Abstract
Significant progress has been made recently in unravelling the embryonic events leading to pituitary morphogenesis, both in vivo and in vitro. This includes dissection of the molecular mechanisms controlling patterning of the ventral diencephalon that regulate formation of the pituitary anlagen or Rathke's pouch. There is also a better characterisation of processes that underlie maintenance of pituitary progenitors, specification of endocrine lineages and the three-dimensional organisation of newly differentiated endocrine cells. Furthermore, a population of adult pituitary stem cells (SCs), originating from embryonic progenitors, have been described and shown to have not only regenerative potential, but also the capacity to induce tumour formation. Finally, the successful recapitulation in vitro of embryonic events leading to generation of endocrine cells from embryonic SCs, and their subsequent transplantation, represents exciting advances towards the use of regenerative medicine to treat endocrine deficits. In this review, an up-to-date description of pituitary morphogenesis will be provided and discussed with particular reference to pituitary SC studies.
Keywords: pituitary, developmental factors, morphogenesis, stem cell
Introduction
The pituitary is a small endocrine gland encased in a depression of the sphenoid bone called the sella turcica, at the base of the skull, just under a part of the brain called the hypothalamus to which it is physically linked by the pituitary stalk. This connection between the pituitary and the hypothalamus is crucial, initially in the embryo for correct morphogenesis of both components of the hypothalamo–pituitary axis, and also postnatally because the hypothalamus controls the secretions of the gland through this stalk. In the embryo, development of the pituitary anlagen or Rathke's pouch (RP) depends on the inductive actions of a restricted domain of the developing ventral diencephalon (VD) with which it is in contact, the infundibulum. It will give rise to the pituitary stalk and neurohypophysis (posterior pituitary). After birth, the different nuclei or groups of neurons that constitute the hypothalamus sense peripheral information, directly and/or indirectly via other parts of the brain, and accordingly secrete specific neurohormones (Fig. 1).
Figure 1.
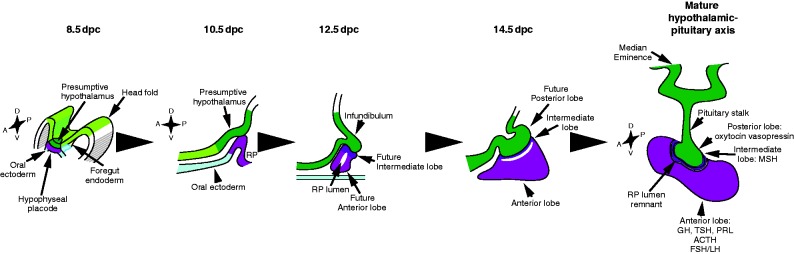
Development and function of the pituitary–hypothalamic axis. Pituitary development is initiated with the appearance of the hypophyseal placode, apposed to the future ventral diencephalon. The placode later invaginates to become Rathke's pouch (RP), still in contact in its dorsal most part with the infundibulum, which lies within the ventral diencephalon. The infundibulum then evaginates towards RP. It will give rise to the median eminence, the pituitary stalk and the posterior lobe, while the anterior and intermediate lobes originate from RP. Postnatally, the hypothalamus centralises peripheral information and controls pituitary endocrine secretions through release of hypophysiotrophic hormones. Hypothalamic peptide hormones can reach the gland directly, such as oxytocin and vasopressin secreted directly in the posterior lobe, or via the hypophyseal portal system; peptides (GnRH, gonadotrophin-releasing hormone; GHRH, GH-releasing hormone; TRH, thyrotropin-releasing hormone; CRH, corticotropin-releasing hormone; and the inhibitory SST, somatostatin) are secreted at the median eminence and collected by capillaries (adapted from Rizzoti & Lovell-Badge (2011)). Anterior pituitary endocrine cells comprise lactotrophs (producing prolactin, Prl), gonadotrophs (producing luteinizing hormone, LH; and follicle stimulating hormone, FSH), thyrotrophs (producing thyroid stimulating hormone, TSH), corticotrophs (producing adrenocorticotrophic hormone, ACTH; proteolytically cleaved from proopiomelanocortin, POMC) and somatotrophs (producing growth hormone, GH).
One important function of the hypothalamo–pituitary axis is to maintain a general internal equilibrium or homoeostasis. However, it also ensures that the organism responds appropriately to changing physiological situations such as puberty, pregnancy, lactation, and also to stress. An important feature of the system is therefore its plasticity with hormonal secretions being constantly modulated. The seven different hormones secreted by the pituitary affect most physiological processes. It is therefore not surprising that pathological situations leading to pituitary hormone imbalance have pleiotropic consequences resulting in significant morbidity and even mortality. Hormone deficiencies (those affecting more than one hormones are described as combined pituitary hormone deficiencies (CPHD)) can be congenital or acquired, and these can have a pituitary or hypothalamic origin and are, in most cases, of unknown aetiology (Kelberman et al. 2009). The most frequent congenital defect in humans is isolated growth hormone (GH) deficiency with a prevalence of one in 3500–10 000 births (Kelberman et al. 2009). Acquired deficits develop mainly as a consequence of pituitary tumours (mostly prolactinomas) and brain damage (Hannon et al. 2013). Substitution or replacement therapies are available to treat deficiencies but these are not optimal because they do not reproduce the physiological secretion patterns, are associated with side effects, and are costly.
Stem cells (SCs) are present in most tissues and organs and are characterised by their capacity to both self-renew and give rise to differentiated progeny, according to the tissue/organ they belong to. Such cells have been recently characterised in the pituitary (Andoniadou et al. 2013, Rizzoti et al. 2013). In organs with a rapid turnover such as the intestine and the haematopoietic system, they are required for tissue homoeostasis and are also recruited in response to physiological challenges, such as injuries. In organs with a low turnover such as the brain and the pituitary (Levy 2002), they can provide a source of differentiating cells to replace those lost through normal processes of attrition, but they mostly appear to be mobilised in response to physiological challenges or to damage (Fu et al. 2012, Langlais et al. 2013, Rizzoti et al. 2013). SCs can also be involved in tumourigenesis, when self-renewal becomes abnormal. In some cases, they can transform into cancer SCs that provide tumours with a pool of reserve cells that can sustain their growth by differentiating into ‘bulk’ tumour cells, and also allow their recurrence after therapy (for review, see Kreso & Dick (2014)). In other cases, SCs can induce formation of tumours in neighbouring cells, and this has been shown in particular in the pituitary (Andoniadou et al. 2013).
Regenerative medicine based on SC therapies holds promise for many diseases and injuries, where lost functions could be restored by supplying SCs or their differentiated derivatives. In the pituitary, the ability to replace missing endocrine cells would represent a significant improvement to treat long-term pituitary deficiencies. Developmental mechanisms are often, but not always (Lepper et al. 2009), re-capitulated in adult SCs for self-renewal, differentiation and also during oncogenic transformation. In this review, an up-to-date description of pituitary morphogenesis will be provided, highlighting the latest developments. Finally, recent pituitary SC studies and advances towards regenerative therapies will be discussed.
Pituitary morphology and morphogenesis
Morphology
The pituitary gland is composed of three different lobes: anterior, intermediate and posterior (Fig. 1). The anterior lobe is the largest and it is richly vascularised by capillaries that deliver hypothalamic inputs and send secreted hormones from five different populations of endocrine cells (Fig. 1). It has been recently demonstrated that endocrine populations form homotypic networks ensuring a robust and coordinated hormonal secretory response to stimulation (for review, see Mollard et al. (2012)). These networks are plastic and can be modulated according to physiological contexts (Hodson et al. 2012). Pituitary SCs/progenitors are localised around the pituitary cleft, a remnant of the RP lumen, and scattered in the anterior lobe (for review, see Rizzoti (2010), Castinetti et al. (2011a) and Vankelecom (2012)). They at least partially overlap with folliculo-stellate cells, a heterogeneous population of dendritic cells (Fauquier et al. 2008, Andoniadou et al. 2013). Pituitary SCs also appear to form a network but its functionality has not yet been studied (Mollard et al. 2012).
The intermediate lobe is located between the anterior and the posterior lobes. It contains only one endocrine population, the melanotrophs. This lobe is poorly vascularised and receives hypothalamic information by direct hypothalamic innervation. Capillaries located between IL and PL collect hormones. It is a useful model for cancer formation in rodents as mutants for several cell cycle regulators display intermediate lobe tumours (for review, see Quereda & Malumbres (2009)). In humans, this lobe is only residual and melanotrophs are mainly found in the skin.
The posterior lobe does not contain any endocrine cells but a population of glial cells called pituicytes and axonal termini from two types of hypothalamic neurons.
Despite being closely associated, the three pituitary lobes do not have the same embryonic origin. Anterior and intermediate lobes, forming the anterior pituitary, are ectodermal derivatives while the posterior lobe has a neuroectodermal origin.
Morphogenesis
Cells secreting peptides related to pituitary hormones exist in invertebrates but the gland is specific to vertebrates (for review, see Patthey et al. (2014)). Pituitary development is initiated during neurulation, from 8 days post coitum (dpc) in mice (all stages refer to mouse development). An ectodermal thickening or hypophyseal placode (HP) appears in the rostral most position, within the anterior neural ridge, which forms just in front of the future VD (Fig. 1). HP forms along with other sensory placodes (olfactory, optic and otic) and it has been proposed that olfactory and HPs were fused into one nasoHP in ancestral craniates. Separation and the subsequent duplication of the olfactory placodes on each side of the median HP have been suggested to represent an important event during evolution, because it allowed development of the future jaws (Khonsari et al. 2013).
By 9 dpc, the future telencephalon has grown relatively faster than the diencephalon and fused rostrally at the midline, so that the future brain is now bent at the level of the diencephalon. As a consequence, the diencephalon is shifted posteriorly. HP has morphed into the pituitary anlagen or RP, an epithelial invagination that forms in continuity with the oral ectoderm and extends dorsally towards VD. RP is located at an interesting junction. It marks the posterior limit of neural crest cell (NCC) contribution to the head mesenchyme; therefore, it is flanked anteriorly by NCC-derived mesenchyme and posteriorly by mesoderm-derived mesenchyme (Jiang et al. 2002). It is in contact with the anterior border of foregut endoderm (Khonsari et al. 2013), and it is also located just in front of the notochord and importantly it is directly in contact with VD. RP is therefore exposed to signals secreted from all the different territories it is in contact with, and these are important for proper morphogenesis.
By 10 dpc, the region of VD directly above RP starts to evaginate towards it. This territory is the infundibulum, which will give rise to the median eminence, pituitary stalk and posterior lobe (for review, see Pearson & Placzek (2013)), while RP will form the anterior and intermediate lobes. The connection between RP and oral ectoderm disappears through apoptosis (Charles et al. 2005). In ancestral vertebrates, this link was maintained as the buccohypophyseal canal, which may have been involved in osmoregulation in some species (Khonsari et al. 2013).
The mature pouch is initially formed of highly proliferative epithelial progenitors arranged around the lumen. Birth dating studies have revealed that there is a main wave of mitotic exit between 11.5 and 13.5 dpc regardless of the endocrine cell type (Davis et al. 2011), while differentiation, characterised by expression of the secreted hormone, occurs at a different time for each population (Japon et al. 1994). Undifferentiated cells exiting the cell cycle adopt more ventral and lateral locations and lose epithelial characteristics, reminiscent of epithelial-to-mesenchymal transition (Himes & Raetzman 2009). All endocrine cell types are present before birth, but cells fully differentiate only postnatally as the secretory apparatus further matures and cell size increases. Homotypic endocrine networks form in the embryo, starting with corticotrophs, where single cells initially arrange into clusters shortly after differentiation. These may serve as a scaffold for the organisation of other endocrine populations (Budry et al. 2011a).
In rodents, the gland will undergo a phase of important growth during the 1st weeks of life. This is characterised by high levels of proliferation, both in progenitors and in endocrine cells. Throughout life, endocrine cells retain the capability to proliferate but they do so very rarely (Levy 2002). After birth, the hypothalamus will take control of pituitary endocrine maturation and secretions, but it is not clear exactly when and how its influence starts to be exerted.
Genetic regulation of pituitary development
Induction of the pituitary primordium, the HP
Just after gastrulation, complex interactions between the head mesoderm and the neural and non-neural ectoderm define a domain at the border of the neural plate. These involve fibroblast growth factor (FGF) signalling inducing neural fate, bone morphogenetic proteins (BMPs) and wingless-related integration site (WNT) activities inducing future epidermal identity in non-neural ectoderm, and active inhibition of BMP and WNT signalling pathways by the neural ectoderm and head mesoderm (for review, see Saint-Jeannet & Moody (2014)). Within this domain, cells are competent to become either placodal or NCC. Rostrally, individual placodes are further specified by surrounding tissues.
While the head ectoderm is generally competent to form pituitary tissue, the inducing ability is restricted to VD, which is required for HP induction and maintenance (elAmraoui & Dubois 1993, Kawamura & Kikuyama 1998, Gleiberman et al. 1999). Foregut endoderm is also likely to be involved in early pituitary development, either directly because it is in contact with the pituitary primordium (Khonsari et al. 2013) or indirectly because it is necessary for anterior neural plate patterning (Thomas & Beddington 1996).
Several transcription factors expressed early in HP are required for later pituitary development. The paired-like homoeodomain transcription factor HESX1 is an important regulator of anterior development (Hermesz et al. 1996, Thomas & Beddington 1996, Dattani et al. 1998). The Sine Oculis homoeodomain proteins SIX3 and SIX6 (Oliver et al. 1995, Jean et al. 1999) are also present in the pan-placodal domain and subsequently in the HP (for review, see Schlosser et al. (2014)). Finally, but not exhaustively, the homoeodomain transcription factors PITX1 and PITX2 are also present at these early stages (for review, see Soukup et al. (2013)).
Formation and maintenance of RP
Signalling pathways
Secreted signals, mainly by VD, are crucial for induction and maintenance of RP (Fig. 2). In addition, several signalling pathways are also active within RP. These will be discussed in this review with an emphasis on recent studies (for review, see Kelberman et al. (2009) and Davis et al. (2013)).
Figure 2.
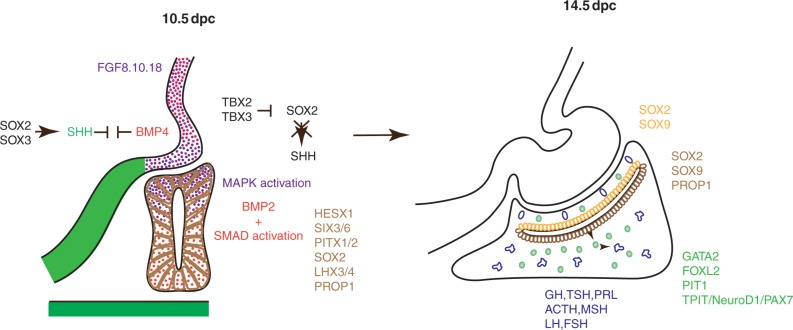
Main signalling pathways and factors required during pituitary development. Opposing activities of BMP4 and SHH within the ventral diencephalon pattern this region and participate in correct morphogenesis of the infundibulum and in consequence positioning of RP. Within the infundibulum, BMP4 and FGF8, FGF10 and FGF18 are required for development of RP. In RP at 10.5 dpc, BMP2 is present and there is a uniform SMAD activation profile, while the FGF pathway is only activated dorsally (Davis et al. 2011). At this stage, different transcription factors required for RP progenitor proliferation and/or maintenance are ubiquitously expressed. Later, at 14.5 dpc, progenitors are confined around the RP lumen, while differentiating and differentiated cells away from the lumen define the developing anterior pituitary.
Bone morphogenetic proteins
BMPs are secreted ligands, which upon binding to receptor serine–threonine kinases initiate an intracellular signalling cascade resulting in activation of SMADs and regulation of gene transcription. BMPs are involved in multiple events in the embryo, and dysregulation of the pathway is linked to diseases and cancers (for review, see Cai et al. (2012)).
BMP4 is the earliest VD signalling molecule known to be extrinsically required for RP development, from 8.5 dpc (Ericson et al. 1998, Takuma et al. 1998). It is maintained in the infundibulum with a sharp ventral boundary up to at least 14.5 dpc (Ericson et al. 1998, Treier et al. 1998, Davis & Camper 2007). In the chick, BMPs are required for infundibular identity and patterning, in particular by inducing expression of the T-box transcription factor TBX2 that represses Sonic Hedgehog (SHH) expression, excluding it from the future infundibulum (Manning et al. 2006). Bmp2 and Bmp7 are first present in ventral mesenchyme at 10.5 dpc, then Bmp2 starts to be expressed in RP, first ventrally and then throughout, decreasing by 14.5 dpc (Ericson et al. 1998, Davis & Camper 2007). The pattern of phosphorylated SMADs between 10.5 and 14.5 dpc correlates with the presence of BMP2 (Davis et al. 2011). Conditional deletion of the gene encoding its receptor, Bmpr1a, in RP at 9.5 dpc results in underdevelopment of the pouch at 10.5 dpc. Therefore, BMP signalling is intrinsically required for RP maintenance and probably for progenitor proliferation (Davis & Camper 2007).
Fibroblast growth factors
FGFs are secreted molecules that bind to receptor tyrosine kinases resulting in activation of multiple signalling cascades through MAPK, PI3 kinase and phospholipase C, affecting, respectively, cell proliferation, survival and motility (for review, see Dorey & Amaya (2010)). They are required for multiple processes during embryogenesis, and dysregulation is linked to cancer and many congenital diseases such as Kallmann syndrome, which affects differentiation of gonadotrophin-releasing hormone (GnRH) neurons (GnRH is a hypothalamic neuropeptide required for reproductive development and function) resulting in hypogonadism (for review, see Chung & Tsai (2010)).
During RP development, Fgf8, Fgf10 and Fgf18 are present in VD, appearing later than Bmp4, from 9.5 dpc up until at least 14.5 dpc (Ericson et al. 1998, Treier et al. 1998, 2001). In chick, FGFs are required within the infundibulum for cell expansion (Pearson et al. 2011). In the murine RP, MAPK activation is observed dorsally between 10.5 and 11.5 dpc in agreement with an infundibular source of FGFs (Davis et al. 2011). Deletion of Fgf10 or Fgfr2IIIb results in formation of a small apoptotic pouch (De Moerlooze et al. 2000, Ohuchi et al. 2000). In contrast, deletion of the paired homoeodomain transcription factor Vax1, normally expressed in VD but excluded from the infundibulum, causes the appearance of a second Fgf10-expressing, but Bmp4-negative, anterior domain, disconnected from the infundibulum. This results in induction of a second RP, leading to duplication of the pituitary (Bharti et al. 2011). FGFs are therefore necessary for dorsal RP progenitor proliferation and maintenance. By analogy to other models, where FGFs act as chemoattractants (for review, see Friedl & Gilmour (2009)), they may also be involved in RP invagination.
Sonic hedgehog
Hedgehog (HH) ligands (Sonic, Desert and Indian hedgehog) not only have been described as morphogens in the embryo, but also act as mitogens, in particular for adult SCs. In the absence of ligand, the receptor Patched inhibits the transmembrane molecule smoothened (SMO), and intracellular GLI repressors silence target genes. In the presence of Hh, SMO allows Gli activators to translocate to the nucleus and activate target gene expression. Dysregulation of SHH signalling results in holoprosencephaly, a syndrome characterised by severe midline defects (Chiang et al. 1996). Postnatally, dysregulation of the pathway is linked to several cancers (for review, see Athar et al. (2014)).
Within the ventro-medial diencephalon, SHH is present anteriorly and laterally to the infundibulum, from which it is excluded (Manning et al. 2006, Trowe et al. 2013). Within the antero-ventral domain, it is required for maintenance of the prospective hypothalamus. Its expression in this domain depends on the HMG box transcription factors Sox2 and Sox3 (Zhao et al. 2012). Exclusion of SHH from the infundibulum is achieved by sequestration of SOX2/SOX3 away from a Shh enhancer by TBX2/TBX3 (Trowe et al. 2013). In the absence of SHH in VD, infundibular Bmp4 and Fgf10 patterns are expanded ventrally, resulting in an anterior shift and duplication of RP. In contrast, when Tbx3 is deleted, the Shh domain is expanded dorsally. Fgf8 and Bmp4 are still expressed while Fgf10 is down-regulated in the infundibulum, where more proliferation is observed. Moreover, evagination fails and consequently RP does not progress beyond 10.5 dpc (Trowe et al. 2013). Altogether, these studies suggest an antagonism between BMP and SHH in VD that is required for infundibular morphogenesis and hence RP positioning and development (Zhao et al. 2012, Trowe et al. 2013).
SHH is also present in the oral ectoderm, but excluded from RP (Treier et al. 2001). It has recently been proposed that SHH is involved in individualisation of RP from the oral ectoderm as dysregulation of the SHH pathway in human and mouse results in maintenance of an ancestral link between RP and oral ectoderm, the buccohypophyseal canal. In consequence, the pituitary in such mutants is ventrally displaced just above the oral cavity and sometimes even connected to it (Khonsari et al. 2013). SHH is therefore likely to be required in the oral ectoderm for basal skull and pituitary morphogenesis.
Within RP, cells express the receptor Patched and the transcriptional regulators GLI1, GLI2 and GLI3; therefore, they are competent to respond to the ligand (Treier et al. 2001). Conditional deletion of Gli2 and Gli3 in RP has revealed that the pathway is involved in RP progenitor proliferation (Wang et al. 2010).
In conclusion, the SHH pathway has different roles, being required in VD, in oral ectoderm and finally within RP for proper pituitary morphogenesis.
WNT
The WNT-secreted molecules play important roles in the embryo and in the adult, regulating in particular proliferation of different SC populations. Dysregulation of the pathway is causative of many diseases, especially colorectal cancers (for review, see Clevers & Nusse (2012)). The canonical WNT pathway is characterised by the central role of the intracellular protein β-catenin. In the absence of the ligand, β-catenin is targeted for degradation. When WNT binds to the co-receptors LRP5/LRP6 and receptor Frizzled, cytoplasmic β-catenin can accumulate and translocate to the nucleus where it displaces the repressor Groucho, associated with the T-cell factor/lymphoid enhancer-binding factor (TCF/LEF) transcription factors. WNT target genes that were silenced can now be activated. A second WNT-activated pathway regulates planar cell polarity, affecting cell morphology and movement, and a third is the related non-canonical Wnt pathway, associated with intracellular Ca2+ release.
WNT5a, along with other members of the pathway, is present throughout VD and RP from 9.5 to 12.5 dpc (Treier et al. 1998, Cha et al. 2004, Potok et al. 2008). In its absence, the β-catenin activation pattern is unaffected, suggesting that Wnt5a does not activate the canonical pathway, but infundibular signalling is ectopically expanded ventrally (Potok et al. 2008). This results in more oral ectoderm being recruited to form RP and with the appearance of extrabifurcations (Cha et al. 2004). Cell fate is unaffected in RP; therefore, WNT5A may be necessary in VD for correct patterning and indirectly affects RP shaping (Potok et al. 2008). TCF4 is also expressed in VD and RP. Deletion of the gene results in a similar phenotype as Wnt5a ablation, but deletion of TCF/LEF factors is not necessarily comparable to loss of ligand or of β-catenin, because these actively repress transcription when the pathway is inactive. Both proteins are probably independently involved within VD in patterning and hence RP positioning.
Within RP, several WNT ligands and members of the pathways are present and active (Olson et al. 2006, Potok et al. 2008). Deletion of Wnt4, which is usually expressed transiently and exclusively in RP, causes a slight reduction in expression of the transcription factor PIT required for emergence of somatotrophs, lactotrophs and thyrotrophs (Potok et al. 2008). Derepression of target genes in Tcf4-deleted mutants may underlie the observed over-proliferation phenotype in the pouch. In these mutants, endocrine cell differentiation can still occur (Brinkmeier et al. 2007). There is, however, little nuclear β-catenin staining in RP (Brinkmeier et al. 2007), and deletion of the gene encoding this does not, at least initially, cause any defect (Olson et al. 2006). β-catenin has nonetheless been demonstrated to participate in Pitx2 activation (Kioussi et al. 2002, Ai et al. 2007), which in turn stimulates progenitor proliferation, but the identity of the Wnt ligand is unknown (Kioussi et al. 2002). Later on, β-catenin can interact with the transcription factor PROP1 (see below) and formation of this complex is important for emergence of endocrine lineages (Olson et al. 2006). Moreover, constitutive activation of the pathway, using a β-catenin degradation resistant form under the control of a portion of the Pitx1 promoter as a transgene, results in loss of the pituitary at 13.5 dpc (Olson et al. 2006). In contrast with this last result, two recent studies have demonstrated that conditional expression of a constitutively active β-catenin mutant protein in response to Cre-mediated activation with either Hesx1Cre or Sox2CreERT2 (both targeted alleles) caused increased proliferation, reduction in GH content, and importantly, development of tumours, similar to human craniopharyngioma (Gaston-Massuet et al. 2011, Andoniadou et al. 2013). In conclusion, the WNT canonical pathway may participate in progenitor proliferation and fate while dysregulation of the pathway is clearly linked to oncogenicity.
NOTCH
The NOTCH signalling pathway has classically been described in the context of lateral inhibition, where cells within a population are selected to adopt certain alternate fates. Briefly, the membrane-bound ligands Delta-like or Jagged activate the transmembrane receptor NOTCH on neighbouring cells; imbalance between secretion of the ligand and activation of the receptor underlines the cell selection process. Binding of the ligand allows for cleavage of the NOTCH intracellular domain (NICD) that can then translocate to the nucleus, where it displaces repressors such as N-COR that are otherwise associated with the transcription factor RBPJκ when the pathway is inactive. This in turn permits transcriptional activation of targets such as the HES bHLH transcription factors. The pathway is required in the embryo in different contexts and dysregulation is associated not only with inherited and degenerative diseases, but also with cancers in humans (for review, see Hori et al. (2013)).
The role of the NOTCH signalling pathway in early VD formation is not fully understood, but it is required for infundibular morphogenesis. Hes1 is expressed throughout the VD and Hes null mutants display reduced evagination and later absence of the PL (Zhu et al. 2006, Kita et al. 2007, Raetzman et al. 2007, Aujla et al. 2011).
Several members of the NOTCH pathway are expressed in RP, becoming largely restricted to perilumenal progenitors as the gland develops (Raetzman et al. 2004, Zhu et al. 2006). Studies of their role demonstrate that they are required for progenitor proliferation and cell fate choice, but different experimental strategies (straightforward deletion, conditional deletion and over activation) lead to different outcomes that are not always reconcilable. Deletion of Hes genes result in increased apoptosis and reduced proliferation, inducing pituitary hypoplasia, but AL cell types can differentiate (Raetzman et al. 2004, 2007, Kita et al. 2007, Monahan et al. 2009), with corticotrophs appearing precociously (Zhu et al. 2006, Kita et al. 2007). The intermediate lobe is more affected, because single or double deletions of the genes encoding the HES1 and HES5 repressors result in either its absence (Zhu et al. 2006, Kita et al. 2007) or, on a different genetic background, in a hypoplastic appearance with ectopic somatotrophs (Raetzman et al. 2007). In contrast, deletion of Rbpjκ in RP does not affect intermediate lobe formation, but, as with Hes gene deletions, it results in premature and increased corticotroph differentiation with overall reduced proliferation (Zhu et al. 2006). Moreover, conditional deletion of either Rbpjκ or Notch2 in RP results in down-regulation of the transcription factor PROP1 (Zhu et al. 2006, Nantie et al. 2014).
The NOTCH pathway is likely to directly activate Prop1 expression and this is necessary for emergence of the Pit1 lineage (see above), the latter being absent in Rbpjκ mutant pituitaries (Zhu et al. 2006). Conversely, regulation of Notch2 by PROP1 has also been suggested (Raetzman et al. 2004). In support of an interaction between the PROP1 and NOTCH pathways, disruption of both Hes1 and Prop1 results in a new phenotype with premature corticotroph and αGSU-positive cell differentiation (Himes & Raetzman 2009). Ectopic activation of NOTCH signalling can be achieved by inducing expression of the NICD. Transgenic expression of NICD under control of a Pit1 (Pou1f1) (Zhu et al. 2006, Goldberg et al. 2011) or a Pomc (Zhu et al. 2006, Goldberg et al. 2011) promoter results in blockade of differentiation. Finally, global impairment of endocrine differentiation results in up-regulation of NOTCH pathway components (Wang et al. 2007, Welcker et al. 2013). Therefore, NOTCH signalling appears to be required early to prevent differentiation, ensuring the generation of sufficient progenitors, and later to promote emergence of the Pit1 lineage (Zhu et al. 2006, Himes & Raetzman 2009), although this later role does not require either HES1 or HES5.
Transcriptional regulators
Several transcriptional regulators are required within RP for proper development. Among them, some are additionally present in VD, where they are necessary for infundibular morphogenesis and therefore indirectly for RP morphogenesis. Finally, others are exclusively present and required in VD and affect indirectly the developing pituitary. All of these will be reviewed in this section.
HESX1
HESX1 is a paired-like homoeodomain transcription factor that acts as a repressor by co-binding TLE1 (a mammalian Groucho family member) and the nuclear co-repressor N-COR (Dasen et al. 2001, Carvalho et al. 2010). Human HESX1 mutations are associated with Septo-optic dysplasia, a rare congenital syndrome characterised by variable CNS midline defects and hypopituitarism (Dattani et al. 1998). HESX1 is essential for correct development of the forebrain, and Hesx1 null mice display postnatal lethality probably caused by CNS defects comprising anophthalmia, a reduced prosencephalon and pituitary dysplasia (Dattani et al. 1998). Loss of HESX1 in the anterior neuroectoderm results in posteriorisation due to lack of repression of the Wnt/β-catenin pathway (Andoniadou et al. 2007, 2011, Matsuda & Kondoh 2014). The development of RP in Hesx1 null embryos is characterised early on by a variable phenotype comprising multiple clefts, correlating with expanded infundibular Fgf10 expression, overproliferation and often misplacement of the gland in the naso-pharyngeal cavity, aspects of which are likely to be explained by loss of the gene in VD (Dasen et al. 2001). After birth, hypoplasia of the pituitary is observed and this is suggested to result from impaired hypothalamic control (Gaston-Massuet et al. 2008).
Hesx1 is expressed in RP progenitors until 13.5 dpc (Hermesz et al. 1996, Thomas & Beddington 1996). Its expression is activated by the Lim homoeodomain transcription factor LHX3, along with PITX2 and GATA2/GATA3 (Chou et al. 2006), while it is down-regulated by the β-catenin/PROP1 complex (Olson et al. 2006). Hesx1 mutant analysis indicates that the interaction of the protein with the repressor TLE1 is crucial for RP development, because the HESX1/TLE1 complex represses expression of Prop1, which is necessary for pituitary development to progress (see below) (Dasen et al. 2001). Conditional deletion of Hesx1 in the CNS vs pituitary has not been performed yet to clearly separate both functions, but it has been proposed that lack of repression of the Wnt pathway, as has been observed in the anterior neutral plate, underlies the pituitary phenotype. However, while in neural ectoderm, this results in posteriorisation, in RP the consequence is hyper-proliferation, which has been observed in some of the mutants (Gaston-Massuet et al. 2008). Despite these defects, endocrine cell differentiation occurs normally in Hesx1 mutants, but with increased numbers (Dasen et al. 2001, Sajedi et al. 2008). HESX1 is therefore an important regulator of early pituitary development and its interaction with PROP1 regulates RP development.
SIX3/SIX6
SIX proteins are members of the Sine Oculis homoeobox transcription factor family, present from flatworms to humans. They are well known for their conserved function in eye development and have other important roles during embryogenesis. As regulators of proliferation, they are also involved in tumourigenesis. They can positively or negatively regulate transcription, as they can, respectively, interact with the Eye-absent (EYA) transcriptional activators or the GROUCHO/TLE and DACH co-repressors (for review, see Kumar (2009)). The closely related Six3 and Six6 are both expressed in VD and RP (Jean et al. 1999, Larder et al. 2011), and SIX6 expression is maintained in the adult pituitary (Li et al. 2002). Deletion of Six6 results in formation of hypomorphic pituitaries and retinas. It has been suggested that SIX6 promotes proliferation of progenitors by repressing expression of the cell cycle negative regulator p27KIP1, and such an interaction has been demonstrated to underlie the Six6−/− retinal phenotype (Li et al. 2002).
Deletion of Six3 is more deleterious and mutants arrest before pituitary development is initiated, and later functions of the gene are not known. However, the phenotype of compound double heterozygous Six3; Hesx1 mutants suggests an interaction of SIX3 with Wnt signalling in RP. In the forebrain, defects in Six3−/− mutants are more severe than those observed in Hesx1 mutants, but they are similar and both are proposed to stem from ectopic Wnt/βcatenin activation (Lavado et al. 2008, Andoniadou et al. 2011). Intriguingly, Six3+/−; Hesx1+/− RP display increased early progenitor proliferation and multiple clefts, comparable to Hesx1−/− mutants. This hyper-proliferation phenotype is proposed to result from increased Wnt signalling (Gaston-Massuet et al. 2008). Therefore, SIX3 would normally serve as a repressor of Wnt activity. Conditional mutation is now required to assess its function directly.
PITX1/PITX2
The paired homoeodomain PITX proteins are important morphogenetic regulators for different organs. They are also well known for their role in left–right asymmetry (for review, see Soukup et al. (2013)). PITX2 mutations are associated with Axenfeld–Rieger syndrome, mainly characterised by eye defects and also comprising pituitary abnormalities. PITX1 and PITX2 are present in the HP, RP and later maintained in differentiated endocrine cells (Gage & Camper 1997, Lanctot et al. 1997). They function redundantly and are required for maintenance of early RP progenitors (Charles et al. 2005). PITX2 appears to be initially more important, because RP development is arrested in Pitx2−/− mutants, while Pitx1 null mutants display a normal RP. Pitx2 has been shown to be a target of Wnt signalling and to induce progenitor proliferation through direct transcriptional activation of cyclins (Kioussi et al. 2002, Ai et al. 2007). In Pitx1; Pitx2 double mutants, there is increased cell death and LHX3, which is also required for progenitor maintenance (Zhao et al. 2006), is completely down-regulated (Charles et al. 2005). Other targets of PITX proteins could underlie this phenotype: indeed, during myogenic differentiation, as metabolic status changes, PITX2 and PITX3 regulate mitochondrial function and anti-oxidant enzymes; in their absence, DNA damage accumulate and apoptosis occurs (L'Honore et al. 2014). Proper Pitx gene dosage is required throughout development for generation and/or maintenance of endocrine cell numbers (Suh et al. 2002). PITX1 and PITX2 are also redundantly required postnatally, for thyrotroph function (Castinetti et al. 2011b). In conclusion, PITX1 and PITX2 are required not only for pituitary morphogenesis but also for endocrine function, reflecting their many interacting partners and, in consequence, target genes.
ISLET 1
Insulin gene enhancer protein ISL-1 (ISLET1) is a LIM-homoeodomain transcription factor, essential for cell fate decisions in different embryonic and SC contexts. ISL1 is expressed throughout the oral ectoderm at 8.5 dpc, maintained in RP at 9.5 dpc and finally further ventrally restricted between 10.5 and 11.5 dpc in prospective rostral tip thyrotrophs (Ericson et al. 1998). In the adult gland, it is maintained in gonadotrophs (Schang et al. 2013). Its expression in RP could be regulated by BMPs (Davis & Camper 2007), where it is proposed to participate in Lhx3 activation, along with PITX1 (Mullen et al. 2012). Isl1 null embryos die at 10 dpc and display a severely hypoplastic RP, suggesting that the protein is initially required for progenitor maintenance (Takuma et al. 1998).
LHX2/LHX3/LHX4
LHX2, LHX3 and LHX4 also belong to the LIM-homoeodomain transcription factor family. LHX2 is expressed throughout VD during RP development and required for infundibulum and hence posterior lobe formation (Zhao et al. 2010). As observed in Tbx3 mutants (Trowe et al. 2013), in Lhx2 null mutants the infundibulum fails to evaginate and proliferate more. Additionally, Bmp and Fgf expression patterns are ventrally expanded. As a consequence, RP fails to expand dorsally and rather develop more rostrally. Importantly, cell differentiation is unaffected and all endocrine cell types are observed (Zhao et al. 2010).
LHX3 and LHX4 are required for both CNS and pituitary development. Mutations in human LHX3 and LHX4 are associated with CPHD. Both genes are first expressed in RP at 9.5 dpc. Lhx4 is subsequently restricted to the future AL then down-regulated at 15.5 dpc, while Lhx3 is maintained throughout in the adult gland (Sheng et al. 1997). LHX4 may participate, along with PROP1 in activation of Lhx3 expression, as Lhx4−/−; Prop1−/− mutants, and in contrast with single mutants, do not express LHX3 (Raetzman et al. 2002). Initially, the two proteins function redundantly, as shown by gene deletion studies (Sheng et al. 1997), where increased apoptosis is a major consequence (Raetzman et al. 2002, Ellsworth et al. 2008). However, while Lhx4−/− hypoplastic pituitaries contain all endocrine cell types, in Lhx3−/− mutants, deficiencies in all cell lineages are observed. This reflects the requirement for LHX3 in the activation of several pituitary hormones, hypothalamic peptide receptors and transcription factors (for review, see Prince et al. (2011)). Additionally, expression of Notch2 is absent in Lhx3 mutants and this could underlie some of the differentiation defects (Ellsworth et al. 2008). Therefore, LHX3 and LHX4 are initially required for progenitor maintenance rather than proliferation and later; LHX3 is also required for proper endocrine differentiation.
RX/RAX
Retina and anterior neural fold homeobox protein (RX/RAX) is a paired-like homoeodomain protein, mostly known for its conserved function for eye morphogenesis. It is expressed early in the anterior neural plate, and some null mutants lack part of the forebrain. It is also maintained later in VD, and its deletion, in a second class of null mutants, causes abnormal morphogenesis with lack of infundibular evagination and down-regulation of Fgf10 expression from 10.5 dpc. Consequently, RP fails to develop beyond this stage and remain fused to oral ectoderm (Medina-Martinez et al. 2009). It is also important for later hypothalamic morphogenesis (Lu et al. 2013).
SOX2/SOX3
The HMG box SOXB1 factors SOX2 and SOX3 are broadly expressed in progenitors in the CNS, where they generally, but not exclusively, promote an undifferentiated proliferative state (for review, see Sarkar & Hochedlinger (2011)). SOX2 is also required for pluripotent cell types in the early embryo, and homozygous null mutant mouse embryos die around implantation. In humans, heterozygous SOX2 loss-of-function mutations are associated with anophthalmia (Fantes et al. 2003) and hypogonadotrophic hypogonadism (reduced gonadal function of hypothalamic origin) (Kelberman et al. 2006), while both SOX3 deletions and duplications are linked to mild hypopituitarism (Laumonnier et al. 2002). SOX proteins act with co-factors; therefore, the specificity of their action often relies on their partner(s). Moreover, there is redundancy within a sub-family. Nevertheless, the ‘dose’ of SOXB1 is important. This is true in VD, where both SOX2 and SOX3 are expressed, while, in RP, SOX2 is the only SOXB1 member to be present. In VD, they have been shown to activate the same targets: Shh, as mentioned earlier (Zhao et al. 2012), and also Six3 and Six6 (Lee et al. 2012, 2013). In the absence of Sox3, the infundibulum does not evaginate fully and cells proliferate less, Fgf8 and Bmp4 domains are expanded anteriorly, and RP is bifurcated (Rizzoti et al. 2004). This is at least partially a consequence of a slight down-regulation of Shh ventrally (Zhao et al. 2012) and possibly Six6, associated with progenitor proliferation in the eye and pituitary (Li et al. 2002). Bifurcations are also observed in Sox2+/− embryos reflecting redundancy within VD (Kelberman et al. 2006). Both murine mutants, Sox3−/− and Sox2+/− are affected by mild hypopituitarism and the gland is slightly hypoplastic (Rizzoti et al. 2004, Kelberman et al. 2006). For SOX3, this is reflecting its role in the hypothalamus where it is maintained, while SOX2 is additionally required in RP (Jayakody et al. 2012). Loss of Sox2 also significantly affects the number of GnRH neurons; therefore, the hypogonadotrophic hypogonadism displayed by human SOX2 patients is likely to be of hypothalamic origin (Jayakody et al. 2012).
In RP, SOX2 is present from at least 9.5 dpc and the protein is maintained in adult pituitary SCs, along with a SOXE sub-family member, SOX9 (Fauquier et al. 2008, Andoniadou et al. 2013, Rizzoti et al. 2013). Deletion of Sox2 in RP at 12.5 dpc results in reduction of proliferation and, in consequence, endocrine cell deficits where later-born cell types, in particular somatotrophs, are more affected. Expression of the transcription factors PROP1 and PIT1 is downregulated in Sox2 mutant embryos, but the molecular mechanisms underlying this are not known (Jayakody et al. 2012). In conclusion, SOX2 is required within RP for progenitor proliferation.
PROP1
PROP1, a paired homoeodomain transcription factor, is the earliest exclusive marker of pituitary identity. In human, mutations are associated with congenital CPHD. In the mouse, PROP1 starts to be expressed at 10 dpc and is then maintained throughout development in SOX2-expressing progenitors except for those in the future IL. Postnatally, it becomes quickly down-regulated and it is present only in a small proportion of SOX2+ cells (Gage et al. 1996, Sornson et al. 1996, Yoshida et al. 2009, 2011). Mice homozygous for the Prop1 null mutant, and the naturally occurring Ames Dwarf mutant, Propdf/df (which results in low DNA-binding activity), both display strong reduction in PIT1 expression and consequently loss of somatotrophs, lactotrophs, thyrotrophs and also gonadotrophs, while Hesx1 expression is maintained beyond its normal temporal limit (Gage et al. 1996, Sornson et al. 1996, Nasonkin et al. 2004). As mentioned earlier, PROP1 can interact with β-catenin and this complex activates Pit1 expression (Olson et al. 2006). There must, nevertheless, be another level of regulation for Pit1 activation as PROP1 is expressed from 10 dpc while Pit1 is first detected at 13.5 dpc. Moreover, PROP1 probably just initiates Pit1 expression and is quickly down-regulated, because a maximum of only 9.7% of PIT1-expressing cells also express PROP1 (Yoshida et al. 2009). Another aspect of PROP1 function is observed in mutants, where progenitors fail to populate the forming AL, inducing formation of a dysmorphic gland at 14.5 dpc. It is only after birth that, as a consequence of a reduced cell pool in AL, both reduction in proliferation and increased apoptosis result in a hypoplastic gland (Ward et al. 2005, 2006). Interaction with NOTCH signalling is proposed to underline the mislocalisation of progenitors (Himes & Raetzman 2009). In summary, PROP1 is initially ubiquitously expressed in progenitors and confers a pituitary identity, while it is required later for morphogenesis of AL.
Commitment and differentiation of RP progenitors
As RP progenitors exit the cell cycle, they express the negative regulators of cell proliferation p57KIP followed, as they differentiate, by up-regulation of p27KIP. Both these proteins prevent cell cycle re-entry (Bilodeau et al. 2009). Analysis of mutants, where either cell cycle exits or differentiation is impaired, further reveals that both processes are regulated independently (for review, see Drouin et al. (2010)). General control of endocrine differentiation relies at least on the presence of the histone demethylase LSD1, acting both in activator and in repressor complexes (Wang et al. 2007), and the zinc finger protein INSM1 (Welcker et al. 2013). Deletion phenotypes of either gene are very similar, with a normal pituitary appearance but a block of endocrine differentiation, up-regulation of NOTCH pathway components (Wang et al. 2007, Welcker et al. 2013) and persistence of SOX2/SOX9 expression (Welcker et al. 2013). In the normal pituitary, emergence of each endocrine lineage relies also on the expression of a lineage-specific transcription factor acting as a switch to promote differentiation of a certain lineage while sometimes repressing emergence of others. Molecular events leading to the differentiation of each pituitary endocrine cell type will be described herein.
Commitment and differentiation of RP progenitors
PIT1 lineage: somatotrophs, lactotrophs and thyrotrophs
The POU homoeodomain protein PIT1 is exclusively expressed in the pituitary from 13.5 dpc (Li et al. 1990, Simmons et al. 1990). Its expression is activated by PROP1 (Olson et al. 2006) and maintained in terminally differentiated somatotrophs, lactotrophs and thyrotrophs, where it regulates expression of hormones and hypothalamic peptide receptors. PIT1 is also necessary for Gh promoter hypomethylation, associated with active transcription; therefore, it also influences accessibility for other regulators (Massah et al. 2014). In Pit1 mouse mutant embryos, emergence of endocrine cells takes place, but these are not maintained postnatally. Proliferation is reduced and apoptosis occurs, leading to GH, PRL and thyroid-stimulating hormone (TSH) deficiencies. The protein therefore appears to be necessary for postnatal expansion/survival probably in response to both hypothalamic and local signals (Ward et al. 2006).
Somatotrophs
In the embryo, PIT1 activates the expression of the bHLH factor Neurod4 at 13.5 dpc. NEUROD4 is required for onset of somatotroph differentiation. In its absence, somatotroph numbers are very low in the embryo, although this improves postnatally. However, these cells never express the receptor for GH-releasing hormone (GHRH, hypothalamic peptide promoting GH secretion), resulting in dwarfism (Zhu et al. 2006). The Notch ligand Dlk1 in also expressed by somatotrophs and loss of the gene leads to a mild reduction in GH (Cheung et al. 2013). Cross-regulatory mechanisms between different cell types also control endocrine populations: after birth, the histone demethylase LSD1 acts as a co-activator on the Gh promoter, while it participates in Prl repression (Wang et al. 2007).
Lactotrophs
The emergence of lactotrophs, mainly postnatally, is thought to largely rely on the concerted transcriptional activities of PIT1 and oestrogen receptors (for review, see Featherstone et al. (2012)). It has been initially proposed that lactotrophs could transdifferentiate from somatotrophs; however, cell lineage tracing experiments have shown that this was not a significant phenomenon and that lactotrophs predominantly differentiate from progenitors (Luque et al. 2007, Castrique et al. 2010, Fu et al. 2012).
Thyrotrophs
Both thyrotrophs and gonadotrophs express αGSU (the common subunit for TSH and gonadotrophins), the earliest marker of endocrine differentiation, appearing at 11.5 dpc. Therefore, early specification processes, comprising regulators of the Cga gene (encoding for αGSU), are shared between the two populations. FOXL2, a forkhead transcription factor, is the first marker of gonadotroph and thyrotroph commitment; expressed ventrally in RP at 10.5 dpc (Treier et al. 1998), it persists in these two endocrine cell types until adulthood. LHX3 and LHX4 are required for its expression (Ellsworth et al. 2006). FOXL2 can regulate Cga expression but does not appear to be essential (Ellsworth et al. 2006). Foxl2−/− pituitaries are hypoplastic; while all endocrine cell types are present, there is an increased cellular density (Justice et al. 2011), suggesting a general defective endocrine maturation, probably of hypothalamic origin. Null pituitaries also present more corticotrophs and somatotrophs in males, and reduced Cga, Fsh (Fshb), Gh and Prl levels in females. This could reflect functions of the protein outside the pituitary; therefore, to better characterise FOXL2 function, the consequences of its conditional deletion in the gonads (see below) and the hypothalamus should be investigated in the pituitary. At 12.5 dpc, the zinc finger transcription factor GATA2, mostly known as an important regulator of haematopoietic SCs, starts to be expressed in the developing pituitary, in the same ventral region as FOXL2. It can also activate expression of Cga, and, along with PIT1, that of TSH (Dasen et al. 1999). It has been proposed that PIT1 blocks a suppressor region in the TSHβ promoter, allowing access and transactivation by GATA2 (Kashiwabara et al. 2009). GATA2 is maintained in thyrotrophs and gonadotrophs (Dasen et al. 1999). Conditional deletion of the gene in αGSU-expressing cells causes a reduction in thyrotroph numbers at birth, suggesting that it plays a role in specification and/or expansion of this population. However, this deficiency is transient, and elevation of Gata3 expression in mutants suggests the existence of a compensatory mechanism (Charles et al. 2006). GATA2 is also required later for mature thyrotroph and also gonadotroph function (Charles et al. 2006). As mentioned earlier, PITX1 and PITX2 are also present in thyrotrophs, where they can activate Gata2 expression (Suh et al. 2002) and are necessary for endocrine function (Castinetti et al. 2011b).
TPIT lineage: corticotrophs and melanotrophs
The T-box transcription factor TPIT (TBX19) is required for corticotroph and melanotroph differentiation (Pulichino et al. 2003a,b). TPIT expression is first and exclusively observed in RP at 12.5 dpc just before differentiation of the first corticotrophs. Its expression is maintained in mature corticotrophs and melanotrophs. TPIT, along with PITX1 and the ETS factor ETV1, controls Pomc expression (Lamolet et al. 2001, Lavoie et al. 2008, Budry et al. 2011b). Mutations in both human and murine TPIT are associated with severe ACTH deficiencies (Pulichino et al. 2003a,b). Corticotroph commitment is nevertheless observed in murine Tpit mutants, but terminal differentiation fails to occur. Moreover, in addition to ‘promoting its own lineage’, TPIT also actively inhibits the emergence of gonadotrophs and rostral-tip thyrotrophs. In vitro TPIT and SF1 mutually repress each others' transcriptional activities, by direct protein–protein interaction. The relevance of this interaction is demonstrated in vivo, in Tpit null IL, by appearance of ectopic gonadotrophs and PIT1-independent thyrotrophs (Pulichino et al. 2003a,b). Finally, network formation is important for optimal POMC expression, because loss of N-cadherin in POMC-expressing cells results in disorganisation of both IL architecture and AL corticotroph network, and a reduction in Pomc levels (Himes et al. 2011).
Corticotrophs
In the context of the pituitary, NeuroD1, a bHLH transcription factor, is transiently expressed only in corticotrophs, where it can also act along with other bHLH proteins and PITX1 to induce the expression of Pomc (Lavoie et al. 2008). However, corticotroph differentiation is only slightly delayed in its absence (Lamolet et al. 2004). Neurod1 is still expressed in Tpit mutants, demonstrating that corticotroph differentiation rather than their commitment is prevented (Pulichino et al. 2003a,b).
Melanotrophs
PAX7, a paired box protein, mostly known for its role in muscle satellite cells, has been recently shown to be responsible for melanotroph specification (Budry et al. 2012). It is expressed in the future IL from 14.5 dpc, where it briefly co-localises with SOX2, and it is maintained while TPIT is up-regulated in differentiating and mature melanotrophs. Deletion of the gene results in a loss of melanotrophs, which are replaced by ectopic corticotrophs. Melanotroph specification is dependent on the chromatin re-modelling properties of PAX7, which allows TPIT to access a subset of melanotroph-specific enhancers (Budry et al. 2012). Finally, proper organisation of IL and its associated dorsal SOX2-expressing progenitor cell layer relies on the presence of the adaptor protein NUMB, probably through its adherens junction-stabilising properties (Moran et al. 2011).
Gonadotrophs
The first prospective gonadotroph-specific marker is probably the GnRH receptor, expressed from 12.5 dpc, well before gonadotrophins at 16 dpc (Wen et al. 2010). Its expression is induced at least in part by LHX3 and ISLET1 (Schang et al. 2013). GnRHR is required in the embryo because emergence of follicle-stimulating hormone (FSH)-positive gonadotrophs depends on GnRH (Wen et al. 2010). FOXL2 and GATA2, as mentioned earlier, are expressed early and maintained in both thyrotroph and gonadotroph populations (Charles et al. 2006, Ellsworth et al. 2006). FOXL2 is an important regulator of ovarian development and, in its absence, fertility is impaired (Uhlenhaut et al. 2009). Conditional deletion of Foxl2 in gonadotrophs indicates that the protein is required, along with SMAD4 for Fsh expression (Fortin et al. 2014). FOXL2 is also likely to be involved in gonadotroph adenoma growth (Chesnokova et al. 2012). GATA2 is not essential for gonadotroph development, but FSH levels are reduced in mutants (Charles et al. 2006). At 14.5 dpc, the orphan nuclear receptor SF1 starts to be expressed in future gonadotrophs and GATA2 is involved in its activation (Dasen et al. 1999). SF1 is expressed and required in the different components of both the reproductive and adrenal hypothalamo–pituitary axis. It can activate expression of Cga, Lh (Lhb), Fsh and Gnrhr along with co-factors such as early growth response 1 (EGR1) and PITX1 (Fortin et al. 2009). Conditional deletion of Sf1 in αGSU-positive cells results in a dramatic reduction in gonadotrophin expression, but high doses of GnRH can partially rescue this phenotype; therefore, responsive gonadotrophs or precursors are still present (Zhao et al. 2001).
Pituitary SCs
Pituitary SCs had been suggested to exist for a long time, but it is only recently that their existence has been convincingly demonstrated (for reviews, see Rizzoti (2010), Castinetti et al. (2011a), Davis et al. (2011) and Vankelecom (2012)). RP progenitors express SOX2 from early stages, and SOX9 is up-regulated in the SOX2-positive cells towards the end of gestation (14.5 dpc). Its up-regulation correlates with reduced levels of both differentiation and proliferation (Rizzoti et al. 2013; C Pires and K Rizzoti, unpublished observations). In the embryonic CNS, SOX9 is required for neural SC generation, (Scott et al. 2010). Future investigations may reveal if it has a similar role in the pituitary, operating a switch from embryonic progenitors towards more quiescent SCs. SOX2; SOX9 double-positive cells are maintained postnatally, mainly in an epithelial layer surrounding the pituitary cleft, but some are also scattered in the AL (Fauquier et al. 2008). SOX2; SOX9 double-positive cells lining the cleft also express the Glial cell line-derived neurotrophic Factor Receptor GFRα2 (Garcia-Lavandeira et al. 2009).
In vitro, SOX2- and SOX9-positive cells isolated from adult pituitaries have the capacity to generate spheres (Fauquier et al. 2008, Rizzoti et al. 2013), a property displayed by progenitor populations in different tissues. Spheres are characterised by self-renewal and differentiation potentials. Pituispheres have a limited self-renewal capacity (for review, see Rizzoti (2010)) and this may reflect sub-optimal conditions, as a transient loss of SOX9 is observed during the early phases of culture (Rizzoti et al. 2013). Pituispheres can generate endocrine cells (Fauquier et al. 2008, Rizzoti et al. 2013), but the molecular mechanisms are unknown. These represent a good model to investigate whether adult progenitors utilise embryonic differentiation processes.
In vivo, lineage-tracing experiments using both Sox2CreERT2- and Sox9CreERT2-targeted alleles revealed that cells expressing either (or both) of them can self-renew and give rise to endocrine cells, both in the embryo and postnatally (Andoniadou et al. 2013, Rizzoti et al. 2013). In the adult, pituitary SCs are mostly quiescent, and they proliferate and differentiate only rarely (Fauquier et al. 2008, Andoniadou et al. 2013, Rizzoti et al. 2013). As mentioned earlier, SOX2 is required for RP progenitor proliferation. In the adult SCs, its role is likely to be different, reflecting interactions with different partners and the effect of niche-derived factors that regulate quiescence vs activation, and self-renewal vs differentiation (for review, see Kamachi & Kondoh (2013)). Additionally, the balance between SOX2 and p27kip is likely to be important in the control of proliferation of pituitary SCs. P27kip is a negative cell cycle regulator but it is also able to recruit co-repressors to down-regulate Sox2 expression in embryonic SCs (ESCs) and this is important for differentiation to proceed. In vivo, p27kip null mice display intermediate lobe tumours and a thicker SC layer lining the cleft. Deletion of one allele of Sox2 on this null background rescues these phenotypes, highlighting the physiological relevance of this interaction (Li et al. 2012). In RP, p27kip and SOX2 are not expressed in the same cells (Bilodeau et al. 2009), but at some point p27kip becomes ubiquitously expressed and it is likely to represent an important regulator of Sox2 expression.
A role for the NOTCH pathway in proliferation of progenitors had been proposed in vitro (Chen et al. 2006). Recently, conditional deletion of NOTCH2 in progenitors confirmed this function as numbers are reduced postnatally, and there is also an abnormal localisation of SOX2- and SOX9-expressing cells in mutants (Nantie et al. 2014). Co-localisation of SOX2 with the transcription factor PROP1 is observed in AL up until 1 week postnatally, after which expression of the later dramatically decreases (Yoshida et al. 2011). This may reflect different rates, or mechanisms, of differentiation. In the adult, there is indeed very little differentiation from SCs under normal conditions (Andoniadou et al. 2013, Rizzoti et al. 2013). In agreement with this observation, an elegant study, where apoptosis is induced upon cell division, demonstrated that corticotroph turnover relies on proliferation of differentiated cells or self-duplication (Langlais et al. 2013). In contrast, physiological challenges such as pituitary target organ ablation efficiently induce proliferation and differentiation of SCs, demonstrating their regenerative potential (Rizzoti et al. 2013).
Tumour-forming potential of the SC compartment has been recently examined postnatally by expressing a degradation-resistant form of β-catenin that was conditionally expressed under the control of Sox2CreERT2 and tamoxifen administration. Tumours are formed, but surprisingly they were not composed of mutant SCs. Instead, the SCs initially form clusters that subsequently induce neighbouring cells to form the tumours in a paracrine manner (Andoniadou et al. 2013).
In conclusion, pituitary SCs do not significantly participate in adult cell turnover but they have a regenerative potential. They have a tumourigenic potential as they can induce tumours if mutated (Andoniadou et al. 2013), but they have not yet been shown to form tumours themselves.
In vitro recapitulation of pituitary ontogeny
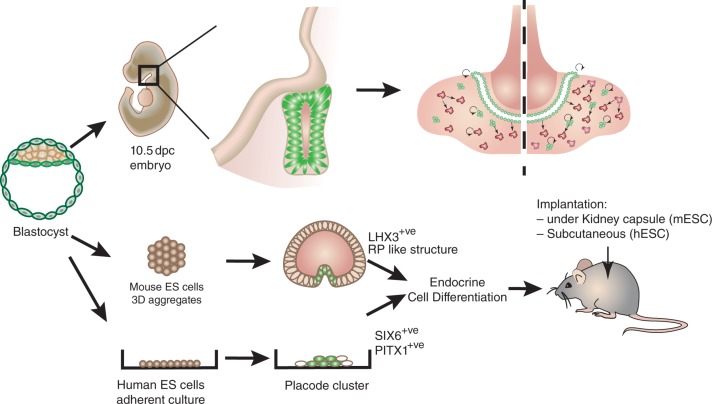
In vitro production of endocrine cells has proved to be difficult and inefficient until recently when two strategies were devised that successfully reproduced some of the embryonic events involved in pituitary development as described earlier, allowing endocrine cells to be obtained from ESCs (Suga et al. 2011, Dincer et al. 2013; Fig. 3). In the late Yoshiki Sasai's laboratory, Wataya et al. (2008) had previously been able to induce hypothalamic identity in 3D mouse ESC aggregates. By further inducing rostral character, they reasoned that it might be possible to obtain ANR-like territory apposed to neuroectoderm-like tissue, where RP may develop. By treating large aggregates with BMPs, which at the placodal stage induce ectodermal identity (for review, see Saint-Jeannet & Moody (2014)), they could induce expression of PITX1 and PITX2 in a superficial layer surrounding a RAX-positive neurectodermal domain. Superposition of these two territories was shown to be essential, and also sufficient for further pituitary-like development, mimicking events in vivo. Subsequent exposure to HH agonist was sufficient to induce LHX3 expression in the PITX2+ layer, initially in cell clusters that thickened, and then invaginated to form spectacular RP-like structures. Inhibition of NOTCH signalling resulted, as again reported in vivo (Zhu et al. 2006, Kita et al. 2007), in differentiation of corticotrophs in these pouches. Their functionality was demonstrated after transplantation in vivo in hypophysectomised mice. Obtention of other endocrine cell types was less efficient, perhaps because the absence of vascularisation compromises endocrine differentiation and maturation, as observed in organoids (for review, see Lancaster & Knoblich (2014)). Activation of Wnt signalling induced PIT1 expression, which could reflect cooperation of β-catenin with PROP1 (Olson et al. 2006). Somatotrophs and lactotrophs were further differentiated using, respectively, glucocorticoids and oestradiol, both supplemented with insulin (Suga et al. 2011). Using human ESCs, Dincer and collaborators, in Studer's laboratory, used a different approach and were able after complex sequential modulation of different pathways to directly induce a placodal fate. Importantly, they succeeded in adherent cultures (Dincer et al. 2013). Briefly, dual SMAD inhibition was initially performed to impart neural fate, and the subsequent de-repression in combination with FGF inhibition was sufficient for acquisition of placodal identity. Modulation of FGF, BMP and SHH pathways further allowed specification of lens, trigeminal and pituitary placode identity. As reported by Suga et al. (2011) treatment with HH agonist resulted initially in HP character (PITX1; SIX6+) and further RP identity (LHX3+). But in contrast with Suga et al. (2011) ACTH expression was then observed without further treatment, while induction of PIT1 and GATA2 expression relied on NOTCH pathway inhibition. In vivo secretion was demonstrated after subcutaneous implantation in mice (Dincer et al. 2013). In both studies, ACTH-positive cells were by far the most efficiently generated cell type. In the future, implantation of cells in the pituitary should be evaluated as hormone secretions are normally controlled locally by the hypothalamus. It will also be necessary to assess the efficiency of these protocols with induced pluripotent SCs.
Figure 3.
In vivo and in vitro regenerative potential of stem cells in the pituitary. In vivo, pituitary SCs can be stimulated by physiological challenge, proliferate and differentiate into the appropriate endocrine cell type. In vitro, different strategies have been successfully devised to obtain transplantable endocrine cells. Using mouse ES cells, Suga et al. (2011) were able to recapitulate early pituitary development and mimic RP morphogenesis in 3D aggregates (Suga et al. 2011). Endocrine cells were further differentiated. Transplantation in hypophysectomised mice demonstrated that these cells were functional. Dincer et al. (2013) were also able to differentiate endocrine cells from human ES cells, in adherent cultures, initially by inducing placodal fate, then hypophyseal identity. Transplantation demonstrated in vivo secretion. These are significant steps towards regenerative medicine to cure long-term pituitary endocrine deficiencies.
Conclusions
Significant advances have been made in recent years and we understand better as to how RP progenitors are controlled and how endocrine cells differentiate, and, importantly, we know that they form endocrine networks. The generation of endocrine cells from ESCs and their subsequent transplantation in vivo represent exciting progress towards the use of regenerative medicine to treat endocrine deficits or manipulate endocrine outputs. We still need a better understanding of how cell fates are specified in vivo, as embryonic progenitors exit the cell cycle, for efficient in vitro generation of all endocrine cell types. In addition, the recent characterisation of pituitary SCs opens the alternative possibility of direct somatic cell conversion into organ-specific SCs for use in regenerative medicine.
Acknowledgements
The author is grateful to Dr S Goldsmith and Dr Robin Lovell-Badge, NIMR, for critical reading of the manuscript, and Photographics section at NIMR for help with preparation of the figures.
Declaration of interest
The author declares that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
References
- Ai D, Wang J, Amen M, Lu MF, Amendt BA, Martin JF. Nuclear factor 1 and T-cell factor/LEF recognition elements regulate Pitx2 transcription in pituitary development. Molecular and Cellular Biology. 2007;27:5765–5775. doi: 10.1128/MCB.01848-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- elAmraoui A, Dubois PM. Experimental evidence for the early commitment of the presumptive adenohypophysis. Neuroendocrinology. 1993;58:609–615. doi: 10.1159/000126599. [DOI] [PubMed] [Google Scholar]
- Andoniadou CL, Signore M, Sajedi E, Gaston-Massuet C, Kelberman D, Burns AJ, Itasaki N, Dattani M, Martinez-Barbera JP. Lack of the murine homeobox gene Hesx1 leads to a posterior transformation of the anterior forebrain. Development. 2007;134:1499–1508. doi: 10.1242/dev.02829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andoniadou CL, Signore M, Young RM, Gaston-Massuet C, Wilson SW, Fuchs E, Martinez-Barbera JP. HESX1- and TCF3-mediated repression of Wnt/β-catenin targets is required for normal development of the anterior forebrain. Development. 2011;138:4931–4942. doi: 10.1242/dev.066597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andoniadou CL, Matsushima D, Mousavy Gharavy SN, Signore M, Mackintosh AI, Schaeffer M, Gaston-Massuet C, Mollard P, Jacques TS, Le Tissier P, et al. Sox2(+) stem/progenitor cells in the adult mouse pituitary support organ homeostasis and have tumor-inducing potential. Cell Stem Cell. 2013;13:433–445. doi: 10.1016/j.stem.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Athar M, Li C, Kim AL, Spiegelman VS, Bickers DR. Sonic hedgehog signaling in basal cell nevus syndrome. Cancer Research. 2014;74:4967–4975. doi: 10.1158/0008-5472.CAN-14-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aujla PK, Bora A, Monahan P, Sweedler JV, Raetzman LT. The Notch effector gene Hes1 regulates migration of hypothalamic neurons, neuropeptide content and axon targeting to the pituitary. Developmental Biology. 2011;353:61–71. doi: 10.1016/j.ydbio.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti K, Gasper M, Bertuzzi S, Arnheiter H. Lack of the ventral anterior homeodomain transcription factor VAX1 leads to induction of a second pituitary. Development. 2011;138:873–878. doi: 10.1242/dev.056465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilodeau S, Roussel-Gervais A, Drouin J. Distinct developmental roles of cell cycle inhibitors p57Kip2 and p27Kip1 distinguish pituitary progenitor cell cycle exit from cell cycle reentry of differentiated cells. Molecular and Cellular Biology. 2009;29:1895–1908. doi: 10.1128/MCB.01885-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmeier ML, Potok MA, Davis SW, Camper SA. TCF4 deficiency expands ventral diencephalon signaling and increases induction of pituitary progenitors. Developmental Biology. 2007;311:396–407. doi: 10.1016/j.ydbio.2007.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budry L, Lafont C, El Yandouzi T, Chauvet N, Conejero G, Drouin J, Mollard P. Related pituitary cell lineages develop into interdigitated 3D cell networks. PNAS. 2011a;108:12515–12520. doi: 10.1073/pnas.1105929108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budry L, Couture C, Balsalobre A, Drouin J. The Ets factor Etv1 interacts with Tpit protein for pituitary pro-opiomelanocortin (POMC) gene transcription. Journal of Biological Chemistry. 2011b;286:25387–25396. doi: 10.1074/jbc.M110.202788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budry L, Balsalobre A, Gauthier Y, Khetchoumian K, L'Honore A, Vallette S, Brue T, Figarella-Branger D, Meij B, Drouin J. The selector gene Pax7 dictates alternate pituitary cell fates through its pioneer action on chromatin remodeling. Genes and Development. 2012;26:2299–2310. doi: 10.1101/gad.200436.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Pardali E, Sanchez-Duffhues G, ten Dijke P. BMP signaling in vascular diseases. FEBS Letters. 2012;586:1993–2002. doi: 10.1016/j.febslet.2012.04.030. [DOI] [PubMed] [Google Scholar]
- Carvalho LR, Brinkmeier ML, Castinetti F, Ellsworth BS, Camper SA. Corepressors TLE1 and TLE3 interact with HESX1 and PROP1. Molecular Endocrinology. 2010;24:754–765. doi: 10.1210/me.2008-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castinetti F, Davis SW, Brue T, Camper SA. Pituitary stem cell update and potential implications for treating hypopituitarism. Endocrine Reviews. 2011a;32:453–471. doi: 10.1210/er.2010-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castinetti F, Brinkmeier ML, Gordon DF, Vella KR, Kerr JM, Mortensen AH, Hollenberg A, Brue T, Ridgway EC, Camper SA. PITX2 and PITX1, regulate thyrotroph function and response to hypothyroidism. Molecular Endocrinology. 2011b;25:1950–1960. doi: 10.1210/me.2010-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrique E, Fernandez-Fuente M, Le Tissier P, Herman A, Levy A. Use of a prolactin-Cre/ROSA-YFP transgenic mouse provides no evidence for lactotroph transdifferentiation after weaning, or increase in lactotroph/somatotroph proportion in lactation. Journal of Endocrinology. 2010;205:49–60. doi: 10.1677/JOE-09-0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha KB, Douglas KR, Potok MA, Liang H, Jones SN, Camper SA. WNT5A signaling affects pituitary gland shape. Mechanisms of Development. 2004;121:183–194. doi: 10.1016/j.mod.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Charles MA, Suh H, Hjalt TA, Drouin J, Camper SA, Gage PJ. PITX genes are required for cell survival and Lhx3 activation. Molecular Endocrinology. 2005;19:1893–1903. doi: 10.1210/me.2005-0052. [DOI] [PubMed] [Google Scholar]
- Charles MA, Saunders TL, Wood WM, Owens K, Parlow AF, Camper SA, Ridgway EC, Gordon DF. Pituitary-specific Gata2 knockout: effects on gonadotrope and thyrotrope function. Molecular Endocrinology. 2006;20:1366–1377. doi: 10.1210/me.2005-0378. [DOI] [PubMed] [Google Scholar]
- Chen J, Crabbe A, Van Duppen V, Vankelecom H. The Notch signaling system is present in the postnatal pituitary: marked expression and regulatory activity in the newly discovered side population. Molecular Endocrinology. 2006;20:3293–3307. doi: 10.1210/me.2006-0293. [DOI] [PubMed] [Google Scholar]
- Chesnokova V, Zonis S, Wawrowsky K, Tani Y, Ben-Shlomo A, Ljubimov V, Mamelak A, Bannykh S, Melmed S. Clusterin and FOXL2 act concordantly to regulate pituitary gonadotroph adenoma growth. Molecular Endocrinology. 2012;26:2092–2103. doi: 10.1210/me.2012-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung LY, Rizzoti K, Lovell-Badge R, Le Tissier PR. Pituitary phenotypes of mice lacking the Notch signalling ligand delta-like 1 homologue. Journal of Neuroendocrinology. 2013;25:391–401. doi: 10.1111/jne.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- Chou SJ, Hermesz E, Hatta T, Feltner D, El-Hodiri HM, Jamrich M, Mahon K. Conserved regulatory elements establish the dynamic expression of Rpx/HesxI in early vertebrate development. Developmental Biology. 2006;292:533–545. doi: 10.1016/j.ydbio.2005.12.053. [DOI] [PubMed] [Google Scholar]
- Chung WC, Tsai PS. Role of fibroblast growth factor signaling in gonadotropin-releasing hormone neuronal system development. Frontiers of Hormone Research. 2010;39:37–50. doi: 10.1159/000312692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Dasen JS, O'Connell SM, Flynn SE, Treier M, Gleiberman AS, Szeto DP, Hooshmand F, Aggarwal AK, Rosenfeld MG. Reciprocal interactions of Pit1 and GATA2 mediate signaling gradient-induced determination of pituitary cell types. Cell. 1999;97:587–598. doi: 10.1016/S0092-8674(00)80770-9. [DOI] [PubMed] [Google Scholar]
- Dasen JS, Barbera JP, Herman TS, Connell SO, Olson L, Ju B, Tollkuhn J, Baek SH, Rose DW, Rosenfeld MG. Temporal regulation of a paired-like homeodomain repressor/TLE corepressor complex and a related activator is required for pituitary organogenesis. Genes and Development. 2001;15:3193–3207. doi: 10.1101/gad.932601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dattani MT, Martinez-Barbera JP, Thomas PQ, Brickman JM, Gupta R, Martensson IL, Toresson H, Fox M, Wales JK, Hindmarsh PC, et al. Mutations in the homeobox gene HESX1/Hesx1 associated with septo-optic dysplasia in human and mouse. Nature Genetics. 1998;19:125–133. doi: 10.1038/477. [DOI] [PubMed] [Google Scholar]
- Davis SW, Camper SA. Noggin regulates Bmp4 activity during pituitary induction. Developmental Biology. 2007;305:145–160. doi: 10.1016/j.ydbio.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SW, Mortensen AH, Camper SA. Birthdating studies reshape models for pituitary gland cell specification. Developmental Biology. 2011;352:215–227. doi: 10.1016/j.ydbio.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SW, Ellsworth BS, Perez Millan MI, Gergics P, Schade V, Foyouzi N, Brinkmeier ML, Mortensen AH, Camper SA. Pituitary gland development and disease: from stem cell to hormone production. Current Topics in Developmental Biology. 2013;106:1–47. doi: 10.1016/B978-0-12-416021-7.00001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Moerlooze L, Spencer-Dene B, Revest J, Hajihosseini M, Rosewell I, Dickson C. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal–epithelial signalling during mouse organogenesis. Development. 2000;127:483–492. doi: 10.1242/dev.127.3.483. [DOI] [PubMed] [Google Scholar]
- Dincer Z, Piao J, Niu L, Ganat Y, Kriks S, Zimmer B, Shi SH, Tabar V, Studer L. Specification of functional cranial placode derivatives from human pluripotent stem cells. Cell Reports. 2013;5:1387–1402. doi: 10.1016/j.celrep.2013.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorey K, Amaya E. FGF signalling: diverse roles during early vertebrate embryogenesis. Development. 2010;137:3731–3742. doi: 10.1242/dev.037689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouin J, Bilodeau S, Roussel-Gervais A. Stem cells, differentiation and cell cycle control in pituitary. Frontiers of Hormone Research. 2010;38:15–24. doi: 10.1159/000318490. [DOI] [PubMed] [Google Scholar]
- Ellsworth BS, Egashira N, Haller JL, Butts DL, Cocquet J, Clay CM, Osamura RY, Camper SA. FOXL2 in the pituitary: molecular, genetic, and developmental analysis. Molecular Endocrinology. 2006;20:2796–2805. doi: 10.1210/me.2005-0303. [DOI] [PubMed] [Google Scholar]
- Ellsworth BS, Butts DL, Camper SA. Mechanisms underlying pituitary hypoplasia and failed cell specification in Lhx3-deficient mice. Developmental Biology. 2008;313:118–129. doi: 10.1016/j.ydbio.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson J, Norlin S, Jessell TM, Edlund T. Integrated FGF and BMP signaling controls the progression of progenitor cell differentiation and the emergence of pattern in the embryonic anterior pituitary. Development. 1998;125:1005–1015. doi: 10.1242/dev.125.6.1005. [DOI] [PubMed] [Google Scholar]
- Fantes J, Ragge NK, Lynch SA, McGill NI, Collin JR, Howard-Peebles PN, Hayward C, Vivian AJ, Williamson K, van Heyningen V, et al. Mutations in SOX2 cause anophthalmia. Nature Genetics. 2003;33:461–463. doi: 10.1038/ng1120. [DOI] [PubMed] [Google Scholar]
- Fauquier T, Rizzoti K, Dattani M, Lovell-Badge R, Robinson IC. SOX2-expressing progenitor cells generate all of the major cell types in the adult mouse pituitary gland. PNAS. 2008;105:2907–2912. doi: 10.1073/pnas.0707886105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone K, White MR, Davis JR. The prolactin gene: a paradigm of tissue-specific gene regulation with complex temporal transcription dynamics. Journal of Neuroendocrinology. 2012;24:977–990. doi: 10.1111/j.1365-2826.2012.02310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin J, Lamba P, Wang Y, Bernard DJ. Conservation of mechanisms mediating gonadotrophin-releasing hormone 1 stimulation of human luteinizing hormone β subunit transcription. Molecular Human Reproduction. 2009;15:77–87. doi: 10.1093/molehr/gan079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin J, Boehm U, Deng CX, Treier M, Bernard DJ. Follicle-stimulating hormone synthesis and fertility depend on SMAD4 and FOXL2. FASEB Journal. 2014;28:3396–3410. doi: 10.1096/fj.14-249532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nature Reviews. Molecular Cell Biology. 2009;10:445–457. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- Fu Q, Gremeaux L, Luque RM, Liekens D, Chen J, Buch T, Waisman A, Kineman R, Vankelecom H. The adult pituitary shows stem/progenitor cell activation in response to injury and is capable of regeneration. Endocrinology. 2012;153:3224–3235. doi: 10.1210/en.2012-1152. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Camper SA. Pituitary homeobox 2, a novel member of the bicoid-related family of homeobox genes, is a potential regulator of anterior structure formation. Human Molecular Genetics. 1997;6:457–464. doi: 10.1093/hmg/6.3.457. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Brinkmeier ML, Scarlett LM, Knapp LT, Camper SA, Mahon KA. The Ames dwarf gene, df, is required early in pituitary ontogeny for the extinction of Rpx transcription and initiation of lineage-specific cell proliferation. Molecular Endocrinology. 1996;10:1570–1581. doi: 10.1210/mend.10.12.8961267. [DOI] [PubMed] [Google Scholar]
- Garcia-Lavandeira M, Quereda V, Flores I, Saez C, Diaz-Rodriguez E, Japon MA, Ryan AK, Blasco MA, Dieguez C, Malumbres M, et al. A GRFa2/Prop1/stem (GPS) cell niche in the pituitary. PLoS ONE. 2009;4:e4815. doi: 10.1371/journal.pone.0004815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston-Massuet C, Andoniadou CL, Signore M, Sajedi E, Bird S, Turner JM, Martinez-Barbera JP. Genetic interaction between the homeobox transcription factors HESX1 and SIX3 is required for normal pituitary development. Developmental Biology. 2008;324:322–333. doi: 10.1016/j.ydbio.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston-Massuet C, Andoniadou CL, Signore M, Jayakody SA, Charolidi N, Kyeyune R, Vernay B, Jacques TS, Taketo MM, Le Tissier P, et al. Increased Wingless (Wnt) signaling in pituitary progenitor/stem cells gives rise to pituitary tumors in mice and humans. PNAS. 2011;108:11482–11487. doi: 10.1073/pnas.1101553108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleiberman AS, Fedtsova NG, Rosenfeld MG. Tissue interactions in the induction of anterior pituitary: role of the ventral diencephalon, mesenchyme, and notochord. Developmental Biology. 1999;213:340–353. doi: 10.1006/dbio.1999.9386. [DOI] [PubMed] [Google Scholar]
- Goldberg LB, Aujla PK, Raetzman LT. Persistent expression of activated Notch inhibits corticotrope and melanotrope differentiation and results in dysfunction of the HPA axis. Developmental Biology. 2011;358:23–32. doi: 10.1016/j.ydbio.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon MJ, Crowley RK, Behan LA, O'Sullivan EP, O'Brien MM, Sherlock M, Rawluk D, O'Dwyer R, Tormey W, Thompson CJ. Acute glucocorticoid deficiency and diabetes insipidus are common after acute traumatic brain injury and predict mortality. Journal of Clinical Endocrinology and Metabolism. 2013;98:3229–3237. doi: 10.1210/jc.2013-1555. [DOI] [PubMed] [Google Scholar]
- Hermesz E, Mackem S, Mahon KA. Rpx: a novel anterior-restricted homeobox gene progressively activated in the prechordal plate, anterior neural plate and Rathke's pouch of the mouse embryo. Development. 1996;122:41–52. doi: 10.1242/dev.122.1.41. [DOI] [PubMed] [Google Scholar]
- Himes AD, Raetzman LT. Premature differentiation and aberrant movement of pituitary cells lacking both Hes1 and Prop1. Developmental Biology. 2009;325:151–161. doi: 10.1016/j.ydbio.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himes AD, Fiddler RM, Raetzman LT. N-cadherin loss in POMC-expressing cells leads to pituitary disorganization. Molecular Endocrinology. 2011;25:482–491. doi: 10.1210/me.2010-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodson DJ, Schaeffer M, Romano N, Fontanaud P, Lafont C, Birkenstock J, Molino F, Christian H, Lockey J, Carmignac D, et al. Existence of long-lasting experience-dependent plasticity in endocrine cell networks. Nature Communications. 2012;3:605. doi: 10.1038/ncomms1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori K, Sen A, S Artavanis-Tsakonas. Notch signaling at a glance. Journal of Cell Science. 2013;126:2135–2140. doi: 10.1242/jcs.127308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japon MA, Rubinstein M, Low MJ. In situ hybridization analysis of anterior pituitary hormone gene expression during fetal mouse development. Journal of Histochemistry and Cytochemistry. 1994;42:1117–1125. doi: 10.1177/42.8.8027530. [DOI] [PubMed] [Google Scholar]
- Jayakody SA, Andoniadou CL, Gaston-Massuet C, Signore M, Cariboni A, Bouloux PM, Le Tissier P, Pevny LH, Dattani MT, Martinez-Barbera JP. SOX2 regulates the hypothalamic-pituitary axis at multiple levels. Journal of Clinical Investigation. 2012;122:3635–3646. doi: 10.1172/JCI64311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean D, Bernier G, Gruss P. Six6 (Optx2) is a novel murine Six3-related homeobox gene that demarcates the presumptive pituitary/hypothalamic axis and the ventral optic stalk. Mechanisms of Development. 1999;84:31–40. doi: 10.1016/S0925-4773(99)00068-4. [DOI] [PubMed] [Google Scholar]
- Jiang X, Iseki S, Maxson RE, Sucov HM, Morriss-Kay GM. Tissue origins and interactions in the mammalian skull vault. Developmental Biology. 2002;241:106–116. doi: 10.1006/dbio.2001.0487. [DOI] [PubMed] [Google Scholar]
- Justice NJ, Blount AL, Pelosi E, Schlessinger D, Vale W, Bilezikjian LM. Impaired FSHβ expression in the pituitaries of Foxl2 mutant animals. Molecular Endocrinology. 2011;25:1404–1415. doi: 10.1210/me.2011-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamachi Y, Kondoh H. Sox proteins: regulators of cell fate specification and differentiation. Development. 2013;140:4129–4144. doi: 10.1242/dev.091793. [DOI] [PubMed] [Google Scholar]
- Kashiwabara Y, Sasaki S, Matsushita A, Nagayama K, Ohba K, Iwaki H, Matsunaga H, Suzuki S, Misawa H, Ishizuka K, et al. Functions of PIT1 in GATA2-dependent transactivation of the thyrotropin β promoter. Journal of Molecular Endocrinology. 2009;42:225–237. doi: 10.1677/JME-08-0099. [DOI] [PubMed] [Google Scholar]
- Kawamura K, Kikuyama S. Morphogenesis of the hypothalamus and hypophysis: their association, dissociation and reassociation before and after "Rathke". Archives of Histology and Cytology. 1998;61:189–198. doi: 10.1679/aohc.61.189. [DOI] [PubMed] [Google Scholar]
- Kelberman D, Rizzoti K, Avilion A, Bitner-Glindzicz M, Cianfarani S, Collins J, Chong WK, Kirk JM, Achermann JC, Ross R, et al. Mutations within Sox2/SOX2 are associated with abnormalities in the hypothalamo–pituitary–gonadal axis in mice and humans. Journal of Clinical Investigation. 2006;116:2442–2455. doi: 10.1172/JCI28658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelberman D, Rizzoti K, Lovell-Badge R, Robinson IC, Dattani MT. Genetic regulation of pituitary gland development in human and mouse. Endocrine Reviews. 2009;30:790–829. doi: 10.1210/er.2009-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khonsari RH, Seppala M, Pradel A, Dutel H, Clement G, Lebedev O, Ghafoor S, Rothova M, Tucker A, Maisey JG, et al. The buccohypophyseal canal is an ancestral vertebrate trait maintained by modulation in sonic hedgehog signaling. BMC Biology. 2013;11:27. doi: 10.1186/1741-7007-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioussi C, Briata P, Baek SH, Rose DW, Hamblet NS, Herman T, Ohgi KA, Lin C, Gleiberman A, Wang J, et al. Identification of a Wnt/Dvl/β-catenin → Pitx2 pathway mediating cell-type-specific proliferation during development. Cell. 2002;111:673–685. doi: 10.1016/S0092-8674(02)01084-X. [DOI] [PubMed] [Google Scholar]
- Kita A, Imayoshi I, Hojo M, Kitagawa M, Kokubu H, Ohsawa R, Ohtsuka T, Kageyama R, Hashimoto N. Hes1 and Hes5 control the progenitor pool, intermediate lobe specification, and posterior lobe formation in the pituitary development. Molecular Endocrinology. 2007;21:1458–1466. doi: 10.1210/me.2007-0039. [DOI] [PubMed] [Google Scholar]
- Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell. 2014;14:275–291. doi: 10.1016/j.stem.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Kumar JP. The sine oculis homeobox (SIX) family of transcription factors as regulators of development and disease. Cellular and Molecular Life Sciences. 2009;66:565–583. doi: 10.1007/s00018-008-8335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamolet B, Pulichino AM, Lamonerie T, Gauthier Y, Brue T, Enjalbert A, Drouin J. A pituitary cell-restricted T box factor, Tpit, activates POMC transcription in cooperation with Pitx homeoproteins. Cell. 2001;104:849–859. doi: 10.1016/S0092-8674(01)00282-3. [DOI] [PubMed] [Google Scholar]
- Lamolet B, Poulin G, Chu K, Guillemot F, Tsai MJ, Drouin J. Tpit-independent function of NeuroD1(β2) in pituitary corticotroph differentiation. Molecular Endocrinology. 2004;18:995–1003. doi: 10.1210/me.2003-0127. [DOI] [PubMed] [Google Scholar]
- Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345:1247125. doi: 10.1126/science.1247125. [DOI] [PubMed] [Google Scholar]
- Lanctot C, Lamolet B, Drouin J. The bicoid-related homeoprotein Ptx1 defines the most anterior domain of the embryo and differentiates posterior from anterior lateral mesoderm. Development. 1997;124:2807–2817. doi: 10.1242/dev.124.14.2807. [DOI] [PubMed] [Google Scholar]
- Langlais D, Couture C, Kmita M, Drouin J. Adult pituitary cell maintenance: lineage-specific contribution of self-duplication. Molecular Endocrinology. 2013;27:1103–1112. doi: 10.1210/me.2012-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larder R, Clark DD, Miller NL, Mellon PL. Hypothalamic dysregulation and infertility in mice lacking the homeodomain protein Six6. Journal of Neuroscience. 2011;31:426–438. doi: 10.1523/JNEUROSCI.1688-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumonnier F, Ronce N, Hamel BC, Thomas P, Lespinasse J, Raynaud M, Paringaux C, Van Bokhoven H, Kalscheuer V, Fryns JP, et al. Transcription factor SOX3 is involved in X-linked mental retardation with growth hormone deficiency. American Journal of Human Genetics. 2002;71:1450–1455. doi: 10.1086/344661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavado A, Lagutin OV, Oliver G. Six3 inactivation causes progressive caudalization and aberrant patterning of the mammalian diencephalon. Development. 2008;135:441–450. doi: 10.1242/dev.010082. [DOI] [PubMed] [Google Scholar]
- Lavoie PL, Budry L, Balsalobre A, Drouin J. Developmental dependence on NurRE and EboxNeuro for expression of pituitary proopiomelanocortin. Molecular Endocrinology. 2008;22:1647–1657. doi: 10.1210/me.2007-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Rizzoti K, Kwon DS, Kim SY, Oh S, Epstein DJ, Son Y, Yoon J, Baek K, Jeong Y. Direct transcriptional regulation of Six6 is controlled by SoxB1 binding to a remote forebrain enhancer. Developmental Biology. 2012;366:393–403. doi: 10.1016/j.ydbio.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Song H, Rizzoti K, Son Y, Yoon J, Baek K, Jeong Y. Genomic code for Sox2 binding uncovers its regulatory role in Six3 activation in the forebrain. Developmental Biology. 2013;381:491–501. doi: 10.1016/j.ydbio.2013.06.016. [DOI] [PubMed] [Google Scholar]
- Lepper C, Conway SJ, Fan CM. Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements. Nature. 2009;460:627–631. doi: 10.1038/nature08209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy A. Physiological implications of pituitary trophic activity. Journal of Endocrinology. 2002;174:147–155. doi: 10.1677/joe.0.1740147. [DOI] [PubMed] [Google Scholar]
- L'Honore A, Commere PH, Ouimette JF, Montarras D, Drouin J, Buckingham M. Redox regulation by Pitx2 and Pitx3 is critical for fetal myogenesis. Developmental Cell. 2014;29:392–405. doi: 10.1016/j.devcel.2014.04.006. [DOI] [PubMed] [Google Scholar]
- Li S, Crenshaw EB, III, Rawson EJ, Simmons DM, Swanson LW, Rosenfeld MG. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature. 1990;347:528–533. doi: 10.1038/347528a0. [DOI] [PubMed] [Google Scholar]
- Li X, Perissi V, Liu F, Rose DW, Rosenfeld MG. Tissue-specific regulation of retinal and pituitary precursor cell proliferation. Science. 2002;297:1180–1183. doi: 10.1126/science.1073263. [DOI] [PubMed] [Google Scholar]
- Li H, Collado M, Villasante A, Matheu A, Lynch CJ, Canamero M, Rizzoti K, Carneiro C, Martinez G, Vidal A, et al. p27(Kip1) directly represses Sox2 during embryonic stem cell differentiation. Cell Stem Cell. 2012;11:845–852. doi: 10.1016/j.stem.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F, Kar D, Gruenig N, Zhang ZW, Cousins N, Rodgers HM, Swindell EC, Jamrich M, Schuurmans C, Mathers PH, et al. Rax is a selector gene for mediobasal hypothalamic cell types. Journal of Neuroscience. 2013;33:259–272. doi: 10.1523/JNEUROSCI.0913-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luque RM, Amargo G, Ishii S, Lobe C, Franks R, Kiyokawa H, Kineman RD. Reporter expression, induced by a growth hormone promoter-driven Cre recombinase (rGHp-Cre) transgene, questions the developmental relationship between somatotropes and lactotropes in the adult mouse pituitary gland. Endocrinology. 2007;148:1946–1953. doi: 10.1210/en.2006-1542. [DOI] [PubMed] [Google Scholar]
- Manning L, Ohyama K, Saeger B, Hatano O, Wilson SA, Logan M, Placzek M. Regional morphogenesis in the hypothalamus: a BMP-Tbx2 pathway coordinates fate and proliferation through Shh downregulation. Developmental Cell. 2006;11:873–885. doi: 10.1016/j.devcel.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Massah S, Hollebakken R, Labrecque MP, Kolybaba AM, Beischlag TV, Prefontaine GG. Epigenetic characterization of the growth hormone gene identifies SmcHD1 as a regulator of autosomal gene clusters. PLoS ONE. 2014;9:e97535. doi: 10.1371/journal.pone.0097535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda K, Kondoh H. Dkk1-dependent inhibition of Wnt signaling activates Hesx1 expression through its 5' enhancer and directs forebrain precursor development. Genes to Cells. 2014;19:374–385. doi: 10.1111/gtc.12136. [DOI] [PubMed] [Google Scholar]
- Medina-Martinez O, Amaya-Manzanares F, Liu C, Mendoza M, Shah R, Zhang L, Behringer RR, Mahon KA, Jamrich M. Cell-autonomous requirement for rx function in the mammalian retina and posterior pituitary. PLoS ONE. 2009;4:e4513. doi: 10.1371/journal.pone.0004513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollard P, Hodson DJ, Lafont C, Rizzoti K, Drouin J. A tridimensional view of pituitary development and function. Trends in Endocrinology and Metabolism. 2012;23:261–269. doi: 10.1016/j.tem.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Monahan P, Rybak S, Raetzman LT. The Notch target gene HES1 regulates cell cycle inhibitor expression in the developing pituitary. Endocrinology. 2009;150:4386–4394. doi: 10.1210/en.2009-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran TB, Goldberg LB, Serviss SL, Raetzman LT. Numb deletion in POMC-expressing cells impairs pituitary intermediate lobe cell adhesion, progenitor cell localization, and neuro-intermediate lobe boundary formation. Molecular Endocrinology. 2011;25:117–127. doi: 10.1210/me.2010-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen RD, Park S, Rhodes SJ. A distal modular enhancer complex acts to control pituitary- and nervous system-specific expression of the LHX3 regulatory gene. Molecular Endocrinology. 2012;26:308–319. doi: 10.1210/me.2011-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nantie LB, Himes AD, Getz DR, Raetzman LT. Notch signaling in postnatal pituitary expansion: proliferation, progenitors, and cell specification. Molecular Endocrinology. 2014;28:731–744. doi: 10.1210/me.2013-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasonkin IO, Ward RD, Raetzman LT, Seasholtz AF, Saunders TL, Gillespie PJ, Camper SA. Pituitary hypoplasia and respiratory distress syndrome in Prop1 knockout mice. Human Molecular Genetics. 2004;13:2727–2735. doi: 10.1093/hmg/ddh311. [DOI] [PubMed] [Google Scholar]
- Ohuchi H, Hori Y, Yamasaki M, Harada H, Sekine K, Kato S, Itoh N. FGF10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochemical and Biophysical Research Communications. 2000;277:643–649. doi: 10.1006/bbrc.2000.3721. [DOI] [PubMed] [Google Scholar]
- Oliver G, Mailhos A, Wehr R, Copeland NG, Jenkins NA, Gruss P. Six3, a murine homologue of the sine oculis gene, demarcates the most anterior border of the developing neural plate and is expressed during eye development. Development. 1995;121:4045–4055. doi: 10.1242/dev.121.12.4045. [DOI] [PubMed] [Google Scholar]
- Olson LE, Tollkuhn J, Scafoglio C, Krones A, Zhang J, Ohgi KA, Wu W, Taketo MM, Kemler R, Grosschedl R, et al. Homeodomain-mediated β-catenin-dependent switching events dictate cell-lineage determination. Cell. 2006;125:593–605. doi: 10.1016/j.cell.2006.02.046. [DOI] [PubMed] [Google Scholar]
- Patthey C, Schlosser G, Shimeld SM. The evolutionary history of vertebrate cranial placodes – I: cell type evolution. Developmental Biology. 2014;389:82–97. doi: 10.1016/j.ydbio.2014.01.017. [DOI] [PubMed] [Google Scholar]
- Pearson CA, Placzek M. Development of the medial hypothalamus: forming a functional hypothalamic-neurohypophyseal interface. Current Topics in Developmental Biology. 2013;106:49–88. doi: 10.1016/B978-0-12-416021-7.00002-X. [DOI] [PubMed] [Google Scholar]
- Pearson CA, Ohyama K, Manning L, Aghamohammadzadeh S, Sang H, Placzek M. FGF-dependent midline-derived progenitor cells in hypothalamic infundibular development. Development. 2011;138:2613–2624. doi: 10.1242/dev.062794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potok MA, Cha KB, Hunt A, Brinkmeier ML, Leitges M, Kispert A, Camper SA. WNT signaling affects gene expression in the ventral diencephalon and pituitary gland growth. Developmental Dynamics. 2008;237:1006–1020. doi: 10.1002/dvdy.21511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince KL, Walvoord EC, Rhodes SJ. The role of homeodomain transcription factors in heritable pituitary disease. Nature Reviews. Endocrinology. 2011;7:727–737. doi: 10.1038/nrendo.2011.119. [DOI] [PubMed] [Google Scholar]
- Pulichino AM, Vallette-Kasic S, Couture C, Gauthier Y, Brue T, David M, Malpuech G, Deal C, Van Vliet G, De Vroede M, et al. Human and mouse TPIT gene mutations cause early onset pituitary ACTH deficiency. Genes and Development. 2003a;17:711–716. doi: 10.1101/gad.1065603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulichino AM, Vallette-Kasic S, Tsai JP, Couture C, Gauthier Y, Drouin J. Tpit determines alternate fates during pituitary cell differentiation. Genes and Development. 2003b;17:738–747. doi: 10.1101/gad.1065703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quereda V, Malumbres M. Cell cycle control of pituitary development and disease. Journal of Molecular Endocrinology. 2009;42:75–86. doi: 10.1677/JME-08-0146. [DOI] [PubMed] [Google Scholar]
- Raetzman LT, Ward R, Camper SA. Lhx4 and Prop1 are required for cell survival and expansion of the pituitary primordia. Development. 2002;129:4229–4239. doi: 10.1242/dev.129.18.4229. [DOI] [PubMed] [Google Scholar]
- Raetzman LT, Ross SA, Cook S, Dunwoodie SL, Camper SA, Thomas PQ. Developmental regulation of Notch signaling genes in the embryonic pituitary: Prop1 deficiency affects Notch2 expression. Developmental Biology. 2004;265:329–340. doi: 10.1016/j.ydbio.2003.09.033. [DOI] [PubMed] [Google Scholar]
- Raetzman LT, Cai JX, Camper SA. Hes1 is required for pituitary growth and melanotrope specification. Developmental Biology. 2007;304:455–466. doi: 10.1016/j.ydbio.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzoti K. Adult pituitary progenitors/stem cells: from in vitro characterization to in vivo function. European Journal of Neuroscience. 2010;32:2053–2062. doi: 10.1111/j.1460-9568.2010.07524.x. [DOI] [PubMed] [Google Scholar]
- Rizzoti K, Lovell-Badge R. Regenerative medicine: organ recital in a dish. Nature. 2011;480:44–46. doi: 10.1038/480044a. [DOI] [PubMed] [Google Scholar]
- Rizzoti K, Brunelli S, Carmignac D, Thomas PQ, Robinson IC, Lovell-Badge R. SOX3 is required during the formation of the hypothalamo–pituitary axis. Nature Genetics. 2004;36:247–255. doi: 10.1038/ng1309. [DOI] [PubMed] [Google Scholar]
- Rizzoti K, Akiyama H, Lovell-Badge R. Mobilized adult pituitary stem cells contribute to endocrine regeneration in response to physiological demand. Cell Stem Cell. 2013;13:419–432. doi: 10.1016/j.stem.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Jeannet JP, Moody SA. Establishing the pre-placodal region and breaking it into placodes with distinct identities. Developmental Biology. 2014;389:13–27. doi: 10.1016/j.ydbio.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajedi E, Gaston-Massuet C, Andoniadou CL, Signore M, Hurd PJ, Dattani M, Martinez-Barbera JP. DNMT1 interacts with the developmental transcriptional repressor HESX1. Biochimica et Biophysica Acta. 2008;1783:131–143. doi: 10.1016/j.bbamcr.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A, Hochedlinger K. A gutsy way to grow: intestinal stem cells as nutrient sensors. Cell. 2011;147:487–489. doi: 10.1016/j.cell.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Schang AL, Granger A, Querat B, Bleux C, Cohen-Tannoudji J, Laverriere JN. GATA2-induced silencing and LIM-homeodomain protein-induced activation are mediated by a bi-functional response element in the rat GnRH receptor gene. Molecular Endocrinology. 2013;27:74–91. doi: 10.1210/me.2012-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosser G, Patthey C, Shimeld SM. The evolutionary history of vertebrate cranial placodes II. Evolution of ectodermal patterning. Developmental Biology. 2014;389:98–119. doi: 10.1016/j.ydbio.2014.01.019. [DOI] [PubMed] [Google Scholar]
- Scott CE, Wynn SL, Sesay A, Cruz C, Cheung M, Gomez Gaviro MV, Booth S, Gao B, Cheah KS, Lovell-Badge R, et al. SOX9 induces and maintains neural stem cells. Nature Neuroscience. 2010;13:1181–1189. doi: 10.1038/nn.2646. [DOI] [PubMed] [Google Scholar]
- Sheng HZ, Moriyama K, Yamashita T, Li H, Potter SS, Mahon KA, Westphal H. Multistep control of pituitary organogenesis. Science. 1997;278:1809–1812. doi: 10.1126/science.278.5344.1809. [DOI] [PubMed] [Google Scholar]
- Simmons DM, Voss JW, Ingraham HA, Holloway JM, Broide RS, Rosenfeld MG, Swanson LW. Pituitary cell phenotypes involve cell-specific Pit-1 mRNA translation and synergistic interactions with other classes of transcription factors. Genes and Development. 1990;4:695–711. doi: 10.1101/gad.4.5.695. [DOI] [PubMed] [Google Scholar]
- Sornson MW, Wu W, Dasen JS, Flynn SE, Norman DJ, O'Connell SM, Gukovsky I, Carriere C, Ryan AK, Miller AP, et al. Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature. 1996;384:327–333. doi: 10.1038/384327a0. [DOI] [PubMed] [Google Scholar]
- Soukup V, Horacek I, Cerny R. Development and evolution of the vertebrate primary mouth. Journal of Anatomy. 2013;222:79–99. doi: 10.1111/j.1469-7580.2012.01540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga H, Kadoshima T, Minaguchi M, Ohgushi M, Soen M, Nakano T, Takata N, Wataya T, Muguruma K, Miyoshi H, et al. Self-formation of functional adenohypophysis in three-dimensional culture. Nature. 2011;480:57–62. doi: 10.1038/nature10637. [DOI] [PubMed] [Google Scholar]
- Suh H, Gage PJ, Drouin J, Camper SA. Pitx2 is required at multiple stages of pituitary organogenesis: pituitary primordium formation and cell specification. Development. 2002;129:329–337. doi: 10.1242/dev.129.2.329. [DOI] [PubMed] [Google Scholar]
- Takuma N, Sheng HZ, Furuta Y, Ward JM, Sharma K, Hogan BL, Pfaff SL, Westphal H, Kimura S, Mahon KA. Formation of Rathke's pouch requires dual induction from the diencephalon. Development. 1998;125:4835–4840. doi: 10.1242/dev.125.23.4835. [DOI] [PubMed] [Google Scholar]
- Thomas P, Beddington R. Anterior primitive endoderm may be responsible for patterning the anterior neural plate in the mouse embryo. Current Biology. 1996;6:1487–1496. doi: 10.1016/S0960-9822(96)00753-1. [DOI] [PubMed] [Google Scholar]
- Treier M, Gleiberman AS, O'Connell SM, Szeto DP, McMahon JA, McMahon AP, Rosenfeld MG. Multistep signaling requirements for pituitary organogenesis in vivo. Genes and Development. 1998;12:1691–1704. doi: 10.1101/gad.12.11.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treier M, O'Connell S, Gleiberman A, Price J, Szeto DP, Burgess R, Chuang PT, McMahon AP, Rosenfeld MG. Hedgehog signaling is required for pituitary gland development. Development. 2001;128:377–386. doi: 10.1242/dev.128.3.377. [DOI] [PubMed] [Google Scholar]
- Trowe MO, Zhao L, Weiss AC, Christoffels V, Epstein DJ, Kispert A. Inhibition of Sox2-dependent activation of Shh in the ventral diencephalon by Tbx3 is required for formation of the neurohypophysis. Development. 2013;140:2299–2309. doi: 10.1242/dev.094524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlenhaut NH, Jakob S, Anlag K, Eisenberger T, Sekido R, Kress J, Treier AC, Klugmann C, Klasen C, Holter NI, et al. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell. 2009;139:1130–1142. doi: 10.1016/j.cell.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Vankelecom H. Pituitary stem cells drop their mask. Current Stem Cell Research & Therapy. 2012;7:36–71. doi: 10.2174/157488812798483467. [DOI] [PubMed] [Google Scholar]
- Wang J, Scully K, Zhu X, Cai L, Zhang J, Prefontaine GG, Krones A, Ohgi KA, Zhu P, Garcia-Bassets I, et al. Opposing LSD1 complexes function in developmental gene activation and repression programmes. Nature. 2007;446:882–887. doi: 10.1038/nature05671. [DOI] [PubMed] [Google Scholar]
- Wang Y, Martin JF, Bai CB. Direct and indirect requirements of Shh/Gli signaling in early pituitary development. Developmental Biology. 2010;348:199–209. doi: 10.1016/j.ydbio.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RD, Raetzman LT, Suh H, Stone BM, Nasonkin IO, Camper SA. Role of PROP1 in pituitary gland growth. Molecular Endocrinology. 2005;19:698–710. doi: 10.1210/me.2004-0341. [DOI] [PubMed] [Google Scholar]
- Ward RD, Stone BM, Raetzman LT, Camper SA. Cell proliferation and vascularization in mouse models of pituitary hormone deficiency. Molecular Endocrinology. 2006;20:1378–1390. doi: 10.1210/me.2005-0409. [DOI] [PubMed] [Google Scholar]
- Wataya T, Ando S, Muguruma K, Ikeda H, Watanabe K, Eiraku M, Kawada M, Takahashi J, Hashimoto N, Sasai Y. Minimization of exogenous signals in ES cell culture induces rostral hypothalamic differentiation. PNAS. 2008;105:11796–11801. doi: 10.1073/pnas.0803078105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welcker JE, Hernandez-Miranda LR, Paul FE, Jia S, Ivanov A, Selbach M, Birchmeier C. Insm1 controls development of pituitary endocrine cells and requires a SNAG domain for function and for recruitment of histone-modifying factors. Development. 2013;140:4947–4958. doi: 10.1242/dev.097642. [DOI] [PubMed] [Google Scholar]
- Wen S, Ai W, Alim Z, Boehm U. Embryonic gonadotropin-releasing hormone signaling is necessary for maturation of the male reproductive axis. PNAS. 2010;107:16372–16377. doi: 10.1073/pnas.1000423107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Kato T, Susa T, Cai LY, Nakayama M, Kato Y. PROP1 coexists with SOX2 and induces PIT1-commitment cells. Biochemical and Biophysical Research Communications. 2009;385:11–15. doi: 10.1016/j.bbrc.2009.05.027. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Kato T, Yako H, Susa T, Cai LY, Osuna M, Inoue K, Kato Y. Significant quantitative and qualitative transition in pituitary stem/progenitor cells occurs during the postnatal development of the rat anterior pituitary. Journal of Neuroendocrinology. 2011;23:933–943. doi: 10.1111/j.1365-2826.2011.02198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Bakke M, Krimkevich Y, Cushman LJ, Parlow AF, Camper SA, Parker KL. Hypomorphic phenotype in mice with pituitary-specific knockout of steroidogenic factor 1. Genesis. 2001;30:65–69. doi: 10.1002/gene.1034. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Morales DC, Hermesz E, Lee WK, Pfaff SL, Westphal H. Reduced expression of the LIM-homeobox gene Lhx3 impairs growth and differentiation of Rathke's pouch and increases cell apoptosis during mouse pituitary development. Mechanisms of Development. 2006;123:605–613. doi: 10.1016/j.mod.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Mailloux CM, Hermesz E, Palkovits M, Westphal H. A role of the LIM-homeobox gene Lhx2 in the regulation of pituitary development. Developmental Biology. 2010;337:313–323. doi: 10.1016/j.ydbio.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Zevallos SE, Rizzoti K, Jeong Y, Lovell-Badge R, Epstein DJ. Disruption of SoxB1-dependent Sonic hedgehog expression in the hypothalamus causes septo-optic dysplasia. Developmental Cell. 2012;22:585–596. doi: 10.1016/j.devcel.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Zhang J, Tollkuhn J, Ohsawa R, Bresnick EH, Guillemot F, Kageyama R, Rosenfeld MG. Sustained Notch signaling in progenitors is required for sequential emergence of distinct cell lineages during organogenesis. Genes and Development. 2006;20:2739–2753. doi: 10.1101/gad.1444706. [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a