Background: The mechanosensitive ion channel PIEZO1 opens when stress is applied to membranes.
Results: PIEZO1 channel activation is inhibited at an extracellular pH of 6.3 with a pK of ≈6.9. Protonation is positively cooperative.
Conclusion: Protonation stabilizes the inactivated state (not the closed state) of the channel.
Significance: During physiological stress that gives rise to low pH, the PIEZO1 ion channel is inhibited.
Keywords: Electrophysiology, Ion Channel, Mechanotransduction, Membrane Protein, pH Regulation
Abstract
PIEZO1 is a recently cloned eukaryotic cation-selective channel that opens with mechanical force. We found that extracellular protonation inhibits channel activation by ≈90% by increased occupancy in the closed or the inactivated state. Titration between pH 6.3 and 8.3 exhibited a pK of ≈6.9. The steepness of the titration data suggests positive cooperativity, implying the involvement of at least two protonation sites. Whole-cell recordings yielded results similar to patches, and pH 6.5 reduced whole-cell currents by >80%. The effects were reversible. To assess whether pH acts on the open or the inactivated state, we tested a double-mutant PIEZO1 that does not inactivate. Cell-attached patches and whole-cell currents from this mutant channel were pH-insensitive. Thus, protonation appears to be associated with domain(s) of the channel involved with inactivation. pH also did not affect mutant channels with point mutations at position 2456 that are known to exhibit slow inactivation. To determine whether the physical properties of the membrane are altered by pH and thereby affect channel gating, we measured patch capacitance during mechanical stimuli at pH 6.5 and 7.3. The rate constants for changes in patch capacitance were independent of pH, suggesting that bilayer mechanics are not involved. In summary, low pH stabilizes the inactivated state. This effect may be important when channels are activated under pathological conditions in which the pH is reduced, such as during ischemia.
Introduction
PIEZO1 is a cation-selective ion channel that responds to mechanical forces in the membrane (1, 2). The protein contains over 2500 amino acids and 24–32 transmembrane helices, making it the largest known transmembrane protein. In solution, it tends to assemble into tetramers to form a complex of ∼1.2 million Da (2). The channel has a characteristic fast inactivation (∼30 ms), and the inactivation is voltage-dependent, slowing with depolarization. The pore itself is cation-selective, with a reversal potential of ∼0 mV in normal saline (1, 3, 4). These properties of PIEZO currents resemble the currents of many endogenous mechanosensitive channels (5).
Because PIEZO1 was only recently cloned, the molecular properties and physiological roles of PIEZO1 and PIEZO2 are still being elucidated. PIEZO1 is involved in nociception, and removing the PIEZO1 ortholog from Drosophila larvae reduces their rollover response to mechanical stimulation (6). PIEZO2 has recently been demonstrated to have a central role in the light-touch response of Merkel cells of the skin (7, 8). A variant of PIEZO2 is also involved in the touch response of zebrafish (9). Recently, a knock-out of PIEZO1 from endothelial cells showed a link of PIEZO1 to shear stress-associated embryonic vascular development (10, 11).
The involvement of PIEZO channels in pathology was first noted for xerocytosis, a human hemolytic anemia that stems from mutations in PIEZO1 (12). The requirement of mechanosensitive channels for erythrocyte volume regulation was previously unknown and unexpected (13).
To understand what effect mutations have on PIEZO1 function, we introduced the xerocytosis mutations into human PIEZO1 and measured its response to mechanical stimuli in patch-clamp experiments. The mutations slow the inactivation rate of the channel (14–16) and also introduce a latency for activation (14).
While exploring the properties of PIEZO1, we found that it is sensitive to extracellular pH. We studied the pH effect in detail in both cell-attached and whole-cell currents from pH 6.3 to 8.3. Acidic solutions depressed the amplitude of the currents in a use-dependent manner, suggesting that the effects are associated with occupancy in the open state, which is also coupled to the inactivated state. Titrating the steady-state currents revealed an effective pK of ∼6.9.
Normal PIEZO1 channel kinetics can be well modeled with three states: open, closed, and inactivated. To help define which state of the channel is affected by protonation, we tested a mutant channel that does not inactivate (17). These currents were pH-independent, suggesting that stabilization of the inactivated state is responsible for the pH effect. To rule out the possible involvement of pH in membrane mechanics with possible effects on channel activity, we measured the changes in patch capacitance during pressure steps at low and high pH and found no effect. The loss of PIEZO1 activity at low pH appears to be an intrinsic property of the channel.
EXPERIMENTAL PROCEDURES
The bath solution contained 168 mm NaCl, 5 mm KCl, 1 mm MgCl2, 1 mm CaCl2, and 10 mm HEPES (pH 7.3; adjusted with NaOH). For cell-attached recordings, the pipette solution contained 160 mm KCl, 0.25 mm EGTA, and 10 mm HEPES, and the pH was adjusted to 6.8, 7.0, 7.3, and 8.3 with KOH and HCl. For pH 6.3 and 6.5, the solutions were buffered with MES. For whole-cell experiments, the pipette solution contained 160 mm KCl, 0.25 mm EGTA, and mm 10 HEPES (pH 7.3), and the cell was perfused with bath solutions at pH 6.5, 7.3, or 8.3.
A Hill plot of the pH effect was generated from the currents of cell-attached patches at different pH solutions in the pipette. The dependent variable (y) was the average peak current after the channels had equilibrated from use dependence. This was plotted against the H+ concentration. Because proton binding results in channel inhibition, the data were fit to Equation 1,
where Vmax is the saturating current, x is the molar concentration, k is the midpoint H+ concentration, and n describes the cooperativity of binding.
HEK293 cells were transfected with 500 ng of cDNA using TransIT-293 reagent (Mirus) according to the manufacturer's protocol and were tested 24–48 h later. Cell-attached patches were mechanically stimulated by applying suction with an HSFC-1 pressure clamp (ALA Scientific Instruments) controlled by QuBIO software.
Whole-cell mechanical stimulation utilized a fire-polished glass pipette (diameter of 2–4 μm) positioned at an angle of 30° with respect to the cover glass. The probe was coarsely positioned ∼20 μm above the cell with an MP-285 manipulator (Sutter Instruments Co.), and from that position, the probe was moved up and down with a trapezoidal waveform in a piezoelectric stage (P-280.20 XYZ NanoPositioner, Physik Instrumente). The indentation depth was controlled using LabVIEW and had 40 nm resolution. The probe velocity was 0.15 μm/ms during transitions, and the stimulus was held constant for 300 ms. Currents were recorded at a membrane potential of −80 mV for cell-attached mode and −60 mV for whole-cell experiments, and all were done at room temperature.
Patch capacitance and conductance were measured as described previously (17–19) using an EG&G 5208 dual-phase lock-in amplifier with sinusoidal stimulation at 1 kHz. The experiments were performed using an Axopatch 200B amplifier (Axon Instruments), sampled at 10 kHz, and filtered at 1 kHz. Data acquisition and stimulation were controlled by QuBIO software.
RESULTS
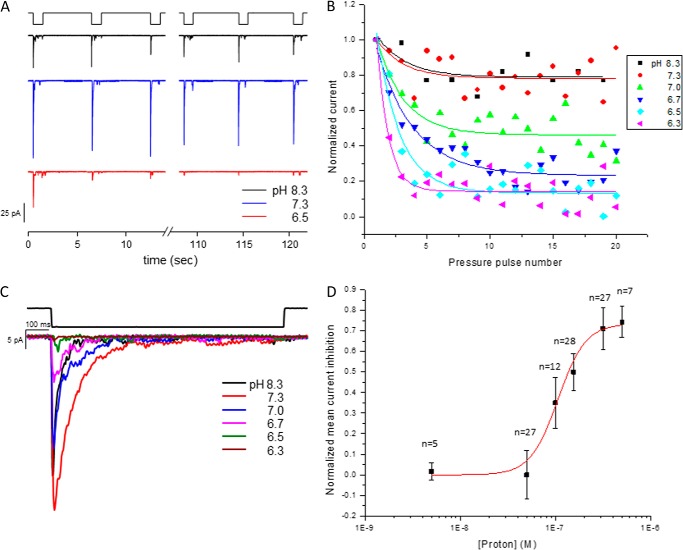
Fig. 1A shows the wild-type channel's response in cell-attached mode using three different pH solutions in the pipette (pH 6.5, 7.3, and 8.3). When a series of suction pulses were applied to the patch at pH 7.3 or 8.3, they produced consistent peak currents from pulse to pulse. At pH 6.5, there was a substantial reduction in channel activity with repeated stimuli, suggesting that the effect of pH is coupled to the open state of the channel. To determine the pH sensitivity of the sites in the channel, we titrated the response with six different pH solutions (Fig. 1B). Channels in cell-attached patches were stimulated by a series of suction pulses with a 5-s rest period between them. The time constant for the rate of decay of the peak current was fit to a first-order exponential. The system equilibrated by about the 10th pulse for all pH values. We found that the effect of use dependence increased at lower pH.
FIGURE 1.
PIEZO1 activity at different pH values in cell-attached mode. HEK293 cells were transfected with a plasmid containing the human PIEZO1 gene, and the cells were tested 24–48 h later. A shows the time course of currents elicited by a negative-pressure pulse train of −30 mm Hg at −80 mV at the indicated pH in the pipette. The suction pulses were 1 s long, followed 5 s of rest. At pH 6.5, there was a significant loss of activity within the first few pulses, and this was not observed at higher pH. B is a plot of the mean peak current from multiple patches with six different pH solutions ranging from pH 6.3 to 8.3. The normalized peak current decay was used to determine the steady-state current. C is a plot of the current for each pH at a membrane voltage of −80 mV and suction of −30 mm Hg. D is the pH profile (titration) for PIEZO1 inhibition derived from the peak currents plotted in C. The data were fit to a Hill plot with a midpoint pH of 6.9 ± 0.8 and n = 3 ± 1 (R2 = 0.98).
We defined the fiducial current for each pH as the mean of the peak currents for pulses 13, 14, and 15. The currents were normalized to the peak current of pulse 1 to reflect factors such as different patch area or channel density. Fig. 1C shows the normalized average response at the indicated pH. The normalized average peak current at each pH was fit to a Hill plot. Because proton binding causes inhibition of current, we plotted the loss of mean current as a function of pH. The midpoint (pK) was pH 6.9 ± 0.7 (Fig. 1D). The apparent Hill coefficient was 3 ± 1.0, indicating positive cooperativity; more than one site is involved.
Because we know that PIEZO1 properties can vary between patches and whole-cell recordings (3), we measured the effect of pH on whole-cell currents. To switch bath solutions, we used a perfusion tip that covered the whole cell (flow rate of 35 nl/s). Because a change in pH caused a drift in the baseline current, we quantitated the data after reaching steady state. Fig. 2A shows whole-cell currents from cells expressing PIEZO1. At low pH (6.7–6.5), the current was <20% of the control current, paralleling the cell-attached patch response. Returning the bath to pH 7.3 reversed the inhibition. Fig. 2B shows the effects on the mean peak currents.
FIGURE 2.
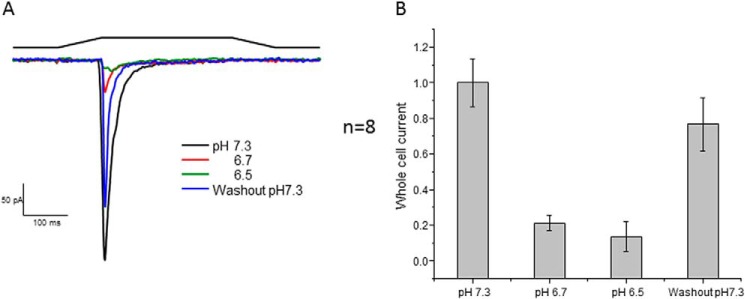
Whole-cell PIEZO1 currents generated at the indicated pH. The stimulus was indentation of the cell by a fire-polished pipette. To change the pH of the cell, we perfused it with the perfusion outlet positioned near the cell. A shows current traces with the stimulus pulses shown above. Returning to the control pH of 7.3 recovered most of the activity. B shows quantification of the change in peak current resulting from the change in pH with the S.D. (membrane potential of −60 mV).
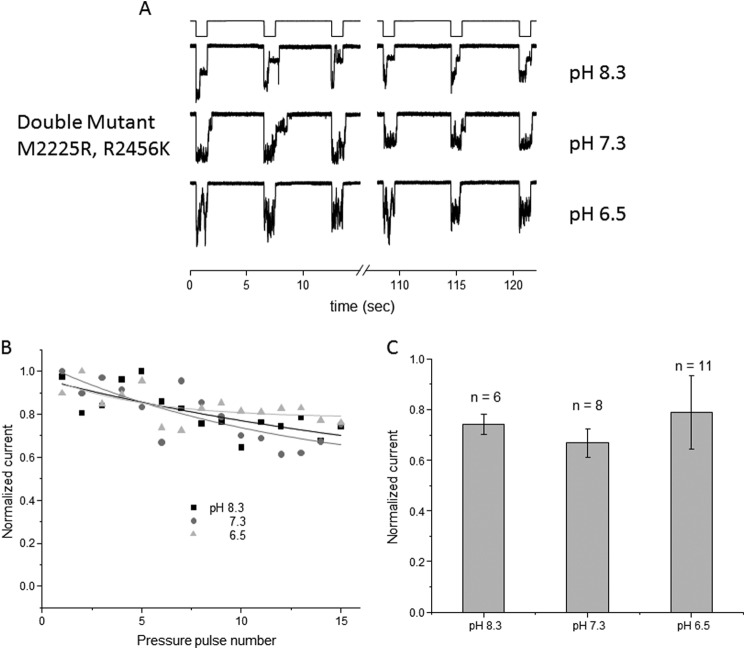
PIEZO1 has been kinetically modeled with three states: closed, open, and inactivated (14, 17). We wanted to determine which state is affected by pH, the open or the inactivated state. We demonstrated previously that inactivation can be removed by introducing two mutations, one at position 2225 and the other at position 2456; this effect is observed in both cell-attached patch and whole-cell modes (17). To ascertain whether pH perturbs inactivation, we used this double-mutant channel (DhPIEZO1 for double-mutant human PIEZO1) and investigated how changes in pH modified its activity. Fig. 3 shows the response of DhPIEZO1 as a function of pH in cell-attached recordings. Fig. 3A compares the channel currents at pH 8.3, 7.3, and 6.5. These data demonstrate that the use-dependent effect was less than observed for the wild-type channel and is pH-insensitive. Fig. 3B plots the current of DhPIEZO1 as a function of the suction pulse number, showing a slow use-dependent decline. Fig. 3C shows the average peak current versus pH.
FIGURE 3.
In cell-attached mode, the non-inactivating form of PIEZO1 (DhPIEZO1) is unaffected by pH. A is a current trace from the non-inactivating form of PIEZO1 at the indicated pipette pH values (membrane potential of −80 mV, suction of −30 mm Hg). Unlike the wild-type channel at low pH, there was only a slow change in peak current from pulse to pulse. The normalized peak current is plotted in B and quantified in C with the S.D.
There was no effect of pH on the whole-cell currents from cells expressing the double-mutant channel (Fig. 4). Fig. 4A shows whole-cell currents at pH 8.3, 7.3, and 6.5 and demonstrates that acidification did not affect use dependence. Fig. 4B summarizes the results.
FIGURE 4.
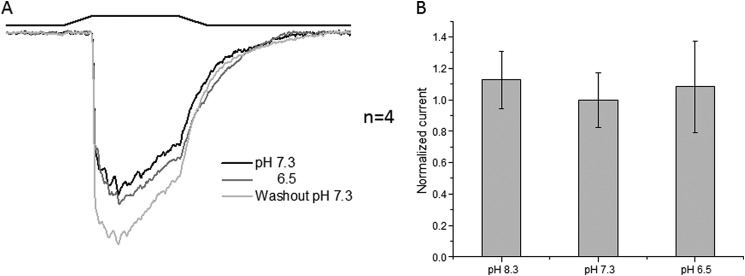
Whole-cell recordings of the non-inactivating form of PIEZO1 perfuse at the indicated pH. A shows the average peak response. Note that the rundown kinetics were no longer sensitive to pH. B shows the average peak response with the S.D. (membrane potential of −60 mV).
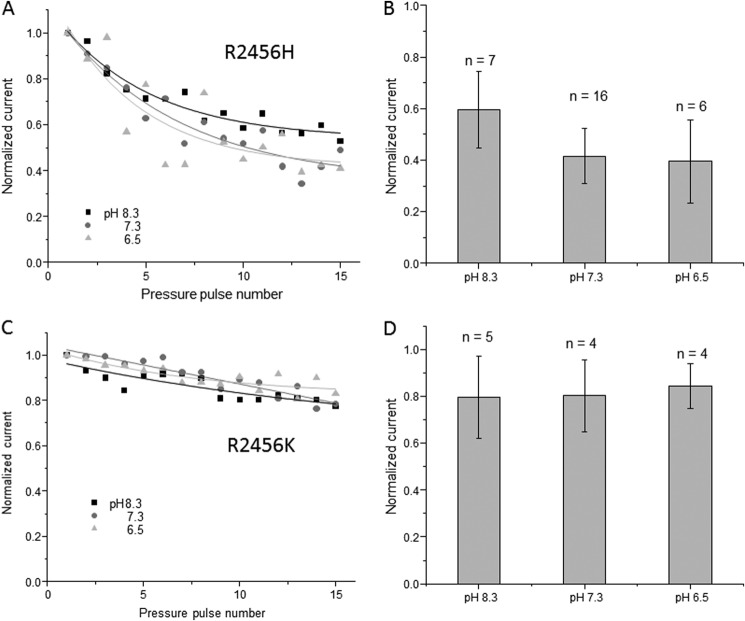
We tested two additional single-site mutations that exhibit slow inactivation (Fig. 5). One mutant, R2456H, is found in human xerocytosis. The second, R2456K, is a conservative mutation but nonetheless exhibits slow inactivation. In both cases, acidification did not affect use dependence on the timescale observed for the wild-type channel (Fig. 5, A and C), although we did see a minor rundown (Fig. 5, B and D). The change in the pH sensitivity of use dependence is easily seen when comparing the channel responses at pH 7.3 and 6.5, where, in the case of the wild-type channel, we would have seen a reduction of >80%.
FIGURE 5.
Effect of pH on cell-attached patch currents from channels with point mutations in arginine at position 2456. Two substitutions, histidine and lysine, have been shown to slow inactivation. A and C show the mean current traces of R2456H and R2456K, respectively, and their response to pipette pH. In both cases, the peak currents displayed little use dependence compared with the wild-type channel. B and D show quantification of the effect of pH on R2456H and R2456K, respectively (membrane voltage of −80 mV, suction of −30 mm Hg).
We wanted to distinguish between possible pH effects on the protein and the membrane itself, so we measured the capacitance and conductance of the patch during activation of the channel (18, 19). Fig. 6 shows that during the pressure step (light gray), the change in membrane capacitance (gray) was accompanied by changes in conductance (black) due to channel activation. However, there was no significant change in capacitance over time. The time course of the capacitance change of the first pulse was nearly identical to later ones. We performed the experiment with the wild-type channel at pH 6.5 (Fig. 6A) and pH 7.3 (Fig. 6B) and found little difference in patch impedance between the two except for the change in patch conductance reflecting the activity of the channels. This supports the idea that protonation effects are related directly to effects on the protein.
FIGURE 6.
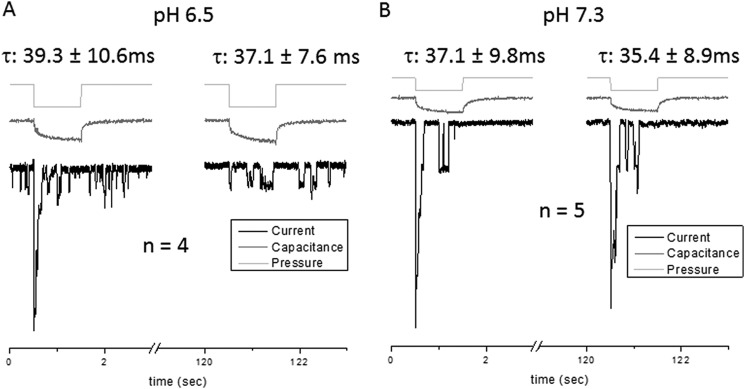
The patch capacitance and presumably the gross mechanics of the membrane are not altered at pH 6.5. We measured the conductance (black) and capacitance (gray) in cell-attached mode at pH 6.5 (A) and compared it with the response at pH 7.3 (B). With suction pulses (light gray), the PIEZO1-driven conductance increased at the first pulse, followed by a decrease in peak conductance with subsequent pulses. The time dependence of the capacitance change with pressure was fit to a first-order exponential to provide fiducial parameters. The rise time constants (τ) for both pH solutions were similar, indicating that pH does not appear to significantly modify the basic mechanics of the patch. Thus, the pH effect on channel kinetics is probably an effect upon the channel itself and not adaptation of the local forces produced by viscoelastic relaxation of the membrane (membrane voltage of −80 mV, suction of −30 mm Hg).
DISCUSSION
Mechanosensitive ion channels are often inactive in resting cells but become active in highly stressed cells or patches (20, 21). They seem to serve as detectors of excessive stress in the bilayer, probably signaling the cytoskeleton for reinforcement (22, 23). Because PIEZO1 is likely to be active under pathological stress (24, 25) and pathology often involves acidification of the environment, we decided to examine the behavior of PIEZO1 under acidic conditions to obtain a better idea of possible in situ behavior.
The experiments shown in Figs. 1 and 2 demonstrate that at low pH (≈6.3), PIEZO1 was inhibited in both cell-attached and whole-cell modes. This effect appears to be associated with the open or the inactivated state of the channel because for cell-attached patches, consecutive stimulating pulses produced use-dependent inactivation, but because normal inactivation is linked to the open state, use dependence could arise from occupying the inactivated state. The pH titration profile of wild-type channels shows a pK of ≈6.9 with an apparent Hill coefficient of ≈3, suggesting that the effect involves more than one protonation site. No one yet knows whether conduction for PIEZO1 channels occurs through a single protein or whether it forms at the interface between multimers, typically thought of as tetramers (2). It remains unclear as to whether protonation of a single protein is involved in the pH effect or whether two or more proteins are involved.
Because PIEZO1 exhibits use-dependent inhibition (via the open state), we wanted to know whether pH modulates the rate of the open to closed or the open to inactivated state. We used a mutant PIEZO channel (M2225R/R2456K) that we have previously shown to eliminate inactivation (17). We first studied this channel while characterizing mutations associated with xerocytosis because it involves individual mutations at M2225R and R2456H. We discovered that a conservative mutation, R2456K, had a more profound effect on inactivation, and by combining M2225R and R2456K (14), we were able to remove any inactivation. The removal of inactivation reduced the three-state model (closed to open to inactivated) to the two-state model (closed to open). Using this non-inactivating channel to study the effect of pH allowed us to explore whether protonation was acting on the inactivated or the open state.
The double-mutant channel was insensitive to pH in both cell-attached and whole-cell modes. This suggests that the titrated residues are in a domain associated with inactivation (Figs. 3 and 4). The effect could be explained by a drop in energy of the inactivated state, slowing the return transition from the inactivated to the open state. When we tested the two mutations (R2456H and R2456K) that slowed inactivation, they also were pH-insensitive (Fig. 5). It is unlikely that the protonation event is directly related to these two residues. The methionine is thought to be on the extracellular side and is not ionizable, whereas the arginine is supposedly on the intracellular side.
An interesting question concerns the role of pH in the physiology of cells in situ. We know that PIEZO1 is present in red blood cells (16), but it is unlikely that the pH effect is important in normal physiology given that blood pH is well regulated. However, acidosis is common in trauma, such as myocardial infarction, where a significant drop in pH is brought on by respiratory acidosis (26). Based on our data, PIEZO1 current would be inhibited by the drop in pH, and this may serve as a protective mechanism to limit excessive cation fluxes. Our data also show that mutant channels linked to xerocytosis inactivate more slowly and are not inhibited by low pH, so if those red cells were exposed to traumatized tissue, there might be increased hemolysis.
Footnotes
This work was supported, in whole or in part, by National Institutes of Health Grant 5R01HL054887-19 (to F. S.) through the NHLBI. This work was also supported by the United States Army Research Office (to F. S.).
REFERENCES
- 1. Coste B., Mathur J., Schmidt M., Earley T. J., Ranade S., Petrus M. J., Dubin A. E., Patapoutian A. (2010) Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330, 55–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coste B., Xiao B., Santos J. S., Syeda R., Grandl J., Spencer K. S., Kim S. E., Schmidt M., Mathur J., Dubin A. E., Montal M., Patapoutian A. (2012) Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 483, 176–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gottlieb P. A., Bae C., Sachs F. (2012) Gating the mechanical channel Piezo1: a comparison between whole-cell and patch recording. Channels 6, 282–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gottlieb P. A., Sachs F. (2012) Piezo1: properties of a cation selective mechanical channel. Channels 6, 214–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Suchyna T. M., Tape S. E., Koeppe R. E., 2nd, Andersen O. S., Sachs F., Gottlieb P. A. (2004) Bilayer-dependent inhibition of mechanosensitive channels by neuroactive peptide enantiomers. Nature 430, 235–240 [DOI] [PubMed] [Google Scholar]
- 6. Kim S. E., Coste B., Chadha A., Cook B., Patapoutian A. (2012) The role of Drosophila Piezo in mechanical nociception. Nature 483, 209–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maksimovic S., Nakatani M., Baba Y., Nelson A. M., Marshall K. L., Wellnitz S. A., Firozi P., Woo S. H., Ranade S., Patapoutian A., Lumpkin E. A. (2014) Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature 509, 617–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Woo S. H., Ranade S., Weyer A. D., Dubin A. E., Baba Y., Qiu Z., Petrus M., Miyamoto T., Reddy K., Lumpkin E. A., Stucky C. L., Patapoutian A. (2014) Piezo2 is required for Merkel-cell mechanotransduction. Nature 509, 622–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Faucherre A., Nargeot J., Mangoni M. E., Jopling C. (2013) piezo2b regulates vertebrate light touch response. J. Neurosci. 33, 17089–17094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ranade S. S., Qiu Z., Woo S. H., Hur S. S., Murthy S. E., Cahalan S. M., Xu J., Mathur J., Bandell M., Coste B., Li Y. S., Chien S., Patapoutian A. (2014) Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc. Natl. Acad. Sci. U.S.A. 111, 10347–10352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li J., Hou B., Tumova S., Muraki K., Bruns A., Ludlow M. J., Sedo A., Hyman A. J., McKeown L., Young R. S., Yuldasheva N. Y., Majeed Y., Wilson L. A., Rode B., Bailey M. A., Kim H. R., Fu Z., Carter D. A., Bilton J., Imrie H., Ajuh P., Dear T. N., Cubbon R. M., Kearney M. T., Prasad K. R., Evans P. C., Ainscough J. F., Beech D. J. (2014) Piezo1 integration of vascular architecture with physiological force. Nature 515, 279–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zarychanski R., Schulz V. P., Houston B. L., Maksimova Y., Houston D. S., Smith B., Rinehart J., Gallagher P. G. (2012) Mutations in the mechanotransduction protein PIEZO1 are associated with hereditary xerocytosis. Blood 120, 1908–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Faucherre A., Kissa K., Nargeot J., Mangoni M. E., Jopling C. (2014) Piezo1 plays a role in erythrocyte volume homeostasis. Haematologica 99, 70–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bae C., Gnanasambandam R., Nicolai C., Sachs F., Gottlieb P. A. (2013) Xerocytosis is caused by mutations that alter the kinetics of the mechanosensitive channel PIEZO1. Proc. Natl. Acad. Sci. U.S.A. 110, E1162–E1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Albuisson J., Murthy S. E., Bandell M., Coste B., Louis-Dit-Picard H., Mathur J., Fénéant-Thibault M., Tertian G., de Jaureguiberry J. P., Syfuss P. Y., Cahalan S., Garçon L., Toutain F., Simon Rohrlich P., Delaunay J., Picard V., Jeunemaitre X., Patapoutian A. (2013) Dehydrated hereditary stomatocytosis linked to gain-of-function mutations in mechanically activated PIEZO1 ion channels. Nat. Commun. 4, 1884–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Andolfo I., Alper S. L., De Franceschi L., Auriemma C., Russo R., De Falco L., Vallefuoco F., Esposito M. R., Vandorpe D. H., Shmukler B. E., Narayan R., Montanaro D., D'Armiento M., Vetro A., Limongelli I., Zuffardi O., Glader B. E., Schrier S. L., Brugnara C., Stewart G. W., Delaunay J., Iolascon A. (2013) Multiple clinical forms of dehydrated hereditary stomatocytosis arise from mutations in PIEZO1. Blood 121, 3925–3935, S1–S12 [DOI] [PubMed] [Google Scholar]
- 17. Bae C., Gottlieb P. A., Sachs F. (2013) Human PIEZO1: removing inactivation. Biophys. J. 105, 880–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Suchyna T. M., Besch S. R., Sachs F. (2004) Dynamic regulation of mechanosensitive channels: capacitance used to monitor patch tension in real time. Phys. Biol. 1, 1–18 [DOI] [PubMed] [Google Scholar]
- 19. Suchyna T. M., Markin V. S., Sachs F. (2009) Biophysics and structure of the patch and the gigaseal. Biophys. J. 97, 738–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yeung E. W., Whitehead N. P., Suchyna T. M., Gottlieb P. A., Sachs F., Allen D. G. (2005) Effects of stretch-activated channel blockers on [Ca2+]i and muscle damage in the mdx mouse. J. Physiol. 562, 367–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Suchyna T. M., Sachs F. (2007) Mechanosensitive channel properties and membrane mechanics in mouse dystrophic myotubes. J. Physiol. 581, 369–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gailly P., De Backer F., Van Schoor M., Gillis J. M. (2007) In situ measurements of calpain activity in isolated muscle fibres from normal and dystrophin-lacking mdx mice. J. Physiol. 582, 1261–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ducret T., Vandebrouck C., Cao M. L., Lebacq J., Gailly P. (2006) Functional role of store-operated and stretch-activated channels in murine adult skeletal muscle fibres. J. Physiol. 575, 913–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sukharev S., Sachs F. (2012) Molecular force transduction by ion channels: diversity and unifying principles. J. Cell Sci. 125, 3075–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sachs F. (2010) Stretch-activated ion channels; what are they? Physiology 25, 50–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chazan J. A., Stenson R., Kurland G. S. (1968) The acidosis of cardiac arrest. N. Engl. J. Med. 278, 360–364 [DOI] [PubMed] [Google Scholar]