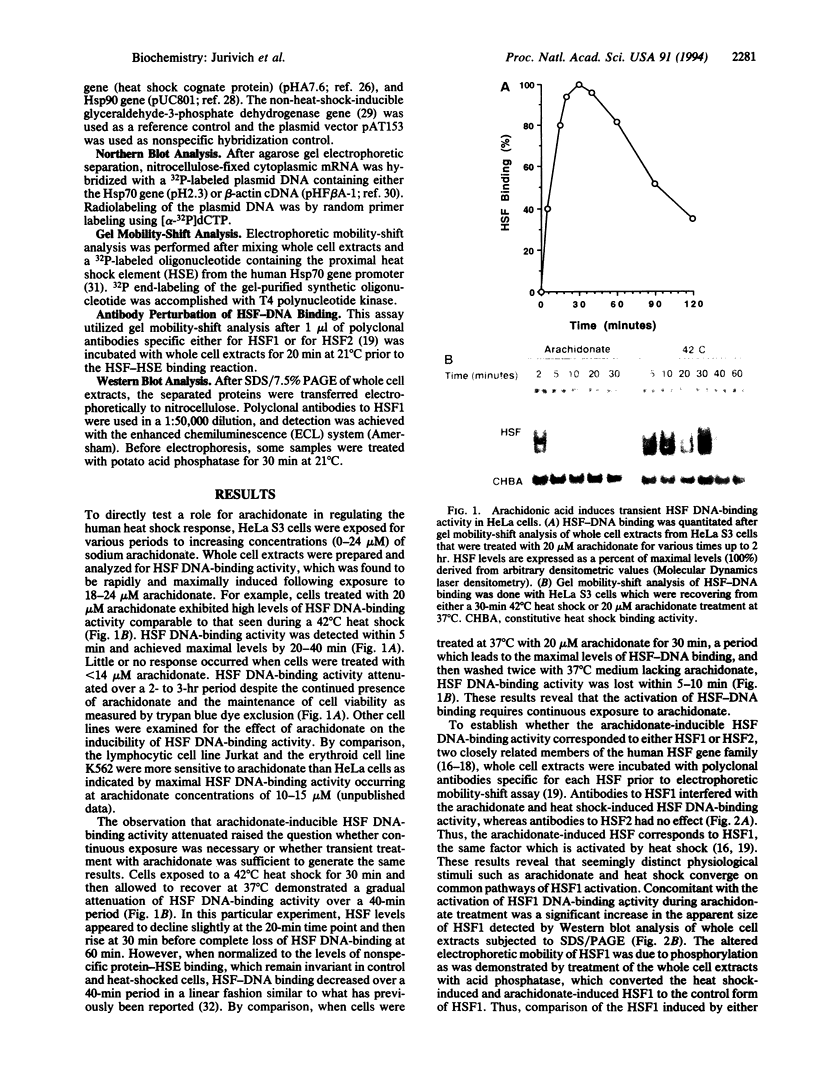
Abstract
Cell and tissue injury activate the inflammatory response through the action(s) of arachidonic acid and its metabolites, leading to the expression of acute-phase proteins and inflammatory cytokines. At the molecular level, little is known how arachidonic acid regulates the inflammatory response. As inflammation is also associated with local increase in tissue temperatures, we examined whether arachidonic acid was directly involved in the heat shock response. Extracellular exposure to arachidonic acid induced heat shock gene transcription in a dose-dependent manner via acquisition of DNA-binding activity and phosphorylation of heat shock factor 1 (HSF1). In addition, exposure of cells to low concentrations of arachidonic acid, which by themselves did not induce HSF1 DNA-binding activity, reduced the temperature threshold for HSF1 activation from elevated temperatures which are not physiologically relevant (> 42 degrees C) to temperatures which can be attained during the febrile response (39-40 degrees C). These results indicate that elevated heat shock gene expression is a direct consequence of an arachidonic acid-mediated cellular response.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abravaya K., Myers M. P., Murphy S. P., Morimoto R. I. The human heat shock protein hsp70 interacts with HSF, the transcription factor that regulates heat shock gene expression. Genes Dev. 1992 Jul;6(7):1153–1164. doi: 10.1101/gad.6.7.1153. [DOI] [PubMed] [Google Scholar]
- Abravaya K., Phillips B., Morimoto R. I. Attenuation of the heat shock response in HeLa cells is mediated by the release of bound heat shock transcription factor and is modulated by changes in growth and in heat shock temperatures. Genes Dev. 1991 Nov;5(11):2117–2127. doi: 10.1101/gad.5.11.2117. [DOI] [PubMed] [Google Scholar]
- Aldashev A. A., Agibetov K. A., Yugai A. A., Shamshiev A. T. Specific proteins synthesized in human lymphocytes during hypoxia. Am J Physiol. 1991 Oct;261(4 Suppl):92–96. doi: 10.1152/ajpheart.1991.261.4.92. [DOI] [PubMed] [Google Scholar]
- Amici C., Sistonen L., Santoro M. G., Morimoto R. I. Antiproliferative prostaglandins activate heat shock transcription factor. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6227–6231. doi: 10.1073/pnas.89.14.6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananthan J., Goldberg A. L., Voellmy R. Abnormal proteins serve as eukaryotic stress signals and trigger the activation of heat shock genes. Science. 1986 Apr 25;232(4749):522–524. doi: 10.1126/science.3083508. [DOI] [PubMed] [Google Scholar]
- Angelidis C. E., Lazaridis I., Pagoulatos G. N. Constitutive expression of heat-shock protein 70 in mammalian cells confers thermoresistance. Eur J Biochem. 1991 Jul 1;199(1):35–39. doi: 10.1111/j.1432-1033.1991.tb16088.x. [DOI] [PubMed] [Google Scholar]
- Baler R., Dahl G., Voellmy R. Activation of human heat shock genes is accompanied by oligomerization, modification, and rapid translocation of heat shock transcription factor HSF1. Mol Cell Biol. 1993 Apr;13(4):2486–2496. doi: 10.1128/mcb.13.4.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji S. S., Theodorakis N. G., Morimoto R. I. Heat shock-induced translational control of HSP70 and globin synthesis in chicken reticulocytes. Mol Cell Biol. 1984 Nov;4(11):2437–2448. doi: 10.1128/mcb.4.11.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M. J., Gershon D., Fargnoli J., Holbrook N. J. Discordant expression of heat shock protein mRNAs in tissues of heat-stressed rats. J Biol Chem. 1990 Sep 5;265(25):15275–15279. [PubMed] [Google Scholar]
- Chan P. H., Chen S. F., Yu A. C. Induction of intracellular superoxide radical formation by arachidonic acid and by polyunsaturated fatty acids in primary astrocytic cultures. J Neurochem. 1988 Apr;50(4):1185–1193. doi: 10.1111/j.1471-4159.1988.tb10591.x. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Leidal K. G., Pearson D. W., Nauseef W. M. NADPH oxidase of human neutrophils. Subcellular localization and characterization of an arachidonate-activatable superoxide-generating system. J Biol Chem. 1987 Mar 25;262(9):4065–4074. [PubMed] [Google Scholar]
- Clerget M., Polla B. S. Erythrophagocytosis induces heat shock protein synthesis by human monocytes-macrophages. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1081–1085. doi: 10.1073/pnas.87.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E. A. Chaperones: helpers along the pathways to protein folding. Science. 1993 Jun 25;260(5116):1902–1903. doi: 10.1126/science.8100364. [DOI] [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidon P. T., Jr, Hightower L. E. Purification and initial characterization of the 71-kilodalton rat heat-shock protein and its cognate as fatty acid binding proteins. Biochemistry. 1986 Jun 3;25(11):3231–3239. doi: 10.1021/bi00359a023. [DOI] [PubMed] [Google Scholar]
- Guidon P. T., Jr, Hightower L. E. The 73 kilodalton heat shock cognate protein purified from rat brain contains nonesterified palmitic and stearic acids. J Cell Physiol. 1986 Aug;128(2):239–245. doi: 10.1002/jcp.1041280215. [DOI] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J. W., McCormick F., Macara I. G. Regulation of Ras-GAP and the neurofibromatosis-1 gene product by eicosanoids. Science. 1991 Apr 26;252(5005):576–579. doi: 10.1126/science.1902323. [DOI] [PubMed] [Google Scholar]
- Hannigan G. E., Williams B. R. Signal transduction by interferon-alpha through arachidonic acid metabolism. Science. 1991 Jan 11;251(4990):204–207. doi: 10.1126/science.1898993. [DOI] [PubMed] [Google Scholar]
- Hickey E., Brandon S. E., Smale G., Lloyd D., Weber L. A. Sequence and regulation of a gene encoding a human 89-kilodalton heat shock protein. Mol Cell Biol. 1989 Jun;9(6):2615–2626. doi: 10.1128/mcb.9.6.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hightower L. E. Cultured animal cells exposed to amino acid analogues or puromycin rapidly synthesize several polypeptides. J Cell Physiol. 1980 Mar;102(3):407–427. doi: 10.1002/jcp.1041020315. [DOI] [PubMed] [Google Scholar]
- Hightower L. E. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell. 1991 Jul 26;66(2):191–197. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- Holbrook N. J., Carlson S. G., Choi A. M., Fargnoli J. Induction of HSP70 gene expression by the antiproliferative prostaglandin PGA2: a growth-dependent response mediated by activation of heat shock transcription factor. Mol Cell Biol. 1992 Apr;12(4):1528–1534. doi: 10.1128/mcb.12.4.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurivich D. A., Sistonen L., Kroes R. A., Morimoto R. I. Effect of sodium salicylate on the human heat shock response. Science. 1992 Mar 6;255(5049):1243–1245. doi: 10.1126/science.1546322. [DOI] [PubMed] [Google Scholar]
- Jättelä M., Wissing D., Bauer P. A., Li G. C. Major heat shock protein hsp70 protects tumor cells from tumor necrosis factor cytotoxicity. EMBO J. 1992 Oct;11(10):3507–3512. doi: 10.1002/j.1460-2075.1992.tb05433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Schoel B., van Embden J. D., Koga T., Wand-Württenberger A., Munk M. E., Steinhoff U. Heat-shock protein 60: implications for pathogenesis of and protection against bacterial infections. Immunol Rev. 1991 Jun;121:67–90. doi: 10.1111/j.1600-065x.1991.tb00823.x. [DOI] [PubMed] [Google Scholar]
- Koide H., Ogita K., Kikkawa U., Nishizuka Y. Isolation and characterization of the epsilon subspecies of protein kinase C from rat brain. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1149–1153. doi: 10.1073/pnas.89.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo T., Towle C. A., Mankin H. J., Treadwell B. V. Stress-induced proteins in chondrocytes from patients with osteoarthritis. Arthritis Rheum. 1985 Oct;28(10):1140–1145. doi: 10.1002/art.1780281010. [DOI] [PubMed] [Google Scholar]
- Landry J., Chrétien P., Laszlo A., Lambert H. Phosphorylation of HSP27 during development and decay of thermotolerance in Chinese hamster cells. J Cell Physiol. 1991 Apr;147(1):93–101. doi: 10.1002/jcp.1041470113. [DOI] [PubMed] [Google Scholar]
- Lapetina E. G., Cuatrecasas P. Rapid inactivation of cyclooxygenase activity after stimulation of intact platelets. Proc Natl Acad Sci U S A. 1979 Jan;76(1):121–125. doi: 10.1073/pnas.76.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson J. S., Schuetz T. J., Kingston R. E. Activation in vitro of sequence-specific DNA binding by a human regulatory factor. Nature. 1988 Sep 22;335(6188):372–375. doi: 10.1038/335372a0. [DOI] [PubMed] [Google Scholar]
- Li G. C., Li L., Liu R. Y., Rehman M., Lee W. M. Heat shock protein hsp70 protects cells from thermal stress even after deletion of its ATP-binding domain. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2036–2040. doi: 10.1073/pnas.89.6.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Lis J., Wu C. Protein traffic on the heat shock promoter: parking, stalling, and trucking along. Cell. 1993 Jul 16;74(1):1–4. doi: 10.1016/0092-8674(93)90286-y. [DOI] [PubMed] [Google Scholar]
- Martin J., Horwich A. L., Hartl F. U. Prevention of protein denaturation under heat stress by the chaperonin Hsp60. Science. 1992 Nov 6;258(5084):995–998. doi: 10.1126/science.1359644. [DOI] [PubMed] [Google Scholar]
- Morimoto R. I. Cells in stress: transcriptional activation of heat shock genes. Science. 1993 Mar 5;259(5100):1409–1410. doi: 10.1126/science.8451637. [DOI] [PubMed] [Google Scholar]
- Morimoto R. I., Sarge K. D., Abravaya K. Transcriptional regulation of heat shock genes. A paradigm for inducible genomic responses. J Biol Chem. 1992 Nov 5;267(31):21987–21990. [PubMed] [Google Scholar]
- Mosser D. D., Duchaine J., Massie B. The DNA-binding activity of the human heat shock transcription factor is regulated in vivo by hsp70. Mol Cell Biol. 1993 Sep;13(9):5427–5438. doi: 10.1128/mcb.13.9.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser D. D., Martin L. H. Induced thermotolerance to apoptosis in a human T lymphocyte cell line. J Cell Physiol. 1992 Jun;151(3):561–570. doi: 10.1002/jcp.1041510316. [DOI] [PubMed] [Google Scholar]
- Mosser D. D., Theodorakis N. G., Morimoto R. I. Coordinate changes in heat shock element-binding activity and HSP70 gene transcription rates in human cells. Mol Cell Biol. 1988 Nov;8(11):4736–4744. doi: 10.1128/mcb.8.11.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno K., Fukushima M., Fujiwara M., Narumiya S. Induction of 68,000-dalton heat shock proteins by cyclopentenone prostaglandins. Its association with prostaglandin-induced G1 block in cell cycle progression. J Biol Chem. 1988 Dec 25;263(36):19764–19770. [PubMed] [Google Scholar]
- Pelham H. R. Heat shock and the sorting of luminal ER proteins. EMBO J. 1989 Nov;8(11):3171–3176. doi: 10.1002/j.1460-2075.1989.tb08475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell. 1986 Sep 26;46(7):959–961. doi: 10.1016/0092-8674(86)90693-8. [DOI] [PubMed] [Google Scholar]
- Perisic O., Xiao H., Lis J. T. Stable binding of Drosophila heat shock factor to head-to-head and tail-to-tail repeats of a conserved 5 bp recognition unit. Cell. 1989 Dec 1;59(5):797–806. doi: 10.1016/0092-8674(89)90603-x. [DOI] [PubMed] [Google Scholar]
- Rabindran S. K., Giorgi G., Clos J., Wu C. Molecular cloning and expression of a human heat shock factor, HSF1. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6906–6910. doi: 10.1073/pnas.88.16.6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabindran S. K., Haroun R. I., Clos J., Wisniewski J., Wu C. Regulation of heat shock factor trimer formation: role of a conserved leucine zipper. Science. 1993 Jan 8;259(5092):230–234. doi: 10.1126/science.8421783. [DOI] [PubMed] [Google Scholar]
- Rotman E. I., Brostrom M. A., Brostrom C. O. Inhibition of protein synthesis in intact mammalian cells by arachidonic acid. Biochem J. 1992 Mar 1;282(Pt 2):487–494. doi: 10.1042/bj2820487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Y., Taulien J., Borkovich K. A., Lindquist S. Hsp104 is required for tolerance to many forms of stress. EMBO J. 1992 Jun;11(6):2357–2364. doi: 10.1002/j.1460-2075.1992.tb05295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro M. G., Garaci E., Amici C. Prostaglandins with antiproliferative activity induce the synthesis of a heat shock protein in human cells. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8407–8411. doi: 10.1073/pnas.86.21.8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarge K. D., Murphy S. P., Morimoto R. I. Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA-binding activity, and nuclear localization and can occur in the absence of stress. Mol Cell Biol. 1993 Mar;13(3):1392–1407. doi: 10.1128/mcb.13.3.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarge K. D., Zimarino V., Holm K., Wu C., Morimoto R. I. Cloning and characterization of two mouse heat shock factors with distinct inducible and constitutive DNA-binding ability. Genes Dev. 1991 Oct;5(10):1902–1911. doi: 10.1101/gad.5.10.1902. [DOI] [PubMed] [Google Scholar]
- Schuetz T. J., Gallo G. J., Sheldon L., Tempst P., Kingston R. E. Isolation of a cDNA for HSF2: evidence for two heat shock factor genes in humans. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6911–6915. doi: 10.1073/pnas.88.16.6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger P. K., Nelson H. C. Trimerization of a yeast transcriptional activator via a coiled-coil motif. Cell. 1989 Dec 1;59(5):807–813. doi: 10.1016/0092-8674(89)90604-1. [DOI] [PubMed] [Google Scholar]
- Sorger P. K., Pelham H. R. Yeast heat shock factor is an essential DNA-binding protein that exhibits temperature-dependent phosphorylation. Cell. 1988 Sep 9;54(6):855–864. doi: 10.1016/s0092-8674(88)91219-6. [DOI] [PubMed] [Google Scholar]
- Westwood J. T., Clos J., Wu C. Stress-induced oligomerization and chromosomal relocalization of heat-shock factor. Nature. 1991 Oct 31;353(6347):822–827. doi: 10.1038/353822a0. [DOI] [PubMed] [Google Scholar]
- Wu B., Hunt C., Morimoto R. Structure and expression of the human gene encoding major heat shock protein HSP70. Mol Cell Biol. 1985 Feb;5(2):330–341. doi: 10.1128/mcb.5.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman L. H., Levine R. A., Farber H. W. Hypoxia induces a specific set of stress proteins in cultured endothelial cells. J Clin Invest. 1991 Mar;87(3):908–914. doi: 10.1172/JCI115097. [DOI] [PMC free article] [PubMed] [Google Scholar]






