Background: The glycosaminoglycan of bikunin is less than 40 monosaccharides in length, but it can reversibly accept two heavy chains (HCs).
Results: TSG-6 can reversibly transfer a single HC to the glycosaminoglycan of bikunin.
Conclusion: The core protein, or sulfated glycosaminoglycan, of bikunin promotes reversible HC transfer to its relatively short CS chain.
Significance: The intracellular assembly of inter-α-inhibitor is likely independent of transesterification by TSG-6.
Keywords: Chondroitin Sulfate, Enzyme, Glycosaminoglycan, Hyaluronan, Proteoglycan
Abstract
We present data that hyaluronan (HA) polysaccharides, about 14–86 monosaccharides in length, are capable of accepting only a single heavy chain (HC) from inter-α-inhibitor via transfer by tumor necrosis factor-stimulated gene 6 (TSG-6) and that this transfer is irreversible. We propose that either the sulfate groups (or the sulfation pattern) at the reducing end of the chondroitin sulfate (CS) chain of bikunin, or the core protein itself, enables the bikunin proteoglycan (PG) to accept more than a single HC and permits TSG-6 to transfer these HCs from its relatively small CS chain to HA. To test these hypotheses, we investigated HC transfer to the intact CS chain of the bikunin PG, and to the free chain of bikunin. We observed that both the free CS chain and the intact bikunin PG were only able to accept a single HC from inter-α-inhibitor via transfer by TSG-6 and that HCs could be swapped from the bikunin PG and its free CS chain to HA. Furthermore, a significant portion of the bikunin PG was unable to accept a single heavy chain. We discuss explanations for these observations, including the intracellular assembly of inter-α-inhibitor. In summary, these data demonstrate that the sulfation of the CS chain of bikunin and/or its core protein promote HC transfer by TSG-6 to its relatively short CS chain, although they are insufficient to enable the CS chain of bikunin to accept more than one HC in the absence of other cofactors.
Introduction
Bikunin is a proteoglycan (PG)2 that has a single chondroitin sulfate (CS) glycosaminoglycan (GAG) chain with 27–39 monosaccharides attached to Ser-10 (1). The structure of this CS chain has been well characterized (2) and possesses a remarkably conserved sulfation pattern in the apparent absence of an instructive template. It has a sulfated domain on the reducing terminus and a non-sulfated domain on the non-reducing terminus. The sulfated domain consists of 12–18 monosaccharides, whereas the non-sulfated domain contains 6–22 monosaccharides, and the sulfation is exclusively at the fourth carbon of its GalNAc residues. During the synthesis of bikunin by hepatocytes, C-terminal aspartate residues of ∼83-kDa proteins, known as “heavy chains” (HC1, HC2), are covalently linked to the sixth carbon of a GlcUA residue on the non-sulfated region of the CS chain, forming what is referred to as a “pre-inter-α-inhibitor” (pre-ΙαΙ) with one HC. Despite the relatively short (6–22 monosaccharides) unsulfated portion of the CS chain, a second HC can be attached to form the full-length ΙαΙ molecule comprised of the 16-kDa bikunin protein “light chain,” with two HCs covalently attached to the unsulfated region on the non-reducing end of the CS chain of bikunin.
During inflammation and developmental processes, the enzyme tumor necrosis factor-stimulated gene 6 (TSG-6) forms a covalent TSG-6-HC and subsequently transfers the HC to the sixth carbon of GlcNAc residues of hyaluronan (HA) to form HC-HA complexes in a novel transesterification reaction that effectively cross-links HA matrices (3) and promotes leukocyte adhesion (4–7). As many as five HCs have been found covalently attached to a single HA molecule 2 kDa in size (8), although the maximum number of HCs that high molecular weight (HMW) HA can accept is still unknown.
We and others have observed that HA oligosaccharides, ranging from 8–22 monosaccharides in length, can accept only a single HC (9, 10). This is interesting considering that the unsulfated portion of the CS chain of bikunin ranges from 6–22 monosaccharides in length, but it is able to accept two HCs. In this manuscript, we present data showing that HA molecules, containing 14–86 monosaccharides, are also only able to accept a single HC. Furthermore, HC transfer to these HA molecules is an irreversible event from which subsequent swapping of HCs do not occur. From these observations, we hypothesized that either the sulfate groups at the reducing terminus of the CS chain of bikunin and/or the bikunin core protein itself enable(s) its unsulfated non-reducing terminus to accept more than a single HC and promote subsequent HC transfer to HA. To test these hypotheses, we performed a series of experiments examining TSG-6-mediated HC transfer to the free CS chain of bikunin and to the intact CS chain on the bikunin PG.
EXPERIMENTAL PROCEDURES
Reagents
Mouse serum, used as our source of ΙαΙ, was purchased from Sigma-Aldrich (catalog no. S7273, St. Louis, MO). Human urine was obtained from non-diseased (control) subjects as described previously and with Cleveland Clinic Institutional Review Board approval and written informed consent (11). Recombinant human TSG-6 was purchased from R&D Systems (catalog no. 2014-TS, Minneapolis, MN) and resuspended at 0.005 mg/ml. An HA oligosaccharide, 14 monosaccharides in length, was provided by Seikagaku Corp. (Japan). Monodispersed HA molecules (3–16 kDa) were provided by Paul DeAngelis (University of Oklahoma Health Sciences Center, Oklahoma City, OK). Select HA, with a monodisperse MW of 1000K, was purchased from Hyalose (Oklahoma City, OK). HMW sodium hyaluronate at 1.7 kDa was purchased from LifeCore Biomedical (catalog no. 80190, Chaska, MN). Plasmas from TSG-6+/+, TSG-6+/−, and TSG-6−/− mice were provided by Georgiana Cheng (Cleveland Clinic, Cleveland, OH). All other chemicals and reagents, unless noted otherwise, were purchased from Sigma-Aldrich.
Bikunin Proteoglycan Preparation
The preparation of the bikunin PG and its CS chain has been described previously (1). Briefly, pharmaceutical-grade bikunin PG (Mochida Pharmaceuticals, Tokyo, Japan) was purified from excipients and buffer salts by dialysis against distilled water using 30-kDa molecular mass centrifugal devices (EMD Millipore, Billerica, MA), followed by lyophilization. The peptido-CS (containing a single serine residue and hereafter referred to as the free CS chain of bikunin) was obtained by proteolytic digestion (actinase E) of the bikunin PG, followed by purification of the CS by anion exchange. Mr dispersion and sulfation sequences of the CS chain of bikunin have been described previously using Fourier transform ion cyclotron resonance mass spectrometry with collision-induced dissociation fragmentation (2).
Reaction Conditions
Reaction conditions had a final volume of 25 μl containing 1 μl of one or more of the following: the bikunin PG (8 mg/ml), the free CS (8 mg/ml), mouse serum, recombinant TSG-6 (0.125 mg/ml), HA14 (1 mg/ml), or HMW HA (1 mg/ml). For optimum TSG-6 activity, 2.5 μl of 1 mm MgCl2 was added to each sample. The reaction was conducted in PBS (pH 7.4). Reaction times varied (4-, 24-, or 76-h reactions), starting with the addition of TSG-6 to the sample. Reactions were stopped by adding EDTA (0.5 μl of 0.5 m EDTA (pH 8.0) was added for each 25 μl of sample). Samples for staining with Alcian blue and silver stain (see below) contained 1 μl of the required reagents diluted to 12 μl in PBS. After reaction completion, 2.5 μl of glycerol was added to each sample.
Western Blot Analysis
Samples were loaded (30 μl/well) onto 4–15% Mini-Protean TGX gels (Bio-Rad; Hercules, CA) and subjected to electrophoresis under denaturing and reducing conditions. The gels were transferred to Bio-Rad nitrocellulose membranes using a Trans-Blot Turbo system. A molecular weight standard (catalog no. 928-40000, Li-Cor, Lincoln, NE) was used in each of the blots. Blots were blocked with Li-Cor blocking buffer (catalog no. 927-40000) for 1 h on an orbital shaker and probed with one or more of the following antibodies: a rabbit polyclonal antibody against ΙαΙ (Dako, Carpinteria, CA) diluted at 1:7500 (with specificity for both mouse and human bikunin and HCs), a rabbit polyclonal antibody against human bikunin (CP6, provided by Anthony J. Day, University of Manchester, Manchester, UK) diluted at 1:1000, and goat polyclonal antibodies against mouse HCs (catalog no. sc-33944/21978, Santa Cruz Biotechnology, Santa Cruz, CA) diluted at 1:1000. Primary antibodies were diluted in blocking solution containing 0.1% Tween 20. The blots were incubated at room temperature with gentle shaking for 1 h and washed for 10 min in 0.1% Tween 20 in PBS (PBST). The blots were then incubated for 45 min at room temperature with an infrared secondary antibody (Li-Cor, catalog nos. 926-68024 and 926-32213) diluted at 1:15,000 in blocking solution containing 0.1% Tween 20 and 0.01% lauryl sulfate. The blots were washed for 10 min in PBST and imaged on a Li-Cor Odyssey infrared imaging system.
Alcian Blue and Silver Staining
Samples with glycerol were loaded onto acrylamide tris acetate gels and were subjected to electrophoresis for 54 min. The gels were removed from their plates and incubated with 50–100 ml of 0.5% Alcian Blue 8GX stain from Sigma-Aldrich (catalog no. SLBG8921V) in 2% acetic acid for 30 min. After staining, the gels were destained with 5% acetic acid until clear, often requiring an overnight wash. When the gels were destained, they were stained with the Invitrogen SilverQuest staining kit (catalog no. 45-1001). Staining was performed according to the recommendations of the manufacturer. After staining, the gels were imaged using the Thermo Scientific myECL imager under white light conditions.
RESULTS
Up to 86 Monosaccharides of Hyaluronan Are Irreversible Heavy Chain Acceptors
We transferred HCs from ΙαΙ in human serum to HA molecules 14, 37, 60, and 86 monosaccharides in length (3–16 kDa) through the addition of recombinant TSG-6 for 4 h and then added 1000-kDa HA (HA1000K) to the reaction mixture for an additional 24 h to test for HC swapping from the smaller HA molecules onto HA1000K (Fig. 1). Only a single HC was transferred to the HA molecules 14–86 monosaccharides in length (3–16 kDa), as indicated by a single HC band in each lane and the absence of a second band ∼83 kDa larger (i.e. the Mr of HCs) that would have been apparent if a second HC had been added. Additionally, these HCs remained bound to the smaller HA molecules after HA1000K was added to the reaction mixture. If the HCs had been swapped onto HA1000K, then the bands in Fig. 1, lanes 3, 5, 7, and 9, would have been significantly weaker than the bands in Fig. 1, lanes 2, 4, 6, and 8, but this did not occur. We conclude that HA, from 14–86 monosaccharides in length, is capable of accepting only a single HC and that this transfer is an irreversible event where subsequent swapping of HCs does not occur.
FIGURE 1.
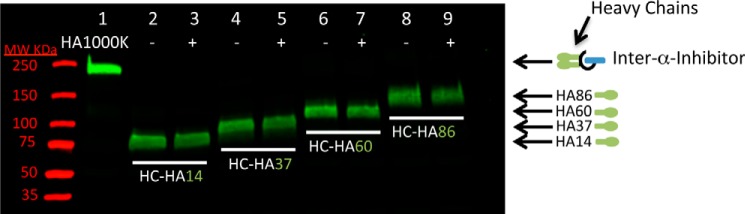
Irreversible transfer of a single heavy chain to hyaluronan polysaccharides up to 86 monosaccharides in length. A Western blot probed with an antibody against ΙαΙ (green) is shown. Lane 1 shows the ΙαΙ band in human serum at 250 kDa. Lanes 2, 4, 6, and 8 show HC transfer from ΙαΙ in human serum to HA polysaccharides 14, 37, 60, and 86 monosaccharides in length, respectively, after a 4-h incubation at 37 °C. In lanes 3, 5, 7, and 9, HCs were first transferred to HA polysaccharides 14, 37, 60, and 86 monosaccharides in length, respectively, for 4 h, and then HA1000K was added to the samples, which were then incubated for another 24 h.
Heavy Chain Transfer to the Free and Intact Chondroitin Sulfate Chains of Bikunin
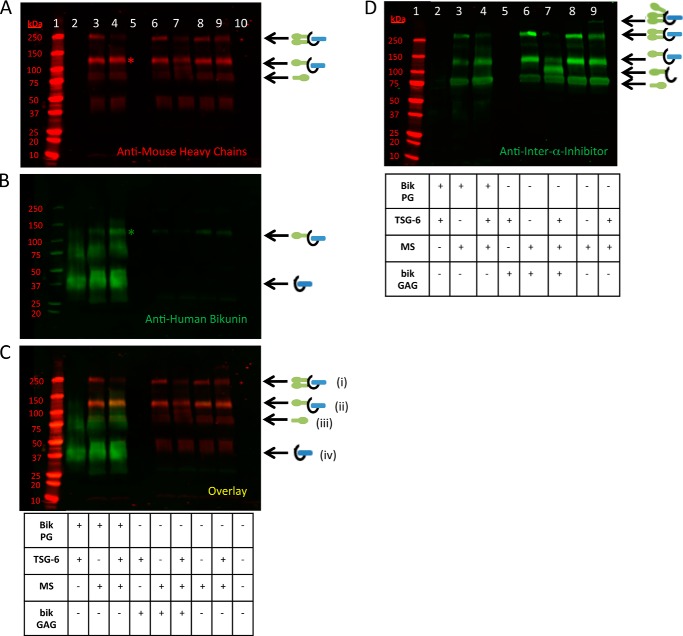
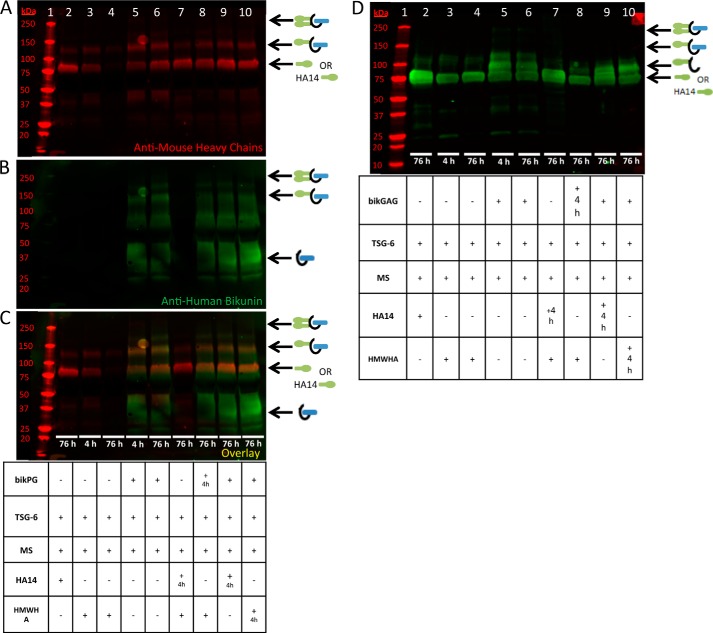
The results of Fig. 1 were intriguing considering that the CS chain of bikunin is only 27–39 monosaccharides in length and that only 6–22 monosaccharides of the non-reducing terminus are the unsulfated sites where HC attachment occurs. To explain these results, we hypothesized that the sulfated domain of the CS chain of bikunin and/or the bikunin core protein itself enabled the chain to accept two HCs during formation of IαI, which could then be transferred to HA by TSG-6. To test this hypothesis, we investigated HC transfer to the intact bikunin PG (purified from human urine) and to its free CS chain. An aliquot of mouse serum was used as our source of ΙαΙ (i.e. the HC donor). Recombinant TSG-6 was added to the reaction mixture to transfer HCs to the CS chain of bikunin by a transesterification reaction. The results are shown in Fig. 2. The appearance of a stronger bikunin band at ∼125 kDa in Fig. 2B, lane 4, compared with lane 3, indicates that HCs were transferred to the CS chain of the intact bikunin PG. Similarly, the appearance of a HC band at ∼95 kDa in Fig. 2, A and D, lanes 7, compared with lanes 6, indicate that HCs were transferred to the free CS chain of bikunin, which is also indicated by the weaker ΙαΙ (250-kDa) band in Fig. 2, A and D, lanes 7, compared with the negative controls in Fig. 2, A and D, lanes 6 and 9. We conclude that both the purified bikunin PG and its free CS chain are able to accept HCs from the ΙαΙ via enzymatic transfer mediated by TSG-6.
FIGURE 2.
Heavy chain transfer to the free and intact chondroitin sulfate chains of bikunin. Shown is a Western blot analysis of samples containing mouse serum incubated at 37 °C for 4 h with recombinant TSG-6 and purified bikunin PG and GAG chain, as listed in the tables. A–C, the same blot probed with an anti-human bikunin antibody (green) and anti-mouse antibodies for HCs 1 and 2 (red). A portrays the red channel only, B portrays the green channel only, and C represents the overlay. Mr standards are shown in lane 1. The samples in lanes 2–4 contain the intact bikunin PG, whereas the samples in lanes 5–7 contain its free CS GAG chains; the samples in lanes 3, 4, and 6–9 contain mouse serum; and the samples in lanes 2, 4, 5, 7, and 9 were treated with recombinant TSG-6. ΙαΙ, with two HCs attached to its single CS chain, appears as a 250-kDa band (i). ΙαΙ, with only one HC attached to its single CS chain, appears as an ∼125-kDa band (ii) (asterisk on lane 4 of A and B). The CS GAG chain is depicted by the curved black line in the models. Individual HC bands from mouse serum appear at ∼83 kDa (iii). The bikunin core protein with the intact CS GAG chain appears as an ∼37-kDa band (iv). MS, mouse serum. D, Western blot from the same treatments in A–C, with the exception that this blot was probed with a polyclonal antibody against ΙαΙ. This blot shows the ∼95-kDa HC-free GAG chain band (lane 7) better than our antibody against the mouse HCs (A).
A Portion of the Bikunin Proteoglycan Is Unable to Accept Heavy Chains
As seen in Fig. 2, the lanes containing the intact bikunin PG show a bright smear from ∼25–50 kDa. This smear contains the bikunin PG, including the bikunin core protein and its attached CS chain without HCs. In Fig. 2, lane 4, a large portion of this PG smear remains despite having transferred HCs to the bikunin PG (as apparent by the appearance of the band at ∼125 kDa in Fig. 2, lane 4; see asterisks in A and B). These data offer two possible explanations. The bikunin PG was in molar excess of the HCs of the ΙαΙ present in the mouse serum, or there is an inactive portion of the bikunin PG that is unable to accept an HC. In the former instance, a large portion of the bikunin PG smear would be present because there is an insufficient number of HCs in our aliquot of mouse serum for HC transfer. In the latter instance, a large portion of the bikunin would remain following HC transfer because there is an inactive portion of the bikunin PG that is unable to accept an HC. To test these hypotheses, we incubated mouse serum with TSG-6 and the intact bikunin PG in a 1:2 serial dilution at 37 °C for 4 h. In Fig. 3B, the 1:1 bikunin PG dilution (lane 5) is identical to the conditions in Fig. 2, lane 4. If our first hypothesis is correct, and the PG was in molar excess of the mouse ΙαΙ, the band at ∼125 kDa, representing the successfully transferred HC attached to the PG, would remain at the same intensity, and the smear representing the bikunin PG would diminish in intensity. However, the intensity of the ∼125-kDa band diminished throughout the dilution (Fig. 3, lanes 5–8), suggesting that our second hypothesis is correct (i.e. there is an inactive portion of the bikunin PG that is unable to accept HCs).
FIGURE 3.
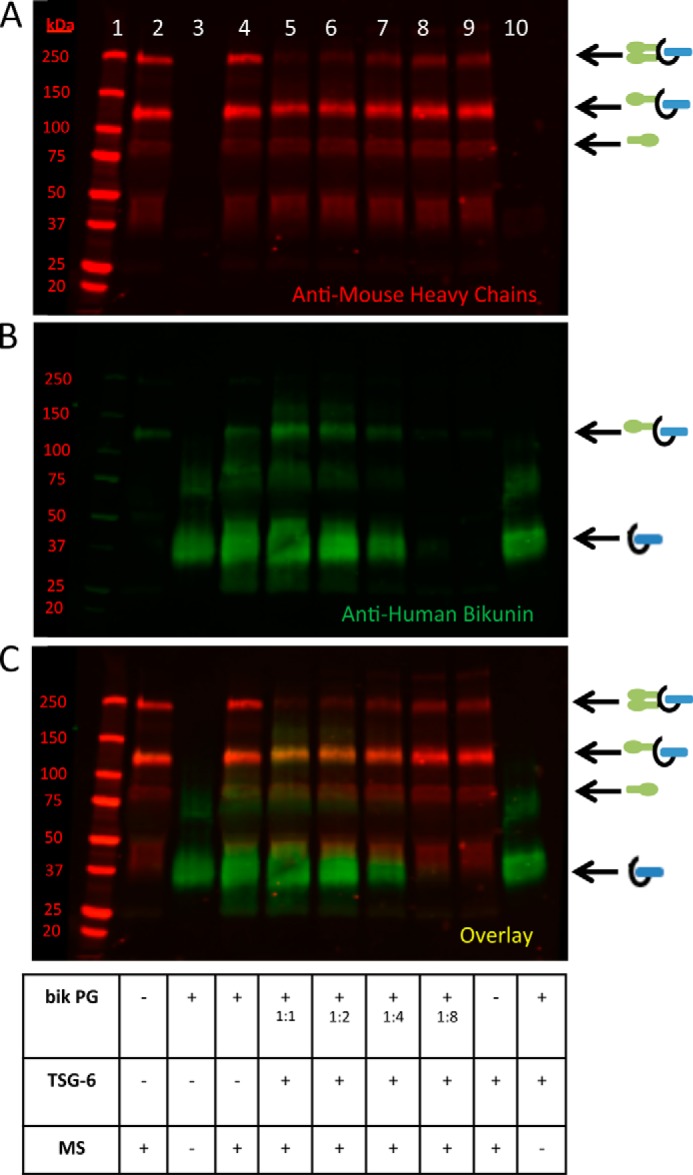
A portion of the bikunin proteoglycan is unable to accept heavy chains. Shown is a Western blot of samples containing mouse serum incubated at 37 °C for 4 h with recombinant TSG-6 and the purified bikunin PG, as listed in the table. A–C represent the same blot, which was probed with an anti-human bikunin antibody (green) and anti-mouse antibodies for HCs 1 and 2 (red). A portrays the red channel only, B portrays the green channel only, and C portrays the overlay. Lane 1 portrays the Mr standards. The sample in lane 2 contains mouse serum alone. A 1:2 serial dilution of the bikunin PG is portrayed in lanes 5–8. Recombinant human TSG-6 was added to the samples in lanes 5–10. The sample in lane 3 contains the bikunin PG only, and the sample lane 10 contains both the PG and TSG-6. MS, mouse serum.
To further test this hypothesis, we evaluated HC transfer to native (not purified) bikunin PG from healthy human urine (Fig. 4). The relative abundance of bikunin PG in human urine, compared with our purified bikunin PG preparation, was determined by Western blot analysis, probing the blot with an antibody against human bikunin (data not shown). The amount of bikunin PG in the purified bikunin PG preparation was about 20 times more concentrated than in the urine we examined. Therefore, in Fig. 4, volumes were adjusted to add nearly equivalent amounts of the bikunin PG. We observed that unpurified urinary bikunin was able to accept an HC via TSG-6-mediated HC transfer from mouse serum-derived ΙαΙ (as evident by the somewhat brighter 125-kDa band in Fig. 4, lane 9, compared with lane 8). This result was comparable with HC transfer to the purified bikunin PG (compare the 125-kDa band in Fig. 4, lane 4, with lane 3). The absence of a 250-kDa band in Fig. 4, lanes 4 and 9, demonstrates that HC transfer to the purified and unpurified bikunin PG was only a single HC. The experiment presented in Fig. 4 was conducted at 37 °C for 4 h. Identical results were determined when the reaction mixture was incubated for a total of 24 h (data not shown). Therefore, the HC transfer reaction appears to have gone to completion despite the observation that HCs were only able to be transferred to a portion of the total bikunin PG, whether purified or unpurified from human urine.
FIGURE 4.
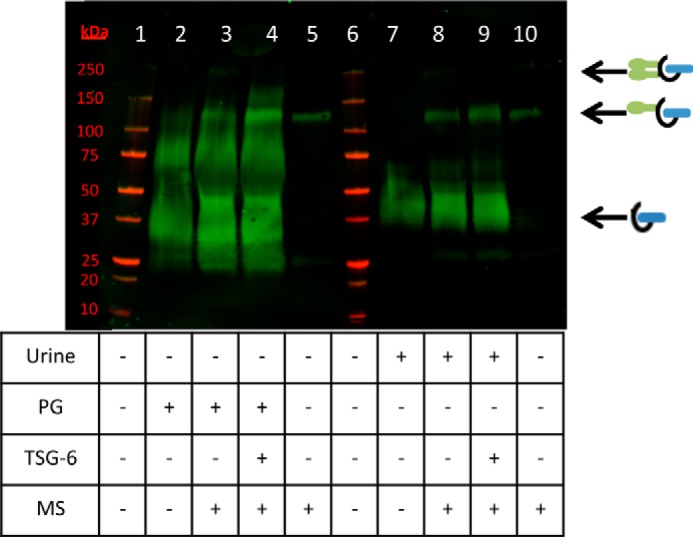
Transferring heavy chains to native bikunin from healthy human urine. Shown is a Western blot of healthy human urine incubated at 37 °C for 4 h with mouse serum, TSG-6, and purified bikunin PG as listed in the table. The blot was probed with an anti-human bikunin antibody. Lanes 1 and 6 portray the molecular weight standards (red). Lanes 2–4 portray the purified bikunin PG, whereas lanes 7–9 portray an aliquot of urine from a healthy (non-asthmatic) patient. Equivalent amounts of the purified PG and the unpurified urinary PG were loaded. Lanes 5 and 10 represent mouse serum only as a negative control. Mouse serum was also added to the reactions portrayed in lanes 3, 4, 8, and 9. TSG-6 was added to the reactions portrayed in lanes 4 and 9. MS, mouse serum.
Collectively, these data indicate that the inability of a considerable portion of our purified bikunin PG preparation to accept a HC is not likely caused by inactivation in the purification process or by a limiting number of available HCs for HC transfer but, rather, that unidentified cofactors are required for HC transfer to a large portion of the bikunin PG, especially with regard to its acceptance of two HCs in the assembly of the ΙαΙ.
Lack of Chain Length Preference for Heavy Chain Transfer to the CS Chain of Bikunin
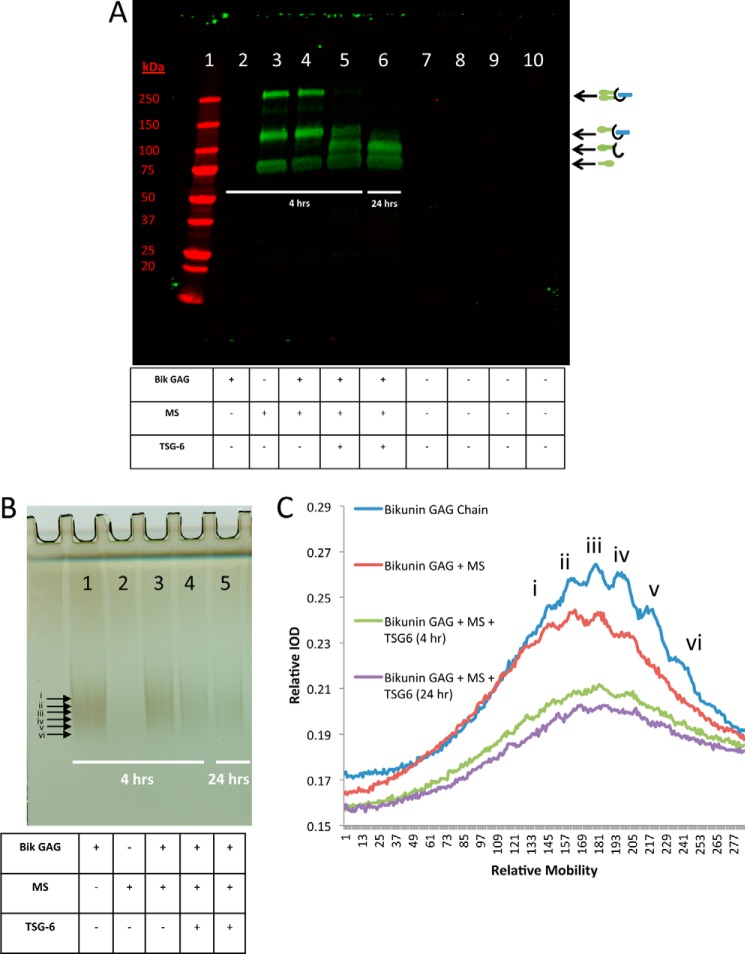
In Figs. 3 and 4, we demonstrated that a relatively large portion of our purified bikunin PG preparation and native (unpurified) bikunin from urine was unable to accept an HC. One possible explanation for this observation is that bikunin PGs with shorter CS chain lengths might be unable to accept HCs. The CS chains of our purified preparation of bikunin PG ranged from 27–39 monosaccharides (2). To test this hypothesis, we transferred HCs to the free bikunin CS chain (Fig. 5A) and separated a portion of this reaction mixture on a silver-stained gel (Fig. 5B) to determine whether there was a size-selective transfer of HCs to larger CS chains. By using this technique, we were able to distinguish six defined bands, representing six different chain lengths of the free CS chains of bikunin (Fig. 5, bands labeled i-vi). As expected, when HCs were transferred to the free CS chains (Fig. 5B, lane 4), the band intensity was weaker than in the absence of TSG-6 (Fig. 5B, lane 3). The difference between HC transfer in 4 h (lane 4) and 24 h (lane 5) was minimal, indicating that the reaction was essentially complete within 4 h. When the lane profiles were plotted (Fig. 5C), there were no obvious differences of relative band intensity molecular weight distributions when comparing the lane profile before (blue and red lines) and after (green and purple lines) HC transfer. Therefore, we conclude that there was not a size-selective chain length preference for HC transfer to the free CS chains of bikunin.
FIGURE 5.
Transferring heavy chains to the free bikunin GAG. A, Western blot of samples containing mouse serum incubated at 37 °C for 4 or 24 h with recombinant TSG-6 and the free bikunin (Bik) CS chain (GAG), as listed in the table. The blot was probed with a rabbit polyclonal antibody against ΙαΙ. Lane 2 portrays the GAG chain alone, whereas lane 3 portrays mouse serum (MS) alone. Lane 4 represents GAG plus mouse serum with no HC transfer. Lane 5 represents the 4-h reaction of GAG with mouse serum and TSG-6, and lane 6 shows the 24-h reaction. The band at 250 kDa represents ΙαΙ with two HCs attached to its single CS chain, and the ∼125-kDa band represents pre-ΙαΙ with only one HC attached to its single CS chain. The free GAG chain with a single HC attached is portrayed as a band above the ∼83-kDa HC band and below the ∼125-kDa pre-ΙαΙ band. B, silver-stained gel of the same samples and in the same order as those presented in A. C, quantitative lane profile of the bands in the lanes from the silver-stained gel in B. Six bands (i–vi) are labeled with arrows on the silver-stained gel (B), and their corresponding peaks are identified on the lane profile (C). IOD, integral optical density.
The Free Chondroitin Sulfate Chain of Bikunin and the Intact Bikunin Proteoglycan Can Only Accept a Single Heavy Chain
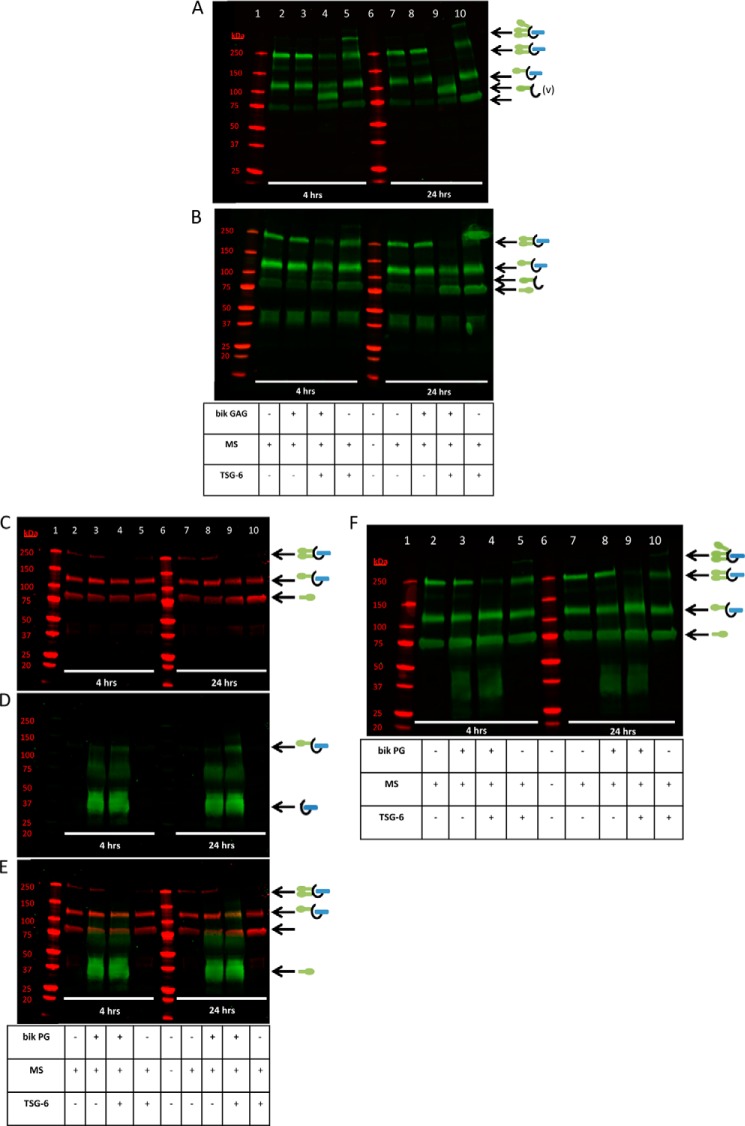
In Fig. 6, we estimated the number of HCs that a single bikunin PG and its free CS chain could accept. Our hypothesis was that they would be able to accept two HCs because the ΙαΙ contains two HCs. To test this hypothesis, we incubated mouse serum (as our ΙαΙ source) with TSG-6 and the free GAG chain (Fig. 6, A and B) or the intact bikunin PG (Fig. 6, C–E) for 4 and 24 h at 37 °C. In Fig. 6, A and B, the samples added to lanes 2–5 are the same as those added to lanes 7–10, with the exception that the incubation of the latter was 24 h, whereas the former was 4 h. In Fig. 6, A and B, lanes 5 and 10 demonstrate shuffling of HCs among ΙαΙ molecules, which occurs when TSG-6 and ΙαΙ are incubated in the absence of HA (9). This shuffling results in ΙαΙ molecules with three or more HCs as well as the subsequent formation of ΙαΙ with only one HC and the release of free HCs. In Fig. 6, A and B, lanes 4 and 9, HC transfer to the free CS chain (Fig. 6, A and B) or the intact bikunin PG (Fig. 6, C–E) was determined. The appearance of a single band at ∼95 kDa in Fig. 6A, lanes 4 and 9, demonstrates that the free CS chain was only able to accept a single HC. If the CS chain had been able to accept two HCs, then a second band would have been visible at ∼175 kDa. Similarly, the bikunin PG, with an intact CS chain, produced a single band at ∼125 kDa, indicating that the bikunin PG was only able to accept a single HC (Fig. 6, C–E).
FIGURE 6.
The intact bikunin proteoglycan and the free chondroitin sulfate chain of bikunin can only accept a single heavy chain. A and B, Western blot of samples containing mouse serum incubated at 37 °C for 4 or 24 h with recombinant TSG-6 and the free bikunin CS chain (GAG), as listed in the table. The samples and lane order in A and B are the same. The blot in A was probed with a rabbit polyclonal antibody against ΙαΙ, and the blot in B was probed with anti-mouse antibodies for HCs 1 and 2. Lane 1 represents Mr standards. The sample in lane 2 contains only mouse serum. The samples in lanes 3, 4, 8, and 9 contain the bikunin PG. All samples contain mouse serum. The samples in lanes 4, 5, 9, and 10 were treated with recombinant human TSG-6. The treatments of samples in lanes 2–5 are identical to the samples in lanes 7–10, aside from incubation times (4 h for lanes 2–5 and 24 h for lanes 7–10). The samples in C–F contain the same treatments as the samples in A and B and are in the same order, but the intact bikunin PG was substituted for the free GAG chain of bikunin in C–F. C–E represent the same blot probed with anti-HC antibodies (C, red), an anti-bikunin antibody (D, green), and the overlay (E). The blot in F contains the same samples and is in the same order as the blots in C–E, but the blot in F was probed with a rabbit polyclonal antibody against ΙαΙ (green). MS, mouse serum.
Preference of Heavy Chain Transfer to Hyaluronan Compared with the Free and Intact Chondroitin Sulfate Chain of Bikunin
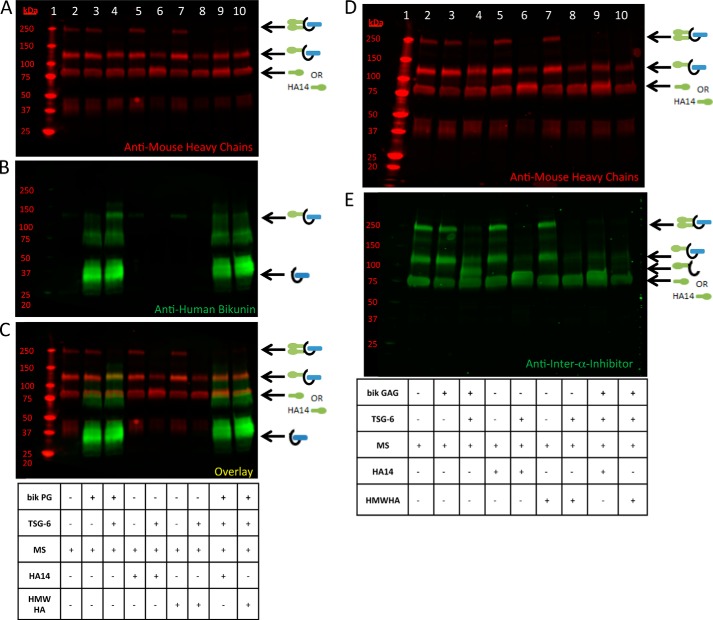
We next determined whether there was a preferential transfer of HCs to HA compared with the bikunin PG or to its free CS chain. To test this hypothesis, we incubated mouse serum (as our ΙαΙ source) with TSG-6 and the intact bikunin PG or its free GAG chain (Fig. 7, A–E) and with two different sizes of HA, an HA oligosaccharide 14 monosaccharides in length (HA14) and HMW HA ∼1700 kDa, for 4 h at 37 °C. Fig. 7 shows the results with the bikunin PG (A–C) and with the free CS chain (D and E). When TSG-6 was added to the sample in Fig. 7, B and C, lane 4 containing mouse serum and the bikunin PG, a band at ∼125 kDa appears, representing the transfer of a single HC to the bikunin PG, as shown in Figs. 1 and 2. When HCs are transferred to HA14 (Fig. 7, lane 6), an ∼83-kDa band, representing a single HC attached to HA14, appears. When HCs are transferred to HMW HA (Fig. 7, lane 8), the ΙαΙ band disappears because the HC-HA band is too large to enter the gel. When the bikunin PG (Fig. 7, A–C) was incubated simultaneously with HA14 and HMW HA (lanes 9 and 10, respectively), the lack of an ∼125-kDa band demonstrates that the bikunin PG was not preferred as an HC acceptor compared with HA. Similarly, when the free CS chain of bikunin (Fig. 7, D and E) was incubated simultaneously with HA14 and HMW HA (lanes 9 and 10, respectively), the lack of the ∼95-kDa band in lanes 9 and 10, compared with lane 4, indicates that the free CS chain of bikunin was not a preferred HC acceptor compared with HA. These data indicate that there is preferential HC transfer from ΙαΙ to HA when both HA and the bikunin PG or its free CS chain are incubated in the same reaction mixture.
FIGURE 7.
Preference of heavy chain transfer to hyaluronan compared with the free and intact chondroitin sulfate chain of bikunin. Shown are Western blots of samples containing mouse serum (MS) incubated at 37 °C for 4 h with recombinant TSG-6 and either the purified bikunin (bik) PG (A–C) or its free GAG chain (D), as listed in the tables. The blot represented by A–C was probed with an anti-human bikunin antibody (B, green) and anti-mouse antibodies for HC 1 and 2 (red). A–C represent the same blot containing the PG treatment. A portrays the red channel only, B portrays the green channel only, and C portrays the overlay. The blot shown in D and E portrays treatments with the free GAG chain of bikunin probed with anti-mouse antibodies for HCs 1 and 2 (D) and an anti-ΙαΙ antibody (E). For both blots, lane 1 represents Mr standards. The samples in lanes 3, 4, 9, and 10 were treated with the bikunin PG (A–C) or its free GAG chain (D). The samples in lanes 4, 6, 8, 9, and 10 were treated with TSG-6. Lanes 2–10 contain mouse serum. The samples in lanes 5, 6, and 9 contain HA14, whereas the samples in lanes 7, 8, and 10 contain HMW HA.
Heavy Chain Swapping from the Free and Intact Chondroitin Sulfate Chains of Bikunin
We then investigated whether HCs can be swapped between HA and the bikunin PG or its free CS chain. On the basis of the preferential transfer of HCs to HA, we hypothesized that HC swapping might occur from the bikunin PG to HA but not from HA to the bikunin PG. To test this hypothesis, we incubated mouse serum (as our ΙαΙ source) with TSG-6, the bikunin PG (Fig. 8, A–C), or its free CS chain (Fig. 8D) with or without HA14 and HMW HA for 4 h (panels A–D, lanes 3 and 5 only) and incubated the reactants for 4 or 76 h at 37 °C. In panels A–C, lanes 5 and 6 show an ∼125-kDa band, indicating HC transfer to the bikunin PG for 4 and 76 h, respectively. When HCs are first transferred to the bikunin PG for 4 h (Fig. 8, panels A–C, lane 5) and then HMW HA is added to the reaction mixture for a total of 76 h (lane 8), the ∼125-kDa HC-bikunin PG band becomes significantly weaker than in the absence of HMW HA, indicating that HCs were removed from the bikunin PG to HMW HA. When HCs are first transferred to HA14 for 4 h (Fig. 8, panels A–C, lane 4) and then the bikunin PG is added to the reaction mixture for a total of 76 h (lane 9), the ∼125-kDa HC-bikunin PG band is much weaker than in the absence of HA14 (lane 5), indicating that the HC remained bound to HA14. When HCs are first transferred to HMW HA for 4 h (Fig. 8, panels A–C, lane 3) and then the bikunin PG is added to the reaction mixture for a total of 76 h (lane 10), the ∼125-kDa HC-bikunin PG band is much weaker than in the absence of HMW HA (lane 5), indicating that the HC remained bound to HMW HA. The same results were observed for HC swapping between the free CS chains of bikunin and HA (Fig. 8D). In summary, HCs that were transferred to the free chain of bikunin could be swapped back to HA14 and HMW HA.
FIGURE 8.
Heavy chain swapping from the free and intact chondroitin sulfate chains of bikunin. Shown is a Western blot of samples containing mouse serum (MS) incubated at 37 °C for 4 or 76 h with recombinant TSG-6 and the purified bikunin PG (bikPG, A–C) and its free GAG chain (D), as listed in the table. A–C represent a blot probed with an anti-human bikunin antibody (green) and anti-mouse antibodies for HCs 1 and 2 (red). A portrays the red channel only, B portrays the green channel only, and C portrays the overlay. Lane 1 portrays the molecular weight standards (also in red). The samples in lanes 3 and 5 were incubated for 4 h only. The samples in lanes 2, 4, and 6–10 were incubated for a total of 76 h. In lanes 7–10, HCs were first transferred to HA14, bikunin PG, HA14, and HMW HA for 4 h, respectively. After the 4-h incubation, HMW HA, HMW HA, bikunin PG, and bikunin PG, were added, respectively, to the reaction mixture and incubated at 37 °C for an additional 72 h (76 h total). The samples and sample order in D are the same as in A–C, but free bikunin GAG was substituted for bikunin PG.
TSG-6 Is Not Required for the Synthesis of Inter-α-Inhibitor
Transferring HCs to the CS chain of the bikunin PG through the action of recombinant TSG-6 raises an interesting question. By what mechanism are HCs originally transferred to the CS chain of bikunin during the intracellular assembly of ΙαΙ? To address this question, we measured ΙαΙ levels in the plasma of +/+, +/−, and −/− TSG-6-deficient mice. As shown in Fig. 9, plasma levels of ΙαΙ were not influenced by the genetic deletion of TSG-6. Therefore, we conclude that TSG-6 is not required for the synthesis of ΙαΙ and that other unidentified enzyme(s) and/or cofactor(s) are responsible for the covalent transfer of HCs to bikunin during the intracellular assembly of ΙαΙ.
FIGURE 9.
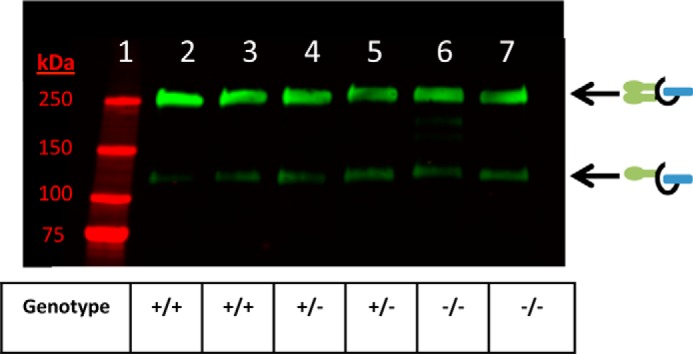
TSG-6 is not required for the synthesis of inter-α-inhibitor. Shown is a Western blot demonstrating levels of ΙαΙ (green) in plasma from wild-type (+/+, lanes 2 and 3), heterozygous (+/−, lanes 4 and 5), and TSG-6 null (−/−, lanes 6 and 7) mice. The blot was probed with a rabbit polyclonal antibody against ΙαΙ.
DISCUSSION
In this manuscript, we compared HC transfer to the intact CS chain of the bikunin PG with its free CS chain. Our hypothesis was that the bikunin core protein, and/or the reducing terminal sulfated domain of its CS chain, would promote the reversible transfer of two HCs to the intact CS chain. The rationale for this hypothesis was on the basis of the observation that HA, up to 86 monosaccharides in length, accepted only a single HC when transferred from ΙαΙ via the enzymatic action of TSG-6 (Fig. 1). Furthermore, this HC transfer was irreversible. The chain length of the CS chain of bikunin varies between 27–39 monosaccharides and is capable of accepting 2 HCs in a triprotein form known as ΙαΙ. Therefore, we hypothesized that the bikunin core protein and/or its sulfated CS domain at the reducing terminus might facilitate reversible HC transfer to its relatively small (<40 monosaccharides) intact CS chain compared with the transfer of only a single HC to larger HA polysaccharides ≤86 monosaccharides in length.
Our observation that the intact, or free, CS chain of bikunin accepts only a single HC raises several interesting questions regarding the intracellular synthesis of ΙαΙ. In humans, ΙαΙ, containing two HCs (either HC1 or HC2), is the predominant bikunin-containing PG in serum, whereas only trace amounts of pre-ΙαΙ, containing one HC, are present in human serum. In contrast, there is significantly more pre-ΙαΙ in mouse serum compared with ΙαΙ (9). The reason for this disparity is unknown, although it may be related to a shorter CS chain length for murine ΙαΙ that prevents the acceptance of more than one HC. Regardless of these species differences, it is clear that, in both mice and humans, ΙαΙ is synthesized and released into the bloodstream at relatively high concentrations (0.1–0.5 mg/ml) (12) with two intact HCs. The intracellular transferase that transfers HCs to the bikunin PG to form ΙαΙ has not been identified but, clearly, does not require TSG-6 (Fig. 9).
We observed that HCs, transferred to the intact, or free, CS chain of bikunin, could be transferred from bikunin to HA. This occurs even though its CS chain is 27–39 monosaccharides in length and its unsulfated non-reducing terminus, where the HCs are attached, is only 6–22 monosaccharides in length. In contrast, we observed that HCs transferred to HA molecules more than twice the length of the CS chain of bikunin, are unable to be swapped between HA molecules (Fig. 1). Therefore, we conclude that the core protein of bikunin, and/or the sulfated domain on its reducing terminus, promotes reversible HC transfer to HA from this relatively short CS chain. This is consistent with previous data that demonstrated that the sulfation of the CS chain of bikunin is involved in HC transfer (13).
The results presented show HC transfer over the full range of bikunin GAG chain sizes (27–39 saccharide residues ranging from 5500–7100 Da) (2). On this basis, we believe that a 27-mer is the minimum CS chain size demonstrated to be competent for HC transfer in this study. It is possible that a smaller bikunin CS chain might work, but such chains would be very hard to isolate or prepare with the requisite bikunin CS structure.
Covalent TSG-6-HC hemiacetal intermediates have been reported (14), and it is not unreasonable to speculate that TSG-6 might be the intracellular transesterase that originally transfers HCs to bikunin to form ΙαΙ during its biosynthesis. However, clearly, this is not the case because plasma, derived from mice lacking TSG-6, has normal levels of circulating ΙαΙ (Fig. 9). Although this observation was implied by the data from Fülöp et al. (15), in the description of the infertility of TSG-6 −/− mice, it has not been specifically demonstrated. The observation that circulating ΙαΙ levels are normal in TSG-6−/− mice is not surprising because the expression of TSG-6 is induced by proinflammatory mediators and is below the limit of detection under basal conditions (16). Our data demonstrate that, even if TSG-6 were the enzyme that originally transferred HCs to the bikunin PG during the intracellular synthesis of ΙαΙ, then it would lack the ability to transfer more than one HC without an intracellular chaperone, or another cofactor, to facilitate this process. Therefore, there is an unidentified HC transferase involved in the synthesis of ΙαΙ independently of the enzyme TSG-6. The mechanism whereby this biosynthetic process occurs is unknown, although it clearly involves a transesterification reaction with a C-terminal aspartate residue (Asp-702) of the HCs to the sixth hydroxyl of GalNAc residues in the CS chain of bikunin (17).
Our observation that a large portion of the bikunin PG was unable to accept even a single HC could simply have been the result of a partial inactivation, or unfolding, of the bikunin protein during its purification. Bikunin was purified from the urine of healthy volunteers by Mochida Pharmaceuticals (Tokyo, Japan) and is used (to treat acute pancreatitis) as an active protease inhibitor, requiring a correctly folded protein. The lyophilized PG was reconstituted in water, dialyzed at 4 °C to remove excipients, and lyophilized again. But our observation that a large portion of native (unpurified) bikunin from human urine was unable to accept a single HC indicates that the inability of TSG-6 to swap HCs from ΙαΙ onto a large portion of bikunin is a quality of this PG rather than due to compromised activity of our purified bikunin preparation.
An alternative explanation for the inability of a large portion of the bikunin PG to accept a HC, or for the active portion to only accept a single HC instead of two HCs, was that CS chain lengths might influence the ability of bikunin to accept HCs. In this model, longer chains might be able to accept one or more HCs, whereas shorter chains might be unable to accept any HCs. The differential CS chain lengths of bikunin (1) might explain why pre-ΙαΙ is more abundant in mouse serum than in human serum, therefore predicting shorter bikunin CS chain lengths in mice compared with humans (9). However, our demonstration that most of the free CS chains of bikunin were able to accept HCs (Fig. 5), and that there did not appear to be a preferred size for HC acceptance, does not support the hypothesis that CS chain length is a significant factor in HC transfer to the GAG chain of bikunin.
The preferential acceptance of HCs to HA compared with the free, or intact, CS chain of bikunin (Fig. 7) was not surprising because HC transfer is known to proceed from the CS chain of ΙαΙ to HA and not the reverse (9). For similar reasons, it was not surprising that TSG-6, after having transferred HCs to the free, or intact, CS chain of bikunin, will proceed to swap them to HA (Fig. 8). Nonetheless, this result emphasizes the importance of stereochemistry at the fourth hydroxyl of HA GlcNAc and CS GalNAc regarding HC transfer.
We conclude that the core protein of the bikunin PG, and/or the sulfation domain on the reducing terminus of its CS chain, promotes reversible HC transfer from CS chains ≤40 monosaccharides, although it does not enable bikunin to accept more than one HC via TSG-6 mediated HC swapping from ΙαΙ in the absence of (an) unidentified cofactor(s). These investigations have also demonstrated that TSG-6 is not likely the primary transesterase involved in the intracellular assembly of ΙαΙ and that the enzyme(s) that mediates this role has yet to be identified.
Acknowledgments
We thank Professor Anthony J. Day (University of Manchester, Manchester, UK) for the anti-human bikunin antibody (CP6). We also thank Paul DeAngelis (University of Oklahoma Health Sciences Center, Oklahoma City, OK) for the HA polysaccharides 3–16 kDa.
- PG
- proteoglycan
- CS
- chondroitin sulfate
- GAG
- glycosaminoglycan
- HC
- heavy chain
- IαI
- inter-α-inhibitor
- HA
- hyaluronan
- HMW
- high molecular weight.
REFERENCES
- 1. Chi L., Wolff J. J., Laremore T. N., Restaino O. F., Xie J., Schiraldi C., Toida T., Amster I. J., Linhardt R. J. (2008) Structural analysis of bikunin glycosaminoglycan. J. Am. Chem. Soc. 130, 2617–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ly M., Leach F. E., 3rd, Laremore T. N., Toida T., Amster I. J., Linhardt R. J. (2011) The proteoglycan bikunin has a defined sequence. Nat. Chem. Biol. 7, 827–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lesley J., Gál I., Mahoney D. J., Cordell M. R., Rugg M. S., Hyman R., Day A. J., Mikecz K. (2004) TSG-6 modulates the interaction between hyaluronan and cell surface CD44. J. Biol. Chem. 279, 25745–25754 [DOI] [PubMed] [Google Scholar]
- 4. Lauer M. E., Cheng G., Swaidani S., Aronica M. A., Weigel P. H., Hascall V. C. (2013) Tumor necrosis factor-stimulated gene-6 (TSG-6) amplifies hyaluronan synthesis by airway smooth muscle cells. J. Biol. Chem. 288, 423–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhuo L., Kanamori A., Kannagi R., Itano N., Wu J., Hamaguchi M., Ishiguro N., Kimata K. (2006) SHAP potentiates the CD44-mediated leukocyte adhesion to the hyaluronan substratum. J. Biol. Chem. 281, 20303–20314 [DOI] [PubMed] [Google Scholar]
- 6. de la Motte C. A., Hascall V. C., Drazba J., Bandyopadhyay S. K., Strong S. A. (2003) Mononuclear leukocytes bind to specific hyaluronan structures on colon mucosal smooth muscle cells treated with polyinosinic acid:polycytidylic acid: inter-α-trypsin inhibitor is crucial to structure and function. Am. J. Pathol. 163, 121–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de La Motte C. A., Hascall V. C., Calabro A., Yen-Lieberman B., Strong S. A. (1999) Mononuclear leukocytes preferentially bind via CD44 to hyaluronan on human intestinal mucosal smooth muscle cells after virus infection or treatment with poly(I.C). J. Biol. Chem. 274, 30747–30755 [DOI] [PubMed] [Google Scholar]
- 8. Yingsung W., Zhuo L., Morgelin M., Yoneda M., Kida D., Watanabe H., Ishiguro N., Iwata H., Kimata K. (2003) Molecular heterogeneity of the SHAP-hyaluronan complex: isolation and characterization of the complex in synovial fluid from patients with rheumatoid arthritis. J. Biol. Chem. 278, 32710–32718 [DOI] [PubMed] [Google Scholar]
- 9. Lauer M. E., Glant T. T., Mikecz K., DeAngelis P. L., Haller F. M., Husni M. E., Hascall V. C., Calabro A. (2013) Irreversible heavy chain transfer to hyaluronan oligosaccharides by tumor necrosis factor-stimulated gene-6. J. Biol. Chem. 288, 205–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mukhopadhyay D., Asari A., Rugg M. S., Day A. J., Fülöp C. (2004) Specificity of the tumor necrosis factor-induced protein 6-mediated heavy chain transfer from inter-α-trypsin inhibitor to hyaluronan: implications for the assembly of the cumulus extracellular matrix. J. Biol. Chem. 279, 11119–11128 [DOI] [PubMed] [Google Scholar]
- 11. Wedes S. H., Wu W., Comhair S. A., McDowell K. M., DiDonato J. A., Erzurum S. C., Hazen S. L. (2011) Urinary bromotyrosine measures asthma control and predicts asthma exacerbations in children. J. Pediatr. 159, 248–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fries E., Kaczmarczyk A. (2003) Inter-α-inhibitor, hyaluronan and inflammation. Acta Biochim. Pol. 50, 735–742 [PubMed] [Google Scholar]
- 13. Lord M. S., Day A. J., Youssef P., Zhuo L., Watanabe H., Caterson B., Whitelock J. M. (2013) Sulfation of the bikunin chondroitin sulfate chain determines heavy chain·hyaluronan complex formation. J. Biol. Chem. 288, 22930–22941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rugg M. S., Willis A. C., Mukhopadhyay D., Hascall V. C., Fries E., Fülöp C., Milner C. M., Day A. J. (2005) Characterization of complexes formed between TSG-6 and inter-α-inhibitor that act as intermediates in the covalent transfer of heavy chains onto hyaluronan. J. Biol. Chem. 280, 25674–25686 [DOI] [PubMed] [Google Scholar]
- 15. Fülöp C., Szántó S., Mukhopadhyay D., Bárdos T., Kamath R. V., Rugg M. S., Day A. J., Salustri A., Hascall V. C., Glant T. T., Mikecz K. (2003) Impaired cumulus mucification and female sterility in tumor necrosis factor-induced protein-6 deficient mice. Development 130, 2253–2261 [DOI] [PubMed] [Google Scholar]
- 16. Lee T. H., Wisniewski H. G., Vilcek J. (1992) A novel secretory tumor necrosis factor-inducible protein (TSG-6) is a member of the family of hyaluronate binding proteins, closely related to the adhesion receptor CD44. J. Cell Biol. 116, 545–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sanggaard K. W., Karring H., Valnickova Z., Thøgersen I. B., Enghild J. J. (2005) The TSG-6 and I α I interaction promotes a transesterification cleaving the protein-glycosaminoglycan-protein (PGP) cross-link. J. Biol. Chem. 280, 11936–11942 [DOI] [PubMed] [Google Scholar]