Abstract
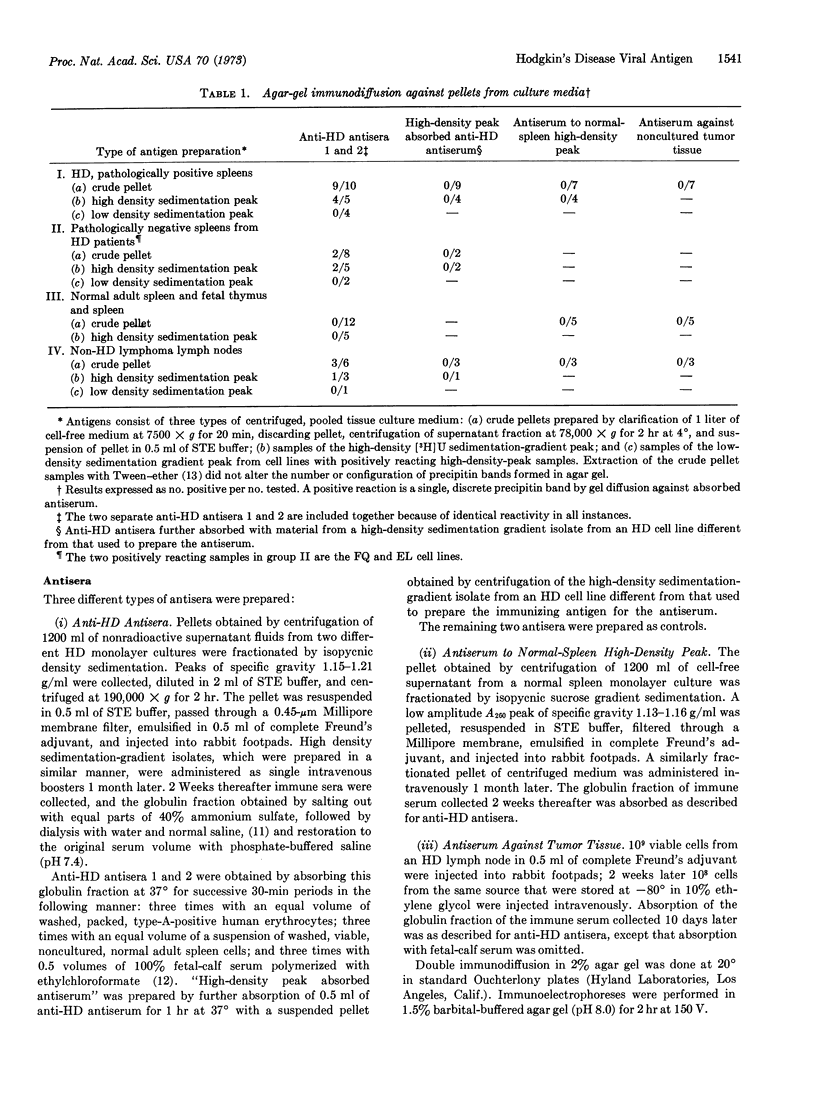
Pellets obtained from supernatant fluids of monolayer cultures of cells from patients with Hodgkin's disease were fractionated by isopycnic density sedimentation. Material in a peak of specific gravity 1.15-1.21 g/ml from two Hodgkin's disease cultures was used to immunize rabbits, and the antisera obtained in this manner were reacted by agar-gel diffusion and immunoelectrophoresis with antigens from the purified peaks and the unfractionated pellets of centrifuged culture medium from all the cultures. The antisera reacted with material from 9 of 10 lines derived from spleens of patients with Hodgkin's disease, 2 of 8 cell lines from histologically negative spleens from patients with Hodgkin's disease and with 3 of 6 lymphoma cell lines not diagnosed as Hodgkin's disease. The antisera did not react with 12 cell cultures prepared from normal adult and fetal spleen and thymus. The antigen from cultures from patients with Hodgkin's disease was not found in material sedimenting at lower specific gravities; it resisted Tweenether solubilization, and migrated as a single band by immunoelectrophoresis. The antigen was not found in disrupted, noncultured tumor cells from patients with Hodgkin's disease, and an antiserum against noncultured, minced tumor tissue did not react with the Hodgkin's disease tissue-culture material. No immunological relationship was found between the tissue culture antigen and Epstein-Barr, RD-114, or Rauscher murine leukemia viruses. The Hodgkin's disease antigen may be a tumorrelated antigen or a component of an oncogenic virus.
Keywords: pulse labeling, density-gradient sedimentation, agar-gel diffusion, immunoelectrophoresis
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander P. Foetal "antigens" in cancer. Nature. 1972 Jan 21;235(5334):137–140. doi: 10.1038/235137a0. [DOI] [PubMed] [Google Scholar]
- Allen D. W., Sarma P. S., Niall H. D., Sauer R. Isolation of a second avian leukosis group-specific antigen (gs-b) from avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1970 Oct;67(2):837–842. doi: 10.1073/pnas.67.2.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avrameas S., Ternynck T. Biologically active water-insoluble protein polymers. I. Their use for isolation of antigens and antibodies. J Biol Chem. 1967 Apr 10;242(7):1651–1659. [PubMed] [Google Scholar]
- Bankole R. O., Bates H. A., Swaim W. R., Amatuzio D. S. Cytoplasmic immunofluorescence of blood cells from myeloma, Hodgkin's disease an cultured cells from normal liver. Br J Cancer. 1972 Feb;26(1):10–14. doi: 10.1038/bjc.1972.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger M. M. A difference in the architecture of the surface membrane of normal and virally transformed cells. Proc Natl Acad Sci U S A. 1969 Mar;62(3):994–1001. doi: 10.1073/pnas.62.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano L. W., Jr, Goldman J. M. Antibody to Herpesvirus hominis types 1 and 2 in patients with Hodgkin's disease and carcinoma of the nasopharynx. Cancer. 1972 Mar;29(3):597–602. doi: 10.1002/1097-0142(197203)29:3<597::aid-cncr2820290311>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- ECKERT E. A., ROTT R., SCHAEFER W. STUDIES ON THE BAI STRAIN A (AVIAN MYELOBLASTOSIS) VIRUS. I. PRODUCTION AND EXAMINATION OF POTENT VIRUS-SPECIFIC COMPLEMENT-FIXING ANTISERA. Virology. 1964 Nov;24:426–433. doi: 10.1016/0042-6822(64)90180-1. [DOI] [PubMed] [Google Scholar]
- Eisinger M., Fox S. M., De Harven E., Biedler J. L., Sanders F. K. Virus-like agents from patients with Hodgkin's disease. Nature. 1971 Sep 10;233(5315):104–108. doi: 10.1038/233104a0. [DOI] [PubMed] [Google Scholar]
- Gilden R. V., Oroszlan S. Group-specific antigens of RNA tumor viruses as markers for subinfectious expression of the RNA virus genome. Proc Natl Acad Sci U S A. 1972 Apr;69(4):1021–1025. doi: 10.1073/pnas.69.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hehlmann R., Kufe D., Spiegelman S. Viral-related RNA in Hodgkins' disease and other human lymphomas. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1727–1731. doi: 10.1073/pnas.69.7.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson B., Klein G., Henle W., Henle G. Epstein-Barr virus (EBV)-associated antibody patterns in malignant lymphoma and leukemia. I. Hodgkin's disease. Int J Cancer. 1970 Nov 15;6(3):450–462. doi: 10.1002/ijc.2910060316. [DOI] [PubMed] [Google Scholar]
- Katz D. H., Order S. E., Graves M., Benacerraf B. Purification of Hodgkin's disease tumor-associated antigens. Proc Natl Acad Sci U S A. 1973 Feb;70(2):396–400. doi: 10.1073/pnas.70.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'CONNOR T. E., RAUSCHER F. J., ZEIGEL R. F. DENSITY GRADIENT CENTRIFUGATION OF A MURINE LEUKEMIA VIRUS. Science. 1964 May 29;144(3622):1144–1147. doi: 10.1126/science.144.3622.1144. [DOI] [PubMed] [Google Scholar]
- Order S. E., Chism S. E., Hellman S. Studies of antigens associated with Hodgkin's disease. Blood. 1972 Nov;40(5):621–633. [PubMed] [Google Scholar]
- Order S. E., Porter M., Hellman S. Hodgkin's disease: evidence for a tumor-associated antigen. N Engl J Med. 1971 Aug 26;285(9):471–474. doi: 10.1056/NEJM197108262850901. [DOI] [PubMed] [Google Scholar]
- Oroszlan S., Bova D., Martin White M. H., Toni R., Foreman C., Gilden R. V. Purification and immunological characterization of the major internal protein of the RD-114 virus. Proc Natl Acad Sci U S A. 1972 May;69(5):1211–1215. doi: 10.1073/pnas.69.5.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oroszlan S., Bova D., Toni R., Gilden R. V. Interactions of immunoglobulins G and M in the detection of the mammalian C-type virus cross-reactive antigen. Science. 1972 Apr 28;176(4033):420–422. doi: 10.1126/science.176.4033.420. [DOI] [PubMed] [Google Scholar]
- Oroszlan S., Fisher C. L., Stanley T. B., Gilden R. V. Proteins of the murine C-type RNA tumour viruses: isolation of a group-specific antigen by isoelectric focusing. J Gen Virol. 1970 Jul;8(1):1–10. doi: 10.1099/0022-1317-8-1-1. [DOI] [PubMed] [Google Scholar]
- Pellegrino M. A., Ferrone S., Mittal K. K., Pellegrino A., Reisfeld R. A. A quantitative study of cross reactivity in the HL-A system with human cultured lymphoid cells and soluble HL-A antigens. Transplantation. 1973 Jan;15(1):42–47. doi: 10.1097/00007890-197301000-00007. [DOI] [PubMed] [Google Scholar]
- Reisfeld R. A., Pellegrino M., Papermaster B. W., Kahan B. D. HL-A antigens from a continuous lymphoid cell line derived from a normal donor. I. Solubilization and serologic characterization. J Immunol. 1970 Mar;104(3):560–565. [PubMed] [Google Scholar]
- Robinson H. L. Isolation of noninfectious particles containing Rous sarcoma virus RNA from the medium of Rous sarcoma virus-transformed nonproducer cells. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1655–1662. doi: 10.1073/pnas.57.6.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart S. E., Mitchell E. Z., Whang J. J., Dunlop W. R., Ben T., Nomura S. Viruses in human tumors. I. Hodgkin's disease. J Natl Cancer Inst. 1969 Jul;43(1):1–14. [PubMed] [Google Scholar]
- Todaro G. J., Huebner R. J. N.A.S. symposium: new evidence as the basis for increased efforts in cancer research. Proc Natl Acad Sci U S A. 1972 Apr;69(4):1009–1015. doi: 10.1073/pnas.69.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Zeve V., Aaronson S. A. Viruses in cell culture derived from human tumour patients. Nature. 1970 Jun 13;226(5250):1047–1049. doi: 10.1038/2261047a0. [DOI] [PubMed] [Google Scholar]
- Vianna N. J., Greenwald P., Brady J., Polan A. K., Dwork A., Mauro J., Davies J. N. Hodgkin's disease: cases with features of a community outbreak. Ann Intern Med. 1972 Aug;77(2):169–180. doi: 10.7326/0003-4819-77-2-169. [DOI] [PubMed] [Google Scholar]
- Wakefield J. D., Thorbecke G. J., Old L. J., Boyse E. A. Production of immunoglobulins and their subunits by human tissue culture cell lines. J Immunol. 1967 Aug;99(2):308–319. [PubMed] [Google Scholar]




