Background: Zwitterionic polysaccharides activate CD4+ T cells via MHCII presentation.
Results: Zwitterionic polysaccharides induce clonal expansion of a subset of anti-inflammatory CD4+ T cells preferentially carrying zwitterionic CDR3 loops.
Conclusion: MHCII-presented polysaccharides are specifically recognized by CD4+ T cells with regulatory T cell activity.
Significance: Zwitterionic polysaccharides are the first non-peptide MHCII-dependent antigens identified that induce clonal T cell expansion.
Keywords: Glycobiology, Immunology, Lymphocyte, Major Histocompatibility Complex (MHC), Polysaccharide
Abstract
For 3 decades, the view of MHCII-dependent antigen presentation has been completely dominated by peptide antigens despite our 2004 discovery in which MHCII was shown to present processed fragments of zwitterionic capsular polysaccharides to T cells. Published findings further demonstrate that polysaccharide A (PSA) from the capsule of Bacteroides fragilis is a potent activator of CD4+ T cells and that these T cells have important biological functions, especially in the maintenance of immunological homeostasis. However, little is known about the nature of T cell recognition of the polysaccharide-MHCII complex or the phenotype of the resulting activated cells. Here, we use next-generation sequencing of the αβT cell receptor of CD4+ T cells from mice stimulated with PSA in comparison with protein antigen simulation and non-immunized controls and found that PSA immunization induced clonal expansion of a small subset of suppressive CD4+CD45RBlow effector/memory T cells. Moreover, the sequences of the complementarity-determining region 3 (CDR3) loop from top clones indicate a lack of specific variable β and joining region use and average CDR3 loop length. There was also a preference for a zwitterionic motif within the CDR3 loop sequences, aligning well with the known requirement for a similar motif within PSA to enable T cell activation. These data support a model in which PSA, and possibly other T cell-dependent polysaccharide antigens, elicits a clonal and therefore specific CD4+ T cell response often characterized by pairing dual-charged CDR3 loop sequences with dual-charged PSA.
Introduction
T cell activation via MHCII-mediated antigen presentation is a mature field in which precise mechanisms are established for MHCII binding of peptide antigens (1), and the fine structural details of many MHCII-peptide-T cell receptor (TCR)2 ternary complexes are well characterized (2). Peptide recognition is exquisitely specific and relies heavily upon clonal expansion of a very low-copy number of randomly generated TCR sequences within the naïve T cell repertoire. Pioneering work in T cell biology revealed that the precursor frequency for any given specificity can be as little as 20–200 copies in the entire body (3). As such, specific clonal expansion generated against a single antigen characteristically generates a small but readily detectable expansion of a limited subset of T cell clones.
In contrast, superantigens, such as the Staphylococcus enterotoxin family, are intact proteins that derive their name from the ability to cause broad nonspecific T cell activation (4). Indeed, up to 50% of all T cells in the body could potentially respond to a superantigen (4). The reason for this massive response is found in the mechanism of binding. Most superantigens associate with peptide-loaded MHCII molecules outside of the canonical peptide-binding groove. In addition, they associate with opposing TCR molecules via interactions with the germ line-encoded portions of specific variable β (Vβ) domains. As such, they cross-link the MHCII-TCR complex in a manner independent of the rearrangements localized in the complementarity-determining region (CDR) loops 1–3, in which antigen specificity is predominantly encoded.
Despite the wealth of information on these classical pathways, the mechanism underlying T cell activation by polysaccharides remains poorly understood. We first discovered the ability of MHCII to present processed fragments of polysaccharides to T cells for recognition and activation in 2004 (5), although the ability to stimulate T cells was first described in 1993 (6). The most important characteristic of these polysaccharide antigens is their zwitterionic nature. Every known T cell-dependent polysaccharide antigen identified to date, including the founding member polysaccharide A (PSA) from the capsule of Bacteroides fragilis, carries both positive and negative charges within the repeating unit structure (6–10). Upon neutralization of either charge, the antigen is rendered inactive (6, 11, 12) due in part to conformational changes that result in loss of MHCII binding (12). Interestingly, we have also found that the glycosylation pattern on MHCII directly impacts MHCII binding by PSA, likely through carbohydrate-carbohydrate interactions with conserved N-linked glycans on MHCII (13–15). Despite these advances in our understanding of MHCII-mediated presentation of these unusual antigens, almost nothing is known about their recognition by their cognate T cell.
Based on the levels of cytokine production and proliferation in T cell responses against PSA (16), it is reasonable to propose that T cell recognition of these “glycoantigens” is more like that of conventional peptide antigens than that of superantigens. However, the T cells that respond to PSA are highly anergic upon secondary exposure to the antigen (16, 17), preventing traditional screening approaches to isolate and characterize antigen-specific clones. This anergy is likely associated with the function of these responding cells, which have been demonstrated to suppress a host of inflammatory conditions in vivo (7, 18–20). A regulatory phenotype is consistent with published data on PSA and the inability to isolate clones because no T cell clones of established regulatory T cells have ever been reported. For this reason, the antigens for natural thymically derived regulatory T cells remain a mystery.
Here, we utilized next-generation sequencing to provide the first genetic-based characterization of the T cell response against the B. fragilis polysaccharide PSA. We found that immunization with PSA expanded a population of CD4+CD45RBlow T cells with an effector/memory surface phenotype (CD62LlowCD44high) and afforded mice robust protection from the induction of airway inflammation. Sequencing of all CDR3 loop sequences among the CD4+ T cell repertoire of immunized mice revealed clonal expansion of a limited number of clones in response to PSA and a conventional protein antigen (ovalbumin) compared with vehicle control naïve mice. Detailed informatic analysis revealed a lack of unusual CDR3 loop lengths among PSA-expanded clones and a lack of preferential Vβ and joining (J) segment use. In contrast, an unusual number of CDR3 loop sequences contained both positively and negatively charged residues, suggesting that recognition by the TCR may be driven in part by electrostatic interactions between MHCII-presented zwitterionic PSA and the opposing CDR3 loop. These data reveal that a polysaccharide antigen elicits a clonal and therefore specific T cell response that aligns with conventional peptide recognition and not superantigen cross-linking, thereby adding a new antigen class to the list of molecules capable of specific T cell recognition and induction of regulatory T cells.
EXPERIMENTAL PROCEDURES
Mice and Bacteria
Wild-type (WT) C57BL/6 breeding pairs were obtained from Jackson ImmunoResearch Laboratories and housed in specific pathogen-free (and B. fragilis-free) conditions according to guidelines established by the Institutional Animal Care and Use Committee of Case Western Reserve University (Cleveland, OH). Experimental mice were 7–12 weeks old. B. fragilis was grown in anaerobic conditions, and PSA was purified as described previously (5, 21). For all PSA exposures, mice were orally gavaged with PSA over 12 days (100 μg/dose in saline every 3 days). Negative controls utilized saline vehicle alone.
Airway Inflammation Model
Mice were sensitized to ovalbumin by intraperitoneal doses of 40 μg of ovalbumin in alum 7 days apart. Seven days following the second injection, the mice received intranasal ovalbumin (40 μg/dose in PBS; Sigma) for 6 consecutive days before being killed on day 7. For intranasal challenge, mice were anesthetized using a tabletop anesthesia system (VetEquip) with 3% isoflurane (Baxter). For T cell function, CD4+ splenocytes were purified with magnetic bead resin (Miltenyi Biotec) from the spleens of PSA- or mock saline-treated mice, and 2 × 106 T cells were transferred into ovalbumin-sensitized recipient mice 24 h prior to the beginning of intranasal ovalbumin challenges. Euthanasia was performed with a mixture of 8.6% ketamine (Fort Dodge Animal Health), 1.7% xylazine (AnaSed), and 2.9% acepromazine (Boehringer Ingelheim) in sterile saline. Mice were dosed at 0.006 ml/g. Mice were then tracheotomized, and lungs were flushed three times with 1 ml of PBS containing 0.6 mm EDTA. Cells from these washes were collected and resuspended in 50 μl of PBS with 0.6 mm EDTA, and differentials were performed by Cytospin and microscopic counting. Lungs were inflated with O.C.T. (Tissue-Tek) and fixed in 10% formalin prior to paraffin embedding and sectioning at the Case Western Reserve University Tissue Procurement and Histology Core Facility.
T Cell Flow Cytometry
CD4+ splenocytes were purified as described above and stained with CD4, CD25, CD45RB, CD62L, and CD44 (eBioscience). For in vitro stimulations, purified T cells were stimulated for 3 days with anti-CD3/CD28 resin (eBioscience). Cytokine levels were analyzed by standard sandwich ELISA (BioLegend). Flow cytometric analysis was performed on a BD Accuri C6 flow cytometer (BD Biosciences) using FCS Express software (De Novo Software).
Histology
H&E staining of lung sections was performed by the Case Western Reserve University Tissue Procurement and Histology Core Facility. Unstained slides were stained with antibodies specific for EpCAM (epithelial cell adhesion molecule; 6 μg/ml, eBioscience) and myeloperoxidase (1:100 dilution, Abcam; anti-rabbit secondary, 1:1,000 dilution, Invitrogen).
Deep Sequencing
CD4+ T cells (106) were isolated from the spleens of treated (ovalbumin and PSA) or untreated (saline) mice and sent to Adaptive Biotechnologies for RNA extraction and deep sequencing of the TCR β-chain using their proprietary assay platform, as reported elsewhere (22). Data analysis was performed using Adaptive Biotechologies immunoSEQ web tools and graphed using GraphPad Prism (version 5.0).
General Data Analyses
All data are shown as means ± S.E. Mice included a minimum of four animals per group per replicate experiment. Graphs and statistical measures were generated with GraphPad Prism (version 5.0). For comparisons between multiple groups, analysis of variance was used, whereas for comparisons between two groups (where appropriate), Student's t test was used.
RESULTS
PSA-responding T Cell Phenotype
WT C57BL/6 mice were orally immunized with 100 μg of PSA five times over 12 days to elicit a potent T cell response in vivo as reported previously (23). On day 15, CD4+ T cells were harvested from both the spleen and mesenteric lymph nodes and either re-stimulated with anti-CD3/CD28 beads or stained for flow cytometry to quantify the impact of PSA immunization. We found that the bulk CD4+ T cell population was skewed away from IFNγ production and toward IL-10 production compared with cells from saline-treated negative control mice (Fig. 1A). Flow cytometry further indicated that exposure to PSA expanded a population of CD45RBlow T cells, many of which also expressed the IL-2 receptor CD25, which is commonly found on regulatory T cells (Fig. 1B). No change in CD25 was seen in CD45RBhigh cells (Fig. 1, B and D). Finally, among the CD4+CD45RBlow T cells, the majority (66.5%) were CD62LlowCD44high effector/memory T cells (Fig. 1, C and D), demonstrating that the in vivo murine T cell response to PSA is dominated by IL-10 skewing through the expansion of CD4+CD45RBlowCD25+/− effector/memory T cells, which is consistent with our previous findings in humans (16).
FIGURE 1.

Mice were orally immunized with PSA. Following the last dose, CD4+ T cells were harvested from the spleen (SPL) and mesenteric lymph nodes (mLN) and either stained for surface markers or re-stimulated with anti-CD3/CD28 beads for 72 h. A, prior exposure PSA skews toward IL-10 and away from IFNγ production. B–D, PSA immunization leads to an expansion of CD4+CD45RBlow cells, which are primarily of the effector/memory (CD62LlowCD44high) subset. *, p < 0.02; **, p < 0.05; ***, p > 0.05. TEM cells, effector/memory T cells.
Deep Sequencing of αβTCR from PSA-expanded T Cells
To characterize the genetic nature of PSA recognition by the αβTCR of T cells, CD4+ T cells were isolated from the spleens of WT mice immunized as described above (Fig. 1) with either PSA or ovalbumin as a conventional antigen control and with saline as a negative/naïve control. Total RNA was isolated, a library for deep sequencing was created for each mouse, and targeted sequencing of the β-chain of the αβTCR was performed as described under “Experimental Procedures.” Table 1 provides the general statistics of the resulting sequencing data, including over 700,000 total and 65,000 unique (found at least once in that sample) productive (no stops) sequences per sample.
TABLE 1.
αβTCR deep sequencing data statistics
The number of sequences from TCR β-chain deep sequencing is reported, including the number of unique, productive, and unique productive sequences; sequences with stops; and unique sequences with stops found in the CD4+ T cell cohort from each immunized mouse reported herein.
| Naïve 1 | Naïve 2 | Ovalbumin 1 | Ovalbumin 2 | Ovalbumin 3 | Ovalbumin 4 | PSA 1 | PSA 2 | PSA 3 | |
|---|---|---|---|---|---|---|---|---|---|
| All sequences | 760,822 | 706,728 | 815,408 | 668,174 | 781,977 | 780,581 | 693,759 | 731,230 | 843,518 |
| Unique sequences | 66,451 | 67,785 | 69,433 | 66,732 | 64,843 | 60,728 | 63,745 | 69,838 | 67,605 |
| All productive sequences | 755,927 | 702,065 | 810,186 | 633,761 | 777,296 | 774,838 | 689,321 | 726,419 | 837,352 |
| Unique productive sequences | 65,909 | 67,234 | 68,857 | 66,131 | 64,313 | 60,234 | 63,209 | 69,264 | 66,989 |
| All sequences with stops | 4,895 | 4,663 | 5,222 | 4,413 | 4,681 | 5,743 | 4,438 | 4,811 | 6,166 |
| Unique sequences with stops | 542 | 551 | 576 | 601 | 530 | 494 | 536 | 574 | 616 |
Informatic analyses of the sequences revealed a lack of significant change in the number of αβTCRs carrying particular Vβ or J segments (Fig. 2), consistent with the notion that neither PSA nor ovalbumin activates T cells based solely upon germ line-encoded sequences. In addition, average CDR3 loop lengths across all sequences in PSA- and ovalbumin-immunized cohorts were indistinguishable from those in the naïve control cohort (Fig. 3), suggesting that although PSA is a much larger structure as presented by MHCII compared with ovalbumin (presented PSA = 5–10 kDa (5, 12), typical presented peptides = 1–2 kDa (1)), the CDR3 loop length is not atypical for conventional recognition.
FIGURE 2.
RNA was purified from CD4+ T cells of mice immunized with saline (A), ovalbumin (B), or PSA (C) and analyzed by deep sequencing of the TCR β-chain. Evaluation of the percentage of each clone with particular Vβ (left) and J (right) segments across all conditions revealed indistinguishable results in all cohorts.
FIGURE 3.
Analysis of the total (left) and unique (right) TCR β-chain CDR3 loop nucleotide sequences in CD4+ T cells from mice immunized with saline (A), ovalbumin (B), or PSA (C) demonstrated indistinguishable lengths among all sequenced clones.
PSA-driven T Cell Clonality
Despite our discovery that PSA (5, 11, 12) and other zwitterionic polysaccharides (9, 10) stimulate CD4+ T cells via presentation by MHCII, the degree to which the cognate T cells recognize the antigen specifically remains highly controversial. To better understand the clonality of the response against PSA, CDR3 loop sequences were compared between the three cohorts to identify those common to both naïve and PSA (or ovalbumin)-immunized mice, as well as those unique to each sample. In comparing the two naïve samples (naïve 1 versus naïve 2), we found 52,528 sequences unique to naïve 1, 54,718 sequences unique to naïve 2, and 13,527 sequences shared between both samples (Fig. 4A). When a naïve sample (naïve 1) was compared with an ovalbumin-immunized sample (ovalbumin 1), we found 55,830 sequences unique to naïve 1, 48,181 sequences unique to ovalbumin 1, and 12,415 sequences in common (Fig. 4B). Finally, when comparing naïve with PSA-immunized samples, we found 55,295 sequences unique to a naïve sample (naïve 1), 50,340 sequences unique to a PSA sample (PSA 1), and 12,950 sequences in common (Fig. 4C). Similar results were found with the other PSA and ovalbumin comparisons.
FIGURE 4.
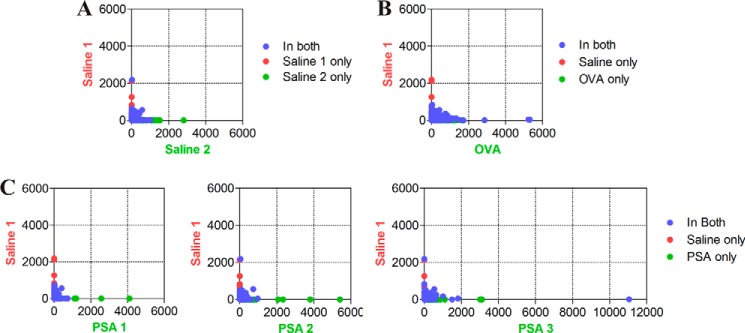
Two-dimensional representation of unique (green/red) and shared (blue) TCR β-chain CDR3 loop sequences among CD4+ T cells isolated from mice immunized with saline (A), ovalbumin (B), or PSA (C). The number of copies of each sequence is plotted on the axes to enable a facile approximation for the TCR β-chain sequence differences and similarities between a non-immunized sample (saline 1) and all other samples. OVA, ovalbumin.
To gain more insight into the most abundant clones, we evaluated the top 50 clones of the combined sequences of naïve, ovalbumin-immunized, and PSA-immunized cohorts. We found that for both ovalbumin- and PSA-immunized groups, a significant increase in a small number of clones was seen above the background of the naïve group (cutoff set at 0.2% of the total), strongly suggesting specific clonal expansion (Fig. 5). Translation of these expanded CDR3 loop sequences is consistent with the overall data, in which there was a lack of significant difference in total CDR3 loop length (Fig. 3).
FIGURE 5.

Analysis of the frequency (percent of total sequences) and amino acid sequence of each TCR β-chain CDR3 loop found in CD4+ T cells isolated from mice immunized with saline (red), ovalbumin (blue), or PSA (green). Those clones rising above the 0.2% background of the naïve controls are shown in color to highlight the degree of clonal expansion accompanying immunization. The top 50 clones for each cohort are plotted for clarity. OVA, ovalbumin.
Interestingly, among the top 50 clones, there was a significantly higher proportion of zwitterionic TCRβ CDR3 amino acid sequences containing both acidic (negatively charged) and basic (positively charged) residues in the PSA-immunized cohorts compared with both naïve and ovalbumin-immunized samples (Fig. 6 and Table 2). Given the zwitterionic nature of the PSA antigen, these data suggest that electrostatic bonds influence PSA recognition.
FIGURE 6.
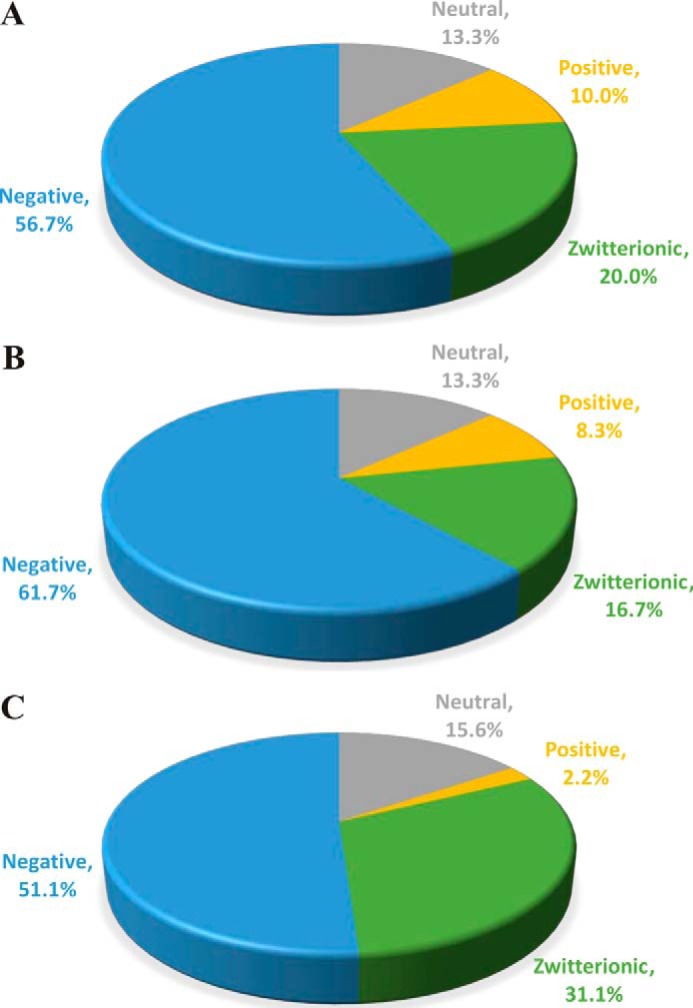
Charge analysis of the top 50 TCR β-chain CDR3 protein sequences in saline-immunized (A), ovalbumin-immunized (B), and PSA-immunized (C) cohorts plotted as a percentage of the top 50 CDR3 loop sequences with neutral (gray), acidic/negative (blue), basic/positive (yellow), and zwitterionic (green) motifs.
TABLE 2.
Zwitterionic TCRβ CDR3 Loops
Shown are the amino acid sequences of all zwitterionic TCRβ CDR3 loops found within the top 50 most frequent clones in each of the saline-, ovalbumin (OVA)-, or PSA-treated populations. Residues are marked with a (+) or (−) to indicate charge to illustrate the charge distribution within the loop.

PSA-expanded T Cells Inhibit Airway Inflammation
To demonstrate biological activity of PSA-specific clonally expanded T cells, WT mice were immunized with PSA as described above (Fig. 1). Splenic CD4+ T cells from these or mock (saline)-treated mice were harvested and adoptively transferred into ovalbumin-sensitized recipients. These mice were then given daily intranasal ovalbumin challenges to induce airway inflammation. On day 7, lungs were lavaged to assess leukocyte infiltration and sectioned for histopathology by H&E staining and confocal microscopy. We found that adoptive transfer of CD4+ T cells from PSA-treated mice robustly inhibited leukocyte infiltration into the airway compared with T cells from saline-treated mice (Fig. 7, A–D). Likewise, PSA-expanded T cells prevented detectable tissue pathology (Fig. 7E), including the infiltration of activated myeloperoxidase-positive leukocytes in and around the airways (Fig. 7F). These data show the suppressive capacity of clonally expanded PSA-responding CD4+ T cells and collectively establish that a clonally expanded antimicrobial polysaccharide T cell response can play important roles in the maintenance of immune homeostasis.
FIGURE 7.
CD4+ T cells from saline- or PSA-immunized mice were adoptively transferred into recipient mice 24 h prior to the induction of airway inflammation via intranasal ovalbumin (i.n. OVA) challenge. Lung lavage isolates of each mouse were analyzed for total white blood cell (WBC) (A), polymorphonuclear cell (PMN) (B), lymphocyte (Lympho) (C), and eosinophil (Eos) (D) infiltration into the airway space. Lungs were also sectioned for histopathology by H&E staining (E) and confocal microscopy (F), revealing the protective efficacy of PSA-responding T cells. Red, myeloperoxidase; green, EpCAM.
DISCUSSION
For over 2 decades, it has been known that zwitterionic polysaccharide glycoantigens like PSA from the commensal organism B. fragilis can activate CD4+ T cells (6, 24). Likewise, it has been known for a decade that this T cell activation is dependent upon processing of these molecules to small fragments (5–10 kDa) by nitric oxide-mediated oxidation and presentation by MHCII molecules (5, 9, 25). Although the mechanisms underlying MHCII binding and presentation have been the subject of intense focus (5, 9–12, 25, 26), the nature of T cell recognition remained in the background of the phenomenology of the response as a whole. For example, PSA-responding T cells have the capacity to prevent abscess and adhesion formation (19, 23) and experimental inflammatory bowel disease (18).
A complicating factor in understanding the nature of T cell recognition of PSA has been the failure to clone PSA-specific T cells. Our studies with human cells revealed that PSA-responding T cells become highly anergic upon re-stimulation with PSA and that this anergy is not fully broken by the addition of growth factors such as IL-2 (16). Moreover, fusion of murine T cells from PSA-immunized mice also results in hybridomas that fail to proliferate, thus preventing cloning and the establishment of a PSA-specific clonal cell line (data not shown).
In this study, we utilized deep RNA sequencing of the TCRβ locus in isolated CD4+ T cells from naïve, PSA-immunized, or control (ovalbumin)-immunized mice to provide the first glimpse of the αβTCRs expanded as a result of PSA exposure. TCRβ was used because expression of a single TCRβ allele prevents expression of the other TCRβ allele within the same cell, yet TCRα does not show this same allelic exclusion. The result is that any mature T cell will express exactly one TCRβ allele, but can conceivably express more than one TCRα allele, thereby making clonality based on TCRα sequencing ambiguous.
We found that PSA induces a clonal expansion of a limited subset of T cells, which mirrors that seen with a conventional antigen. The response is characterized by average TCRβ CDR3 loop length and a lack of particular Vβ and J region use. A hallmark difference between nonspecific recognition of superantigens and the specific recognition of conventional peptide antigens is the use and distribution of Vβ segments in the responding T cell repertoire. Superantigens cross-link MHCII and Vβ domains in a manner independent of CDR loop rearrangements. As a result, these antigens activate essentially all T cells with an αβTCR containing specific Vβ domains. In contrast, conventional antigen recognition is driven by the CDR rearrangements and not specific Vβ or J domains. Given the lack of particular Vβ and J region use in response to PSA, our findings support the conclusion that recognition is driven by antigen-specific interactions, not by the nonspecific cross-linking seen with superantigens.
The T cell response also shows a propensity to generate zwitterionic CDR3 loop sequences, at least within the TCR β-chain. This is intriguing because the impact of PSA charge neutralization on PSA-mediated T cell activation has been known for some time (6, 20). Our work has shown that the loss of charges on PSA alters its conformation and significantly reduces interactions with MHCII (12). In fact, the defining characteristic of the PSA family of T cell-dependent antigens is the presence of an alternating charge motif within the polysaccharide repeating unit (5, 6, 8–10, 12, 20, 24). The correlation between the recognition of a zwitterionic antigen and a propensity for zwitterionic TCRβ CDR3 loop sequences raises the possibility that electrostatic interactions are a key feature in the recognition of these antigens by T cells, although further studies are needed to analyze the TCRα CDR3 loop sequences to determine what additional patterns may be critical for this response.
Finally, we have demonstrated that the clonally expanded population of T cells in PSA-immunized mice can robustly prevent the onset of inflammation in the lung. This finding is consistent with the anti-inflammatory activity of PSA (18, 19, 23). Because PSA is derived from a commensal organism, the ability to help maintain immune homeostasis via T cell expansion and skewing aligns our findings with the growing evidence for the protective efficacy of the microbiota in disease (27). Moreover, the apparent specificity of PSA recognition suggests that the anti-inflammatory activity of these T cells is characteristic of so-called bystander suppression, in which the regulatory T cell inhibits the activation of any other T cell regardless of its cognate antigen. This is supported by our findings with human cells, which suppress other T cell responses in vitro via IL-10 secretion (16).
In summary, we have demonstrated that the zwitterionic polysaccharide PSA induces a specific clonal population of CD4+CD45RBlow effector/memory cells with in vivo regulatory activity characterized by IL-10-skewed cytokine production and protection of airway inflammation. PSA is therefore the first non-peptide MHCII-dependent antigen identified that is specifically recognized by CD4+ T cells, thereby expanding the paradigm of T cell biology to include polysaccharide antigens.
Acknowledgments
We thank Drs. Elizabeth Pierce and Tracey Bonfield for assistance with the murine airway inflammation model and Lori S. C. Kreisman for critical reading and assistance in the preparation of this manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants GM082916 and OD004225 (to B. A. C.). This work was also supported by American Asthma Foundation Grant 10-0187 (to B. A. C.).
- TCR
- T cell receptor
- Vβ
- variable β
- J
- joining
- CDR
- complementarity-determining region
- PSA
- polysaccharide A.
REFERENCES
- 1. Neefjes J., Jongsma M. L., Paul P., Bakke O. (2011) Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat. Rev. Immunol. 11, 823–836 [DOI] [PubMed] [Google Scholar]
- 2. Turner S. J., Doherty P. C., McCluskey J., Rossjohn J. (2006) Structural determinants of T-cell receptor bias in immunity. Nat. Rev. Immunol. 6, 883–894 [DOI] [PubMed] [Google Scholar]
- 3. Moon J. J., Chu H. H., Pepper M., McSorley S. J., Jameson S. C., Kedl R. M., Jenkins M. K. (2007) Naive CD4+ T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity 27, 203–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Choi Y. W., Kotzin B., Herron L., Callahan J., Marrack P., Kappler J. (1989) Interaction of Staphylococcus aureus toxin “superantigens” with human T cells. Proc. Natl. Acad. Sci. U.S.A. 86, 8941–8945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cobb B. A., Wang Q., Tzianabos A. O., Kasper D. L. (2004) Polysaccharide processing and presentation by the MHCII pathway. Cell 117, 677–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tzianabos A. O., Onderdonk A. B., Rosner B., Cisneros R. L., Kasper D. L. (1993) Structural features of polysaccharides that induce intra-abdominal abscesses. Science 262, 416–419 [DOI] [PubMed] [Google Scholar]
- 7. Ruiz-Perez B., Chung D. R., Sharpe A. H., Yagita H., Kalka-Moll W. M., Sayegh M. H., Kasper D. L., Tzianabos A. O. (2005) Modulation of surgical fibrosis by microbial zwitterionic polysaccharides. Proc. Natl. Acad. Sci. U.S.A. 102, 16753–16758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tzianabos A. O., Wang J. Y., Lee J. C. (2001) Structural rationale for the modulation of abscess formation by Staphylococcus aureus capsular polysaccharides. Proc. Natl. Acad. Sci. U.S.A. 98, 9365–9370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Velez C. D., Lewis C. J., Kasper D. L., Cobb B. A. (2009) Type I Streptococcus pneumoniae carbohydrate utilizes a nitric oxide and MHC II-dependent pathway for antigen presentation. Immunology 127, 73–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Young N. M., Kreisman L. S., Stupak J., MacLean L. L., Cobb B. A., Richards J. C. (2011) Structural characterization and MHCII-dependent immunological properties of the zwitterionic O-chain antigen of Morganella morganii. Glycobiology 21, 1266–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cobb B. A., Kasper D. L. (2008) Characteristics of carbohydrate antigen binding to the presentation protein HLA-DR. Glycobiology 18, 707–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kreisman L. S., Friedman J. H., Neaga A., Cobb B. A. (2007) Structure and function relations with a T-cell-activating polysaccharide antigen using circular dichroism. Glycobiology 17, 46–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ryan S. O., Leal S. M., Jr., Abbott D. W., Pearlman E., Cobb B. A. (2014) Mgat2 ablation in the myeloid lineage leads to defective glycoantigen T cell responses. Glycobiology 24, 262–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ryan S. O., Bonomo J. A., Zhao F., Cobb B. A. (2011) MHCII glycosylation modulates Bacteroides fragilis carbohydrate antigen presentation. J. Exp. Med. 208, 1041–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ryan S. O., Cobb B. A. (2012) Roles for major histocompatibility complex glycosylation in immune function. Semin. Immunopathol. 34, 425–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kreisman L. S., Cobb B. A. (2011) Glycoantigens induce human peripheral Tr1 cell differentiation with gut-homing specialization. J. Biol. Chem. 286, 8810–8818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stingele F., Corthésy B., Kusy N., Porcelli S. A., Kasper D. L., Tzianabos A. O. (2004) Zwitterionic polysaccharides stimulate T cells with no preferential Vβ usage and promote anergy, resulting in protection against experimental abscess formation. J. Immunol. 172, 1483–1490 [DOI] [PubMed] [Google Scholar]
- 18. Mazmanian S. K., Round J. L., Kasper D. L. (2008) A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 453, 620–625 [DOI] [PubMed] [Google Scholar]
- 19. Tzianabos A. O., Gibson F. C., 3rd, Cisneros R. L., Kasper D. L. (1998) Protection against experimental intraabdominal sepsis by two polysaccharide immunomodulators. J. Infect. Dis. 178, 200–206 [DOI] [PubMed] [Google Scholar]
- 20. Tzianabos A. O., Onderdonk A. B., Zaleznik D. F., Smith R. S., Kasper D. L. (1994) Structural characteristics of polysaccharides that induce protection against intra-abdominal abscess formation. Infect. Immun. 62, 4881–4886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tzianabos A. O., Pantosti A., Baumann H., Brisson J. R., Jennings H. J., Kasper D. L. (1992) The capsular polysaccharide of Bacteroides fragilis comprises two ionically linked polysaccharides. J. Biol. Chem. 267, 18230–18235 [PubMed] [Google Scholar]
- 22. Marrero I., Hamm D. E., Davies J. D. (2013) High-throughput sequencing of islet-infiltrating memory CD4+ T cells reveals a similar pattern of TCR Vβ usage in prediabetic and diabetic NOD mice. PLoS ONE 8, e76546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tzianabos A. O., Kasper D. L., Cisneros R. L., Smith R. S., Onderdonk A. B. (1995) Polysaccharide-mediated protection against abscess formation in experimental intra-abdominal sepsis. J. Clin. Invest. 96, 2727–2731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brubaker J. O., Li Q., Tzianabos A. O., Kasper D. L., Finberg R. W. (1999) Mitogenic activity of purified capsular polysaccharide A from Bacteroides fragilis: differential stimulatory effect on mouse and rat lymphocytes in vitro. J. Immunol. 162, 2235–2242 [PubMed] [Google Scholar]
- 25. Lewis C. J., Cobb B. A. (2011) Adaptive immune defects against glycoantigens in chronic granulomatous disease via dysregulated nitric oxide production. Eur. J. Immunol. 41, 2562–2572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang Q., McLoughlin R. M., Cobb B. A., Charrel-Dennis M., Zaleski K. J., Golenbock D., Tzianabos A. O., Kasper D. L. (2006) A bacterial carbohydrate links innate and adaptive responses through Toll-like receptor 2. J. Exp. Med. 203, 2853–2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Blaser M. J. (2014) The microbiome revolution. J. Clin. Invest. 124, 4162–4165 [DOI] [PMC free article] [PubMed] [Google Scholar]





