoter was inserted by excising the EcoRI/NdeI fragment from plasmid mRFP-C1, in which the reading frame had been shifted by digestion with XhoI, filling in recessive ends with Klenow polymerase and religation. The variants encoding mutations E113R, E200R, and E287R were obtained by the same procedure using the corresponding myc-PEX7 plasmids with the mutations (10).
Two-hybrid Plasmids
GAL4DBD-HA-PEX5L (pM-HA-PEX5L) was obtained by combining (i) the vector backbone of the empty plasmid pM digested with SalI and NdeI, (ii) a pM variant containing an additional HA tag behind the GAL4-DBD digested with NdeI and BamHI, and (iii) the ORF of PEX5L obtained by digestion of CD2 with BglII/SalI. VP16AD-HA-PEX5L (pVP16-HA-PEX5L) was generated by digesting a variant of empty plasmid pVP16 that also harbors the HA tag insertion (including a NotI site) with SalI and NotI and inserting the ORF of PEX5L by digestion of pM-HA-HsPEX5 with SalI/NotI. GAL4DBD-PEX7 was generated by digesting empty plasmid pM with EcoRI and XbaI and ligating with the ORF of PEX7 that was obtained by excision from expression plasmid myc-PEX7 (10) as the EcoRI/XbaI fragment. Variants of GAL4DBD-PEX7 were obtained by the same procedure using ORFs from myc-HsPEX7 variants using plasmids encoding the corresponding mutations (plasmids are listed in supplemental Table S1).
Mammalian Two-hybrid Assay/Luciferase Assay
COS7 cells were transfected in 24-well plates using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions (1.5 μl of Lipofectamine) with the following plasmids: 0.1 μg of luciferase reporter plasmid pFR-Luc (Stratagene) and 0.05 μg of pCMV-β-Gal (P204, Promega) for normalization were combined with the appropriate combination of either (i) for 2 plasmids of 0.35 μg of bait (GAL4DBD encoding plasmids) and 0.35 μg of prey (VP16AD encoding plasmids) or (ii) for 3 plasmids with each 0.23 μg of bait, 0.23 μg of prey, and 0.23 μg of expression vector. After 48 h, the cells were washed once with PBS and incubated with 50 μl of lysis buffer (100 mm phosphate buffer, pH 7.8, 0.5% Triton X-100, cOmplete protease inhibitor mixture (Roche Applied Science)) for 20 min. The extracts were centrifuged for 20 min at 15,300 × g, and the supernatant was measured. The luciferase assay was performed according to the protocol of the MatchmakerTM system (Clontech) using the pRF-Luc vector (Stratagene) as reporter plasmid for the detection of interaction by luminescence-based luciferase activity measurements (SynergyH4 reader; BioTek) and using plasmid pCMV-β-Gal for normalization of transfection efficiency by measuring β-galactosidase activity (34).
RESULTS
Identification of a Mutation in Human PEX7 That Interferes with PEX5L Binding
In the predicted structure of human PEX7, a highly conserved glutamate residue at position 287 is located in proximity to the PTS2-binding groove (Fig. 1A) and the charge-inverting mutation E287R reduced the affinity of PEX7 to the cargo protein, although binding was retained (10). This raised the question, whether a PEX7 variant harboring this mutation is functional and able to compensate for PEX7 deficiency. Thus, we cotransfected human skin fibroblasts obtained from a healthy human subject (Fig. 1B) or from an RCDP1 patient lacking functional PEX7 (Fig. 1, C–E) with the PTS2-reporter protein (PTS2thiolase-EGFP) and either an empty vector (Fig. 1, B and C), an expression plasmid encoding myc-tagged human PEX7 (myc-PEX7) (Fig. 1D) or a variant thereof harboring the E287R mutation (myc-PEX7E287R) (Fig. 1E). In cells transfected with the empty plasmid the reporter protein appeared punctate in cells from the healthy person and colocalized with the peroxisomal marker protein PMP70 (Fig. 1B), whereas in cells from the RCDP1 patient the protein was evenly distributed across the cell (Fig. 1C). In cells from an RCDP1 patient expressing myc-PEX7 the reporter protein showed a punctate staining pattern colocalizing with the peroxisomal marker PMP70 indicating peroxisomal import (Fig. 1D), but in cells expressing the mutated myc-PEX7E287R form the reporter protein was again evenly distributed across the cell (Fig. 1E). This demonstrates that the E287R mutation destroys the ability of PEX7 to restore PTS2-mediated import in RCDP1 cells although cargo binding is retained in this PEX7 variant. Because the transport of cargo-loaded PEX7 to the surface of peroxisomes requires interaction between PEX7 and the long isoform of PEX5 (PEX5L), we hypothesized that the E287R mutation interferes with the binding to PEX5L. Thus, we tested the interaction between PEX7 and PEX5L in a mammalian two-hybrid assay. When COS7 cells were cotransfected with expression plasmids encoding PEX5L fused to the C terminus of the VP16-activation domain (prey) (VP16AD-PEX5L) and PEX7 fused to the GAL4-DNA-binding domain (bait) (GAL4DBD-PEX7) together with a luciferase reporter plasmid (five copies of the GAL4-binding element in front of luciferase, UAS5xGAL4-BE-luciferase) and a β-galactosidase reporter plasmid for normalization, we obtained a significant and specific increase in the relative luciferase activity compared with controls indicating a specific interaction between the proteins (Fig. 1F). However, when VP16AD-PEX5L was cotransfected with the PEX7 variant harboring the E287R mutation the reporter protein activity was comparable with the background level suggesting that an interaction between the proteins does not occur (Fig. 1F). These results indicate that the E287R mutation interferes with the interaction between receptor and co-receptor and, thus, the inability of PEX7E287R to restore PTS2-mediated import is caused by a defect in peroxisomal transport of the receptor.
Binding of Co-receptor PEX5L Increases the Interaction Strength between PEX7 and the PTS2-carrying Cargo
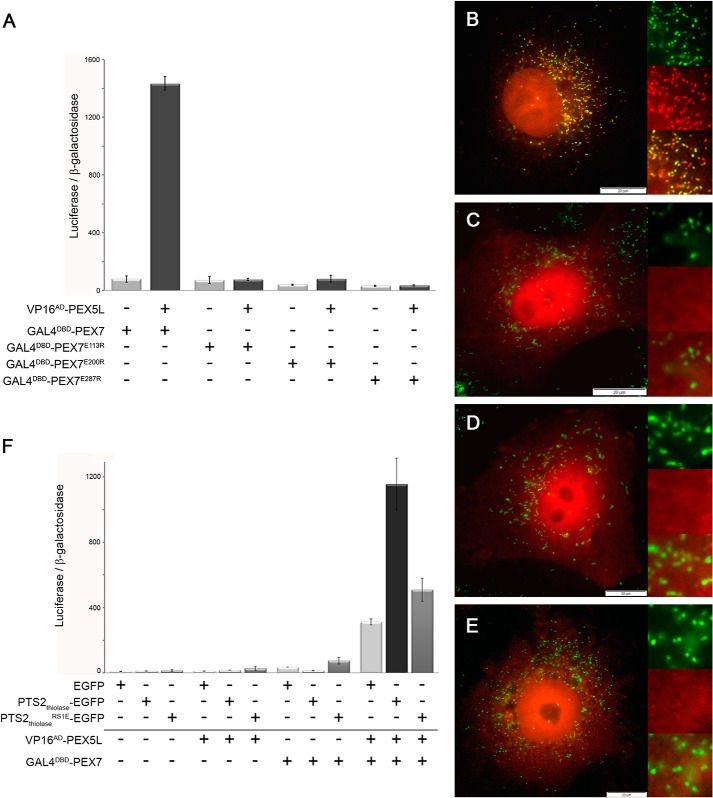
To confirm the reduced interaction between PEX7E287R and the prototypic cargo protein (PTS2thiolase-EGFP) relative to native PEX7, we again performed a mammalian two-hybrid assay using GAL4DBD-PEX7 and VP16AD-PTS2thiolase-EGFP, thus inverting bait and prey compared with our previous work (10). We found that the specific interaction between PTS2-carrying protein and PEX7 was well reflected by the high relative luciferase reporter activity upon cotransfection of the encoding plasmids, whereas controls using empty plasmids showed low activity levels (Fig. 2A). Furthermore, we found that PEX7 retained its ability to interact with PTS2-carrying proteins upon introduction of the E287R mutation (PEX7E287R), although the apparent interaction strength3 was reduced to about half (Fig. 2A). This demonstrates that PEX7E287R can still interact with the cargo protein and, thus, the protein is neither misfolded nor degraded. Moreover, it suggests that the binding of endogenous PEX5L strengthens the interaction between PEX7 and cargo, which cannot occur in the PEX7 variant with the mutation E287R.
FIGURE 2.
Co-receptor binding stabilizes the interaction between PEX7 and its cargo. A, mammalian two-hybrid assay measuring the interaction strength between the PTS2-reporter protein PTS2thiolase-EGFP (VP16AD-PTS2thiolase-EGFP) and either native PEX7 (GAL4DBD-PEX7) or a variant thereof harboring the mutation E287R (GAL4DBD-PEX7E287R). B, modified mammalian two-hybrid assay measuring the interaction strength between GAL4DBD-PTS2thiolase-EGFP and VP16AD-PEX7 or VP16AD-PEX7E287R in the absence or presence of ectopically expressed co-receptor PEX5L. C, modified mammalian two-hybrid assay measuring the interaction strength between VP16AD-PTS2thiolase-EGFP and either GAL4DBD-PEX7 or variants thereof harboring mutations E113R (GAL4DBD-PEX7E113R), E200R (GAL4DBD-PEX7E200R), or E287R (GAL4DBD-PEX7E287R) in the absence or presence of the co-receptor PEX5L. COS7 cells were transfected with expression plasmids for bait and prey proteins, the luciferase and β-galactosidase reporter plasmids, and the expression plasmid encoding PEX5L or the respective empty vector. The ratio of luciferase activity and β-galactosidase activity is indicated.
To corroborate the hypothesis of a stable trimeric complex consisting of cargo, receptor, and co-receptor we performed a modified version of the mammalian two-hybrid assay, in which a putatively stabilizing factor is co-transfected together with the bait and prey encoding plasmids. The involvement of such a third interaction partner that modifies the interaction between bait and prey proteins is a well known effect in two-hybrid assays, which can also generate false positives due to an incorrect attribution of direct interactions. However, the power of this approach for the investigation of the contribution of a protein in trimer-complex formation has hardly been exploited. Provided that the interaction strength between two proteins is markedly increased in the presence of the third protein, the reporter protein activity should become higher upon ectopic expression of this protein. Here, this approach was prototypically used to investigate the stabilizing effect of PEX5L on the interaction between PTS2-carrying cargo and PEX7.
Therefore, we co-transfected COS7 cells with the reporter plasmids for luciferase and β-galactosidase together with expression plasmids encoding GAL4DBD-PTS2thiolase-EGFP and VP16AD-PEX7, and in addition either an expression plasmid for PEX5L or an empty vector. We observed that the apparent interaction strength between PTS2thiolase-EGFP and PEX7 was drastically increased by the addition of PEX5L, which supports the hypothesis that PEX5L binding stabilizes the interaction between cargo and receptor (Fig. 2B). In contrast, PEX5L overexpression did not have a stabilizing effect on the interaction between PTS2thiolase-EGFP and PEX7E287R, which cannot bind PEX5L. Instead, PEX5L appeared to even have a destabilizing effect on the interaction. This probably reflects a dominant negative effect exerted by the sequestration of GAL4DBD-PTS2thiolase-EGFP into a stable, but transcriptionally inactive protein complex together with PEX5L and endogenous PEX7, whereas VP16AD-PEX7E287R cannot form a stable trimeric complex. Finally, the question was, whether the mutations in the cargo-binding groove of PEX7 (PEX7E113R and PEX7E200R) (10) can be overcome by the stabilizing effect of highly abundant co-receptor PEX5L. Thus, we co-transfected the VP16AD-PTS2thiolase-EGFP together with PEX7 variants GAL4DBD-PEX7E113R and GAL4DBD-PEX7E200R and compared the stabilizing effects of PEX5L overexpression. We found that overexpression of PEX5L also slightly increased the apparent interaction strength between these PEX7 variants and the PTS2-carrying cargo protein (Fig. 2C), but the values remained low compared with the unaffected PEX7 protein. This indicated that in the presence of PEX5L the number of transcriptionally active complexes is increased and that this effect is retained in PEX7 variants, which show no detectable interaction with the cargo. Thus, the stabilizing effect of overexpressed PEX5L generates a more sensitive detection system for low-abundant PEX7-cargo complexes, which are also present in case of PEX7 variants that harbor a mutation in the PTS2-binding groove. However, the number of dimers, which are putative targets for the stabilizing effect of PEX5L, is expected to be very low for these PEX7 variants and thus the apparent complex stability remains low.
Altogether, these results corroborated the hypothesis that binding of the co-receptor PEX5L drastically stabilizes the interaction between PEX7 and its PTS2-carrying cargo protein, but is not essential. However, it remained unclear whether cargo binding has to precede co-receptor binding or whether trimer formation involves a preformed receptor·co-receptor complex.
Interaction between PEX7 and PEX5L Requires Cargo Binding
To discriminate between these possibilities we investigated whether cargo binding to PEX7 is a prerequisite for the interaction between PEX7 and the co-receptor PEX5L. Therefore, we performed a mammalian two-hybrid assay to compare the apparent interaction strength between PEX5L and PEX7 with that of the PEX7 variants, in which mutations in the PTS2-binding groove (E113R, E200R) interfere with cargo binding. The variant that cannot bind PEX5L (E287R) served as control. COS7 cells were cotransfected with the reporter plasmids (luciferase and β-galactosidase) together with VP16AD-PEX5L and GAL4DBD-PEX7 or with the corresponding PEX7 variants (GAL4DBD-PEX7E113R, GAL4DBD-PEX7E200R, and GAL4DBD-PEX7E287R). We found that the relative luciferase activity reflecting the apparent interaction strength between PEX5L and PEX7 was reduced to a background level by the mutations in the cargo-binding domain (Fig. 3A). This suggested that the binding of endogenous PTS2-carrying proteins contributes to the receptor-co-receptor interaction even if the relative cytosolic abundance of these proteins might be low under standard conditions. Moreover, it demonstrates that cargo binding is required for an effective interaction between PEX7 and PEX5L and thus a cargo-independent interaction between receptor and co-receptor appears improbable. This suggests a sequential binding of cargo and co-receptor to PEX7 in a defined two-step process.
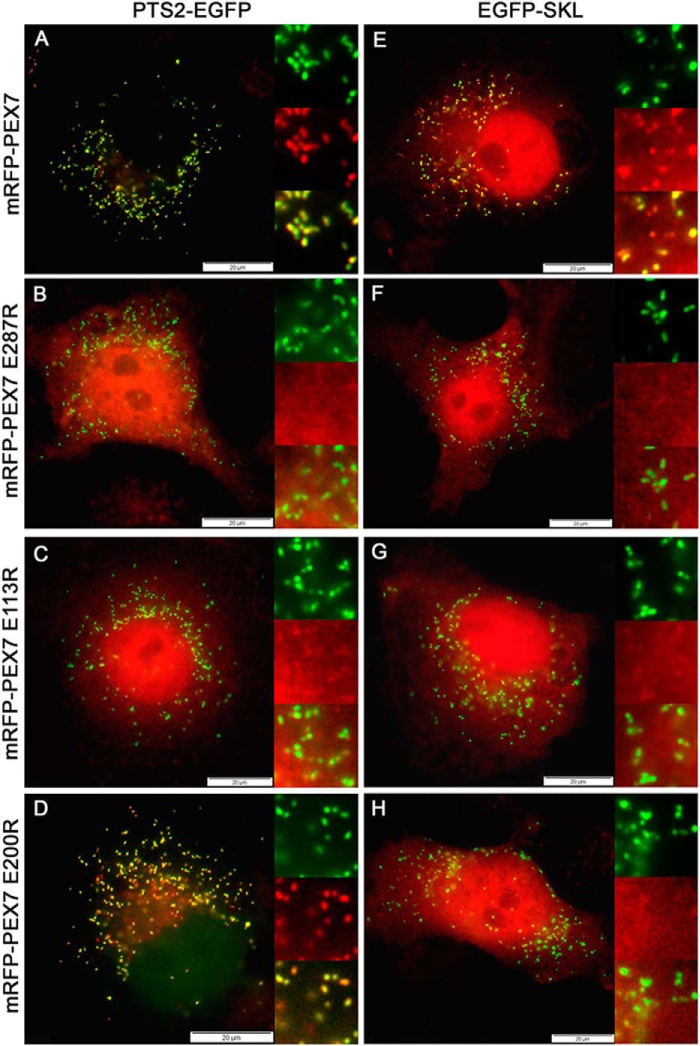
FIGURE 3.
Binding of cargo protein to PEX7 is required for its interaction with PEX5L. A, mammalian two-hybrid assay: COS7 cells were transfected with the luciferase and β-galactosidase reporter plasmids and either the empty plasmids or VP16AD-HA-PEX5L and GAL4DBD-PEX7 or mutated variants thereof that either severely interfere with cargo binding (E113R, E200R) or directly affect the interaction between PEX5L and PEX7 (E287R). B–E, immunofluorescence microscopy of COS7 cells transfected with mRFP-PEX7 (B) or variants thereof that harbor mutations interfering with cargo binding, mRFP-PEX7E113R (C) or mRFP-PEX7E200R (D), or interfering with PEX5L binding, mRFP-PEX7E287R (E). mRFP autofluorescence (red) and α-PMP70 antibody staining (green) were used for detection of the cellular location of the protein. F, modified mammalian two-hybrid assay: COS7 cells were co-transfected with bait and prey plasmids encoding HA-PEX5L and PEX7 together with expression plasmids for EGFP (light gray), the PTS2-carrying cargo protein PTS2thiolase-EGFP (dark gray), or a variant of the PTS2-carrying reporter protein harboring a mutation in the PTS2 (PTS2thiolaseRS1E-EGFP) (gray). The ratio of luciferase activity and β-galactosidase activity is indicated.
Cargo Binding Is Necessary for Peroxisomal Transport of PEX7
As peroxisomal targeting of PEX7 requires interaction with PEX5L and cargo binding is necessary for their interaction, the inability of PEX7 variants to bind cargo should block peroxisomal targeting of the receptor. However, even native myc-PEX7 was nearly exclusively found in the cytosol (Ref. 35, and our own observation) and cannot serve as a reporter protein. Thus, we took advantage of the observation that PEX7 was found at peroxisomes, when it was tagged at its N terminus with a large protein such as EGFP (35), possibly due to an inhibitory effect on receptor recycling. Based on this, we generated a PEX7 variant, which is extended by the monomeric red fluorescent protein (mRFP) at its N terminus (mRFP-PEX7). When this protein was expressed in COS7 cells and its subcellular localization was investigated by immunofluorescence microscopy, we found that mRFP-PEX7 appeared as punctate structures colocalizing with PMP70 against a cytosolic background (Fig. 3B). However, the mRFP-PEX7 variant that cannot interact with PEX5L due to the E287R mutation (mRFP-PEX7E287R) was evenly distributed across the cytosol (Fig. 3E) demonstrating that PEX5L binding is required for peroxisomal targeting of mRFP-PEX7. Next, we expressed the mRFP-PEX7 variants that cannot interact with PTS2-cargo proteins (mRFP-PEX7E113R, mRFP-PEX7E200R) and found that both variants were evenly distributed across the cytosol, but could not be detected in punctate structures (Fig. 3, C and D). This confirms that cargo binding is a prerequisite for peroxisomal targeting of the receptor, which is induced by endogenous PTS2-carrying proteins.
Binding to the Cargo Affects the Interaction between PEX7 and PEX5L
However, we assumed that the amount of endogenously expressed PTS2-carrying proteins that support the interaction between PEX7 and PEX5L in the two-hybrid assay is comparably low and, consequently, a high level of ectopically expressed cargo protein should increase the interaction between PEX7 and PEX5L. Therefore, we again performed the modified version of the mammalian two-hybrid assay using VP16AD-PEX5L and GAL4DBD-PEX7 and investigated the effect of ectopically expressed PTS2-carrying cargo proteins on the apparent interaction strength between PEX5L and PEX7. Thus, COS7 cells were co-transfected with the reporter plasmids and expression plasmids for the above mentioned bait and prey proteins together with an expression plasmid for PTS2thiolase-EGFP. As control we used expression plasmids for EGFP alone or for a variant of PTS2thiolase-EGFP in which arginine at the key residue S1 was substituted by glutamate (PTS2thiolaseRS1E-EGFP), which destroys the PTS2. We found that in the presence of the PTS2-cargo protein PTS2thiolase-EGFP the apparent interaction strength between PEX7 and PEX5L was markedly higher than in the presence of EGFP alone or of PTS2thiolaseRS1E-EGFP (Fig. 3F). These results confirmed the hypothesis that the amount of available PTS2-carrying cargo determines the apparent interaction strength between PEX7 and PEX5L. Moreover, the results are in agreement with sequential binding of cargo and co-receptor, provided that only the level of cargo-loaded PEX7 limits the formation of trimeric complexes, which is reflected by the apparent PEX7-PEX5L interaction strength.
Formation of PEX7-Cargo Dimers Limits the Generation of Trimeric Complexes
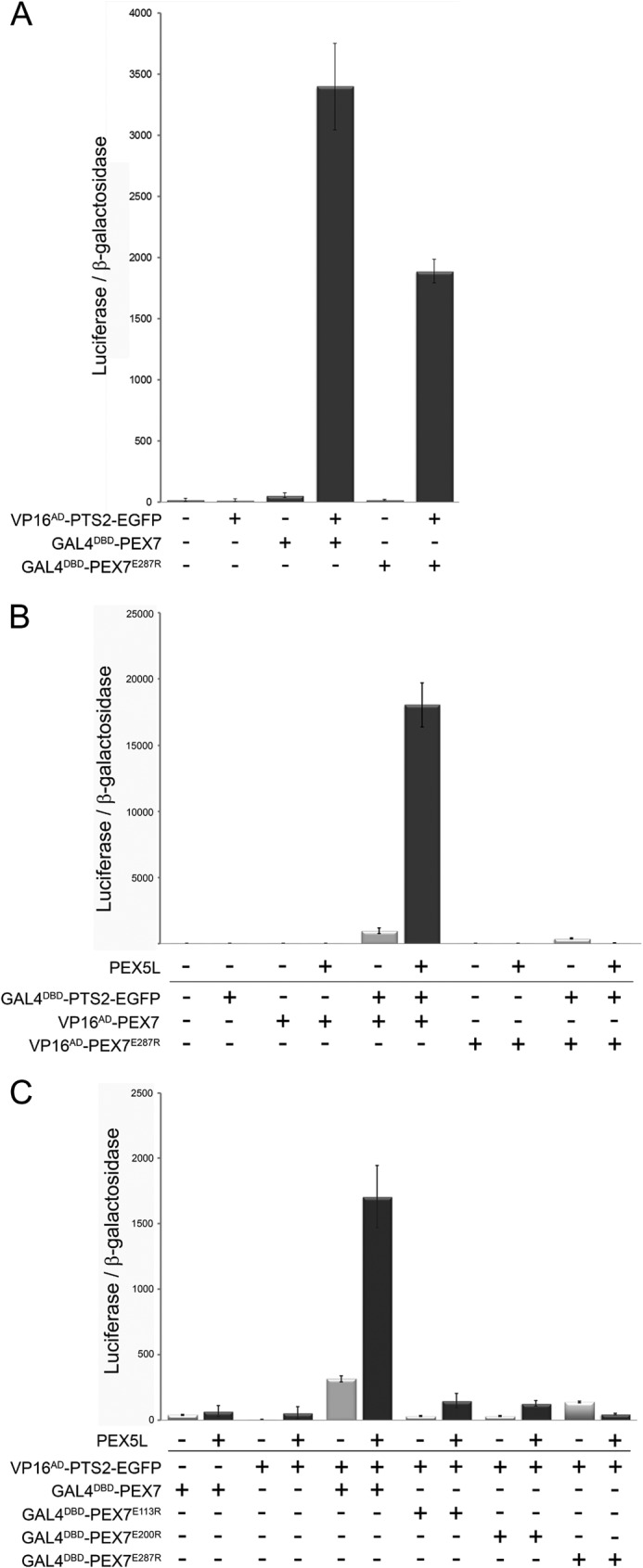
To corroborate this interpretation, we investigated whether cargo overexpression can also overcome the effects of the mutations in the PTS2-binding domain of PEX7 that abrogate the interaction between PEX7 and PEX5L (Fig. 3A), because cargo binding is only drastically reduced, but not completely blocked in these PEX7 variants (Fig. 2C). However, in the latter experiment overexpression of the co-receptor increased the level of the third interaction partner that snaps pre-formed dimer complexes, but does not change the amount of these dimers. In contrast, the number of pre-formed dimeric complexes should be increased when the PEX7 variants are exposed to high levels of ectopically expressed cargo protein, whereas the number of snapping PEX5L is not limiting. Consequently, the increase of the apparent interaction strength between PEX5L and these PEX7 variants should be much higher and might be limited by the levels of receptor and co-receptor. In contrast, interaction with the PEX7 variant harboring the mutation in the PEX5L-binding site (E287R) should not be affected and serves as control. Thus, COS7 cells were co-transfected with the reporter proteins, the vectors encoding VP16AD-PEX5L and GAL4DBD-PEX7 or the variants thereof (PEX7E113R, PEX7E200R, and PEX7E287R) together with the cargo protein (PTS2thiolase-EGFP) or a variant thereof, in which the PTS2-signal is substituted by an arbitrary sequence (PTS2emtpy-EGFP) (Fig. 4A). We observed that cargo overexpression increased the apparent interaction strength between PEX5L and the PEX7 variants affected in cargo binding close to the control level (E200R) or at least to more than half of the value (E113R), whereas the interaction between PEX5L and PEX7 harboring the mutation in the PEX5L binding site (E287R) was not restored. This result confirmed that a high level of cargo protein can suppress the effect of mutations in the PTS2-binding groove on the apparent PEX5L-PEX7 interaction suggesting that the level of PEX7-cargo dimers is rate-limiting for the apparent interaction between PEX5L and PEX7. Nonetheless, the apparent interaction strength between PEX5L and PEX7 harboring the E113R mutation was surprisingly high taking into account the inability of this variant to compensate for PEX7 deficiency (10). Provided that the amount of endogenous PTS2-carrying proteins is sufficiently high to mediate PEX5L binding of native PEX7, but not of PEX7E113R (Fig. 4A, bright gray), then these interactions should be differentially dependent on the amount of ectopically expressed cargo. Therefore, we investigated the effect of a stepwise reduction (serial dilution) of ectopically expressed PTS2-carrying cargo protein on the apparent interaction strength between PEX5L and PEX7 or PEX7E113R, respectively (Fig. 4B). We found that for native PEX7 a relatively low level of PTS2-EGFP is sufficient to reach the maximum value of the apparent interaction strength (about 1:27 dilutions are stimulating to a similar extent). In contrast, the interaction of PEX7E113R was highly sensitive to a reduction of the PTS2-EGFP level (already a dilution of 1:3 reduced the apparent interaction strength to less than half of the value). Thus, already at a dilution of 1:9 we observed drastic differences in the apparent interaction strength of PEX5L with PEX7 and PEX7E113R, respectively. Altogether, these results support the hypothesis that the primary interaction of PEX7 with the cargo protein is the rate-limiting step for trimer formation. Endogenous PTS2-carrying proteins solely contribute to the apparent interaction strength of PEX5L with PEX7, but not with PEX7E113R, because this mutation drastically reduces the affinity of PEX7 to standard PTS2.
FIGURE 4.
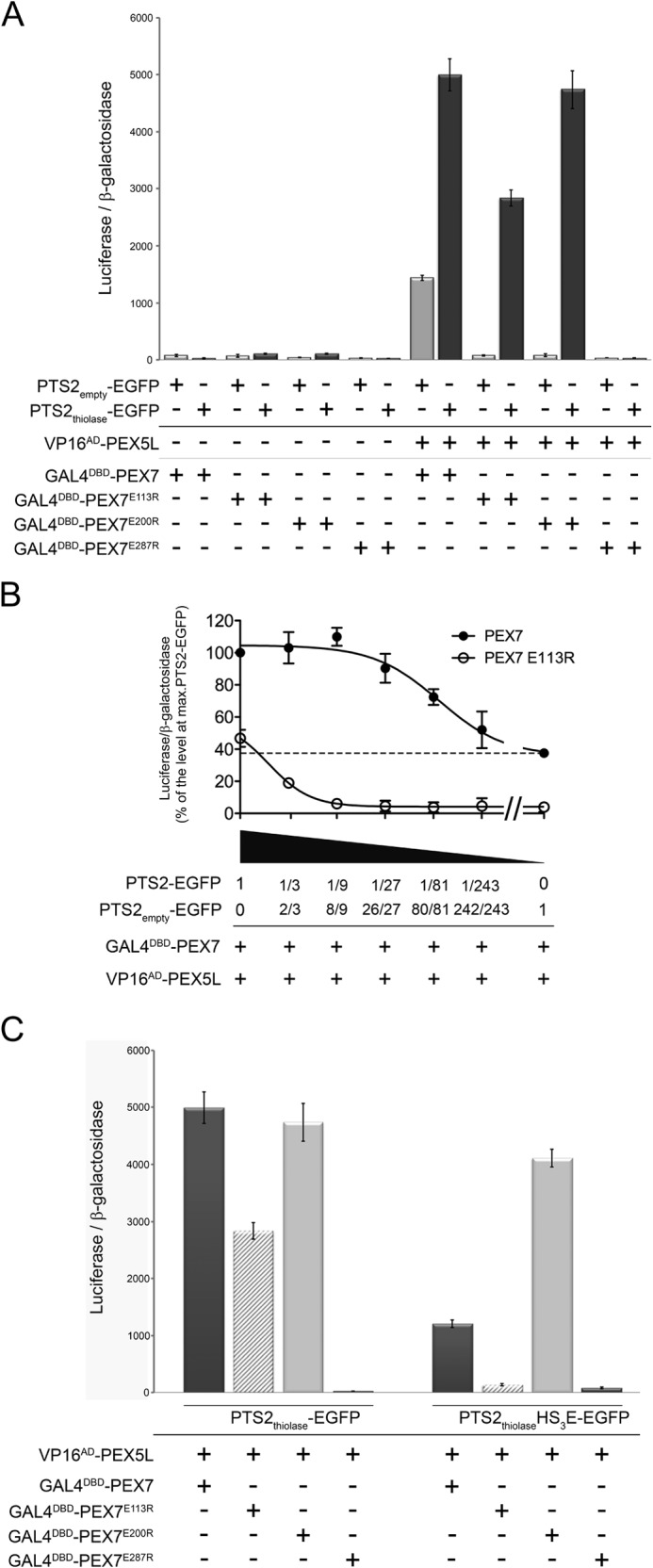
Complex formation between cargo and PEX7 determines the apparent interaction strength between PEX7 and PEX5L. A–C, modified mammalian two-hybrid assay in COS7 cells. A, cells were transfected with the reporter plasmids together with bait and prey plasmids encoding VP16AD-HA-PEX5L and GAL4DBD-PEX7 or the respective variants thereof (PEX7E113R, PEX7E200R, or PEX7E287R) and either an expression plasmid encoding a prototypic PTS2-carrying protein (PTS2thiolase-EGFP) or a variant thereof lacking the PTS2 (PTS2empty-EGFP). B, cells were transfected as described before in A using expression plasmids that encode VP16AD-HA-PEX5L and GAL4DBD-PEX7 (filled circles) or GAL4DBD-PEX7E113R (open circles) together with different amounts of the expression plasmid encoding PTS2-EGFP. For serial dilution a variant of PTS2-EGFP lacking the core PTS2 (PTS2empty-EGFP) was used. The values are presented as percentage of the apparent interaction strength between PEX5L and native PEX7 in the presence of the same level of PTS2-EGFP as in A. The dashed line represents the apparent interaction of native PEX7 without ectopically expressed cargo protein. C, a charge inverting mutation in the PTS2-signal causes amplifying or compensatory effects toward different mutations in PEX7. Cells were transfected as above, but co-transfected with the expression plasmid for either PTS2thiolase-EGFP or a variant thereof that harbors the PTS2 mutation HS3E (PTS2thiolaseHS3E-EGFP), which severely decreases the affinity to native PEX7, but allows the interaction with PEX7E200R. The ratio of luciferase activity and β-galactosidase activity is indicated.
However, even this mutation in the PTS2 binding groove can be suppressed by a high level of cargo protein, because of the rapid conversion of the receptor-cargo dimer into a very stable trimeric complex. Therefore, we next investigated whether the number of directly interacting residues at the interface between the signal-binding groove of PEX7 and the PTS2 is critical for the apparent interaction strength between PEX7 and PEX5L. The affinity between PEX7 and a PTS2 signal should be determined by the fitting between the five key residues of PTS2 signals (S1, S2, X3, S3, and S4) and the corresponding residues in the cargo-binding groove of the receptor, although other residues might be involved. Therefore, we compared the stabilizing effect of standard cargo protein PTS2thiolase-EGFP with a modified variant thereof, in which histidine at position S3 was substituted by glutamate (HS3E). This PTS2 variant appeared especially suitable, because on the one hand this mutation abolished the ability of the nonapeptide to act as a PTS2, but on the other hand this ability could be restored by the expression of PEX7E200R, which acts as a receptor variant with a compensatory charge inverting mutation (cross-complementation) (10). Consequently, this modified PTS2-reporter protein (PTS2thiolaseHS3E-EGFP) has an optimal fitting with PEX7E200R, the fitting with native PEX7 lacks one interaction and the fitting with PEX7E113R lacks two interactions. We performed a modified two-hybrid experiment to measure the apparent interaction between VP16AD-PEX5L and the different GAL4DBD-PEX7 variants as described for Fig. 4A, but co-transfected expression plasmids encoding either PTS2thiolase-EGFP or the variant PTS2thiolaseHS3E-EGFP (Fig. 4C). We observed that the apparent interaction strength between PEX5L and the PEX7 variant with optimal fitting (PEX7E200R) was similar when comparing the effect exerted by this PTS2 variant with the effect of the optimal PTS2. However, the apparent interaction strength between PEX5L and native PEX7 was only modestly stabilized by this PTS2 variant and the interaction of PEX5L with PEX7E113R remained at the level of the PEX7 variant that cannot interact with PEX5L. This demonstrated that the stabilizing effect was more sensitive to mutations in the PTS2 signal compared with mutations in the PTS2-binding groove, which might be due to the fact that a residue of the PTS2 signal can interact with more than one residue of the receptor.
Binding of the Cargo Protein Induces Peroxisomal Targeting of PEX7
To confirm that the sequential binding of cargo and co-receptor protein to PEX7 is reflected by the induction of its peroxisomal transport, we investigated whether the subcellular location of mRFP-PEX7 or its variants changes upon overexpression of the cargo protein PTS2thiolase-EGFP. Ectopic overexpression of an EGFP variant that is imported into the peroxisomal matrix via the PTS1-dependent import pathway (EGFP-SKL) and, thus, does not interact with PEX7 served as control. Therefore, we co-transfected COS7 cells with an expression plasmid encoding mRFP-PEX7 together with an expression plasmid that encodes either PTS2thiolase-EGFP or EGFP-SKL. When we determined the subcellular distribution of mRFP-PEX7 by autofluorescence microscopy, we found that in cells expressing the PTS2-carrying cargo protein mRFP-PEX7 appeared in an intense punctate pattern colocalizing with the cargo protein with hardly any cytosolic staining (Fig. 5A). In contrast, mRFP-PEX7 appeared slightly punctate against a strong cytosolic background when cells express PTS1-carrying EGFP (Fig. 5E). The amount of mRFP-PEX7 that is not imported into peroxisomes and thus distributed across the cytosol and the nucleus can be best evaluated by comparing the intensity of the red mRFP-signal that is observable in the area of the nucleus. Red nuclear staining was clearly recognizable in cells with a weak peroxisomal transport of PEX7, but was hardly visible in cells with efficient peroxisomal transport. This latter pattern resembled localization of the protein in the absence of ectopically expressed peroxisomal protein (Fig. 3B). In contrast, the expression of PTS2thiolase-EGFP did not change localization of the mRFP-PEX7 variant, which cannot interact with PEX5L due to the E287R mutation (Fig. 5B). However, when the effect of PTS2-carrying cargo on subcellular localization of the mRFP-PEX7 variants harboring mutations in the cargo-binding site (E113R, E200R) was investigated, we found that overexpression of the PTS2-EGFP-induced peroxisomal transport of mRFP-PEX7 harboring the E200R mutation (Fig. 5D), but not the one harboring the E113R mutation (Fig. 5C). PTS2-less EGFP-SKL did not induce peroxisomal targeting of any mRFP-PEX7 variant (Fig. 5, F–H). These results suggest that peroxisomal transport of PEX7 is increased by high levels of PTS2-carrying cargo protein due to an induction of the interaction between PEX7 and the co-receptor PEX5L. Moreover, the results confirm that the absence of peroxisomal targeting of a PEX7 variant with severely reduced cargo-binding properties is overcome by overexpression of cargo protein, as suggested by the modified two-hybrid assay.
FIGURE 5.
Cargo binding induces transport of PEX7 to the peroxisomal surface. A–H, subcellular localization of PEX7 upon cargo binding: COS7 cells were co-transfected with mRFP-PEX7 (A and E), mRFP-PEX7E287R (B and F), mRFP-PEX7E113R (C and G), or mRFP-PEX7E200R (D and H) together with PTS2thiolase-EGFP (A, C, E, and G) or EGFP-SKL (B, D, F, and H) and the subcellular location of proteins was determined using the autofluorescence of mRFP (red) and EGFP (green).
DISCUSSION
In mammalian cells PTS2-harboring proteins are shuttled to the peroxisomal membrane by the concerted activity of the receptor PEX7 and the co-receptor PEX5L. In this article we identify the first point mutation in HsPEX7 that interferes with PEX5L binding and demonstrate that PEX5L binding is required to stabilize the interaction between PEX7 and PTS2-carrying proteins. This extends the previously described function of PEX5L in transporting PEX7-cargo complexes to the peroxisomal surface. Because cargo binding has to precede the interaction between PEX7 and PEX5L, a defined sequence of interactions governs the peroxisomal import of PTS2-carrying proteins and avoids peroxisomal transport of cargo-free PEX7 (futile cycles).
The cone-like structure of PEX7 is shaped by the characteristic WD-40 motifs, whereupon its top side appears enriched in evolutionary conserved residues. These conserved residues are involved in the formation of the binding groove for PTS2 helices, but also of the binding site for co-receptors such as PEX5L in mammals. We demonstrate that the latter interaction is abrogated upon charge inversion at a conserved glutamate residue of human PEX7 (E287R) that has been previously described on the top side of PEX7 next to glutamate 113 (Glu113) and glutamate 200 (Glu200), which are involved in cargo binding (10). Based on the observation that this mutation also reduces the interaction of PEX7 with a PTS2-carrying reporter protein, we hypothesized that binding of endogenous PEX5L strengthens the interaction between PEX7 and the cargo. Thus, we investigated the stabilizing effect of the co-receptor PEX5L on the receptor-cargo dimer using a modified variant of the mammalian two-hybrid assay, in which the stabilizing effect of an ectopically expressed third binding partner on the interaction strength between bait and prey is measured. We found that the apparent interaction strength between PEX7 and a cargo protein was drastically increased (about 20-fold) upon ectopic expression of PEX5L, when using the change in relative luciferase activity as measure of the interaction strength. This effect was not observed in the PEX7 variant that cannot interact with PEX5L (PEX7E287R), and even a dominant negative effect was observed that probably arises from the sequestration of the bait protein (GAL4DBD-PTS2-EGFP) by the concerted action of endogenous PEX7 and the ectopically expressed PEX5L. These results suggest that the basic interaction between PTS2-carrying cargo protein and the receptor PEX7 is drastically strengthened by the formation of a trimeric complex with PEX5L, but would also be compatible with other mechanisms by which PEX5L stabilizes the dimer. PEX5L could either induce a conformational change in PEX7 that tightens the grip of the binding groove around the PTS2-helix, or it could close the binding groove of PEX7 without a specific interaction with individual residues of the PTS2-helix, but physically prohibiting its release. Finally, PEX5L could engage in direct interactions with the PTS2-helix rendering PEX5L a part of a bipartite receptor model together with PEX7. The latter model appears less likely considering the low conservation of physical properties at those positions within naturally occurring PTS2 motifs that are averted from PEX7 and their insensitivity to mutations at these positions (10). However, the recently elucidated structure of a similar trimeric complex in yeast consisting of the PTS2-carrying protein Fox3p (thiolase), Pex7p, and parts of the co-receptor Pex21p suggests a direct interaction between individual residues of Pex21p and the PTS2-helix (16). However, this might be specific for yeast, because in this organism thiolase represents the nearly exclusive substrate for the PTS2-dependent import pathway.
A model of sequential binding, in which PEX5L binding succeeds the primary interaction between PEX7 and its PTS2-carrying cargo is corroborated by our investigation of the interaction between PEX7 and PEX5L and its dependence on cargo binding. We demonstrate that PEX7 variants that hardly bind cargo (PEX7E113R or PEX7E200R) show no detectable interaction with PEX5L, which indicates that binding of endogenous PTS2-carrying proteins contributes to the basic interaction between PEX7 and PEX5L. Furthermore, it suggests that cargo binding is a prerequisite for the formation of the PEX7·PEX5L complex and renders a cargo-less dimer not plausible. Conversely, the ectopic expression of cargo protein drastically increases the apparent interaction strength between PEX7 and PEX5L in the modified version of the mammalian two-hybrid assay, which supports the hypothesis of an exceedingly stable trimer compared with the dimeric forms. A similar observation was reported in vitro for the corresponding trimeric protein complex in the yeast S. cerevisiae, which appears drastically more stable than the dimeric complexes, when mixtures of purified proteins were analyzed by pulldown experiments (16). Moreover, in yeast cells lacking the predominant PTS2-carrying protein (Fox3p), less Pex7p was bound to the co-receptor Pex18p indicating that cargo binding supports the interaction between receptor and co-receptor (36). Altogether, the interaction between PEX7 and its cargo is stabilized upon ectopic expression of co-receptor PEX5L and the interaction between PEX7 and PEX5L is stabilized upon ectopic expression of a prototypical cargo protein. However, it needs to be stressed that the sequence of binding events cannot be delineated from the relative stability of the di- and trimeric complexes, but an analysis of the effects caused by mutations in PEX7 allows some hypotheses. The mutation E287R only reduces the apparent interaction strength between this PEX7 variant and cargo proteins, but prohibits co-receptor binding. Accordingly, a dominant negative effect of PEX7E287R on the import of PTS2-EGFP into peroxisomes is not expected, because the competitive binding of a cargo protein results only in the formation of an instable dimer, but cannot effectively sequester PTS2-EGFP. However, a direct stabilizing effect of PEX5L on the receptor-cargo protein interaction is demonstrated by the ectopic expression of the co-receptor, which does not act on PEX7E287R. Furthermore, a loss of the cargo-receptor interaction due to mutations in the PTS2-binding site cannot be compensated by overexpression of PEX5L suggesting that in the absence of primary cargo binding the stabilizing effect of PEX5L cannot be observed (Fig. 3C). However, when the apparent interaction strength between PEX7 and PEX5L has been reduced to the background level due to mutations in the PTS2-binding groove of PEX7 (E113R, E200R) this interaction can be restored to a certain extent upon ectopic overexpression of cargo protein (Fig. 4A). On the one hand this demonstrates that these PEX7 variants are properly folded for interaction with cargo protein and with the co-receptor, but on the other hand it demonstrates that the effect of a mutation in the PTS2-binding groove of PEX7 can be compensated by a high level of cargo protein. However, the compensatory effect of ectopic cargo expression is dose dependent and less effective for PEX7E113R, which reflects the lower affinity of this variant for PTS2-EGFP. This result is in agreement with a model, in which cargo binding is the rate-limiting step in the formation of the trimeric complex. If the individual mutations in the binding groove drastically reduce the affinity of these PEX7 variants for cargo proteins, the formation rate of dimeric complexes is drastically reduced and PEX5L cannot bind to cargo-less PEX7. However, drastic overexpression of cargo protein facilitates the formation of a sufficiently high cargo-receptor complex level to allow the fixation in a trimeric complex by PEX5L. This model is also corroborated by the investigation of the stabilizing effects exerted by a cargo protein variant harboring the HS3E mutation in the PTS2 on the interaction between PEX5L and PEX7 variants. Thereby, we observed that the fitting between cargo and receptor is predictive for the apparent interaction strength between PEX7 and PEX5L.
Thus, in the course of PTS2-mediated peroxisomal protein import the sequential assembly of a highly stable, trimeric complex consisting of cargo protein, PEX7, and PEX5L is required for the peroxisomal transport of this complex (schematically depicted in Fig. 6). This model is reflected by our investigation of peroxisomal targeting of the receptor PEX7 using an N terminally tagged version that can be detected at peroxisomes. We demonstrate that cargo binding is necessary for peroxisomal transport, because PEX7 variants harboring a mutation in the cargo-binding groove are solely cytosolic. Furthermore, peroxisomal targeting of the receptor can be increased by ectopic expression of cargo protein, which can actually overcome a mutation in the signal-binding domain. The observation reflects the relative interaction strength between the PEX7 variant and PEX5L.
FIGURE 6.
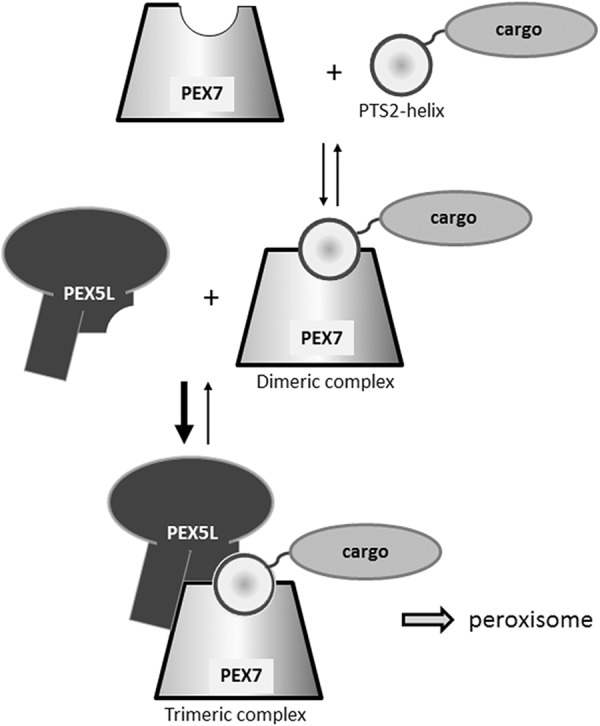
Hypothetical model of PTS2-mediated peroxisomal protein import. The primary interaction between a PTS2-carrying cargo protein and PEX7 is drastically stabilized by this incorporation into a stable trimeric complex with the co-receptor PEX5L. The latter subsequently mediates the transfer of the complex to the peroxisomal surface.
Our results are not compatible with a simplistic model, in which PTS2-carrying cargo proteins are just gathered by PEX7 and imported in a piggyback-like mechanism, because binding of the co-receptor drastically stabilizes the receptor·cargo complex and, thus, acts as a second step in cargo recognition. The necessity of a sequential assembly also prevents peroxisomal transport of cargo-less PEX7 and, thus, futile cycles of the receptor at the peroxisomal membrane. This assembly functionally corresponds to the conformational change in PEX5 upon cargo binding (37) that reflects the necessity of cargo binding for an efficient integration of PEX5 into the peroxisomal membrane (38). A similar mechanistic coupling between the recognition of a targeting signal and the transport of the receptor to the organellar destination is found in the co-translational transport of proteins destined for the endoplasmic reticulum or secretion. There, the signal peptide is recognized by the signal recognition particle, which interrupts translation and initiates the transport of the complex consisting of the ribosome, the signal recognition particle, and the signal peptide together with the mRNA to the docking site on the endoplasmic reticulum, SEC61 (2).
However, our findings also shed light on the second important step specific for PTS2-dependent protein import, namely the release of cargo inside peroxisomes. The dramatic difference in stability between dimeric and trimeric complexes implies that the disassembly of the complex and the release of the cargo within peroxisomes can be initiated by each of the interacting partners. Possible mechanisms involve processing of the N terminus of the cargo by the peroxisomal protease TYSND1, a conformational change in PEX5L resembling the conformational change upon release of PTS1-carrying cargo proteins, or any change in PEX7 that changes the interaction with the PTS2-helix or PEX5L. Altogether, our investigation provides detailed insight into the sequential assembly of PTS2-carrying proteins, PEX7 and PEX5L into a trimeric complex that is required for peroxisomal transport.
Acknowledgments
We thank Manuela Haberl for technical assistance, Bastian Hoesel for supporting two-hybrid luciferase measurements, Sonja Forss-Petter and Fabian Dorninger for critically reading the manuscript, Andreas Hartig and Petra Scholze for helpful discussions, and Marc Fransen for providing a plasmid.
This work was supported in part by the European Union Project “Peroxisomes” LSHG-C/2004-512018 and Austrian Science Fund (FWF) Projects P15510-B14 and P21950-B20.

This article contains supplemental Table S1.
We use the term apparent interaction strength to emphasize that the relative luciferase activity only correlates with the stability of the transcriptionally active nuclear complex consisting of bait and prey proteins and thus reflects the actual interaction strength between the tested proteins only indirectly.
- PTS
- peroxisomal targeting signal
- RCDP1
- rhizomelic chondrodysplasia punctata type 1
- EGFP
- enhanced green fluorescent protein
- UAS
- upstream activating sequence
- RFP
- red fluorescent protein.
REFERENCES
- 1. Blobel G., Dobberstein B. (1975) Transfer of proteins across membranes: I. presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J. Cell Biol. 67, 835–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Akopian D., Shen K., Zhang X., Shan S. O. (2013) Signal recognition particle: an essential protein-targeting machine. Annu. Rev. Biochem. 82, 693–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chacinska A., Koehler C. M., Milenkovic D., Lithgow T., Pfanner N. (2009) Importing mitochondrial proteins: machineries and mechanisms. Cell 138, 628–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brocard C., Hartig A. (2006) Peroxisome targeting signal 1: is it really a simple tripeptide? Biochim. Biophys. Acta 1763, 1565–1573 [DOI] [PubMed] [Google Scholar]
- 5. Wanders R. J., Waterham H. R. (2006) Biochemistry of mammalian peroxisomes revisited. Annu. Rev. Biochem. 75, 295–332 [DOI] [PubMed] [Google Scholar]
- 6. Steinberg S. J., Dodt G., Raymond G. V., Braverman N. E., Moser A. B., Moser H. W. (2006) Peroxisome biogenesis disorders. Biochim. Biophys. Acta 1763, 1733–1748 [DOI] [PubMed] [Google Scholar]
- 7. Wanders R. J., Waterham H. R. (2006) Peroxisomal disorders: the single peroxisomal enzyme deficiencies. Biochim. Biophys. Acta 1763, 1707–1720 [DOI] [PubMed] [Google Scholar]
- 8. Reumann S. (2004) Specification of the peroxisome targeting signals type 1 and type 2 of plant peroxisomes by bioinformatics analyses. Plant Physiol. 135, 783–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Glover J. R., Andrews D. W., Subramani S., Rachubinski R. A. (1994) Mutagenesis of the amino targeting signal of Saccharomyces cerevisiae 3-ketoacyl-CoA thiolase reveals conserved amino acids required for import into peroxisomes in vivo. J. Biol. Chem. 269, 7558–7563 [PubMed] [Google Scholar]
- 10. Kunze M., Neuberger G., Maurer-Stroh S., Ma J., Eck T., Braverman N., Schmid J. A., Eisenhaber F., Berger J. (2011) Structural requirements for interaction of peroxisomal targeting signal 2 and its receptor PEX7. J. Biol. Chem. 286, 45048–45062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Petriv O. I., Tang L., Titorenko V. I., Rachubinski R. A. (2004) A new definition for the consensus sequence of the peroxisome targeting signal type 2. J. Mol. Biol. 341, 119–134 [DOI] [PubMed] [Google Scholar]
- 12. van der Voorn L., Ploegh H. L. (1992) The WD-40 repeat. FEBS Lett. 307, 131–134 [DOI] [PubMed] [Google Scholar]
- 13. Braverman N., Steel G., Obie C., Moser A., Moser H., Gould S. J., Valle D. (1997) Human PEX7 encodes the peroxisomal PTS2 receptor and is responsible for rhizomelic chondrodysplasia punctata. Nat. Genet. 15, 369–376 [DOI] [PubMed] [Google Scholar]
- 14. Braverman N., Chen L., Lin P., Obie C., Steel G., Douglas P., Chakraborty P. K., Clarke J. T., Boneh A., Moser A., Moser H., Valle D. (2002) Mutation analysis of PEX7 in 60 probands with rhizomelic chondrodysplasia punctata and functional correlations of genotype with phenotype. Hum. Mutat. 20, 284–297 [DOI] [PubMed] [Google Scholar]
- 15. Stanley W. A., Fodor K., Marti-Renom M. A., Schliebs W., Wilmanns M. (2007) Protein translocation into peroxisomes by ring-shaped import receptors. FEBS Lett. 581, 4795–4802 [DOI] [PubMed] [Google Scholar]
- 16. Pan D., Nakatsu T., Kato H. (2013) Crystal structure of peroxisomal targeting signal-2 bound to its receptor complex Pex7p-Pex21p. Nat. Struct. Mol. Biol. 20, 987–993 [DOI] [PubMed] [Google Scholar]
- 17. Dodt G., Warren D., Becker E., Rehling P., Gould S. J. (2001) Domain mapping of human PEX5 reveals functional and structural similarities to Saccharomyces cerevisiae Pex18p and Pex21p. J. Biol. Chem. 276, 41769–41781 [DOI] [PubMed] [Google Scholar]
- 18. Matsumura T., Otera H., Fujiki Y. (2000) Disruption of the interaction of the longer isoform of Pex5p, Pex5pL, with Pex7p abolishes peroxisome targeting signal type 2 protein import in mammals: study with a novel Pex5-impaired Chinese hamster ovary cell mutant. J. Biol. Chem. 275, 21715–21721 [DOI] [PubMed] [Google Scholar]
- 19. Woodward A. W., Bartel B. (2005) The Arabidopsis peroxisomal targeting signal type 2 receptor PEX7 is necessary for peroxisome function and dependent on PEX5. Mol. Biol. Cell 16, 573–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Titorenko V. I., Smith J. J., Szilard R. K., Rachubinski R. A. (1998) Pex20p of the yeast Yarrowia lipolytica is required for the oligomerization of thiolase in the cytosol and for its targeting to the peroxisome. J. Cell Biol. 142, 403–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Purdue P. E., Yang X., Lazarow P. B. (1998) Pex18p and Pex21p, a novel pair of related peroxins essential for peroxisomal targeting by the PTS2 pathway. J. Cell Biol. 143, 1859–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Einwächter H., Sowinski S., Kunau W. H., Schliebs W. (2001) Yarrowia lipolytica Pex20p, Saccharomyces cerevisiae Pex18p/Pex21p and mammalian Pex5pL fulfil a common function in the early steps of the peroxisomal PTS2 import pathway. EMBO Rep 2, 1035–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schliebs W., Kunau W. H. (2006) PTS2 co-receptors: diverse proteins with common features. Biochim. Biophys. Acta 1763, 1605–1612 [DOI] [PubMed] [Google Scholar]
- 24. Lee J. R., Jang H. H., Park J. H., Jung J. H., Lee S. S., Park S. K., Chi Y. H., Moon J. C., Lee Y. M., Kim S. Y., Kim J. Y., Yun D. J., Cho M. J., Lee K. O., Lee S. Y. (2006) Cloning of two splice variants of the rice PTS1 receptor, OsPex5pL and OsPex5pS, and their functional characterization using pex5-deficient yeast and Arabidopsis. Plant J. 47, 457–466 [DOI] [PubMed] [Google Scholar]
- 25. Kerssen D., Hambruch E., Klaas W., Platta H. W., de Kruijff B., Erdmann R., Kunau W. H., Schliebs W. (2006) Membrane association of the cycling peroxisome import receptor Pex5p. J. Biol. Chem. 281, 27003–27015 [DOI] [PubMed] [Google Scholar]
- 26. Otera H., Setoguchi K., Hamasaki M., Kumashiro T., Shimizu N., Fujiki Y. (2002) Peroxisomal targeting signal receptor Pex5p interacts with cargoes and import machinery components in a spatiotemporally differentiated manner: conserved Pex5p WXXXF/Y motifs are critical for matrix protein import. Mol. Cell. Biol. 22, 1639–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grou C. P., Carvalho A. F., Pinto M. P., Wiese S., Piechura H., Meyer H. E., Warscheid B., Sá-Miranda C., Azevedo J. E. (2008) Members of the E2D (UbcH5) family mediate the ubiquitination of the conserved cysteine of Pex5p, the peroxisomal import receptor. J. Biol. Chem. 283, 14190–14197 [DOI] [PubMed] [Google Scholar]
- 28. Liu X., Subramani S. (2013) Unique requirements for mono- and polyubiquitination of the peroxisomal targeting signal co-receptor, Pex20. J. Biol. Chem. 288, 7230–7240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Glover J. R., Andrews D. W., Rachubinski R. A. (1994) Saccharomyces cerevisiae peroxisomal thiolase is imported as a dimer. Proc. Natl. Acad. Sci. U.S.A. 91, 10541–10545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Islinger M., Li K. W., Seitz J., Völkl A., Lüers G. H. (2009) Hitchhiking of Cu/Zn superoxide dismutase to peroxisomes: evidence for a natural piggyback import mechanism in mammals. Traffic 10, 1711–1721 [DOI] [PubMed] [Google Scholar]
- 31. McNew J. A., Goodman J. M. (1994) An oligomeric protein is imported into peroxisomes in vivo. J. Cell Biol. 127, 1245–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Girzalsky W., Saffian D., Erdmann R. (2010) Peroxisomal protein translocation. Biochim. Biophys. Acta 1803, 724–731 [DOI] [PubMed] [Google Scholar]
- 33. Wiesinger C., Kunze M., Regelsberger G., Forss-Petter S., Berger J. (2013) Impaired very long-chain acyl-CoA β-oxidation in human X-linked adrenoleukodystrophy fibroblasts is a direct consequence of ABCD1 transporter dysfunction. J. Biol. Chem. 288, 19269–19279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sughra K., Birbach A., de Martin R., Schmid J. A. (2010) Interaction of the TNFR-receptor associated factor TRAF1 with IκB kinase-2 and TRAF2 indicates a regulatory function for NF-κB signaling. PLoS One 5, e12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ghys K., Fransen M., Mannaerts G. P., Van Veldhoven P. P. (2002) Functional studies on human Pex7p: subcellular localization and interaction with proteins containing a peroxisome-targeting signal type 2 and other peroxins. Biochem. J. 365, 41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Grunau S., Schliebs W., Linnepe R., Neufeld C., Cizmowski C., Reinartz B., Meyer H. E., Warscheid B., Girzalsky W., Erdmann R. (2009) Peroxisomal targeting of PTS2 pre-import complexes in the yeast Saccharomyces cerevisiae. Traffic 10, 451–460 [DOI] [PubMed] [Google Scholar]
- 37. Stanley W. A., Filipp F. V., Kursula P., Schüller N., Erdmann R., Schliebs W., Sattler M., Wilmanns M. (2006) Recognition of a functional peroxisome type 1 target by the dynamic import receptor pex5p. Mol. Cell 24, 653–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gouveia A. M., Guimarães C. P., Oliveira M. E., Sá-Miranda C., Azevedo J. E. (2003) Insertion of Pex5p into the peroxisomal membrane is cargo protein-dependent. J. Biol. Chem. 278, 4389–4392 [DOI] [PubMed] [Google Scholar]