Abstract
IMPORTANCE
Hypothermia at 33.5°C for 72 hours for neonatal hypoxic ischemic encephalopathy reduces death or disability to 44% to 55%; longer cooling and deeper cooling are neuroprotective in animal models.
OBJECTIVE
To determine if longer duration cooling (120 hours), deeper cooling (32.0°C), or both are superior to cooling at 33.5°C for 72 hours in neonates who are full-term with moderate or severe hypoxic ischemic encephalopathy.
DESIGN, SETTING, AND PARTICIPANTS
Arandomized, 2 × 2 factorial design clinical trial performed in 18 US centers in the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network between October 2010 and November 2013.
INTERVENTIONS
Neonates were assigned to 4 hypothermia groups; 33.5°C for 72 hours, 32.0°C for 72 hours, 33.5°C for 120 hours, and 32.0°C for 120 hours.
MAIN OUTCOMES AND MEASURES
The primary outcome of death or disability at 18 to 22 months is ongoing. The independent data and safety monitoring committee paused the trial to evaluate safety (cardiac arrhythmia, persistent acidosis, major vessel thrombosis and bleeding, and death in the neonatal intensive care unit [NICU]) after the first 50 neonates were enrolled, then after every subsequent 25 neonates. The trial was closed for emerging safety profile and futility analysis after the eighth review with 364 neonates enrolled (of 726 planned). This report focuses on safety and NICU deaths by marginal comparisons of 72 hours’ vs 120 hours’ duration and 33.5°C depth vs 32.0°C depth (predefined secondary outcomes).
RESULTS
The NICU death rates were 7 of 95 neonates (7%) for the 33.5°C for 72 hours group, 13 of 90 neonates (14%) for the 32.0°C for 72 hours group, 15 of 96 neonates (16%) for the 33.5°C for 120 hours group, and 14 of 83 neonates (17%) for the 32.0°C for 120 hours group. The adjusted risk ratio (RR) for NICU deaths for the 120 hours group vs 72 hours group was 1.37 (95% CI, 0.92–2.04) and for the 32.0°C group vs 33.5°C group was 1.24 (95% CI, 0.69–2.25). Safety outcomes were similar between the 120 hours group vs 72 hours group and the 32.0°C group vs 33.5°C group, except major bleeding occurred among 1% in the 120 hours group vs 3% in the 72 hours group (RR, 0.25 [95% CI, 0.07–0.91]). Futility analysis determined that the probability of detecting a statistically significant benefit for longer cooling, deeper cooling, or both for NICU death was less than 2%.
CONCLUSIONS AND RELEVANCE
Among neonates who were full-term with moderate or severe hypoxic ischemic encephalopathy, longer cooling, deeper cooling, or both compared with hypothermia at 33.5°C for 72 hours did not reduce NICU death. These results have implications for patient care and design of future trials.
Hypoxic ischemic encephalopathy due to acute perinatal asphyxia is an important cause of childhood neurodevelopmental deficits among infants who were born at full-term. Five randomized clinical trials of induced hypothermia at 33.0°C to 34.0°C for 72 hours have demonstrated a decrease in death or disability up to 24 months of age.1–7 This neuroprotection continues to childhood8–10; however, the rate of death or disability in the cooled group remains high (range, 44%–55%).1–7
Preclinical animal studies have demonstrated that brain injury following hypoxic ischemia continues and evolves over days and weeks after the initial injury. Deeper cooling compared with normothermia has been noted to minimize brain swelling, preserve cerebral energy metabolism, and suppress oxidative metabolism, and longer cooling may protect against apoptosis and inflammation initiated during reperfusion.11–17 These studies suggest that cooling below 33.5°C and beyond 72 hours may provide greater neuroprotection.
Methods
The study protocol was approved by the institutional review board at each site. Written informed consent was obtained from a parent or guardian. This study was a multicenter randomized clinical trial of whole-body hypothermia among infants who were born at full-term with moderate or severe hypoxic ischemic encephalopathy to evaluate the safety and effectiveness of deeper cooling, longer cooling, or both. This study was conducted at all the 18 US sites participating in the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Multicenter Neonatal Research Network between October 20, 2010, and November 27, 2013. RTI International (trade name for the Research Triangle Institute) was the data coordinating center for the Neonatal Research Network.
Criteria for eligibility and details of cooling and rewarming were similar to the first NICHD hypothermia randomized clinical trial.2 Each principal investigator certified additional neonatologists to perform the neurological examinations, and a training session for research personnel was held to standardize study procedures.2
Neonates were screened for eligibility if they had a gestational age of at least 36 weeks and were admitted to the neonatal intensive care unit (NICU) within 6 hours of birth with either poor respiratory effort at birth, a need for resuscitation, or a diagnosis of encephalopathy.
Neonates were evaluated according to physiological criteria and subsequently by a neurological examination.2 Eligibility criteria included a pH of 7.0 or less or a base deficit of 16 mmol/L or more in a sample of umbilical cord blood or any blood during the first hour after birth. If, during this interval, a pH was between 7.01 and 7.15, a base deficit was between 10 mmol/L and 15.9 mmol/L, or a blood gas was not available, additional criteria were required. These included an acute perinatal event (eg, late or variable decelerations, cord prolapse, cord rupture, uterine rupture, maternal trauma, hemorrhage, or acute cardiorespiratory arrest) and either a 10-minute Apgar score of 5 or less or assisted ventilation initiated at birth and continued for at least 10 minutes.
Once these criteria were met, all neonates underwent a standardized neurological examination. Neonates were candidates for the study when seizures or moderate or severe encephalopathy was present.2 Encephalopathy was defined as the presence of either moderate or severe signs in at least 3 of the following 6 categories: (1) level of consciousness (moderate is lethargic, severe is stupor or coma), (2) spontaneous activity (moderate is decreased activity, severe is no activity), (3) posture (moderate is distal flexion or complete extension, severe is decerebrate), (4) tone (moderate is hypotonia, severe is flaccid), (5) primitive reflexes (moderate is a weak suck [or incomplete Moro reflex], severe is an absent suck [or absent Moro reflex]), and (6) autonomic nervous system; either pupils (moderate is constricted, severe is deviated, dilated, or nonreactive to light), heart rate (moderate is bradycardia, severe is variable heart rate), or respiration (moderate is periodic breathing, severe is apnea). The number of moderate or severe signs determined the extent of the encephalopathy; if signs were equally distributed, the designation was based on the level of consciousness. Exclusion criteria were an inability to randomize within 6 hours of birth, a major congenital abnormality, severe growth restriction (birth weight <1800 g), a core temperature less than 32.5°C for at least 2 hours at the time of random assignment, and refusal of consent by a parent or an attending neonatologist; moribund neonates for whom no further treatment was planned also were excluded.
Treatment
Neonates were randomly assigned by telephone by the data coordinating center. Assignments were stratified according to center and level of encephalopathy (moderate or severe) in a 2 × 2 factorial design to 33.5°C or 32.0°C and to 72 hours or 120 hours. Assignments were generated by a random, permuted block algorithm with a block size of 4 or 8. As cooling for neonatal hypoxic ischemic encephalopathy was usual care in all sites, clinical cooling to 33.5°C was initiated when the neonate met criteria for cooling while trial consent was being sought.
Neonates were placed on a blanket, 25 in × 33 in (64 cm × 84 cm), precooled to 5°C (Blanketrol II Hyper-Hypothermia system, Cincinnati Sub-Zero). A second blanket, 25 in × 64 in (64 cm × 163 cm), was attached to the cooling system. Water circulated simultaneously through both blankets to diminish the variability in the esophageal temperature.2 An esophageal probe was inserted and the core temperature was lowered to 33.5°C by the blanket’s servomechanism in both 33.5°C study groups. To dampen overshoot during induction of cooling in the 32.0°C groups, the esophageal temperature was initially lowered to 33.5°C and, once stable for15 minutes at this temperature, lowered further to 32.0°C. Neither an overhead warmer nor any other heat source was used during cooling. Abdominal wall skin temperature was monitored with a skin probe by means of either the radiant warmer (with the heater turned off) or a temperature-monitoring unit (Mon-a-therm, Mallinckrodt Medical). Esophageal and skin temperatures were monitored continuously and recorded every 15 minutes for the first 4 hours, every hour for the next 8 hours, and every 4 hours during the remaining period of cooling.
After completing either 72 or 120 hours of hypothermia, the set point of the automatic control of the cooling system was increased by 0.5°C per hour until the esophageal temperature reached 36.5°C to 37.0°C for 4 hours. The esophageal probe was removed, and skin temperature was used to control the radiant warmer. If the skin and set point temperature remained low when the esophageal probe was removed, the warmer control temperature was set at 0.5°C higher than the skin temperature and was increased 0.5°C every hour until the warmer set point reached 36.5°C. Temperatures were monitored for the first 10 days and hyperthermia treated as per usual care at the center. Blood gas determinations were corrected for body temperature. Neonates received the center’s routine clinical care, including monitoring of vital signs and surveillance for organ dysfunction. Information on the race of the neonate was obtained by maternal interview to evaluate distribution across study groups.
Monitoring of Study
An independent data and safety monitoring committee (DSMC) appointed by the director of the NICHD monitored interim data and evaluated safety. As specified in the protocol, the study was paused for evaluation of safety (head sonograms for thrombosis or hemorrhage) after the first 50 neonates were enrolled. Once approved for resumption, safety reviews were planned after every 25 neonates were enrolled, with the data coordinating center to conduct these interim safety analyses and convey them to the DSMC if any safety concerns were apparent.
Outcomes
The primary outcome was death or disability (moderate or severe) at 18 to 22 months.18 Data collection at these ages has not yet been completed and will be reported later. The NICHD Neonatal Research Network steering committee of principal investigators decided to report the secondary outcomes during hospitalization because of the implications of the results for patient care and the design of future trials. Secondary outcomes reported here include deaths in the NICU, number of neonates with support withdrawn, frequency of adverse events, and clinical seizures. Adverse events monitored included cardiac arrhythmia, persistent acidosis, major vessel thrombosis or bleeding, and alteration of skin integrity (eTable 1 in the Supplement). Other adverse events (eTable 2 in the Supplement) and hospital outcomes were predefined. Any equipment malfunction that caused interruption of cooling was noted. All serious adverse events were reported within 72 hours to the data center and site institutional review board per local guidelines.
Statistical Analysis
Sample size calculations assumed that there were no large statistical interactions between depth and duration of cooling. The primary goal was the marginal comparison of (1) cooling to 33.5°C vs 32.0°C and (2) cooling for 72 hours vs 120 hours. A requirement for a sample size of 726 neonates (363 per marginal group comparison) was based on a 2-tailed α of .05, a statistical power of 80%, a 5% loss to follow-up, and a comparison of death or disability of 37.5% and 27.5% in the 2 marginal comparative groups (based on assumed outcome rates of 45% in the 33.5°C for 72 hours group, 30% in the 32.0°C for 72 hours and 33.5°C for 120 hours groups, and 25% in the 32.0°C for 120 hours group). The outcome rate of death or moderate or severe disability at 18 months of age of 45% for infants cooled in the 33.5°C for 72 hours group is based on our first trial.2
All data analyses were performed according to the intention-to-treat principle. There was no missing data for safety outcomes and minimal missing data for in-hospital outcomes, except bradycardia (190 missing in-hospital outcomes), which was not collected for the entire study period. The statistical software used was SAS (SAS Institute), version 9.3.
Maternal and neonatal variables were compared using the Fisher exact test for categorical variables, and the median or Wilcoxon 2-sample t test for continuous variables. Binary (yes or no) safety and other in-hospital outcomes were assessed using generalized estimating equations models for binary data, using a log link, to obtain adjusted relative risk estimates for the treatment effect adjusted for level of encephalopathy at random assignment, while accounting for any intracenter correlations in the outcomes. Continuous outcomes were similarly assessed using linear regression accounting for intracenter correlations, with log transformations used wherever necessary. To account for differential exposure periods, safety outcomes assessed during the intervention were compared between the 72 hours and 120 hours groups using Poisson regression in a generalized estimating equations framework to model the number of such events that occurred during the intervention, with an offset for the exposure time (3 days for the 72 hours group and 5 days for the 120 hours group), while adjusting for level of encephalopathy and intracenter correlation. Treatment interactions between the 2 factors (deeper and longer cooling) in this factorial design and in-hospital mortality also were assessed. All reported P values are 2-sided and not adjusted for multiple comparisons. A P value less than .05 was considered significant. Stopping rules for safety in the form of Pocock bounds19 were calculated to reflect the number of planned interim safety looks. Interim futility analyses requested by the DSMC were conducted by estimating the conditional power to detect a statistically significant treatment effect, given the available data, and assuming the hypothesized effect size for the unobserved data.
Results
The study was initiated on October 20, 2010. As specified in the protocol, patient accrual was paused from August 6, 2011, to September 15, 2011, after 50 neonates were enrolled and head sonograms evaluated for the presence of thrombosis or hemorrhage. The DSMC concluded that the trial should continue. The DSMC requested performance of head sonograms for the next 50 neonates. In addition, the DSMC requested data collection on bradycardia (sustained heart rate of <70 beats/min). At the third DSMC review, after 100 neonates were enrolled, the DSMC determined that head sonogram monitoring could cease (no safety concerns, particularly thrombosis) and recommended the study move forward. At the fourth review, detailed narratives were requested on all in-hospital deaths. The study continued following the next 3 DSMC reviews. At the eighth review on November 27, 2013, after 364 neonates were enrolled, although the interim safety data did not satisfy the prespecified safety stopping bounds, upon review of the emerging safety profile and the futility analyses, the DSMC recommended to the director of NICHD that the trial be closed to further enrollment.
At study closure, 1261 neonates were screened, 514 were eligible, and 364 were enrolled (Figure 1). One hundred eighty five neonates were assigned to the 72 hours group and 179 to the 120 hours group; 191 were assigned to the 33.5°C group and 173 to the 32.0°C group.
Figure 1.
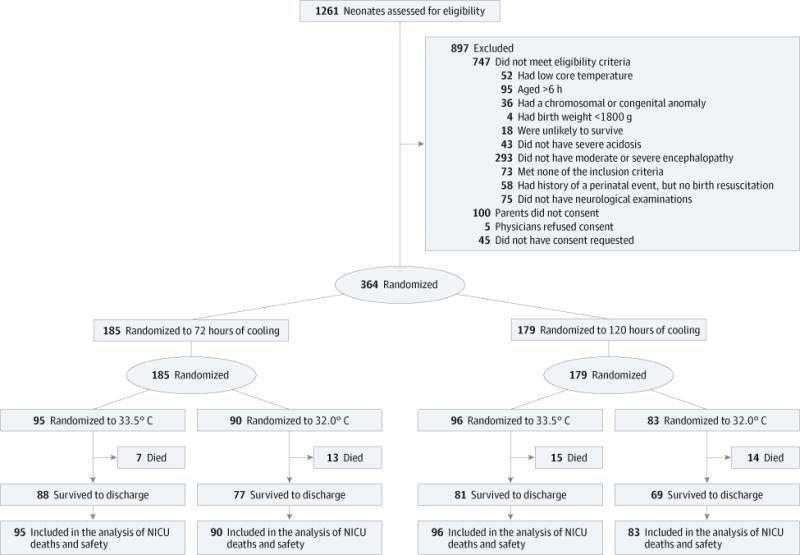
Flow of Neonates Through the Trial
Maternal and Neonatal Characteristics
Baseline maternal and neonatal characteristics were similar in the 72 hours and 120 hours groups and the 33.5°C and 32.0°C groups, except that among the 33.5°C and the 32.0°C groups, birth weight was higher in the 32.0°C group compared with the 33.5°C group, P = .02 (Table 1).
Table 1.
Maternal and Neonatal Characteristicsa
| Duration of Cooling, No. (%) | Depth of Cooling, No. (%) | |||
|---|---|---|---|---|
| 72 h (n = 185) | 120 h (n = 179) | 33.5°C (n = 191) | 32.0°C (n = 173) | |
| Maternal | ||||
| Raceb | ||||
| Black | 57 (31) | 57 (33) | 61 (32) | 53 (31) |
| White | 116 (63) | 103 (59) | 115 (61) | 104 (62) |
| Otherb | 11 (6) | 14 (8) | 13 (7) | 12 (7) |
| Maternal age, mean (SD), y | 27.9 (6.7) | 27.9 (6.9) | 28.3 (6.5) | 27.4 (7.1) |
| Married | 95 (52) | 91 (51) | 102 (54) | 84 (49) |
| Gravida, median (IQR) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 2 (1–3) |
| Parity, median (IQR) | 1 (1–2) | 1 (1–3) | 1 (1–3) | 1 (1–2) |
| Pregnancy complications | ||||
| Chronic hypertension | 37 (20) | 36 (20) | 35 (18) | 38 (22) |
| Antepartum hemorrhage | 23 (13) | 17 (10) | 21 (11) | 19 (11) |
| Thyroid dysfunction | 11 (6) | 3 (2) | 6 (3) | 8 (5) |
| Diabetes | 21 (11) | 25 (14) | 22 (12) | 24 (14) |
| Intrapartum complications | ||||
| Fetal decelerations | 142 (77) | 140 (80) | 149 (79) | 133 (77) |
| Cord prolapse, rupture, compression | 27 (15) | 22 (12) | 29 (15) | 20 (12) |
| Uterine rupture | 11 (6) | 11 (6) | 9 (5) | 13 (8) |
| Maternal pyrexia (≥37.6°C) | 23 (13) | 18 (10) | 17 (9) | 24 (14) |
| Shoulder dystocia | 14 (8) | 15 (8) | 14 (7) | 15 (9) |
| Maternal hemorrhage | 27 (15) | 28 (16) | 30 (16) | 25 (14) |
| Rupture of membranes (spontaneous or induced) | ||||
| No rupture | 46 (26) | 51 (30) | 56 (30) | 41 (25) |
| ≤18h | 116 (64) | 100 (58) | 112 (60) | 104 (63) |
| >18 h | 18 (10) | 20 (12) | 18 (10) | 20 (12) |
| Rupture of membranes, h | ||||
| Mean (SD) | 11.8 (20.4) | 10.5 (16.4) | 11.4 (17.6) | 10.9 (19.5) |
| Median (IQR) | 7.6 (2.5–14.7) | 5.9 (2.0–13.7) | 6.4 (2.4–14.1) | 7.3 (1.6–14.6) |
| Emergency cesarean delivery | 117 (63) | 114 (64) | 119 (62) | 112 (65) |
| Neonatal | ||||
| Age at randomization, h | 5.0 (1.1) | 4.9 (1.4) | 4.9 (1.1) | 4.9 (1.4) |
| Transferred from birth hospital | 119 (64) | 115 (64) | 124 (65) | 110 (64) |
| Male | 105 (57) | 107 (60) | 103 (54) | 109 (63) |
| Apgarscore ≤5 | ||||
| 5 min after birth | 155 (84) | 151 (85) | 162 (85) | 144 (84) |
| 10 min after birth | 107 (66) | 116 (72) | 115 (70) | 108 (68) |
| Birth weight, g | 3293 (538) | 3427 (651) | 3292 (608) | 3432 (582) |
| Length, cm | 50.5 (2.9) | 50.8 (3.1) | 50.5 (2.9) | 50.8 (3.1) |
| Head circumference, cm | 34.0 (1.9) | 34.3 (1.7) | 34.0 (1.6) | 34.2 (2.0) |
| Intubation in delivery room | 143 (77) | 143 (80) | 152 (80) | 134 (78) |
| Continued resuscitation at 10 min | 155 (84) | 160 (90) | 168 (88) | 147 (85) |
| Time to spontaneous respiration >10 min | 72 (41) | 78 (47) | 83 (47) | 67 (41) |
| Cord blood | ||||
| pH | 6.94 (0.2) | 6.95 (0.2) | 6.94 (0.2) | 6.95 (0.2) |
| Base deficit | 16.2 (7.8) | 15.9 (6.7) | 16.1 (7.5) | 16.0 (7.0) |
| Seizuresc | 48 (26) | 57 (32) | 58 (30) | 47 (27) |
| Moderate encephalopathy | 144 (78) | 136 (76) | 152 (80) | 128 (74) |
| Severe encephalopathy | 41 (22) | 43 (24) | 39 (20) | 45 (26) |
| Inotropic supportc | 42 (23) | 34 (19) | 35 (18) | 41 (24) |
| Anticonvulsantsc | 32 (19) | 25 (17) | 31 (19) | 26 (17) |
Abbreviation: IQR, interquartile range.
Percentages are based on the number of mothers or neonates for whom data were available. Because of rounding, not all percentages sum to 100.
Other race includes American Indian or Alaskan Native, Asian, Native Hawaiian or other Pacific Islander, and multiracial.
Data are for this characteristic at the time of randomization.
NICU Deaths
Mortality in the NICU was 7% (7 of 95 neonates) for the 33.5°C for 72 hours group, 14% (13 of 90 neonates) for the 32.0°C for 72 hours group, 16% (15 of 96 neonates) for the 33.5°C for 120 hours group, and 17% (14 of 83 neonates) for the 32.0°C for 120 hours group (Figure 2).
Figure 2. Survival for the Hypothermia Groups.
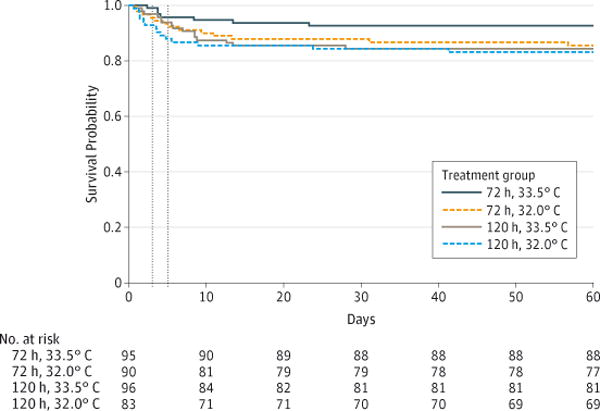
Dotted lines represent day 3 (72 hours) and day 5 (120 hours).
There were20 deaths of 185 neonates (11%) in the 72 hours group compared with 29 deaths of 179 neonates (16%) in the 120 hours group (Table 2), and the number of neonates for whom support was withdrawn was 15 of 20 neonates (75%) in the 72 hours group compared with 26 of 29 neonates (90%) in the 120 hours group. There were 22 deaths of 191 neonates (12%) in the 33.5°C group compared with 27 deaths of 173 neonates (16%) in the 32.0°C group, and the number of neonates for whom support was withdrawn was 19 of 22 (86%) in the 33.5°C group compared with 22 of 27 (81%) in the 32.0°C group. Support was withdrawn prior to the end of the designated cooling period for 1 neonate in the 33.5°C for 72 hours group, 1 neonate in the 32.0°C for 72 hours group, 6 neonates in the 33.5°C for 120 hours group, and 9 neonates in the 32.0°C for 120 hours group. The causes of death for the 7 neonates in the 33.5°C for 72 hours group were asphyxia brain injury (5 neonates), persistent pulmonary hypertension (1 neonate), and other causes (1 neonate). For the 13 neonates in the 32.0°C for 72 hours group, causes of death included asphyxia brain injury (7 neonates), multiorgan failure (2 neonates), sepsis (1 neonate), and other (3 neonates). In the 33.5°C for 120 hours group, the causes of death among 15 neonates were asphyxia brain injury (7 neonates), multiorgan failure (6 neonates), persistent pulmonary hypertension (1 neonate), and other causes (1 neonate). For the 32.0°C for 120 hours group, the causes of death for the 14 neonates were asphyxia brain injury (5 neonates), multi-organ failure (4 neonates), persistent pulmonary hypertension (1 neonate), pneumonia (1 neonate), and other causes (3 neonates).
Table 2.
Outcomes Assessed for Safety Among Neonates in the Hypothermia Groups
| Duration of Cooling | Depth of Cooling | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neonates, No. (%) | Unadjusted RR (95 % CI) |
P Value | Adjusted RR (95% CI) |
P Value | Neonates, No. (%) | Unadjusted RR (95 % CI) |
P Value | Adjusted RR (95% CI) |
P Value | |||
| 72 h (n = 185) |
120 h (n = 179) |
33.5°C (n = 191) |
32.0°C (n = 173) |
|||||||||
| All NICU deaths | 20 (11) | 29 (16) | 1.50 (0.88–2.55) |
.14 | 1.37 (0.92–2.04) |
.12 | 22 (12) | 27 (16) | 1.35 (0.80–2.29) |
.26 | 1.24 (0.69–2.25) |
.47 |
| All in-hospital deaths or ECMO | 29 (16) | 40 (22) | 1.43 (0.93–2.19) |
.11 | 1.33 (0.96–1.83) |
.09 | 29 (15) | 40 (23) | 1.52 (0.99–2.34) |
.06 | 1.35 (0.83–2.19) |
.22 |
| All deaths within 120 h of initiation of cooling | 11 (6) | 16 (9) | 1.50 (0.72–3.15) |
.28 | 1.36 (0.75–2.48) |
.31 | 10 (5) | 17 (10) | 1.88 (0.88–3.99) |
.10 | 1.59 (0.77–3.30) |
.21 |
Abbreviations: ECMO, extracorporeal membrane oxygenation; RR, relative risk.
Results are from generalized estimating equations regression (log-binomial), adjusted for level of hypoxic ischemic encephalopathy and intracenter correlations.
Among neonates with moderate hypoxic ischemic encephalopathy, death in the NICU occurred in 6 of 144 neonates (4%) in the 72 hours group compared with 11 of 136 neonates (8%) in the 120 hours group and in 10 of 152 neonates (7%) in the 33.5°C group compared with 7 of 128 neonates (5%) in the 32.0°C group. Among neonates with severe hypoxic ischemic encephalopathy, deaths in the NICU occurred in 14 of 41 neonates (34%) in the 72 hours group compared with 18 of 43 neonates (42%) in the 120 hours group and in 12 of 39 neonates (31%) in the 33.5°C group compared with 20 of 45 neonates (44%) in the 32.0°C group.
The NICU mortality rates for the 120 hours and 72 hours groups were not significantly different (16% for 120 hours group vs 11% for 72 hours group, P = .12); the rates for the 32.0°C group vs the 33.5°C group (16% for 32.0°C group vs 12% for 33.5°C group, P = .47) were also not significantly different. The adjusted risk ratio for NICU mortality for the 120 hours group vs 72 hours group was 1.37 (95% CI, 0.92–2.04) and for the 32.0°C group vs 33.5°C group was 1.24 (95% CI, 0.69–2.25, Table 2).
Extracorporeal membrane oxygenation (ECMO) was selected post hoc by the DSMC for evaluating safety based on reports received from participating centers; the outcome of death or ECMO was not associated with length or depth of cooling following adjusted analysis; for the 120 hours group vs 72 hours group the risk ratio was 1.33 (95% CI, 0.96–1.83, P = .09) and for the 32.0°C group vs 33.5°C group was 1.35 (95% CI, 0.83–2.19, P = .22). There was no interaction between depth and duration of cooling for in-hospital mortality adjusted for level of encephalopathy and center (P = .21).
Futility analysis revealed that the probability of detecting a statistically significant treatment benefit of longer or deeper cooling for in-hospital mortality was less than 2%.
Safety
Predefined safety events during study intervention were similar in the 72 hours and 120 hours groups and the 33.5°C and 32.0°C groups, except that among the 72 hours and the 120 hours groups, the frequency of major bleeding was higher in the 72 hours group compared with the 120 hours group (3% for 72 hours group vs 1% for 120 hours group, P = .04, Table 2). The temperature profile of the 4 groups is shown in Figure 3.
Figure 3.
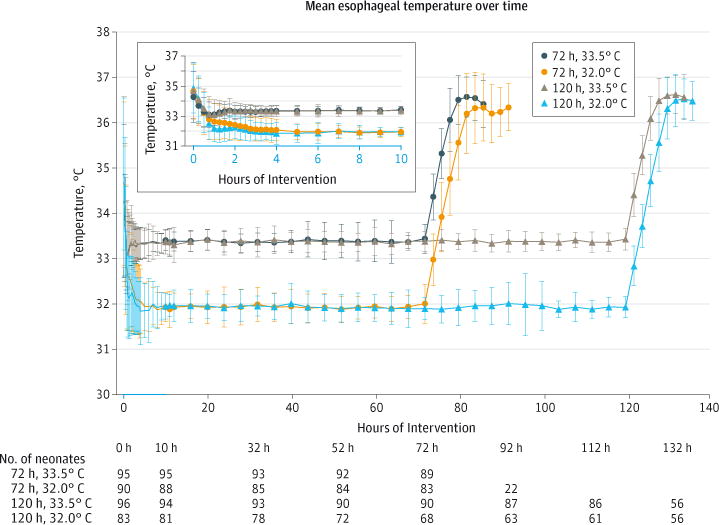
Mean (SD) Esophageal Temperatures During the Intervention Period
During the hospital course, defined as the period between birth and death or discharge, in the 120 hours group compared with the 72 hours group, the incidence of arrhythmia (7% for 120 hours group vs 1% for 72 hours group, P = .02) and anuria (9% for 120 hours group vs 3% for 72 hours group, P = .01) were higher and hospital stay was longer (mean [SD], 26.4 days [34.1] for 120 hours group vs 21.6 days [15.3] for 72 hours group, P = .002). In the 32.0°C group compared with the 33.5°C group, a higher incidence of bradycardia (23% for 32.0°C group vs 1% for 33.5°C group, P = <.001), inhaled nitric oxide (34% for 32.0°C group vs 24% for 33.5°C group, P = .03) and ECMO (9% for 32.0°C group vs 4% for 33.5°C group, P = .005), and moredays of oxygen (mean [SD], 8.8 [8.5] for 32.0°C group vs 8.0 [9.7] for 33.5°C group, P = .02), were noted (Table 3).
Table 3.
Hospital Course and Statusat Discharge for Neonates in the Hypothermia Groupsa
| Duration of Cooling | Depth of Cooling | |||||||
|---|---|---|---|---|---|---|---|---|
| Neonates, No. (%) | P Valueb | Adjusted RR (95% CI) |
Neonates, No. (%) | P Valueb | Adjusted RR (95% CI) |
|||
| 72 h (n = 185) |
120 h (n = 179) |
33.5°C (n = 191) |
32.0°C (n = 173) |
|||||
| During Study Intervention | ||||||||
| Arrhythmia requiring therapyc | 2 (1) | 8 (4) | .16 | 3.12 (0.65–15.0) |
2 (1) | 8 (5) | .11 | 5.75 (0.68–48.7) |
| Acidosis lasting >3 hc | 2 (1) | 5 (3) | .85 | 0.87 (0.22–3.45) |
3 (2) | 4 (2) | .98 | 0.98 (0.23–4.14) |
| Major bleedingc | 5 (3) | 2 (1) | .04 | 0.25 (0.07–0.91) |
4 (2) | 3 (2) | .77 | 0.82 (0.22–3.04) |
| Death after initiation of cooling | ||||||||
| Within 72 h | 5 (3) | 9 (5) | .38 | 1.66 (0.53–5.24) |
4 (2) | 10 (6) | .07 | 2.33 (0.92–5.86) |
| Within 120 h | 11 (6) | 16 (9) | .31 | 1.36 (0.75–2.48) |
10 (5) | 17 (10) | .21 | 1.59 (0.77–3.30) |
| Skin erythema, cyanosis, sclerema, subcutaneous fat necrosisc | 6 (3) | 10 (6) | .42 | 1.34 (0.66–2.71) |
8 (4) | 8 (5) | .66 | 0.78 (0.26–2.36) |
| During Hospital Coursed | ||||||||
| Hypotension | 63 (34) | 55 (31) | .28 | 0.86 (0.66–1.13) |
58 (30) | 60 (35) | .43 | 1.13 (0.83–1.53) |
| Inotropic agentsc,e | 87 (47) | 93 (52) | .25 | 0.84 (0.63–1.13) |
88 (46) | 92 (53) | .32 | 1.13 (0.89–1.43) |
| Blood transfusionsc,e | 54 (29) | 63 (35) | .15 | 0.70 (0.44–1.14) |
57 (30) | 60 (35) | .11 | 1.31 (0.94–1.82) |
| Platelet transfusionsc,e | 44 (24) | 53 (30) | .21 | 0.85 (0.65–1.10) |
48 (25) | 49 (28) | .39 | 1.12 (0.87–1.43) |
| Persistent pulmonary hypertension | 47 (25) | 60 (34) | .13 | 1.23 (0.94–1.61) |
48 (25) | 59 (34) | .06 | 1.33 (0.98–1.79) |
| Inhaled nitric oxide therapy | 45 (24) | 60 (34) | .07 | 1.30 (0.97–1.72) |
46 (24) | 59 (34) | .03 | 1.36 (1.04–1.77) |
| ECMO | 10 (5) | 12 (7) | .52 | 1.26 (0.62–2.55) |
7 (4) | 15 (9) | .005 | 2.37 (1.30–4.30) |
| Documented seizures | 93 (50) | 84 (47) | .29 | 0.90 (0.75–1.09) |
94 (49) | 83 (48) | .49 | 0.94 (0.77–1.13) |
| Arrhythmia | 2 (1) | 12 (7) | .02 | 6.11 (1.26–29.5) |
5 (3) | 9 (5) | .21 | 1.82 (0.72–4.62) |
| Sustained heart rate <50 beats/minc | 10 (11) | 12 (15) | .88 | 1.06 (0.48–2.37) |
1 (1) | 21 (23) | .0007 | 6.93 (2.25–21.3) |
| Oliguria | 49 (26) | 46 (26) | .90 | 0.97 (0.66–1.44) |
48 (25) | 47 (27) | .88 | 1.04 (0.65–1.66) |
| Anuria | 5 (3) | 16 (9) | .01 | 3.24 (1.29–8.12) |
8 (4) | 13 (8) | .18 | 1.56 (0.82–2.98) |
| Hepatic dysfunctionf | 38 (21) | 52 (29) | .06 | 1.35 (0.99–1.85) |
45 (24) | 45 (26) | .70 | 1.06 (0.80–1.40) |
| Infection | ||||||||
| Positive culture during interventionc | 0 (0) | 4 (2) | .06g | 1 (1) | 3 (2) | .26h | 3.67 (0.38–35.3) |
|
| Septicemia after rewarming | 5 (3) | 8 (4) | .19 | 1.61 (0.79–3.28) |
5 (3) | 8 (5) | .06 | 1.74 (0.98–3.09) |
| Disseminated intravascular coagulopathy | 25 (14) | 29 (16) | .46 | 1.16 (0.78–1.72) |
29 (15) | 25 (14) | .67 | 0.90 (0.57–1.44) |
| Blood glucose <30 mg/dL | 30 (16) | 20 (11) | .20 | 0.68 (0.37–1.23) |
24 (13) | 26 (15) | .71 | 1.13 (0.60–2.11) |
| Blood calcium <8 mmol/L | 45 (24) | 45 (25) | .90 | 0.98 (0.74–1.30) |
49 (26) | 41 (24) | .75 | 0.93 (0.62–1.41) |
| Skin changes | 12 (6) | 16 (9) | .40 | 1.37 (0.66–2.86) |
12 (6) | 16 (9) | .29 | 1.46 (0.72–2.95) |
| Days of ventilationi | ||||||||
| Mean (SD) | 5.9 (7.3) | 6.8 (8.0) | .06 | 6.1 (7.6) | 6.6 (7.8) | .74 | ||
| Median (IQR) | 4 (1–8) | 5 (2–10) | 1.78 (0.97–3.27) |
3 (1–9) | 4 (1–10) | 1.09 (0.67–1.77) |
||
| Days receiving oxygeni | ||||||||
| Mean (SD) | 7.6 (8.2) | 9.2 (10.1) | .58 | 8.0 (9.7) | 8.8 (8.5) | .02 | ||
| Median (IQR) | 5 (2–10) | 6 (2–14) | 1.12 (0.75–1.66) |
5 (1–12) | 7 (2–13) | 1.29 (1.04–1.59) |
||
| Gastric fundoplication | 4 | 1 | 4 | 1 | ||||
| Gastrostomy | 13 | 11 | 18 | 6 | ||||
| Tracheostomy | 1 | 3 | 3 | 1 | ||||
| NICU death | 20 (11) | 29 (16) | .12 | 1.37 (0.92–2.04) |
22 (12) | 27 (16) | .47 | 1.24 (0.69–2.25) |
| Among survivors | ||||||||
| Length of stay, di | ||||||||
| Mean (SD) | 21.6 (15.3) | 26.4 (34.1) | .002 | 22.0 (15.7) | 26.0 (34.2) | .21 | ||
| Median (IQR) | 17 (11–27) | 20 (14–27) | 1.19 (1.07–1.33) |
17 (11–26) | 20 (13–28) | 1.11 (0.94–1.32) |
||
| Discharged with home therapy | 66 (40) | 52 (35) | .18 | 0.85 (0.68–1.08) |
57 (34) | 61 (42) | .24 | 1.16 (0.90–1.50) |
| Ventilator | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||
| Oxygen | 6 (9) | 3 (6) | .51 | 0.56 (0.10–3.16) |
7 (12) | 2 (3) | .40 | 0.36 (0.03–4.03) |
| Gavage tube | 18 (27) | 16 (31) | .56 | 1.11 (0.78–1.59) |
16 (28) | 18 (30) | .69 | 1.09 (0.71–1.68) |
| Gastrostomy tube | 16 (24) | 10 (19) | .15 | 0.70 (0.42–1.14) |
18 (32) | 8 (13) | .07 | 0.49 (0.22–1.05) |
| Anticonvulsant medication | 41 (62) | 30 (58) | .73 | 0.95 (0.73–1.25) |
38 (67) | 33 (54) | .23 | 0.84 (0.63–1.12) |
Abbreviations: ECMO, extracorporeal membrane oxygenation; GEE, generalized estimating equations; HIE, hypoxic ischemic encephalopathy; IQR, interquartile range; NICU, neonatal intensive care unit; RR, relative risk.
Percentages are based on the number of mothers or neonates for whom data were available. Missing data for Table 3: hypotension (2 missing), bradycardia (190 missing; n = 174), septicemia (1 missing), hypocalcemia (1 missing), length of hospital stay (3 missing among 315 survivors), discharged with home therapy (2 missing among 315 survivors).
Unless otherwise noted, all P values are from GEE models for binary data, using a log link, adjusted for level of HIE and intracenter correlation.
These P values are from Poisson regression for the total number of events during intervention period, with offset for differential exposure times (72 h vs 120 h). These are adjusted for level of HIE and intracenter correlation in a GEE model.
The hospital course was defined as the period between birth and death or discharge.
Administration of inotropic agents, blood transfusions, and platelet transfusion were recorded during study intervention.
Hepatic dysfunction was defined as an aspartate aminotransferase level above 200 IU and an alanine aminotransferase level above 100 IU.
GEE model did not converge when accounting for intracenter correlation; P value given is adjusted for level of HIE only.
Unadjusted P value from the Fisher exact test for any positive culture during intervention; the adjusted model did not converge.
These P values are linear regression after log-transformation. These are adjusted for level of HIE and intracenter correlation in a GEE model.
Discussion
Hypothermia for 72 hours duration to a depth of 33.0°C to 34.0°C is now usual care for neonates who are full-term with moderate or severe encephalopathy.20 This study of whole-body hypothermia to an esophageal temperature of 32.0°C or continued for 120 hours was designed to evaluate potential benefit of longer cooling, deeper cooling, or both. The trial was closed to patient enrollment because of safety and futility concerns. Had it been continued to its full sample size, the likelihood of this trial favoring longer cooling, deeper cooling, or both was found to be small. The NICU mortality rates for the deeper or longer cooling groups were not less than that of the usual care group; the risk ratios and upper bound of the 95% CIs of greater than 2 suggest deeper or longer cooling may be associated with an increase in mortality. Longer duration of cooling was associated with more arrhythmia and anuria and longer hospital days, whereas deeper cooling was associated with higher use of inhaled nitric oxide therapy, ECMO, more days of oxygen, and higher incidence of bradycardia. The primary outcome evaluations of this trial are still ongoing; we wish to report these safety data at the present time to dissuade drifts in adherence to established protocols of hypothermia for neonatal hypoxic ischemic encephalopathy.
The NICU mortality rate for neonates assigned to the 33.5°C for 72 hours group was 7%; this rate is unexpectedly low.2 The mortality rates in the longer and deeper cooling groups (14%–17%) were similar to that in the cooled group of our previous trial (19%).2 Comparisons with other trials are limited because NICU mortality data are not provided for these trials.1,3–5 The eligibility criteria for this trial were the same as our previous trial and neonates who were overcooled were excluded. However, the rate of severe encephalopathy was lower in this trial than among cooled neonates in our first trial (23% in current trial vs 32% in the previous trial), and the neonates in this trial appeared less critically ill at enrollment with lower base deficit, less intubation at birth, and lower frequency of seizures.2 In this study there was a higher frequency of maternal diabetes than our first trial2 and neonates in the 32.0°C group had a higher birth weight than neonates in the 33.5°C group; it has been reported that larger neonates are more difficult to cool than smaller neonates.21,22 Last, in this study, any elevations of temperature following rewarming were treated according to the study protocol, unlike in the previous study.2 Elevations of temperature are associated with a higher mortality rate and a higher disability rate in infancy and childhood among neonates with hypoxic ischemic encephalopathy.23,24
In this study, cooling to 32.0°C was associated with significantly more inhaled nitric oxide and ECMO therapy. Our study protocol did not include ECMO as an adverse event because ECMO was rarely used in the early trials.1–6 A previous report noted an increased risk of pulmonary hypertensive crisis requiring ECMO among neonates undergoing hypothermia for hypoxic ischemic encephalopathy.25 Extracorporeal membrane oxygenation has not been uniformly used in this population; however, this appears to be changing. A recent meta-analyses of 4 trials reported risk ratios of 1.36 (95% CI, 0.94–1.97) for pulmonary hypertension with hypothermia of 33.0° to 34.0°C, whereas 3 trials noted no significant effect of hypothermia on inhaled nitric oxide therapy.7 In an observational study of neonatal hypoxic ischemic encephalopathy where 29 neonates were treated with whole-body hypothermia to a core temperature of 30.0°C to 33.0°C and28 neonates cooled to a temperature of 33.0°C to 34.0°C, pulmonary function was similar in the 2 groups but additional safety data provided were limited.26,27
The limitations of this study are that information on initiation of cooling during transport of neonates to the Neonatal Research Network centers was not known nor was the frequency of electroencephalographic seizures at random assignment. There were no adjustments made for multiple comparisons.
Currently deeper or longer cooling are not administered as part of usual care; however, there are a few reports of core temperatures recorded below 33.5°C during therapeutic hypothermia.28–30 In addition, a single report has been published regarding cooling continued beyond 72 hours.31 Our findings suggest that cooling for 120 hours or to 32.0°C, or both, may be deleterious. For clinicians using hypothermia, we suggest that deviations from the current published regimen of cooling of 33.0°C to 34.0°C for 72 hours have the potential for harm.19
Conclusions
Among neonates of at least 36 weeks’ gestational age with moderate or severe hypoxic ischemic encephalopathy, deeper cooling or longer duration of cooling compared with hypothermia at 33.5°C for 72 hours did not reduce NICU death. These results have implications for patient care and the design of future trials.
Supplementary Material
Acknowledgments
Funding/Support: The National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Center for Advancing Translational Sciences provided grant support for the Neonatal Research Network’s Optimizing Cooling trial through cooperative agreements.
Role of the Funders/Sponsors: The NICHD program scientist had input into the design and conduct of the study, collection, analysis, and interpretation of the data; preparation, review and approval of the manuscript and decision to submit the manuscript for publication. Data collected at participating sites of the NICHD Neonatal Research Network (NRN) were transmitted to RTI International, the data coordinating center for the network, which stored, managed, and analyzed the data for this study.
Author Contributions
Dr Das and Mr McDonald had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Shankaran, Laptook, Pappas, Tyson, Poindexter, Schibler, Ehrenkranz, Chalak, Carlo, Higgins.
Acquisition, analysis, or interpretation of data: Shankaran, Pappas, McDonald, Das, Tyson, Poindexter, Schibler, Bell, Heyne, Pedroza, Bara, Van Meurs, Grisby, Huitema, Garg, Shepherd, Chalak, Hamrick, Khan, Reynolds, Laughon, Truog, Dysart, Walsh, Watterberg, Higgins.
Drafting of the manuscript: Shankaran, Laptook, Tyson, Van Meurs, Huitema, Garg.
Critical revision of the manuscript for important intellectual content: Shankaran, Pappas, McDonald, Das, Tyson, Poindexter, Schibler, Bell, Heyne, Pedroza, Bara, Van Meurs, Grisby, Ehrenkranz, Shepherd, Chalak, Hamrick, Khan, Reynolds, Laughon, Truog, Dysart, Carlo, Walsh, Watterberg, Higgins.
Statistical analysis: Shankaran, McDonald, Das, Tyson, Pedroza.
Obtained funding: Shankaran, Schibler, Bell, Walsh.
Administrative, technical, or material support: Shankaran, Tyson, Bara, Huitema, Garg, Chalak, Hamrick, Khan, Laughon, Carlo, Higgins.
Study supervision: Shankaran, Das, Poindexter, Schibler, Van Meurs, Truog, Higgins.
Conflict of Interest Disclosures
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Laughon reports consulting for Abbvie, Astellas, Discovery Labs, and Pfizer. No other disclosures were reported.
Disclaimer
The comments and views of the authors do not necessarily represent the views of the NICHD.
Additional Contributions
We thank our medical and nursing colleagues and the infants and their parents who agreed to take part in this study.
Group Information
Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network: NRN Steering Committee Chairs: Michael S. Caplan, MD (University of Chicago); Richard A. Polin, MD (Columbia University). Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island (U10 HD27904): Martin Keszler, MD; Betty R. Vohr, MD; Angelita M. Hensman, MS, RNC-NIC; Elisa Vierira, RN, BSN; Emilee Little, RN, BSN; Ross Sommers, MD; Birju Shah, MD; Nicholas Guerina, MD; Leslie T. McKinley, MS, RD; Melinda Caskey, MD; Andrea Halbrook; Robert T. Burke, MD, MPH. Case Western Reserve University, Rainbow Babies & Children’s Hospital (U10 HD21364, M01 RR80): Anna Maria Hibbs, MD; Nancy S. Newman, RN; Arlene Zadell, RN. Children’s Mercy Hospital and University of Missouri Kansas City School of Medicine (U10 HD68284): Eugenia K. Pallotto, MD; Howard W. Kilbride, MD; Cheri Gauldin, RN, BSN, CCRC; Anne Holmes, RN, MSN, MBA-HCM, CCRC; Kathy Johnson, RN, CCRC. Cincinnati Children’s Hospital Medical Center, University of Cincinnati Medical Center, and Good Samaritan Hospital (U10 HD27853, UL1 TR77): Suhas G. Kallapur, MD; Barbara Alexander, RN; Estelle E. Fischer, MHSA, MBA; Teresa L. Gratton, PA; Lenora Jackson, CRC; Jennifer Jennings, RN, BSN; Kristin Kirker, CRC; Greg Muthig, BA; Sandra Wuertz, RN, BSN, CLC. Duke University School of Medicine, University Hospital, University of North Carolina, and Duke Regional Hospital (U10 HD40492, UL1 RR24128): Ronald N. Goldberg, MD; Joanne Finkle, RN, JD; Kimberley A. Fisher, PhD, FNP-BC, IBCLC; Sandra Grimes, RN, BSN; Matthew M. Laughon, MD, MPH; Carl L. Bose, MD; Janice Bernhardt, MS, RN; Cindy Clark, RN. Emory University, Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory University Hospital Midtown (U10 HD27851, UL1 TR454): Barbara J. Stoll, MD; David P. Carlton, MD; Ellen C. Hale, RN, BS, CCRC; Yvonne Loggins, RN. Eunice Kennedy Shriver National Institute of Child Health and Human Development: Stephanie Wilson Archer, MA. Indiana University, Riley Hospital for Children at Indiana University Health and Methodist Hospital (U10 HD27856, UL1 TR6): Gregory M. Sokol, MD; Leslie Dawn Wilson, BSN, CCRC; Dianne E. Herron, RN; Susan Gunn, NNP; Lucy Smiley, CCRC. Nationwide Children’s Hospital and the Ohio State University Medical Center (U10 HD68278): Sudarshan R. Jadcherla, MD; Pablo J. Sánchez, MD; Patricia Luzader, RN; Christine A. Fortney, PhD, RN; Gail E. Besner; Nehal A. Parikh, MD. RTI International (U10 HD36790): Dennis Wallace, PhD; Kristin M. Zaterka-Baxter, RN, BSN, CCRP; Margaret Crawford, BS, CCRP; Jenna Gabrio, BS, CCRP; Marie G. Gantz, PhD; Jamie E. Newman, PhD, MPH; Jeanette O’Donnell Auman, BS; Carolyn M. Petrie Huitema, MS, CCRP; Tracy L. Nolen, DrPH. Stanford University and Lucile Packard Children’s Hospital (U10 HD27880, M01 RR70, UL1 TR93): David K. Stevenson, MD; M. Bethany Ball, BS, CCRC; Marian M. Adams, MD; Alexis S. Davis, MD, MS (Epi); Carol Kibler, RN; Jeffrey R. Parker, RRT; Melinda S. Proud, RCP; Ronald J. Wong, BS. University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (U10 HD34216, M01 RR32): Namasivayam Ambalavanan, MD; Monica V. Collins, RN, BSN, MAEd; Shirley S. Cosby, RN, BSN. University of California, Los Angeles, Mattel Children’s Hospital, Santa Monica Hospital, Los Robles Hospital and Medical Center, and Olive View Medical Center (U10 HD68270): Uday Devaskar, MD; Meena Garg, MD; Teresa Chanlaw, MPH; Rachel Geller, RN, BSN. University of Iowa and Mercy Medical Center (U10 HD53109, UL1 TR442): Dan L. Ellsbury, MD; Tarah T. Colaizy, MD, MPH; Jane E. Brumbaugh, MD; Karen J. Johnson, RN, BSN; Donia B. Campbell, RNC-NIC; Jacky R. Walker, RN; Jonathan M. Klein, MD; Jeffrey L. Segar, MD; John M. Dagle, MD, PhD; Julie B. Lindower, MD, MPH; Steven J. McElroy, MD; Glenda K. Rabe, MD; Robert D. Roghair, MD; Lauritz R. Meyer, MD; Cary R. Murphy, MD; Vipinchandra Bhavsar, MB, BS. University of New Mexico Health Sciences Center (U10 HD53089, UL1 TR41): Kristi L. Watterberg, MD; Robin K. Ohls, MD; Conra Backstrom Lacy, RN; Sandra Beauman, MSN; Carol Hartenberger, BSN, MPH. University of Pennsylvania, Hospital of the University of Pennsylvania, Pennsylvania Hospital, and Children’s Hospital of Philadelphia (U10 HD68244): Barbara Schmidt, MD, MSc; Haresh Kirpalani, MB, MSc; Sara B. DeMauro, MD, MSCE; Aasma S. Chaudhary, BS, RRT; Soraya Abbasi, MD; Toni Mancini, RN, BSN, CCRC; Dara M. Cucinotta, RN. University of Rochester Medical Center, Golisano Children’s Hospital, and the State University New York at Buffalo Women’s and Children’s Hospital of Buffalo (U10 HD68263, UL1 TR42): Nirupama Laroia, MD; Carl T. D’Angio, MD; Ronnie Guillet, MD, PhD; Satyan Lakshminrusimha, MD; Karen Wynn, NNP, RN; Holly I. M. Wadkins; Ann Marie Scorsone, MS; Patrick Conway, MS; Michael G. Sacilowski, BS; Stephanie Guilford, BS; Ashley Williams, MS Ed. University of Texas Southwestern Medical Center at Dallas, Parkland Health & Hospital System, and Children’s Medical Center Dallas (U10 HD40689, M01 RR633): Myra Wyckoff, MD; Pablo J. Sánchez, MD; Luc P. Brion, MD; Diana M. Vasil, RNC-NIC; Lijun Chen, PhD, RN; Emma Ramon, RN. University of Texas Health Science Center at Houston Medical School and Children’s Memorial Hermann Hospital (U10 HD21373): Kathleen A. Kennedy, MD, MPH; Georgia E. McDavid, RN; Julie Arldt-McAlister, RN, BSN; Katrina Burson, RN, BSN; Carmen Garcia, RN, CCRP; Karen Martin, RN; Shawna Rodgers, RN, BSN; Patti L. Pierce Tate, RCP; Sharon L. Wright, MT (ASCP). Wayne State University, University of Michigan, Hutzel Women’s Hospital, and Children’s Hospital of Michigan (U10 HD21385): Beena G. Sood, MD, MS; John Barks, MD; Rebecca Bara, RN BSN; Maria Batts, RRT; Lilia De Jesus, MD; Kimberly Hayes-Hart, RN, MSN, NNP-BC; Mary E. Johnson, RN, BSN; Girija Natarajan, MD; Lisa Sulkowski, BS; Laura Sumner, RN, BSN; Nicole Walker, BA; Kathleen Weingarden, RN, BSN; Mary Christensen, RT; Stephanie A. Wiggins, MS. Data and Safety Monitoring Committee: Christine A. Gleason, MD (University of Washington); Robert J. Boyle, MD (University of Virginia Health System); Traci Clemons, PhD (EMMES Corporation); Mary E. D’Alton, MD (Columbia University); Abhik Das, PhD (RTI International); Carol K. Redmond, ScD (University of Pittsburg); Michael G. Ross, MD, MPH (UCLA School of Medicine and Public Health); Steven J. Weiner, MS (George Washington University); Marian Willinger, PhD (Eunice Kennedy Shriver National Institute of Child Health and Human Development).
Footnotes
TRIAL REGISTRATION clinicaltrials.gov Identifier: NCT01192776
Author Audio Interview at jama.com
Supplemental content at jama.com
References
- 1.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy. Lancet. 2005;365(9460):663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 2.Shankaran S, Laptook AR, Ehrenkranz RA, et al. National Institute of Child Health and Human Development Neonatal Research Network Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353(15):1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 3.Azzopardi DV, Strohm B, Edwards AD, et al. TOBY Study Group Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361(14):1349–1358. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 4.Simbruner G, Mittal RA, Rohlmann F, Muche R, neo.nEURO.network Trial Participants Systemic hypothermia after neonatal encephalopathy. Pediatrics. 2010;126(4):e771–e778. doi: 10.1542/peds.2009-2441. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs SE, Morley CJ, Inder TE, et al. Infant Cooling Evaluation Collaboration Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy. Arch Pediatr Adolesc Med. 2011;165(8):692–700. doi: 10.1001/archpediatrics.2011.43. [DOI] [PubMed] [Google Scholar]
- 6.Tagin MA, Woolcott CG, Vincer MJ, Whyte RK, Stinson DA. Hypothermia for neonatal hypoxic ischemic encephalopathy. Arch Pediatr Adolesc Med. 2012;166(6):558–566. doi: 10.1001/archpediatrics.2011.1772. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2013(1):CD003311. doi: 10.1002/14651858.CD003311.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guillet R, Edwards AD, Thoresen M, et al. CoolCap Trial Group Seven- to 8-year follow-up of the CoolCap trial of head cooling for neonatal encephalopathy. Pediatr Res. 2012;71(2):205–209. doi: 10.1038/pr.2011.30. [DOI] [PubMed] [Google Scholar]
- 9.Shankaran S, Pappas A, McDonald SA, et al. Eunice Kennedy Shriver NICHD Neonatal Research Network Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med. 2012;366(22):2085–2092. doi: 10.1056/NEJMoa1112066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azzopardi D, Strohm B, Marlow N, et al. TOBY Study Group Effects of hypothermia for perinatal asphyxia on childhood outcomes. N Engl J Med. 2014;371(2):140–149. doi: 10.1056/NEJMoa1315788. [DOI] [PubMed] [Google Scholar]
- 11.Busto R, Dietrich WD, Globus MY, Valdés I, Scheinberg P, Ginsberg MD. Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury. J Cereb Blood Flow Metab. 1987;7(6):729–738. doi: 10.1038/jcbfm.1987.127. [DOI] [PubMed] [Google Scholar]
- 12.Thoresen M, Penrice J, Lorek A, et al. Mild hypothermia after severe transient hypoxia-ischemia ameliorates delayed cerebral energy failure in the newborn piglet. Pediatr Res. 1995;37(5):667–670. doi: 10.1203/00006450-199505000-00019. [DOI] [PubMed] [Google Scholar]
- 13.Williams GD, Dardzinski BJ, Buckalew AR, Smith MB. Modest hypothermia preserves cerebral energy metabolism during hypoxia-ischemia and correlates with brain damage: a 31P nuclear magnetic resonance study in unanesthetized neonatal rats. Pediatr Res. 1997;42(5):700–708. doi: 10.1203/00006450-199711000-00024. [DOI] [PubMed] [Google Scholar]
- 14.Iwata O, Thornton JS, Sellwood MW, et al. Depth of delayed cooling alters neuroprotection pattern after hypoxia-ischemia. Ann Neurol. 2005;58(1):75–87. doi: 10.1002/ana.20528. [DOI] [PubMed] [Google Scholar]
- 15.Bennet L, Roelfsema V, George S, Dean JM, Emerald BS, Gunn AJ. The effect of cerebral hypothermia on white and grey matter injury induced by severe hypoxia in preterm fetal sheep. J Physiol. 2007;578(Pt 2):491–506. doi: 10.1113/jphysiol.2006.119602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perlman JM. Summary proceedings from the neurology group on hypoxic-ischemic encephalopathy. Pediatrics. 2006;117(3 Pt 2):S28–S33. doi: 10.1542/peds.2005-0620E. [DOI] [PubMed] [Google Scholar]
- 17.Johnston MV, Fatemi A, Wilson MA, Northington F. Treatment advances in neonatal neuroprotection and neurointensive care. Lancet Neurol. 2011;10(4):372–382. doi: 10.1016/S1474-4422(11)70016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39(4):214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 19.Pocock SJ. Group sequential methods in the design and analysis of clinical trials. Biometrika. 1977;64(2):191–199. doi: 10.1093/biomet/64.2.191. [DOI] [Google Scholar]
- 20.Papile LA, Baley JE, Benitz W, et al. Committee on Fetus and Newborn Hypothermia and neonatal encephalopathy. Pediatrics. 2014;133(6):1146–1150. doi: 10.1542/peds.2014-0899. [DOI] [PubMed] [Google Scholar]
- 21.Wyatt JS, Gluckman PD, Liu PY, et al. CoolCap Study Group Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics. 2007;119(5):912–921. doi: 10.1542/peds.2006-2839. [DOI] [PubMed] [Google Scholar]
- 22.Shankaran S, Pappas A, Laptook AR, et al. NICHD Neonatal Research Network Outcomes of safety and effectiveness in a multicenter randomized, controlled trial of whole-body hypothermia for neonatal hypoxic-ischemic encephalopathy. Pediatrics. 2008;122(4):e791–e798. doi: 10.1542/peds.2008-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laptook A, Tyson J, Shankaran S, et al. National Institute of Child Health and Human Development Neonatal Research Network Elevated temperature after hypoxic-ischemic encephalopathy. Pediatrics. 2008;122(3):491–499. doi: 10.1542/peds.2007-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laptook AR, McDonald SA, Shankaran S, et al. Extended Hypothermia Follow-up Subcommittee of the National Institute of Child Health and Human Development Neonatal Research Network Elevated temperature and 6- to 7-year outcome of neonatal encephalopathy. Ann Neurol. 2013;73(4):520–528. doi: 10.1002/ana.23843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah SK, Khan AM, Cox CS., Jr Pulmonary hypertensive crisis requiring ECMO associated with rewarming from whole body hypothermia for hypoxic ischemic encephalopathy. Eur J Pediatr Surg. 2010;20(3):205–206. doi: 10.1055/s-0029-1241872. [DOI] [PubMed] [Google Scholar]
- 26.Cavallaro G, Filippi L, Cristofori G, et al. Does pulmonary function change during whole-body deep hypothermia? Arch Dis Child Fetal Neonatal Ed. 2011;96(5):F374–F377. doi: 10.1136/adc.2009.181826. [DOI] [PubMed] [Google Scholar]
- 27.Compagnoni G, Bottura C, Cavallaro G, Cristofori G, Lista G, Mosca F. Safety of deep hypothermia in treating neonatal asphyxia. Neonatology. 2008;93(4):230–235. doi: 10.1159/000111101. [DOI] [PubMed] [Google Scholar]
- 28.O’Reilly KM, Tooley J, Winterbottom S. Therapeutic hypothermia during neonatal transport. Acta Paediatr. 2011;100(8):1084–1086. doi: 10.1111/j.1651-2227.2011.02249.x. discussion e49. [DOI] [PubMed] [Google Scholar]
- 29.Hallberg B, Olson L, Bartocci M, Edqvist I, Blennow M. Passive induction of hypothermia during transport of asphyxiated infants. Acta Paediatr. 2009;98(6):942–946. doi: 10.1111/j.1651-2227.2009.01303.x. [DOI] [PubMed] [Google Scholar]
- 30.Kendall GS, Kapetanakis A, Ratnavel N, Azzopardi D, Robertson NJ, Cooling on Retrieval Study Group Passive cooling for initiation of therapeutic hypothermia in neonatal encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2010;95(6):F408–F412. doi: 10.1136/adc.2010.187211. [DOI] [PubMed] [Google Scholar]
- 31.Kendall GS, Mathieson S, Meek J, Rennie JM. Recooling for rebound seizures after rewarming in neonatal encephalopathy. Pediatrics. 2012;130(2):e451–e455. doi: 10.1542/peds.2011-3496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


