Abstract
Organocatalytic reactions of 3-olefinic oxindoles and pentane-1,5-dial were investigated to provide access to substituted spirocyclohexane oxindoles via Michael/Aldol cascade reactions. Of particular interest, we have examined the stereochemical outcome of electron withdrawing and electron-donating groups on the oxindole ring nitrogen. Interestingly, we have observed that the N-protecting group on the oxindole has critical effect on aldol ring closure leading to ultimate stereochemical outcome of the hydroxyl center. The overall process is quite efficient and afforded products with multiple stereocenters in high yields and excellent enantioselectivities (>99% ee).
Keywords: Organocatalyst, Spiroindole, Michael reaction, Aldol reaction, Asymmetric catalysis
Spiro[cyclohexane-1,3′-indolin]-2′-one is an important structural motif for many bioactive natural products as well as for medicinal agents.1,2 Over the years, various stereoselective synthetic protocols have been developed.3-5 However, enantioselective and efficient methods for construction of these structural features did not emerge until recently.6-9 Organocatalytic cascade reactions leading to functionalized cyclohexane rings were earlier developed by Enders et. al.10 In the context of our design and synthesis of molecular probes, we have incorporated a spiro-indoline structural template as a P2′-ligand in the HIV-1 protease active site.11 For further access to spiroindoline scaffolds, we have investigated the feasibility of organocatalytic Michael/aldol cascade reactions involving 3-olefinic oxindole and pentane-1,5-dialdehyde.12-15 Recently, Wang and co-workers have reported a related work and their report prompted us to disclose our investigation in this area.16 Herein, we report (R)-diphenylprolinol silyl ether-catalyzed tandem Michael/aldol reaction leading to the synthesis of a variety of spiro[cyclohexane-1,3′-indolin]-2′-one derivatives with four/five contiguous chiral centers in excellent yield and optical purity. Interestingly, protecting groups on the indolin-2-one nitrogen played important roles in the stereochemical outcome of the final aldol ring closure. The electron-withdrawing N-protecting group in the presence of (R)-catalyst provided spiro[cyclohexane-1,3′-indoline] derivatives with 6-(R)-hydroxy configuration. Whereas, the electron-donating N-protecting group furnished spiro[cyclohexane-1,3′-indoline] derivatives with 6-(S)-hydroxy configuration as the major product.
We initially investigated a catalytic domino reaction of 3-olefinic oxindole 1a and pentane-1,5-dial 212,13 using organocalatyst A (10 mol%) in THF at ambient temperature, under an aerobic atmosphere as shown in Scheme 1. The desired tandem Michael/aldol product 5a was isolated in 41% yield. As shown in Table 1, the ratio of diastereomers was moderate (3.5:1:0.5:0.3). However, the major product 5a showed excellent enantioselectivity (96% ee, Table 1, entry 1) in HPLC analysis.17 The overall process presumably proceeded through Michael reaction resulting in intermediate 3 followed by an intramolecular aldol reaction providing intermediate 4. Addition of an acidic co-catalyst, such as ortho-fluorobenzoic acid, trifluoroacetic acid or acetic acid, failed to improve the yield (Table 1, entries 2-4). We investigated other organic solvents such as CH2Cl2, toluene, N,N-dimethylformamide (DMF), hexane, and CH3CN (entries 5-9). As it turned out that the choice of solvent was crucial to this Michael-Aldol tandem reaction and the best results were obtained using DMF as the solvent, affording 5a as the major diastereomer (6:1:0.5:0.4) in 80% yield and showed excellent enantioselectivity for the major diastereomer (>99% ee, entry 8). An attempt to perform the reaction at lower temperature resulted in low conversion (entry 10).
Scheme 1.
Michael/aldol cascade with oxindole 1a and dialdehyde 2.
Table 1.
Michael/Aldol Reaction Optimizationa

| |||||
|---|---|---|---|---|---|
|
| |||||
| entry | solvent | T (h) | yieldb (%) |
dr c |
ee (%)d |
| 1 | THF | 48 | 41 | 3.5:1:0.5:0.3 | 96 |
| 2e | THF | 48 | <10 | nd | n. d. |
| 3f | THF | 48 | <5 | nd | n. d. |
| 4g | THF | 48 | <5 | n. d. | n. d. |
| 5 | CH2Cl2 | 48 | <5 | n. d. | n. d. |
| 6 | toluene | 48 | <5 | n. d. | n. d. |
| 7 | hexane | 48 | <5 | n. d. | n. d. |
| 8 | DMF | 20 | 80 | 6:1:0.5:0.4 | >99 |
| 9 | CH3CN | 48 | <5 | n. d. | n. d. |
| 10h | DMF | 48 | 16 | 7:1:0.5:04 | 99 |
Unless otherwise noted, the reaction was performed by employing 1a (0.1 mmol), dialdehyde 2 (50% in water, 0.3 mmol), and organocatalyst A (0.01 mmol) in the indicated solvent (0.5 mL) at room temperature. n. d. = not determined.
Isolated yield.
Determined by 1H NMR spectroscopy.
Determined by HPLC of the major diastereomer.
Ortho-fluorobenzoic acid (0.01 mmol) was added.
Trifluoroacetic acid (0.01 mmol) was added.
Acetic acid (0.01 mmol) was added.
This reaction was performed at 0 °C.
We subsequently explored substrate scope and limitations under the optimized reaction conditions (Figure 1). As evidenced by the results in Table 2, the reaction proceeded smoothly with both aromatic and aliphatic substituents to give desired products in high yields. The substituents on the aryl ring have little effect on the enantioselectivity of the major isomer. Monosubstituted phenyl methyleneindol-inones proceeded with excellent enantioselectivity ranging from 98 to 99% ee (entries 1-7). However, electronic properties of aryl substituents showed definite effect on diastereoselectivity. Electron-donating groups resulted in better diastereoselectivity. As shown, a high diastereomeric ratio of 7:1:0.5:0.4 was observed for 3-methoxyphenyl substituted methyleneindolinone, whereas a dr of 3:1:0.7:0.5 was observed for 3-nitro-phenyl substituted methyleneindolinone (entry 3 vs 7). Reactions with both heterocyclic and alkyl methyleneindolinones with aromatic substituents also proceeded with high yields and excellent enantioselectivity (entries 8 and 9). We further examined the cyclization reaction using an N-acetyl group in indole in place of an N-Boc protecting group. This resulted in product 5j in excellent yield with excellent enantioselectivity; however, the diastereomeric ratio (3:1:0.8:0.2; entry 10) was lower compared to the Boc-protected derivative (entry 1).
Figure 1.
Structure and enantioselectivity of major isomer
Table 2.
Enantioselective syntheses of Spirooxindoles a

| |||
|---|---|---|---|
|
| |||
| Entry | R1 | Pdt (Y%)b | Drc (ee %)d |
| 1 | Ph | 5a (80) | 6:1:0.5:0.4 (99) |
| 2 | 4-Me-Ph | 5b (82) | 6:1:0.5:0.3 (99) |
| 3 | 3-OMePh | 5c (82) | 7:1:0.5:0.4 (98) |
| 4 | 2-OMe-Ph | 5d (84) | 5.5:1:0.5 (99) |
| 5 | 3-Cl-Ph | 5e (74) | 5.2:1:0.5:0.5 (99) |
| 6 | 4-NO2-Ph | 5f (80) | 3:1:0.5 (99) |
| 7 | 3-NO2-Ph | 5g (74) | 3:1:0.7:0.5 (99) |
| 8e | 2-furyl | 5h (80) | 6:1:0.5:0.3 (98) |
| 9e | Pr | 5i (54) | 6:1:0.7 (95) |
| 10f | Ph | 5j (90) | 3:1:0.8:0.2 (95) |
General reaction conditions: oxindole (0.1 mmol), dialdehyde 2 (50% in water, 0.3 mmol), and organocatalyst A (0.01 mmol) in DMF (0.5 mL) at 23°C.
Isolated combined yield.
The ee was determined by chiral-phase HPLC analysis of major diastereomer.
Determined by 1H NMR of crude product.
The reaction was performed for 3 days.
Oxindole is protected as NAc.
We then examined the effect of electron-donating N-protecting groups on the oxindole. Interestingly, this resulted in aldol ring closure with opposite hydroxyl stereochemistry as the major diastereomer. The results in Table 3 show various N-alkyl protecting groups, such as methyl, allyl and benzyl groups, provided products 5k-5m as the major diastereomers in excellent yields (70-84%) and excellent enantioselectivity for the major diastereomer (>99%, entries 1-3). Furthermore, both electron-donating (Me) and electron-withdrawing (Cl) substituents at the C4-position of the phenyl group afforded the corresponding products in high yields with excellent enantioselectivity for the major diastereomer (>99% ee, entries 4 and 5). Interestingly, the electron-withdrawing 4-NO2 derivative furnished moderate diastereoselectivity (entry 6) compared to 4-Cl substituent (entry 4). However, the major isomer showed excellent enantioselectivity.
Table 3.
Syntheses of Spirooxindoles with N-alkyl groupsa

| ||||
|---|---|---|---|---|
|
| ||||
| entry | R1 | R2 | Pdt(Y%) b | drc (ee %)d |
| 1 | Ph | Me | 5k (70) | 6.5:1:0.5 (99) |
| 2 | Ph | Bn | 5l (82) | 6:1:0.5:0.3 (99) |
| 3 | Ph | allyl | 5m (84) | 7:1:0.4 (99) |
| 4 | p-ClPh | allyl | 5n (74) | 6.5:1:0.3:0.2 (99) |
| 5 | p-MePh | allyl | 5o (74) | 7:1:0.5:0.5 (99) |
| 6 | pNO2Ph | allyl | 5p (70) | 2:1:0.5 (99) |
General reaction conditions: the reaction was performed by employing 3-olefinic oxindole (0.1 mmol), dialdehyde 2 (50% in water, 0.3 mmol), and organocatalyst A (0.01 mmol) in DMF (0.5 mL) at room temperature for 2 days.
Isolated yield.
Determined by 1H NMR of crude product.
The ee was determined by chiral-phase HPLC analysis of major diastereomer.
We have also explored this Michael/aldol reaction with 3-methylpentane-1,5-dial 618 as shown in Scheme 2. This reaction proceeded smoothly and afforded spirocyclic oxindole 5q with five consecutive stereogenic centers in 65% yield and excellent enantioselectivity (99% ee). Interestingly, the aldehyde functionality at the C3-position was isomerized from the axial to equatorial position leading to the formation of the more stable C2–C3 anti-product.
Scheme 2.
Reaction of oxindole 1a and 3-methylpentane-1,5-dial 6
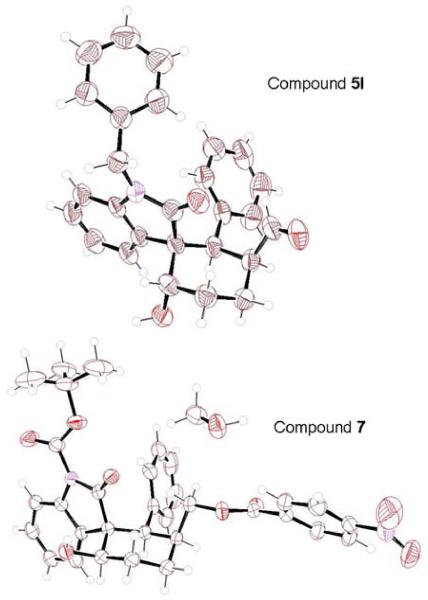
We have carried out the reaction of oxindole 1a and dialdehyde 2 up to a gram scale providing 5a in 75% yield (Scheme 3). This product showed excellent enantioselectivity as well. The relative configurations of compounds were assigned by extensive NOE analyses of compounds 5a, 5d, 5o and 5q. The absolute configurations of the tandem Michael/aldol products were determined unambiguously through X-ray crystallographic analysis. As shown in Scheme 3, benzoate derivative 7 was prepared by reduction of 5a with NaBH4 followed by reaction of the resulting alcohol with 4–nitrobenzoyl chloride in the presence of Et3N in 56% yield over 2-steps. This was later recrystallized from methanol (23 °C). Subsequent single crystal X-ray crystallographic analysis supported the assignment of the relative and absolute stereochemistry shown in Figure 2 (see supporting information for the details of X-ray analysis).19
Scheme 3.
Synthesis of benzoate 7
Figure 2.
ORTEP drawing of 5l and 7
In conclusion, we have developed a diastereoselective organocatalytic Michael/aldol cascade reaction that provided convenient access to functionalized spirooxindoles with up to five consecutive stereogenic centers, including a spiro quaternary center. The products were obtained in excellent yield and the major diastereomer showed excellent enantioselectivity. In addition, depending upon our selection of N-protecting group on the oxindole, we were able to effectively control the stereochemical outcome of the hydroxyl center upon aldol ring closure. Further studies and applications of this methodology are the subject of current investigation in our laboratory.
Supplementary Material
Acknowledgments
Financial support of this work was provided by the National Institutes of Health and Purdue University.
Footnotes
Supplementary data (experimental procedures and 1H, 13C-NMR data) associated with this article can be found in the online version.
References and notes
- 1.For selected examples, see: Serradeil-Le Gal C, Lacour C, Valette G, Garcia G, Foulon L, Galindo G, Bankir L, Pouzet B, Guillon G, Barberis C, Chicot D, Jard S, Vilain P, Garcia C, Marty E, Raufaste D, Brossard G, Nisato D, Maffrand JP, Le Fur G. J. Clin. Invest. 1996;98:2729. doi: 10.1172/JCI119098. Venkatesan H, Davis MC, Altas Y, Snyder JP, Liotta DC. J. Org. Chem. 2001;66:3653. doi: 10.1021/jo0004658.
- 2.(a) Beccalli EM, Clerici F, Gelmi ML. Tetrahedron. 2003;59:4615. [Google Scholar]; (b) Liu J-J, Zhang Z. PCT Int. Appl. WO2008/055812. Hoffmann-LaRoche AG; 2008. “Spiroindolinone derivatives”. [Google Scholar]
- 3.For selected reviews, see: Dounay AB, Overman LE. Chem. Rev. 2003;103:2945. doi: 10.1021/cr020039h. For selected publications, see: Madin A, ODonnell CJ, Oh T, Old DW, Overman LE, Sharp MJ. J. Am. Chem. Soc. 2005;127:18054. doi: 10.1021/ja055711h.
- 4.Trost BM, Cramer N, Silverman SM. J. Am. Chem. Soc. 2007;129:12396. doi: 10.1021/ja075335w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Hojo D, Noguchi K, Hirano M, Tanaka K. Angew. Chem, Int. Ed. 2008;47:5820. doi: 10.1002/anie.200801642. [DOI] [PubMed] [Google Scholar]; (b) Shintani R, Hayashi S-Y, Murakami M, Takeda M, Hayashi T. Org. Lett. 2009;11:3754. doi: 10.1021/ol901348f. [DOI] [PubMed] [Google Scholar]
- 6.Bencivenni G, Wu LY, Mazzanti A, Giannichi B, Pesciaioli F, Song MP, Bartoli G, Melchiorre P. Angew. Chem. Int. Ed. 2009;48:7200. doi: 10.1002/anie.200903192. [DOI] [PubMed] [Google Scholar]
- 7.(a) Wei Q, Gong L-Z. Org. Lett. 2010;12:1008. doi: 10.1021/ol100020v. [DOI] [PubMed] [Google Scholar]; (b) Companyo X, Zea A, Alba A-NR, Mazzanti A, Moyano A, Rios R. Chem Commum. 2010;46:6953. doi: 10.1039/c0cc01522a. [DOI] [PubMed] [Google Scholar]; (c) Jiang K, Jia Z-J, Chen S, Wu L, Chen Y-C. Chem.Eur. J. 2010;16:2852. doi: 10.1002/chem.200903009. [DOI] [PubMed] [Google Scholar]
- 8.(a) Wang L-L, Peng L, Bai J-F, Huang QC, Xu X-Y, Wang L-X. Chem. Commum. 2010;46:8064. doi: 10.1039/c0cc03032e. [DOI] [PubMed] [Google Scholar]; (b) Jia Z-J, Jiang H, Li J-L, Gschwend B, Li Q-Z, Yin X, Grouleff J, Chen Y-C, Jørgensen KA. J. Am. Chem. Soc. 2011;133:5053. doi: 10.1021/ja1112194. [DOI] [PubMed] [Google Scholar]; (c) Liu Y, Nappi M, Arceo E, Vera S, Melchiorre P. J. Am. Chem. Soc. 2011;133:15212. doi: 10.1021/ja206517s. [DOI] [PubMed] [Google Scholar]
- 9.Tan B, Hernandez-Torres G, Barbas CF., III J. Am. Chem. Soc. 2011;133:12354. doi: 10.1021/ja203812h. [DOI] [PubMed] [Google Scholar]
- 10.(a) Enders D, Huttl MRM, Grondal C, Raabe G. Nature. 2006;441:861. doi: 10.1038/nature04820. [DOI] [PubMed] [Google Scholar]; (b) Enders D, Huttl MRM, Runsink G, Raabe G. Angew. Chem, Int. Ed. 2007;46:467. doi: 10.1002/anie.200603434. [DOI] [PubMed] [Google Scholar]
- 11.Ghosh AK, Schiltz G, Perali RS, Leschenko S, Kay S, Walters DE, Koh Y, Maeda K, Mitsuya H. Bioorg. Med. Chem. Lett. 2006;16:1869. doi: 10.1016/j.bmcl.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi Y, Okano T, Aratake S, Hazelard D. Angew. Chem. Int. Ed. 2007;46:4922. doi: 10.1002/anie.200700909. [DOI] [PubMed] [Google Scholar]
- 13.Chintala P, Ghosh SK, Long E, Headley AD, Ni B. Adv. Synth. Catal. 2011;353:2905. [Google Scholar]
- 14.Zhao G-L, Dziedzic P, Ulla F, Eriksson L, Cordova A. Tetrahedron Lett. 2009;50:3458. [Google Scholar]
- 15.Pellissier H. Adv. Synth. Catal. 2012;354:237. [Google Scholar]
- 16.Huang X-F, Liu Z-M, Geng Z-C, Zhang S-Y, Wang Y, Wang X-W. Org. Biomol. Chem. 2012;10:8794. doi: 10.1039/c2ob26205c. [DOI] [PubMed] [Google Scholar]
- 17.For details, please see supplementary data.
- 18.Sobhani S, Maleki MF. Synlet. 2010:383. [Google Scholar]
- 19.CCDC 897144 (5l) and CCDC 897143 (7) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.