Abstract
Background
Calcium sensitising inotropes are increasingly being used in cardiac surgical patients. Theoretically, increasing contractile protein sensitivity to Ca2+ prevents the Ca2+ elevation associated arrhythmogenicity and potentiates the inotropic effect of catecholamines. On the other hand, we hypothesised that Ca2+ sensitisation exacerbates post-ischaemic myocardial stunning by impairing diastolic relaxation, which might have deleterious effects in postoperative cardiac surgical patients.
Methods
In an isolated rabbit heart model, 45 min normothermic ischaemia with potassium-induced cardioplegic arrest was followed by 120 min reperfusion. Isovolumetric left ventricular (LV) function and myocardial oxygen consumption (MvO2) were measured, and cytosolic Ca2+ was monitored by rhod-2 surface spectrofluorometry. During reperfusion, ORG 30029 (250 μM) and levosimendan (0.5 μM) were used as Ca2+ sensitisers (ORG, n = 6, Levo, n = 6), Ca2+ de-sensitisation was induced with butanedione-monoxime (5 mM, BDM, n = 6), and dopamine (20 nM) served as a representative catecholamine (n = 6). To counteract the PDE III inhibiting properties of ORG and Levo, IGF-1 (0.1 μM) and parathyroid hormone (0.05 μM) were used.
Results
As expected, ischaemia/reperfusion induced moderate cytosolic calcium overload. Dopamine increased LV contractility and MvO2 by augmenting the amplitude of the Ca2+ transient, but relaxation was unchanged due to faster diastolic Ca2+ removal. Dopamine-induced Ca2+ handling was unchanged after uncoupling the Mg-ATPase with BDM, and MvO2 decreased in proportion with the reduced LV mechanical work load. ORG improved contractility without apparent effects on Ca2+ handling, and MvO2 remained constant despite increased contractile work. Conversely, ORG induced a rightward shift of the diastolic pressure-volume relationship in post-ischaemic hearts (diastolic pressure at 0.8 ml balloon volume 14.3 ± 5 mmHg, p = 0.01 vs control), but not in non-ischaemic control hearts. With levosimendan, the Ca2+ sensitising effects were less pronounced (7.6 ± 3 mmHg, p = 0.4 vs control). By counteracting the PDE inhibiting effects of ORG and Levo using parathyroid hormone and IGF-1, the negative lusotropic effects of Ca2+ sensitisation were unmasked.
Conclusions
Calcium sensitisation improves systolic function and energetic efficiency. However, Ca2+ sensitisers should be used with caution during post-ischaemic reperfusion, as they may exacerbate myocardial stunning and thus impair cardiac output.
Keywords: Calcium sensitiser, Catecholamines, Ischaemia
1. Introduction
While pharmacologic treatment of post-operative pump failure remains largely based on catecholamines, novel types of inotropic agents have also found widespread clinical applications [1,2]. In principle, PDE III inhibitors increase contractility by targeting the same molecular end-effectors in cardiomyocytes via an increase of cyclic adenosine monophosphate (cAMP), but much of their clinical benefit is due to a potent afterload reduction. In the immediate postoperative situation, this can lead to a sustained loss of blood pressure that needs to be counteracted with vasoconstricting agents. Both, catecholamines and PDE III, inhibitors have in common that they improve contractility by increasing the cytosolic Ca2+ concentration and the amplitude and slope of the calcium transient, using the physiological adrenergic downstream effectors that regulate rapid changes in myocardial contractility. The net increase in intracellular calcium cycling, however, consumes additional energy resources, resulting in an increase of myocardial oxygen consumption that is disproportionate to that required by contractile work and may even activate cell-death-signalling cascades [3]. At least in theory, this effect is even more problematic when myocardial calcium cycling is already exacerbated, not only as in acute myocardial infarction but also as in surgical post-ischaemia settings. Another potential problem of post-ischaemic calcium overload is the impairment of diastolic relaxation when cytosolic Ca2+ is elevated, but adrenergic inotropes theoretically counteract this effect by protein kinase A-mediated reduction of contractile protein Ca2+ sensitivity. In the 1990s, the concept of increasing contractile protein Ca2+ sensitivity to improve contractility without affecting intracellular Ca2+ cycling had been promoted, and some of those drugs have reached the clinical arena [4,5]. In patients with chronic heart failure, agents such as levosimendan are being used with increasing frequency, and several studies have evaluated Ca2+ sensitisers in experimental models and in patients with post-surgical contractile failure. Since the role of Ca2+ sensitisers in the clinical setting has not been clearly defined yet, we sought to test the hypothesis that Ca2+ sensitisation can potentially impair diastolic relaxation and thus the global function of post-ischaemic hearts, when cytosolic Ca2+ is already elevated. The rationale for doing so is that, theoretically, increased Ca2+ affinity of the EF and Ca2+ binding site on troponin C has a positive inotropic effect but, at the same time, impairs relaxation. In the clinical situation, this can impede not only diastolic filling of the heart but, via the Frank-Starling mechanism, also systolic function.
2. Methods
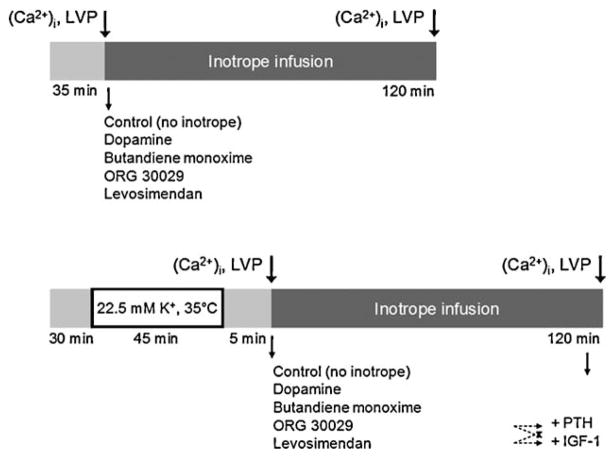
New Zealand white rabbits (2.5 kg) were anaesthetised by intravenous injection of ketamine (100 mg kg−1) and heparin. The heart was rapidly excised, the aorta was cannulated and perfused in the Langendorff mode at 80 mmHg constant perfusion pressure with modified Krebs-Henseleit buffer, as previously described [3,6,7]. Temperature was maintained at 37° C and monitored with a probe placed in the right ventricle. The cava and pulmonary veins were closed and the pulmonary artery cannulated for collection of the venous effluent. A fluid-filled balloon connected to a Millar micromanometry catheter was placed in the left ventricle (LV) via the left atrium. The hearts were not paced. After 30 min stabilisation, the aorta was clamped and the hearts were subjected to 45 min global ischaemia at 35° C in a heated chamber filled with humidified room air with cardioplegic arrest induced by oxygenated, warm Krebs-Henseleit buffer with 22.5 mM K+. Left ventricular pressure was measured pre-ischaemia and in 30 min intervals during reperfusion (Fig. 1). The LV balloon was filled stepwise in increments of 0.1 ml and diastolic and systolic pressure was recorded. No pre-filling of the balloon up to a pressure of 0 mmHg was performed; all balloon volumes given represent the actual filling. The data were used to plot the diastolic and systolic pressure-volume relationships. Between measurements, the balloon was deflated and the heart was beating unloaded. Coronary flow was measured by timed collection of the coronary effluent. Myocardial oxygen consumption (MvO2) was derived from the arteriovenous difference in O2 tension (Stat Profile Plus 9, Nova Biochemical, Waltham, MA, USA), multiplied by coronary flow and divided by dry heart weight.
Fig. 1.
Schematic depiction of the experimental protocol. LVP, left ventricular pressure; PTH, parathormone; IGF-1, insulin-like growth factor-1.
2.1. Cytosolic Ca2+
Measurement of beat-to-beat intracellular calcium transients was performed in separate sets of intact perfused hearts, as we have previously described and validated in detail [3,6–9]. For quantitative measurement of cytosolic Ca2+ at the end of the reperfusion period, the hearts were saturated with Ca2+-sensitive dye rhod-2-AM (Molecular Probes, Eugene, OR, USA) after 100 min reperfusion by perfusion with the cell-permeable acetoxymethylester (rhod-2-AM; 0.5 mg/0.25 ml DMSO infused over 2 min at 37° C without re-circulation). Dye loading was followed by a 15-min washout period. A modified spectrofluorometer (SLM-Aminco, Springfield, IL, USA) provided excitation light at 524 nm and recorded emission light at 589 nm. Tissue absorbance was quantified using the ratio of scattered excitation light at 524 nm (peak rhod-2 absorbance in myocardial tissue) and 589 nm. The change in absorbance over time was then used to account for differences in dye loading or changes in tissue dye concentration. At the end of each experiment 2,2′-dithiodipyridine (100 μM) was infused over a period of 2 min to induce calcium release from the sarcoplasmic reticulum. This was immediately followed by bolus injection of calcium ionophore A23187 (calcimycin) in 10 ml 10% calcium solution to maximise calcium entry from the extracellular space and maximum fluorescence (Fmax) was determined to calculate systolic and diastolic calcium concentration using the following equation:
where [Ca2+]i is the free cytosolic calcium concentration, Kd is the dissociation constant for rhod-2 with calcium at nor-mothermia and pH 7.1, Ft is fluorescence at a specific time point, F0 is autofluorescence measured before dye loading, At and Amax are tissue light absorbance at the specific time point or at the end of the experiment, respectively. In this model, rhod-2 has no measurable effect on cardiac function [7].
In a subset of hearts (n = 3 per group), a small amount of rhod-2-AM (0.1 mg in 0.1 ml DMSO) was administered just prior to the onset of the inotrope infusion. This allowed for qualitative registration of Ca2+ transients for approximately 15 min.
2.2. Experimental groups
Control hearts (n = 6) were subjected to 45 min warm ischaemia with cardioplegic arrest followed by unmodified reperfusion for 120 min. Calcium sensitisation was induced by adding the calcium sensitising agent ORG 30029 (250 μM, n = 6) to the perfusate 5 min after start of reperfusion and given throughout the entire reperfusion period. To test a drug with calcium sensitising properties that is clinically available, levosimendan (0.5 μM) was used (n = 6). The concentrations we used in this study were determined in previous experiments and chosen so that a similar effect on LV contractility was induced. They are not comparable to the drug concentrations that would be used in in vivo models or in the clinical setting. To unmask the effect of true Ca2+ sensitisation by counteracting the PDE III-inhibiting effects of ORG and Levo, the known PDE activators parathyroid hormone (PTH, 0.05 μM, n = 5) and insulin like growth factor-1 (IGF-1, 0.1 μM, n = 5) were added to the perfusion buffer at the end of the reperfusion period in a separate set of experiments.
For comparison with a clinical routine catecholamine, 20 nM dopamine used in other hearts (n = 6). In an attempt to determine the effects of reverse manipulation of contractile protein calcium sensitivity, 2,3-butanedione monoxime (BDM) 5 mM was added (n = 5). BDM directly inhibits the myosin Mg-ATPase and hence decreases cross-bridge cycling and myocardial contractility. This concentration of BDM was chosen because we could previously show that it has no effect on intracellular calcium handling but results in an approximately 30% decrease in contractility.
All inotropes were also given to non-ischaemic hearts (n = 4 each group). Here, the respective agents were added to the buffer after 30 min stabilisation, and perfusion continued for 120 min.
2.3. Animal care
All animals received humane care in compliance with the ‘Principles of Laboratory Animal Care’ formulated by the National Society for Medical Research and the ‘Guide for the Care and Use of Laboratory Animals’ prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH Publication No. 85-23, revised 1996). The protocol was reviewed and approved by the Internal Animal Care and Use Committee at Children’s Hospital Boston.
2.4. Statistical analysis
The sample size was chosen to be able to detect a difference in LV contractile function between dopamine-treated post-ischaemic hearts and post-ischaemic control hearts with a confidence level of 95% (normal distribution provided). The expected difference in mean value and confidence intervals were taken from previous experiments in similar models. Analysis of calcium recordings was performed using Sigma Plot software (version 4.0, SPSS Inc., Chicago, IL, USA). Data are expressed as the mean ± standard deviation and statistical analysis was performed using the SPSS software package (version 17.0, SPSS Inc., Chicago, IL, USA). All statistically relevant experimental data were normally distributed, and hence parametric tests were used. Data of unrelated experimental groups (intracellular Ca2+, LV pressure at 0.8 ml balloon volume) were analysed by analysis of variance (ANOVA) followed by Dunnet’s post hoc test for multiple comparisons with a reference group. When the effects of different inotropes were compared, ‘control’ served as the reference group. In the experiments with IGF-1 or PTH, ‘Levo’ or ‘ORG’ were the respective reference groups. For comparison of the LV pressure-volume relationships under the influence of inotropes, the slope of each individual experiment was computed by linear regression (data series with R2 < 0.6 were excluded), mean values and confidence intervals were calculated for each group, and the entire data set was analysed by ANOVA followed by Dunnet’s post hoc test for multiple comparisons with ‘control’ as the reference group. The same process was performed in order to compare energetic efficiency (RPP/MvO2) and contractile calcium responsiveness (dP/[Ca2+]i) data between dopamine and calcium-sensitiser treated hearts.
3. Results
3.1. Inotrope effects in non-ischaemic hearts
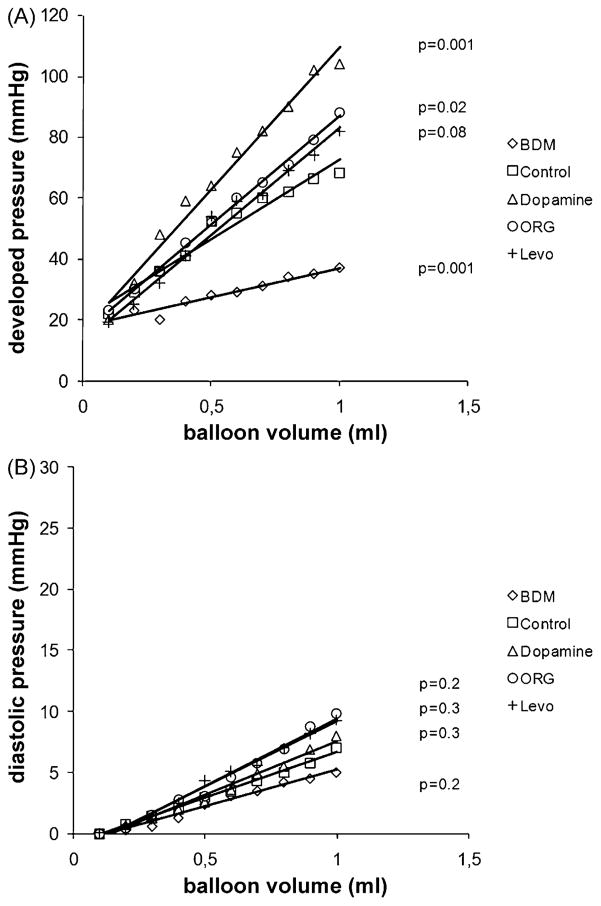
As expected, dopamine as well as ORG and Levo exerted a positive inotropic effect, as evidenced by a leftward shift of the isovolumetric LV pressure-volume curve (Fig. 2A). Not surprisingly, contractile protein Ca2+ de-sensitisation by Mg-ATPase inhibition with BDM was negative inotropic. Diastolic relaxation of the LV myocardium, indicated by the diastolic pressure-LV volume curve, was facilitated by BDM, but was not significantly affected by any of the inotropes (Fig. 2B). The data, thus, confirm the notion that, in non-ischaemic hearts, Ca2+ sensitisers can improve contractility without impairing relaxation.
Fig. 2.
(A) Isovolumetric systolic pressure-volume relationship (developed pressure, devP) in non-ischaemic isolated rabbit hearts treated with the calcium de-sensitiser butanedione monoxime (BDM), dopamine, and the calcium sensitisers ORG 30029 (ORG) and levosimendan (Levo), n = 6 per group. The LV balloon was inflated in increments of 0.1 ml. (B) Diastolic pressure-volume relationship in the same non-ischaemic hearts. The p-values are based on the comparison of the slopes of the pressure-volume relationships by ANOVA with Dunnet’s post hoc test for multiple comparisons (unpaired), with ‘control’ as the reference group.
3.2. Inotrope effects in post-ischaemic hearts
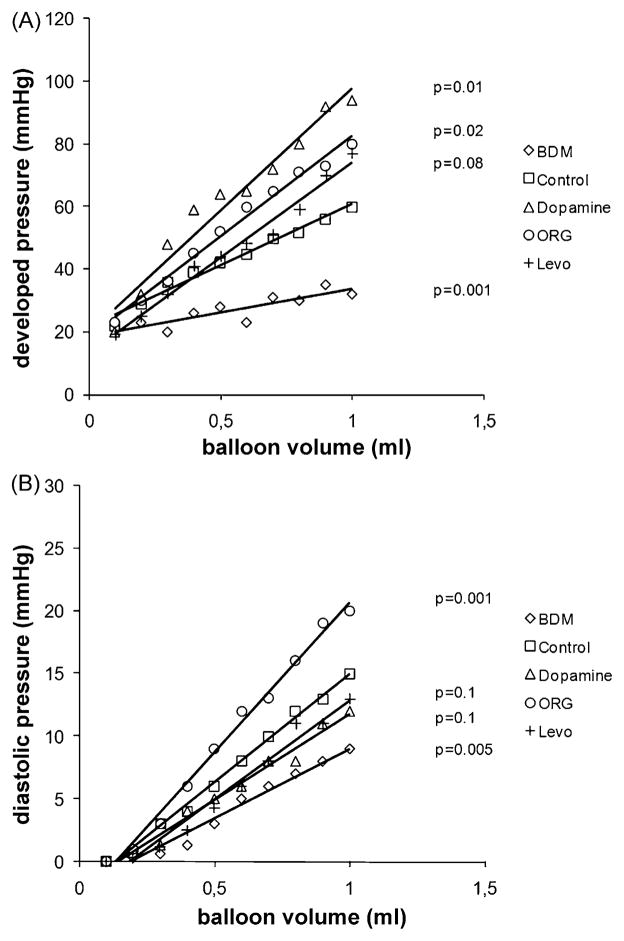
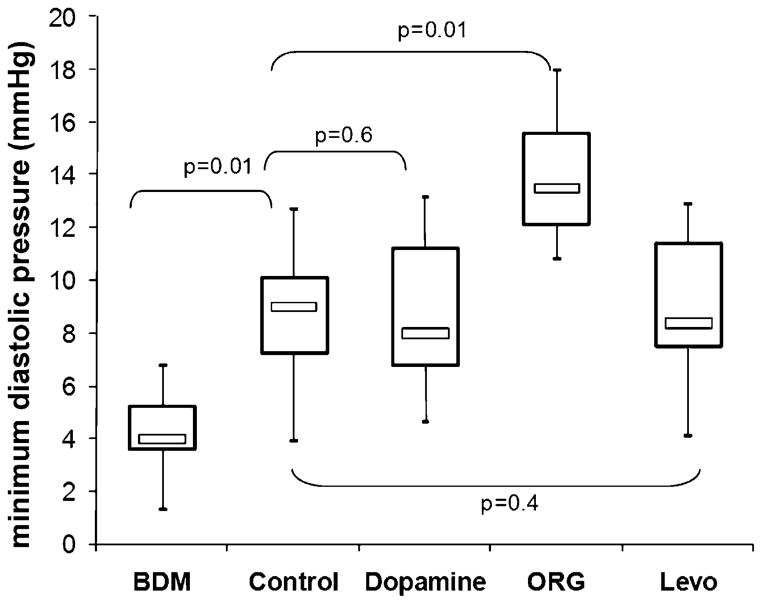
Isovolumetric contractility in post-ischaemic control hearts was moderately impaired as compared to non-ischaemic hearts, and maximum developed pressure at 0.8-ml balloon filling volume was 23% lower (62 ± 12 mmHg vs 47 ± 15 mmHg, p = 0.01). Similar to the effects observed in non-ischaemic hearts, both Ca2+ sensitisers induced a leftward shift of the systolic pressure-volume relationship (Fig. 3A), illustrating their potent positive inotropic effects even in hearts that had been subjected to ischaemia-reperfusion injury. Again, Mg-ATPase inhibition by BDM immediately reduced contractility. Diastolic relaxation in control hearts remained impaired after 120-min reperfusion (Fig. 3B), with a 86% higher minimum diastolic pressure at 0.8 ml than in non-ischaemic control hearts (4.9 ± 1.9 mmHg vs 9.1 ± 4.2 mmHg, p = 0.001). In hearts treated with Levo or dopamine, diastolic pressure tended to be lower than in control hearts (Fig. 3B), but the difference did not reach statistical significance (dopamine, 8.0 ± 2.5 mmHg p = 0.6 vs control; Levo, 7.6 ± 2.8 mmHg, p = 0.4 vs control). ORG, however, led to a clear leftward shift of the diastolic pressure-volume relationship (Fig. 3B). This impairment of relaxation is also demonstrated in Fig. 4 (14.3 ± 4.6 mmHg, p = 0.01 vs control at 0.8 ml balloon filling volume).
Fig. 3.
(A) Isovolumetric systolic pressure-volume relationship (developed pressure, devP) in post-ischaemic isolated rabbit hearts treated with the calcium desensitiser butanedione monoxime (BDM), dopamine, and the calcium sensitisers ORG 30029 (ORG) and levosimendan (Levo), n = 6 per group. The LV balloon was inflated in increments of 0.1 ml. (B) Diastolic pressure-volume relationship in the same post-ischaemic hearts. The p-values are based on the comparison of the slopes of the pressure-volume relationships by ANOVA with Dunnet’s post hoc test for multiple comparisons (unpaired), with ‘control’ as the reference group.
Fig. 4.
Minimum diastolic pressure at a filling volume of 0.8 ml in post-ischaemic hearts treated with the calcium desensitiser butanedione monoxime (BDM), dopamine, and the calcium sensitisers ORG 30029 (ORG) and levosi-mendan (Levo), n = 6 per group. The boxplots indicate minimum, maximum (whiskers), interquartile range (box) and median (bar). The p-values represent the results of ANOVA with Dunnet’s post hoc test for multiple comparisons (unpaired), with ‘control’ as the reference group.
3.3. Cytosolic calcium
The intensity change of the rhod-2 signal was first recorded during the onset of inotrope infusion during early reperfusion. Because Fmax and Amax were recorded for calculation of the absolute cytosolic Ca2+ concentration nearly 120 min later, the early Ca2+ induced fluorescence can only be interpreted in qualitative fashion, that is, did Ca2+ rise or fall. The Ca2+ dependent rhod-2 signal clearly increased upon infusion on dopamine, while it remained largely unchanged with BDM and ORG. Infusion of Levo, however, also resulted in an increase in signal intensity, implying that Levo also induces an acute rise in cytosolic Ca2+, probably via its PDE III inhibiting properties (data not shown).
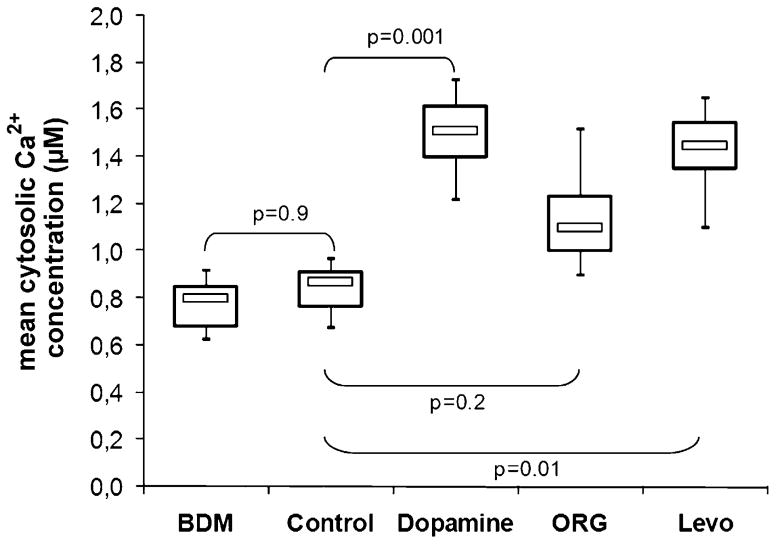
At the end of the reperfusion period, the cytosolic Ca2+ concentration was quantitatively measured by rhod-2 spectrofluorometry in the intact isolated heart. As compared with non-ischaemic control hearts, Ca2+ was significantly higher in post-ischaemic hearts (mean Ca2+ = 0.84 ± 0.32 μM vs 0.52 ± 0.21 μM, p = 0.012). The behaviour of cytosolic Ca2+ in response to inotropes is shown in Fig. 5. All positive inotropic drugs increased the mean cytosolic Ca2+ concentration, but the increase was less pronounced in ORG treated hearts. When the data are expressed as the percent increase in Ca2+, ORG induced a 47% ± 14% rise in Ca2+, while Ca2+ rose by 71% ± 21% or 82% ± 26% with Levo or dopamine, respectively.
Fig. 5.
Mean cytosolic Ca2+ concentration in post-ischaemic rabbit hearts treated with butanedione monoxime (BDM), dopamine, and the calcium sensitisers ORG 30029 (ORG) and levosimendan (Levo), n = 6 per group. Control, untreated post-ischaemic hearts. The p-values represent the results of ANOVA with Dunnet’s post hoc test for multiple comparisons (unpaired), with ‘control’ as the reference group.
3.4. Calcium sensitivity
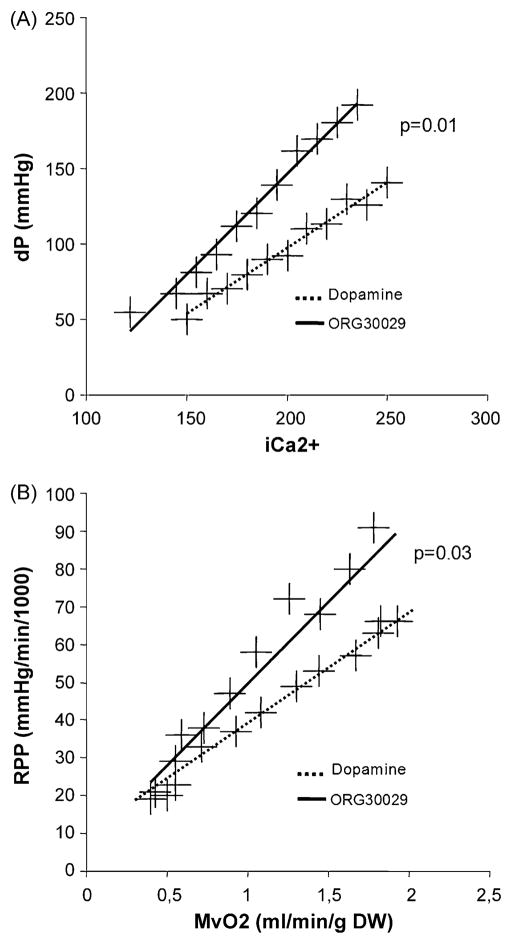
When the LV developed pressure data at different balloon volumes are plotted as a function of the corresponding mean Ca2+ concentration, we observed that ischaemia/reperfusion injury reduces contractile protein Ca2+ sensitivity in control hearts (rightward shift of the devP/Ca2+ relationship), and that dopamine or Levo stimulation leaves the Ca2+ responsiveness largely unchanged. ORG treatment, however, induced a clear leftward shift of the devP/Ca2+ relationship, indicating a Ca2+ sensitising effect (Fig. 6A). A more direct and detailed measurement of contractile protein Ca2+ sensitivity would require to work isolated myofibril preparations or with tetanised whole hearts, neither of which we could perform in the current series of experiments [8,9].
Fig. 6.
(A) Calcium-force relationship in post-ischaemic isolated rabbit hearts treated with a standard catecholamine (dopamine) or the calcium sensitiser ORG 30029, n = 6 per group. Depicted is systolic developed pressure as a function of free cytosolic Ca2+ measured by rhod-2 spectrofluorometry. The Ca2+ sensitising effect of ORG is indicated by the rightward shift of the curve, i.e. more force is produced for a given amount of Ca2+. (B) Metabolic efficiency of post-ischaemic isolated rabbit hearts treated with a standard catecholamine (dopamine) or the calcium sensitiser ORG 30029. Depicted is myocardial work (rate-pressure product) as function of myocardial oxygen consumption (MvO2). The rightward shift of the curve in response to ORG indicates that contractile efficiency is increased, whereas there is energy wasting under the influence of dopamine. The p-values are based on the comparison of the slopes of the pressure-volume relationships by ANOVA with Dunnet’s post hoc test for multiple comparisons (unpaired), with ‘dopamine’ as the reference group.
3.5. Metabolic efficiency
Myocardial work was expressed as the rate–pressure product (RPP) and was plotted as a function of myocardial oxygen consumption (MvO2). When comparing contractile efficiency under the influence of dopamine and ORG (Fig. 6B), it is evident by the leftward shift of the RPP/MvO2 relationship that ORG increases contractility in a metabolically more efficient manner. The RPP/MvO2 relationship under the influence of Levo was not significantly different from that of dopamine (data not shown).
3.6. PDE activation
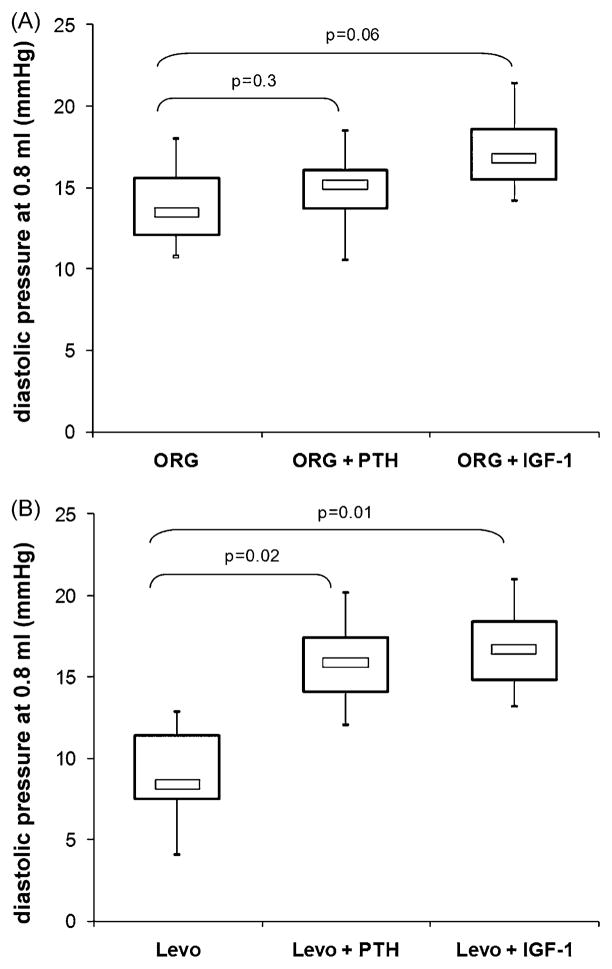
To distinguish between the direct calcium-sensitising effects of ORG and Levo and those induced by an increase in cAMP induced by PDE III inhibition, we sought to neutralise the inotrope-induced PDE III inhibition with IGF-1 or parathyroid hormone, both known to directly activate PDE III [10,11]. As seen in Fig. 7A, the already high diastolic pressure with ORG was not further elevated by IGF-1 or PTH, implying that PDE III inhibition does not play an important role here. In contrast, both IGF-1 and PTH induced a significant increase in diastolic pressure in Levo treated hearts (Fig. 7B: 7.6 ± 2.3 mmHg vs 16.1 ± 5.2 mmHg, p = 0.02 (PTH) and 15.4 ± 5.5 mmHg, p = 0.01 (IGF)). This phenomenon can be interpreted as an unmasking of the true Ca2+-sensitising effects of Levo by a neutralisation of the PDE III inhibition, indeed resulting in impaired relaxation in post-ischaemic hearts.
Fig. 7.
Minimum diastolic pressure at a balloon filling volume of 0.8 ml in post-ischaemic hearts treated with the calcium sensitisers ORG 30029 (ORG, A) or levosimendan (Levo, B). n = 5 per group to counteract their PDE III-inhibiting effect, parathyroid hormone (PTH) or insulin-like growth factor-1 (IGF-1) were given at the end of the reperfusion period. Thus, the impact of Ca2+ sensitisation on diastolic relaxation was unmasked. The p-values represent the results of ANOVA with Dunnet’s post hoc test for multiple comparisons (unpaired), with ‘Levo’ or ‘ORG’ as the respective reference group.
4. Discussion
Our experiments confirmed the notion that Ca2+-sensitis-ing inotropes improve contractility in non-ischaemic and post-ischaemic hearts. Ca2+ sensitisation alone, however, can impair diastolic function, especially when intracellular calcium cycling is already increased. These effects are clearly observable when ORG 30029 is used, a first-generation Ca2+ sensitiser that has not been further developed into a clinically used drug. In the case of levosimendan, its potent PDE III-inhibiting effects seem to over-ride the negative lusitropic actions of Ca2+ sensitisation, probably via the classic pathway of protein kinase A phosphorylation of contractile protein subunits. This mechanism is believed to preserve diastolic function in the presence of catecholamine induced cytosolic Ca2+ elevation. We hence believe that Ca2+ sensitisation should always be done with caution when Ca2+ overload of the myocardium is suspected.
It has long been established that high-dose or long-term therapeutic application of β1-stimulating catecholamines can have detrimental effects such as arrhythmogenicity, tachycardia, increased energy demand of the myocardium, induction of pro-apoptotic signalling cascades [3] or increased afterload via concomitant α-stimulation. Many of these problems are at least in part caused by the inevitable increase in cytosolic Ca2+, the main trigger of positive inotropy [12]. In principle, this also applies to specific PDE III inhibitors such as milrinone, but here the afterload-reducing effects have much more impact on the overall clinical performance (inodilators). In theory, Ca2+ sensitisers that increase the affinity of troponin C or decrease that of troponin I for calcium and thereby elicit stronger contractile force for a given amount of Ca2+ offer an elegant solution [13]. Not only can the negative effects of elevated cytosolic Ca2+ be avoided, but myocardial work can become also more efficient in terms of energy metabolism, because less energy-rich phosphates are wasted on the removal of Ca2+ ions against immense concentration gradients. The saved energy can thus be used by the Mg-ATPase for the actual contractile work. At least, with respect to ORG 30029, we could clearly demonstrate this effect in our experiments, and other groups have found similar phenomena in different model, including large animals [14–18].
On the other hand, based on our current understanding of myocardial physiology, an artificial increase in contractile protein sensitivity to calcium that is not counteracted by PKA phosphorylation should impair diastolic relaxation of the myofilaments. When Ca2+ sensitisers were newly introduced in the 1990, this had been the subject of substantial controversy [19,20]. Partly because of these concerns, most of the first-generation Ca2+ sensitisers that were evaluated in the 1990s have not reached the clinical application phase. Second-generation Ca2+ sensitisers such as levosimendan or pimobendan have been more successful, primarily because it could reproducibly be demonstrated that they do not impair but perhaps even improve diastolic function in vivo [15–17]. This phenomenon is readily explained by the potent PDE III inhibiting effects of the recent Ca2+ sensitisers, prompting some to include them in the group of clinical ‘inodilators’. However, we could show that when the PDE III inhibition induced cAMP elevation and PKA activation is neutralised by PDE III agonists, the Ca2+ sensitising effects on relaxation (negative lusitropic) dominate. This is not to say that we would warn against the use of novel Ca2+ sensitisers, which have been shown to be effective even in postoperative cardiac surgical patients. However, diastolic function should always be monitored carefully, especially if more potent ‘true’ Ca2+ sensitisers will be available in the future. Moreover, simultaneous application of cAMP elevating drugs is perhaps advisable, since here the PKA induced depression of contractile protein Ca2+ sensitivity may be protective. In addition, current Ca2+ sensitisers also seem to have beneficial pre-conditioning effects on the myocardium that is challenged by ischaemia or oxidative stress [21,22], on which novel strategies for myocardial protection may be built.
4.1. Limitations of the study
The experimental model we used is highly artificial since crystalloid buffer perfused isolated hearts were used. We tried to design the ischaemia reperfusion protocol as close to the clinical setting during cardiac surgery as possible, including normothermic cardioplegic arrest. However, a more physiologic model was not possible because we wanted to monitor cytosolic calcium. This also implies that the inotrope concentrations we needed were empirically determined by monitoring the effect on LV function while increasing the infusion rate and are not comparable with those used in vivo and in patients, respectively. This may have important implications for the balance between Ca2+ sensitising and PDE III inhibiting actions, which are known to be highly dose dependent.
Appendix A. Conference discussion
Dr D. Nordhaug (Trondheim, Norway):I have a few questions and a comment. First: you warned that diastolic function may be impaired by calcium sensitisation. However, you have only presented pressure volume data, and I am wondering whether you had the opportunity in your instrumentation to look at other diastolic indices describing other phases of the diastole, for instance the derivative of the pressure decay, the time constant of relaxation, τ, and so on? That is my first question.
My second question is: levosimendan is known to carry phosphodiesterase inhibiting effects, at higher doses at least, and it appears to act mainly as a calcium sensitiser at low doses. I am wondering what the rationale for your dose was? And importantly, have you got any idea of what this dose corresponds to in a clinical setting? One of the main problems with using levosimendan clinically is that we don’t know the balance between the phosphodiesterase inhibiting effects and the calcium sensitisation at the doses we use clinically. Did you use a high or a low dose compared to a clinical setting?
My third question is: the lack of impact on diastolic function by levosimendan may be due to phosphodiesterase inhibition, as you suggest. However, there is also evidence for a calcium dependent calcium sensitisation, so that during diastole when calcium is low, it actually doesn’t work as a calcium sensitiser. That is probably specific for levosimendan compared to other calcium sensitisers. Have you looked into that? That is my last question.
My comment is that in your paper you speculate about possible preconditioning properties of levosimendan, and I would very much like to have a similarly thoroughly and well-conducted study like this performed where you investigate levosimendan given before ischaemia and look into the effects of that.
Dr Choi: To start with the second question, which was referring to the dosage that we used. Of course, one of the limitations of this model is that it is an animal model, and the metabolism of all drugs, is different compared to patients. The dosage that we actually used was based on previous trial and error experiments. We tried different concentrations, and the concentration which has been used here, was 0.5 μM of levosimendan, which resulted in significant differences in systolic and diastolic function. By exceeding this concentration we did not see any further differences, but by staying below this concentration, we did not observe any significant effects. This applies to all the drugs that we used in this experiment.
Your other question was regarding?
Dr Nordhaug: Other indices of diastolic function.
Dr Choi: Yes, the diastolic function. Typically for a Langendorff experiment we had the balloon placed in the left ventricle which was the only instrument of determining the diastolic function; by just determining the diastolic pressure depending on the different preloads of the balloon, starting at 0.1 ml and increasing the volume incrementally up to 1 ml. All diastolic pressure measurements have been carried out at 0.8 ml in all hearts which have been of similar size in order to have comparable results at the end.
We did not look into a calcium related calcium sensitisation of the myocyte. What we did, we measured calcium concentration during all phases by direct fluorescence spectroscopy of the whole heart using the rhod-2, and that was just referring to the calcium concentrations and the efficiency. And what we saw here was just the developed pressure over the intracellular calcium concentration, which showed a direct left shift in the hearts that had been treated with calcium sensitisers, in this case with ORG.
Dr G. Lutter (Kiel, Germany): I also have a question concerning the inhibiting effect of levosimendan on the myocardial phosphodiesterase. Can it be blocked differently, how is it done, and with which substances is it tested?
Dr Choi: The phosphodiesterase-inhibiting effect, which is like the same effect of e.g. milrinone, is just one of the features of levosimendan in this case. In order to counteract that effect, we treated the hearts with IGF-1 or parathyroid hormone.
I am sorry, then, I didn’t get the question properly.
Dr Lutter: No, I thought you meant other substances that also block phosphodiesterase.
Dr Choi: No.
Dr Lutter: Sorry, I misunderstood.
Dr Choi: Yes.
Dr C. Vahl (Mainz, Germany): I want to congratulate you for this interesting and I think important data. I only want to offer perhaps a different interpretation. Your BDM data may be interpreted as an indicator that passive resting force may be really active and that a Frank-Starling mechanism may be active in nature. This could mean that calcium sensitisers may have an effect of the restoration of the Frank-Starling mechanism, the active component, and for that reason this may be one effect that may be extremely beneficial even in those hearts with too much intracellular calcium concentration. Could you agree with this kind of interpretation, just relating to your BDM data that showed that your passive resting tension declines? And this seems to indicate that this is not passive. But you can only detach active cross bridges if you add BDM.
Dr Choi: That’s right.
Dr Vahl: And you may reattach them giving calcium sensitisers, and this may be interpreted as restoration of the Frank-Starling mechanism, perhaps.
Dr Choi: I am afraid I can’t comment on this question. I am very sorry.
Dr Lutter: I think this is a very interesting comment. The problem is that you did not look for Frank-Starling mechanisms in the hearts, right?
Dr Choi: No, not at all.
Footnotes
Presented at the 22nd Annual Meeting of the European Association for Cardio-thoracic Surgery, Lisbon, Portugal, September 14–17, 2008.
References
- 1.Mebazaa A, Nieminen MS, Packer M, Cohen-Solal A, Kleber FX, Pocock SJ, Thakkar R, Padley RJ, Poder P, Kivikko M. Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE Randomized Trial. J Am Med Assoc. 2007;297:1883–91. doi: 10.1001/jama.297.17.1883. [DOI] [PubMed] [Google Scholar]
- 2.Tokuda Y, Grant PW, Wolfenden HD, Manganas C, Lyon WJ, Murala JS. Levosimendan for patients with impaired left ventricular function undergoing cardiac surgery. Interact Cardiovasc Thorac Surg. 2006;5:322–6. doi: 10.1510/icvts.2005.122390. [DOI] [PubMed] [Google Scholar]
- 3.Stamm C, Friehs I, Cowan DB, Cao-Danh H, Choi YH, Duebener LF, McGowan FX, del Nido PJ. Dopamine treatment of postischemic contractile dysfunction rapidly induces calcium-dependent pro-apoptotic signaling. Circulation. 2002;106:I290–298. [PubMed] [Google Scholar]
- 4.Edes I, Kiss E, Kitada Y, Powers FM, Papp JG, Kranias EG, Solaro RJ. Effects of Levosimendan, a cardiotonic agent targeted to troponin C, on cardiac function and on phosphorylation and Ca2+ sensitivity of cardiac myofibrils and sarcoplasmic reticulum in guinea pig heart. Circulation Res. 1995;77:107–13. doi: 10.1161/01.res.77.1.107. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe A, Tomoike H, Endoh M. Ca2+ sensitizer Org-30029 reverses acidosis- and BDM-induced contractile depression in canine myocardium. Am J Physiol. 1996;271:H1829–1839. doi: 10.1152/ajpheart.1996.271.5.H1829. [DOI] [PubMed] [Google Scholar]
- 6.Stamm C, del Nido PJ. Protein kinase C and myocardial calcium handling during ischemia and reperfusion: lessons learned using Rhod-2 spectrofluorometry. Thorac Cardiovasc Surg. 2004;52:127–34. doi: 10.1055/s-2004-817978. [DOI] [PubMed] [Google Scholar]
- 7.Stamm C, Friehs I, Choi YH, Zurakowski D, McGowan FX, del Nido PJ. Cytosolic calcium in the ischemic rabbit heart: assessment by pH- and temperature-adjusted rhod-2 spectrofluorometry. Cardiovasc Res. 2003;59:695–704. doi: 10.1016/s0008-6363(03)00467-x. [DOI] [PubMed] [Google Scholar]
- 8.Stamm C, Cowan DB, Friehs I, Noria S, del Nido PJ, McGowan FX., Jr Rapid endotoxin-induced alterations in myocardial calcium handling: obligatory role of cardiac TNF-alpha. Anesthesiology. 2001;95:1396–405. doi: 10.1097/00000542-200112000-00019. [DOI] [PubMed] [Google Scholar]
- 9.Stamm C, Friehs I, Cowan DB, Cao-Danh H, Noria S, Munakata M, McGowan FX, Jr, del Nido PJ. Post-ischemic PKC inhibition impairs myocardial calcium handling and increases contractile protein calcium sensitivity. Cardiovasc Res. 2001;51:108–21. doi: 10.1016/s0008-6363(01)00249-8. [DOI] [PubMed] [Google Scholar]
- 10.Ahlstrom M, Lamberg-Allardt C. Rapid protein kinase A-mediated activation of cyclic AMP-phosphodiesterase by parathyroid hormone in UMR-106 osteoblast-like cells. J Bone Miner Res. 1997;12:172–8. doi: 10.1359/jbmr.1997.12.2.172. [DOI] [PubMed] [Google Scholar]
- 11.Ahmad M, Flatt PR, Furman BL, Pyne NJ. The role of the cyclic GMP-inhibited cyclic AMP-specific phosphodiesterase (PDE3) in regulating clonal BRIN-BD11 insulin secreting cell survival. Cell Signal. 2000;12:541–8. doi: 10.1016/s0898-6568(00)00093-0. [DOI] [PubMed] [Google Scholar]
- 12.Grandis DJ, MacGowan GA, Koretsky AP. Comparison of the effects of ORG 30029, dobutamine and high perfusate calcium on function and metabolism in rat heart. J Mol Cell Cardiol. 1998;30:2605–12. doi: 10.1006/jmcc.1998.0817. [DOI] [PubMed] [Google Scholar]
- 13.Robertson IM, Baryshnikova OK, Li MX, Sykes BD. Defining the binding site of levosimendan and its analogues in a regulatory cardiac troponin C–troponin I complex. Biochemistry. 2008;47:7485–95. doi: 10.1021/bi800438k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banfor PN, Preusser LC, Campbell TJ, Marsh KC, Polakowski JS, Reinhart GA, Cox BF, Fryer RM. Comparative effects of levosimendan, OR-1896, OR-1855, dobutamine, and milrinone on vascular resistance, indexes of cardiac function, and O2 consumption in dogs. Am J Physiol. 2008;294:H238–48. doi: 10.1152/ajpheart.01181.2007. [DOI] [PubMed] [Google Scholar]
- 15.Barraud D, Faivre V, Damy T, Welschbillig S, Gayat E, Heymes C, Payen D, Shah AM, Mebazaa A. Levosimendan restores both systolic and diastolic cardiac performance in lipopolysaccharide-treated rabbits: comparison with dobutamine and milrinone. Crit Care Med. 2007;35:1376–82. doi: 10.1097/01.CCM.0000261889.18102.84. [DOI] [PubMed] [Google Scholar]
- 16.Brendt P, Behrends M, Peters J. Myocardial stunning following no flow ischaemia is diminished by levosimendan or cariporide, without benefits of combined administration. Resuscitation. 2008;76:95–102. doi: 10.1016/j.resuscitation.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 17.Meyer K, Klocke RC, Schipke JD, Gams E, Korbmacher B. Ca2+ sensitizer superior to catecholamine during myocardial stunning? Eur J Cardiothorac Surg. 2008;34:326–31. doi: 10.1016/j.ejcts.2008.04.042. [DOI] [PubMed] [Google Scholar]
- 18.Yapici D, Altunkan Z, Ozeren M, Bilgin E, Balli E, Tamer L, Doruk N, Birbicer H, Apa D, Oral U. Effects of levosimendan on myocardial ischaemia–reperfusion injury. Eur J Anaesthesiol. 2008;25:8–14. doi: 10.1017/S0265021507002736. [DOI] [PubMed] [Google Scholar]
- 19.Haikala H, Nissinen E, Etemadzadeh E, Levijoki J, Linden IB. Troponin C-mediated calcium sensitization induced by levosimendan does not impair relaxation. J Cardiovasc Pharmacol. 1995;25:794–801. doi: 10.1097/00005344-199505000-00016. [DOI] [PubMed] [Google Scholar]
- 20.Hajjar RJ, Schmidt U, Helm P, Gwathmey JK. Ca++ sensitizers impair cardiac relaxation in failing human myocardium. J Pharmacol Exp Ther. 1997;280:247–54. [PubMed] [Google Scholar]
- 21.Lepran I, Pollesello P, Vajda S, Varro A, Papp JG. Preconditioning effects of levosimendan in a rabbit cardiac ischemia–reperfusion model. J Cardiovasc Pharmacol. 2006;48:148–52. doi: 10.1097/01.fjc.0000246151.39758.2a. [DOI] [PubMed] [Google Scholar]
- 22.Sahin AS, Gormus N, Duman A. Preconditioning with levosimendan pre-vents contractile dysfunction due to H2O2-induced oxidative stress in human myocardium. J Cardiovasc Pharmacol. 2007;50:419–23. doi: 10.1097/FJC.0b013e318123fbf9. [DOI] [PubMed] [Google Scholar]