Abstract
The capacity of HeLa cell mitochondria, either isolated or in intact cells, to incorporate different labeled amino acids into proteins was investigated. Eight amino acids (alanine, arginine, aspartic acid, cysteine, glutamic acid, glutamine, glycine, and lysine), which include most of the charged polar ones, showed a very low amount, if any at all, of chloramphenicol-sensitive incorporation, relative to that expected for an “average” HeLa-cell protein. By contrast, the most hydrophobic amino acids (leucine, isoleucine, valine, phenylalanine, and methionine) were the most actively incorporated by HeLa mitochondria. The available evidence suggests that pool effects cannot account for this general pattern of utilization of amino acids; furthermore, this pattern is in good agreement with the known hydrophobic properties of proteins synthesized in mitochondria.
Keywords: chloramphenicol, endoplasmic reticulum, amino-acid pools, mitochondrial tRNA
Full text
PDF
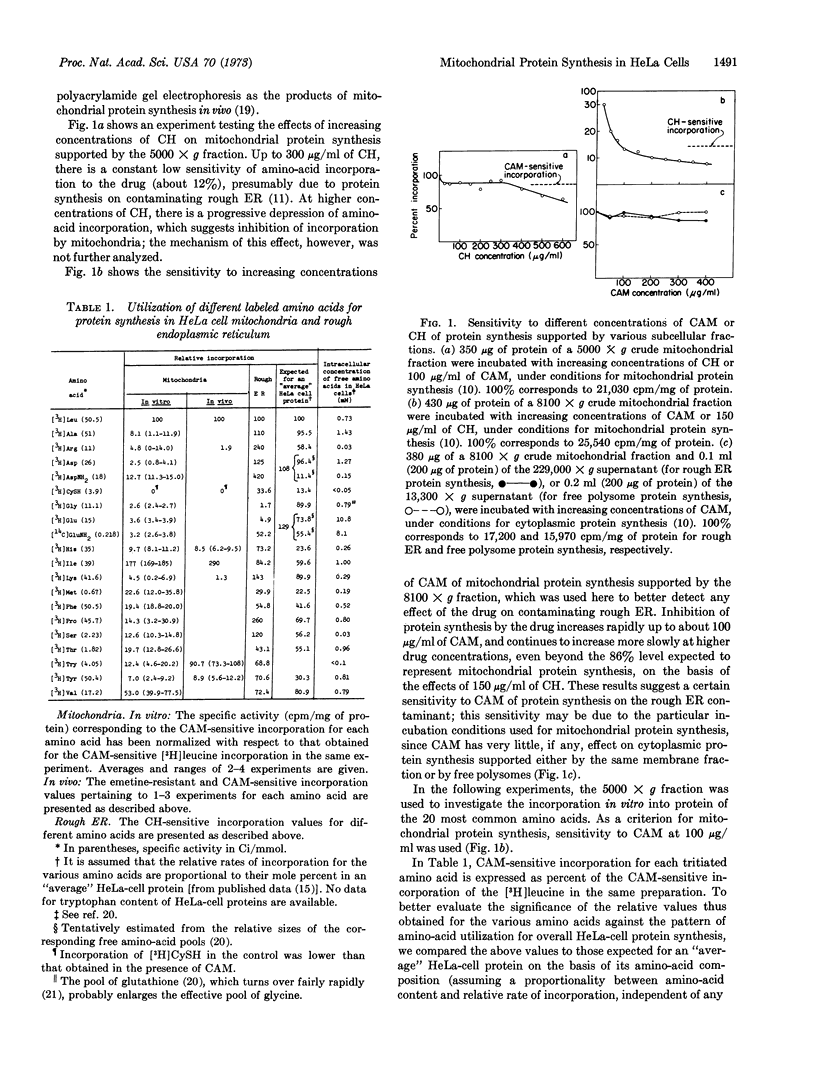
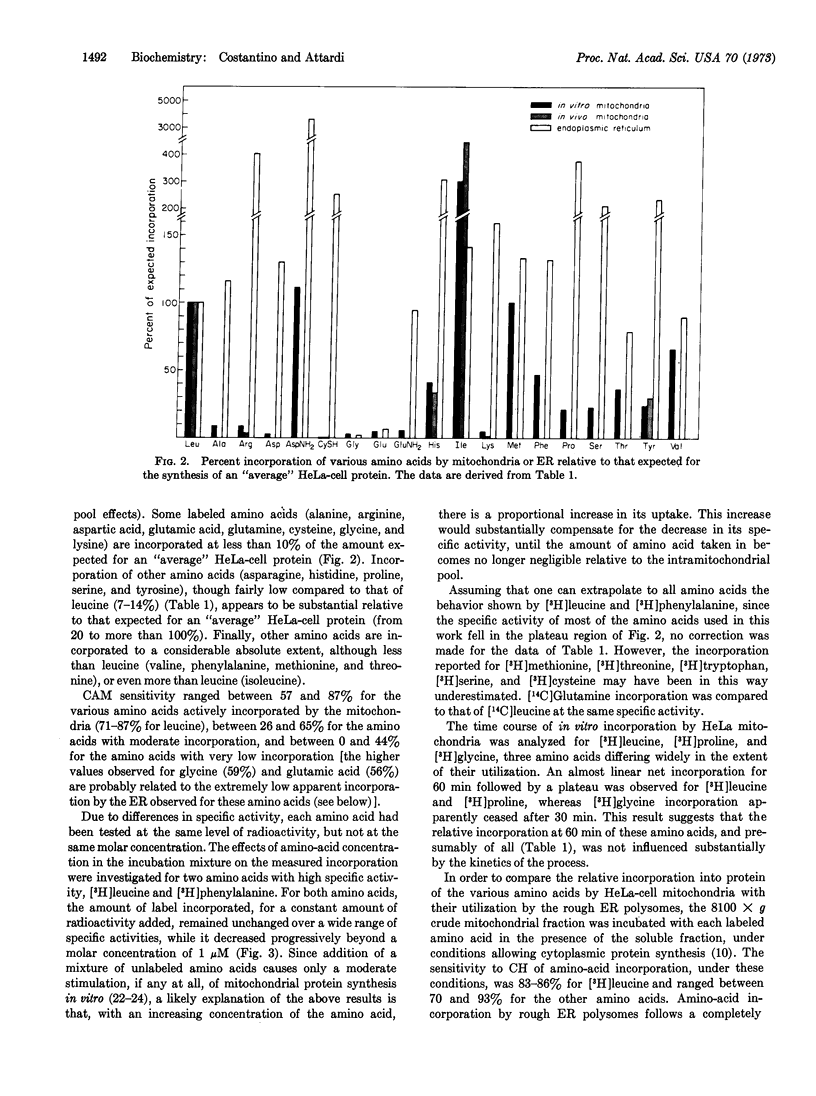
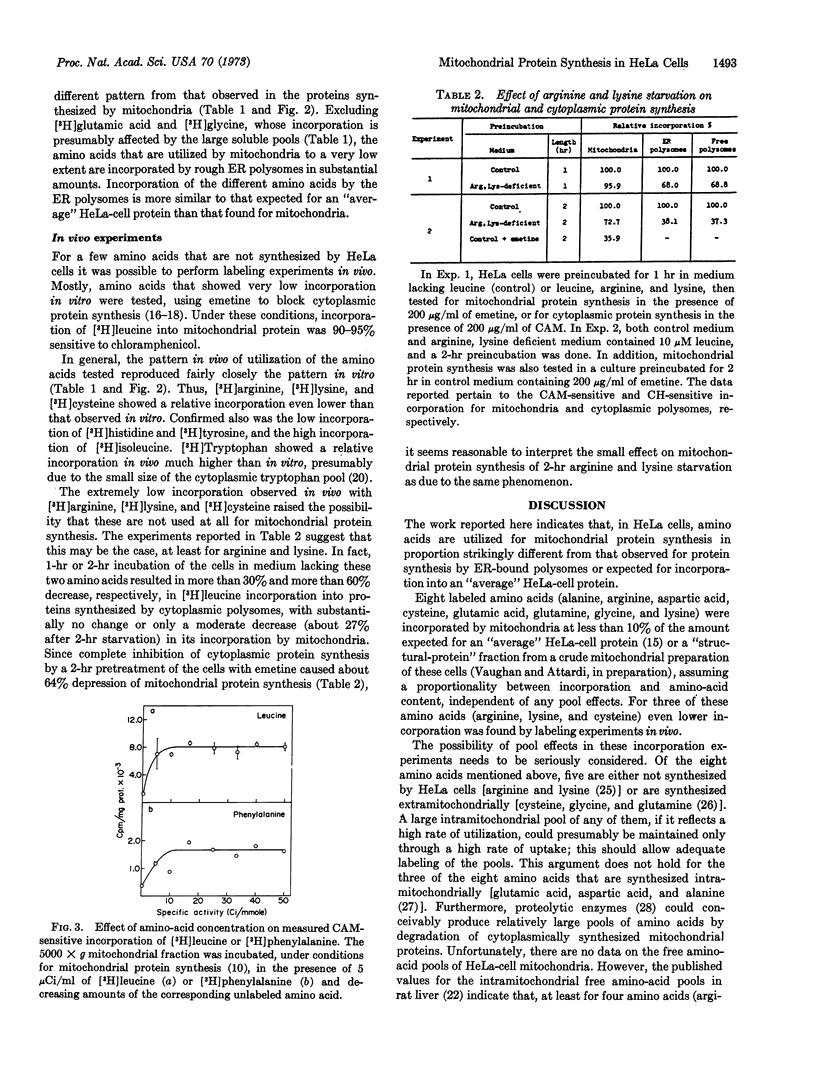

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBERTI K. G., BARTLEY W. FURTHER OBSERVATIONS ON THE PRODUCTION OF AMINO ACIDS BY RAT-LIVER MITOCHONDRIA AND OTHER SUBCELLULAR FRACTIONS. Biochem J. 1965 Jun;95:641–656. doi: 10.1042/bj0950641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni Y., Attardi G. Expression of the mitochondria genome in HeLa cells. IV. Titration of mitochondrial genes for 16 s, 12 s and 4 s RNA. J Mol Biol. 1971 Jan 28;55(2):271–276. doi: 10.1016/0022-2836(71)90197-5. [DOI] [PubMed] [Google Scholar]
- Amaldi F., Attardi G. Partial sequence analysis of ribosomal RNA from HeLa cells. I. Oligonucleotide pattern of 28 s and 18 s RNA after pancreatic ribonuclease digestion. J Mol Biol. 1968 May 14;33(3):737–755. doi: 10.1016/0022-2836(68)90317-3. [DOI] [PubMed] [Google Scholar]
- Ashwell M., Work T. S. The biogenesis of mitochondria. Annu Rev Biochem. 1970;39:251–290. doi: 10.1146/annurev.bi.39.070170.001343. [DOI] [PubMed] [Google Scholar]
- Attardi B., Cravioto B., Attardi G. Membrane-bound ribosomes in HeLa cells. I. Their proportion to total cell ribosomes and their association with messenger RNA. J Mol Biol. 1969 Aug 28;44(1):47–70. doi: 10.1016/0022-2836(69)90404-5. [DOI] [PubMed] [Google Scholar]
- Barnett W. E., Brown D. H. Mitochondrial transfer ribonucleic acids. Proc Natl Acad Sci U S A. 1967 Feb;57(2):452–458. doi: 10.1073/pnas.57.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P., Grivell L. A. Mitochondrial ribosomes. FEBS Lett. 1971 Feb 19;13(2):73–88. doi: 10.1016/0014-5793(71)80204-1. [DOI] [PubMed] [Google Scholar]
- Borst P. Mitochondrial nucleic acids. Annu Rev Biochem. 1972;41:333–376. doi: 10.1146/annurev.bi.41.070172.002001. [DOI] [PubMed] [Google Scholar]
- Buck C. A., Nass M. M. Studies on mitochondrial tRNA from animal cells. I. A comparison of mitochondrial and cytoplasmic trna and aminoacyl-tRNA synthetases. J Mol Biol. 1969 Apr 14;41(1):67–82. doi: 10.1016/0022-2836(69)90126-0. [DOI] [PubMed] [Google Scholar]
- Casey J., Cohen M., Rabinowitz M., Fukuhara H., Getz G. S. Hybridization of mitochondrial transfer RNA's with mitochondrial and nuclear DNA of grande (wild type) yeast. J Mol Biol. 1972 Feb 14;63(3):431–440. doi: 10.1016/0022-2836(72)90438-x. [DOI] [PubMed] [Google Scholar]
- Dawid I. B. Mitochondrial RNA In Xenopus laevis. I. The expression of the mitochondrial genome. J Mol Biol. 1972 Jan 28;63(2):201–216. doi: 10.1016/0022-2836(72)90370-1. [DOI] [PubMed] [Google Scholar]
- EAGLE H. The specific amino acid requirements of a human carcinoma cell (Stain HeLa) in tissue culture. J Exp Med. 1955 Jul 1;102(1):37–48. doi: 10.1084/jem.102.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firkin F. C., Linnane A. W. Differential effects of chloramphenicol on the growth and respiration of mammalian cells. Biochem Biophys Res Commun. 1968 Aug 13;32(3):398–402. doi: 10.1016/0006-291x(68)90674-8. [DOI] [PubMed] [Google Scholar]
- Galper J. B., Darnell J. E. The presence of N-formyl-methionyl-tRNA in HeLa cell mitochondria. Biochem Biophys Res Commun. 1969 Jan 27;34(2):205–214. doi: 10.1016/0006-291x(69)90633-0. [DOI] [PubMed] [Google Scholar]
- Grollman A. P. Structural basis for inhibition of protein synthesis by emetine and cycloheximide based on an analogy between ipecac alkaloids and glutarimide antibiotics. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1867–1874. doi: 10.1073/pnas.56.6.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENRIQUES S. B., HENRIQUES O. B., MANDELBAUM F. R. Incorporation of glycine into glutathione and fibrinogen of rats under adrenaline treatment. Biochem J. 1957 Jun;66(2):222–227. doi: 10.1042/bj0660222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KROON A. M. PROTEIN SYNTHESIS IN MITOCHONDRIA. II. A COMPARISON OF MITOCHONDRIA FROM LIVER AND HEART WITH SPECIAL REFERENCE TO THE ROLE OF OXIDATIVE PHOSPHORYLATION. Biochim Biophys Acta. 1964 Sep 11;91:145–154. [PubMed] [Google Scholar]
- Kroon A. M. Protein synthesis in mitochondria. 3. On the effects of inhibitors on the incorporation of amino acids into protein by intact mitochondria and digitonin fractions. Biochim Biophys Acta. 1965 Oct 11;108(2):275–284. doi: 10.1016/0005-2787(65)90012-2. [DOI] [PubMed] [Google Scholar]
- LEVINTOW L., DARNELL J. E., Jr A simplified procedure for purification of large amounts of poliovirus: characterization and amino acid analysis of Type 1 poliovirus. J Biol Chem. 1960 Jan;235:70–73. [PubMed] [Google Scholar]
- Lederman M., Attardi G. In vitro protein synthesis in a mitochondrial fraction from HeLa cells: sensitivity to antibiotic and ethidium bromide. Biochem Biophys Res Commun. 1970 Sep 30;40(6):1492–1500. doi: 10.1016/0006-291x(70)90037-9. [DOI] [PubMed] [Google Scholar]
- Nass M. M., Buck C. A. Studies on mitochondrial tRNA from animal cells. II. Hybridization of aminoacyl-tRNA from rat liver mitochondria with heavy and light complementary strands of mitochondrial DNA. J Mol Biol. 1970 Dec 14;54(2):187–198. doi: 10.1016/0022-2836(70)90426-2. [DOI] [PubMed] [Google Scholar]
- Ojala D., Attardi G. Expression of the mitochondrial genome in HeLa cells. X. Properties of mitochondrial polysomes. J Mol Biol. 1972 Mar 28;65(2):273–289. doi: 10.1016/0022-2836(72)90282-3. [DOI] [PubMed] [Google Scholar]
- PIEZ K. A., EAGLE H. The free amino acid pool of cultured human cells. J Biol Chem. 1958 Mar;231(1):533–545. [PubMed] [Google Scholar]
- Perlman S., Penman S. Mitochondrial protein synthesis: resistance to emetine and response to RNA synthesis inhibitors. Biochem Biophys Res Commun. 1970 Aug 24;40(4):941–948. doi: 10.1016/0006-291x(70)90994-0. [DOI] [PubMed] [Google Scholar]
- Perlman S., Penman S. Protein-synthesizing structures associated with mitochondria. Nature. 1970 Jul 11;227(5254):133–137. doi: 10.1038/227133a0. [DOI] [PubMed] [Google Scholar]
- Reijnders L., Borst P. The number of 4-S RNA genes on yeast mitochondrial DNA. Biochem Biophys Res Commun. 1972 Apr 14;47(1):126–133. doi: 10.1016/s0006-291x(72)80019-6. [DOI] [PubMed] [Google Scholar]
- TRUMAN D. E., KORNER A. Incorporation of amino acids into the protein of isolated mitochondria. A search for optimum conditions and a relationship to oxidative phosphorylation. Biochem J. 1962 Jun;83:588–596. doi: 10.1042/bj0830588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeldon L. W., Lehninger A. L. Energy-linked synthesis and decay of membrane proteins in isolated rat liver mitochondria. Biochemistry. 1966 Nov;5(11):3533–3545. doi: 10.1021/bi00875a021. [DOI] [PubMed] [Google Scholar]
- Wu M., Davidson N., Attardi G., Aloni Y. Expression of the mitochondrial genome in HeLa cells. XIV. The relative positions of the 4 S RNA genes and of the ribosomal RNA genes in mitochondrial DNA. J Mol Biol. 1972 Oct 28;71(1):81–93. doi: 10.1016/0022-2836(72)90402-0. [DOI] [PubMed] [Google Scholar]