Summary
Background
Weanimix is an important food for children in Ghana. Mothers are trained to prepare homemade weanimix from beans, groundnuts and maize for their infants. Groundnuts and maize are prone to aflatoxin contamination while fumonisin contaminates maize. Aflatoxin, is produced by the Asperguillus fungi while fumonisin, is produced by Fusarium fungi. These mycotoxins occur in tropical areas worldwide due to favorable climate for their growth.
Objective
The objective of the study was to determine the levels of aflatoxin and fumonisin in homemade weanimix in the Ejura-Sekyedumase district in the Ashanti Region of Ghana.
Methods
Thirty six homemade weanimix samples (50g each) were collected from households. Aflatoxin and fumonisin were measured using a fluorometric procedure described by the Association of Official Analytical Chemist (AOAC official method 993.31, V1 series 4).
Results
Aflatoxin and fumonisin were detected in all 36 samples, range 7.9–500ppb. Fumonisin levels range: 0.74–11.0ppm). Thirty (83.3%) of the thirty six samples were over the action limit of 20ppb for aflatoxin with an overall mean of 145.2 ppb whiles 58.3% of the samples had fumonisins above the action limit of 4 ppm with an overall mean of 4.7 ppm.
Conclusion
There were significant aflatoxin and fumonisin contamination of homemade weanimix. Children fed on this nutritional food were being exposed to unacceptable levels of aflatoxin and fumonisin. Therefore there is a critical need to educate mothers on the dangers of mycotoxin exposure and to develop strategies to eliminate exposure of children fed homemade weanimix to aflatoxin and fumonisin.
Keywords: Aflatoxin, Fumonisin, Home-made Weanimix, infants
Introduction
Human exposures to mycotoxins have raised worldwide concerns due to their negative effect on health. In sub-Sahara Africa, aflatoxins and fumonisins contamination of food have been associated with increased incidence of hepatocellular carcinoma in the presence of hepatitis B virus (HBV) infection1 and esophageal cancer respectively.2 Aflatoxins B1 (AFB1) being the most potent are produced on various food crops including maize and groundnuts by Aspergillus flavus and Aspergillus parasiticus. Fumonisins B1 (FB1) are produced primarily in maize by Fusarium verticulliode and F. monoliforme.3
Environmental conditions typical of tropical countries favour the formation of both of these mycotoxins in food crops.Acute exposure to AFB1 in excess of 2ppm is known to cause nonspecific liver damage, maliase and death in few days. 4,5 Chronic AFB1 exposure via consumption of contaminated foods (20ppb or more) is associated with immune suppression 6 and nutritional deficiencies. 7 FB1 also suppresses the immune system, causes deficiency in nutrients such as folic acid 8 and the modification of sphingomyelin metabolism .9
AFB1 exposure in children increases significantly following weaning and stunting in children.10 Studies have shown that, saliva IgA (sIgA) was markedly lower in children with detectable AFB1 level of 50.4µg AFlysine/mg protein compared with those with non-detectable levels.7
These observations indicate that exposure to AFB1 and FB1 could negatively affect the Expanded Programme of Immunization (EPI), recovery from illness and development with consequences for achieving Primary Health Care goals in tropical countries, Ghana included.
Ghanaian foods prepared to eat11 or unprepared obtained from both urban and rural areas and sold in public markets 12 were shown to be highly contaminated with aflatoxins with levels ranging from (5.7–22,168 ppb) in groundnuts and groundnuts products. Co-exposure to AFB1 and FB1 has been demonstrated in persons living at Ejura-Sekyedumase district in the Ashanti region, Ghana. 13,14 In an effort to increase nutritional status and child growth, mothers of the children are taught to prepare a nutritional food, “weanimix” which is designed for children who are newly weaned from breastfeeding (approximately 6 months-2 years in age).
It is worth noting that maize and groundnuts, usually contaminated with AFB1 and FB1, are important food crops for homemade weanimix in Ghana. However data is not readily available on the safety, with respect to mycotoxins, of homemade weanimix fed to children in selected communities in Ghana. In this study, we seek to find out the extent of dietary intake of AFB1 and FB1 in homemade weanimix fed to children in selected communities in Ghana.
Materials and Methods
The study site and population
The study was carried out at Ejura-Sekyedumase district in the Ashanti Region of Ghana and located in the transition zone between the Northern and Southern zones of the country. Previous work in this district showed high exposure rates of aflatoxins with 100% of people (n=180) screened testing positive for the AFB1-albumin biomarker of exposure and 75% testing positive for urinary biomarkers of exposure. 15 Thirty-six mothers who were trained by the Nutrition Department of the Ejura-Sekyedumase District hospital on weanimix preparation were recruited and consented for their participation in the study. Study participants were from the following three communities in the district, Hiawoawu, Dromankuma and Ejura town.
Materials
Vicam AflaTest and FumoniTest kits were used according to the Association of Official Analytical Chemists' method (AOAC) for aflatoxin and fumonisin analysis in weanimix, respectively (AOAC official method 993.31, V1 series 4). Sodium chloride (NaCl) and HPLC grade methanol were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO). AflaTest and FumoniTest immunoaffinity columns were purchased from VICAM (Watertown, MA, USA). All other chemicals and reagents used were of highest purity available and commercially purchased.
Homemade weanimix preparation
The mothers prepared weanimix by mixing groundnuts, beans and maize, in the ratio 0.5:0.5:4.0 respectively. The food items were purchased on the open market at Ejura. The mixture was roasted for about 20 minutes and milled to obtain the homemade weanimix powder. The latter was used to prepare porridge for feeding of children.
A 100g sample of the homemade weanimix powder was collected from each of the 36 mothers participating in the study and kept in sterile zip-locked bags at room temperature. The samples were labeled with individual identity codes and transported at room temperature to the Noguchi Memorial Institute for Medical Research in a sealed sterile transport box for analysis of aflatoxins and fumonisins.
Sample processing and analysis
Weanimix Aflatoxin Analysis (Aflatest)
Briefly, 25g of the weanimix powder was blended with 5g NaCl and 125ml of the extraction solution (70% methanol) in a covered blender jar at high speed for 2 minutes. The extract was then filtered twice; first through fluted filter paper and then with a glass micro-fiber filter (90mm, 1.0µm). The eluent was collected in a clean beaker. Deionized water (30 ml) was added to 15 ml of the eluent and mixed thoroughly.
The resulting diluent was then filtered again through a glass microfiber filtered. Fifteen milliliters (15 ml) of the filtered eluent was passed through Aflatest column at the rate of 1–2 drops/second. The column was washed twice with 10 ml deionized water at the rate of 1–2 drops/second. This was followed by elution with 1.0 ml HPLC grade methanol at the rate of 1–2 drops/second and collected in a glass cuvette. Aflatest developer (VICAM) was prepared daily. One milliliter (1ml) of the developer was mixed thoroughly with the eluent and the concentrations of aflatoxins were measured at a fluorescence detection of 425 nm using a calibrated fluorometer (VICAM, series 4, detection limit was 2 ppb).
Fumonisin analysis
The procedure for fumonisin determination was similar for the aflatoxin but with minor changes. Briefly, 50g of milled sample was blended with 5g NaCl and 100ml of the extraction solution (80% methanol) in a covered blender jar at high speed for 1 minute. The extract was then filtered twice, diluted 4-fold with 0.1% Tween-20 and refiltered and was passed through Fumonitest column. This was eluted with 1.0 ml HPLC grade methanol and mixed with the fumonitest developer.
After 4 minutes the concentration of total fumonisin was measured at a fluorescence detection of 483nm with detection limit of 0.2 ppm (2mg/kg).
Statistical analysis
Data from the three communities were analyzed with JMP version 9 (SAS Institute Inc. Cary, NC USA). The data was subjected to simple descriptive statistics, which looked at the range, the mean and the variance.
Results
A total of 36 weanimix samples were collected from 3 different communities in the Ejura-Sekyedumase district (12 samples from each community) and analyzed for total aflatoxins and fumonisins. Of those samples all (100%) were positive for aflatoxin with 83.34% of the samples exceeding a concentration of 20 ppb; the action limit set forth by the U.S. Food and Drug Administration (U.S. FDA). Fumonisin was also present in all weanimix samples collected with 58.3% over the U.S. FDA limit of 4 ppm.
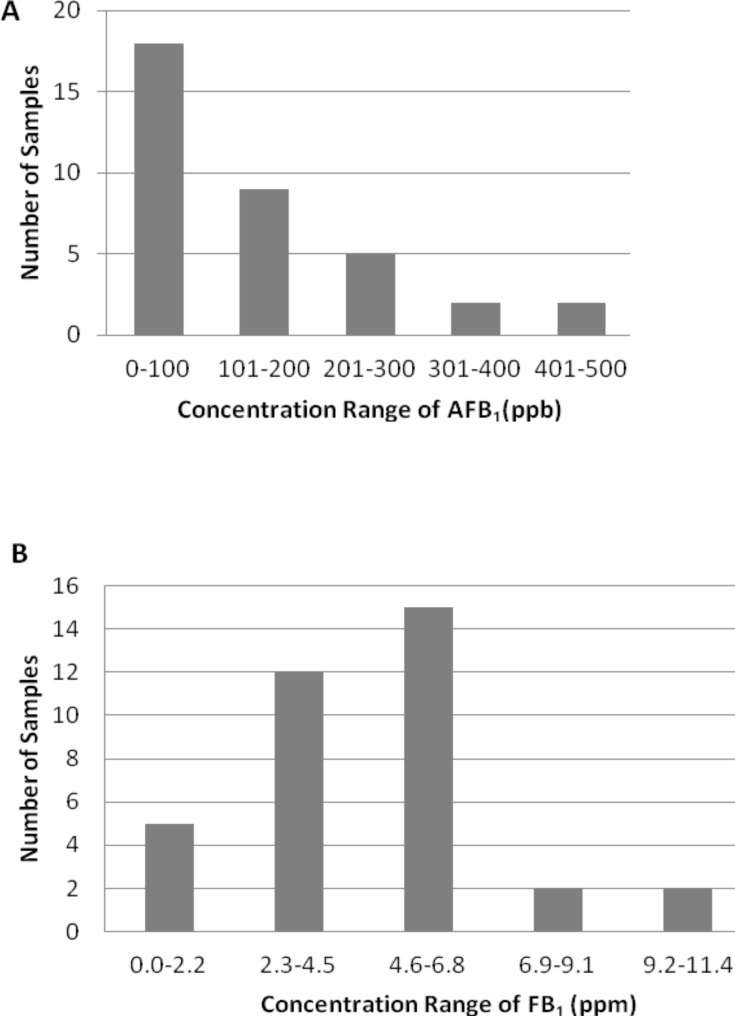
The range of aflatoxin concentrations from the three communities was 7.9–500 ppb with 9 samples over 200 ppb (Figure 1a) and fumonisins ranged from 0.75–11.0 ppm (Figure 1b).
Figure 1.
Range and Concentration of Aflatoxin and Fumonisin in Weanimix. (A) Aflatoxin (AFB1) concentration. (B) Fumonisin (FB1) concentrations.
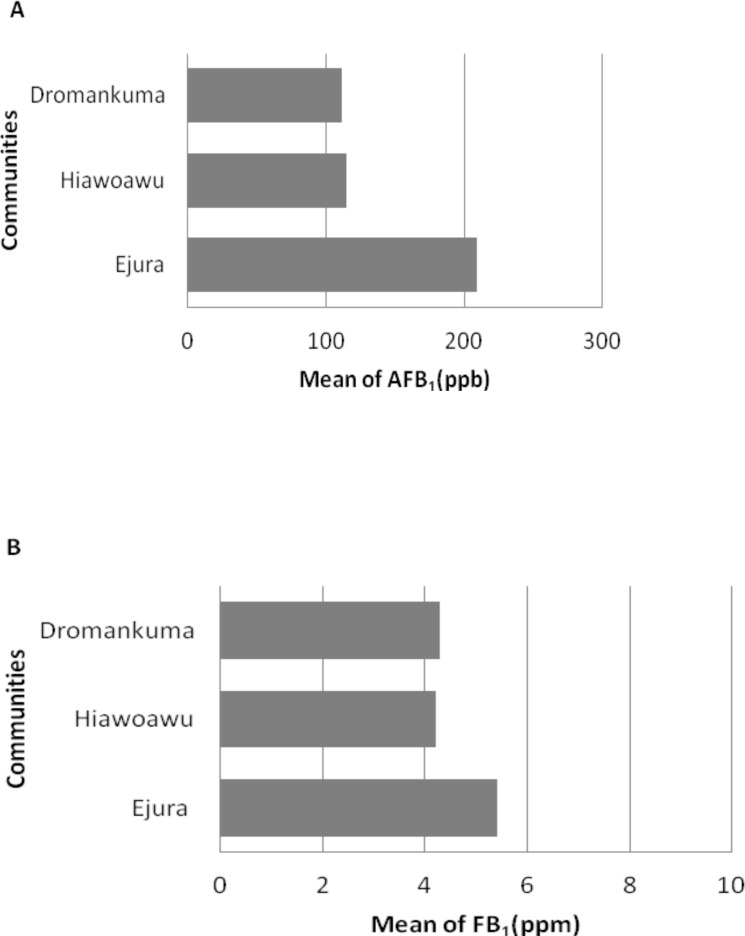
Aflatoxin concentrations in weanimix varied from community to community with Ejura town having the highest with mean value of 209.4 ppb and a range of 23–470 ppb, Hiawoawu had a range of 7.9–380 ppb with a mean concentration of 115.2 ppb and Dromankuma had a range of 14–500ppb with a mean concentration of 111.1 ppb (Figure 2a). The mean aflatoxin level in the homemade food for children at Dromankuma (111.1ppb) was significantly (p< 0.05) lower when compared with the level (209.4ppb) obtained at Ejura town. The data on fumonisin showed no significant (p> 0.05) difference in the fumonisin concentrations between the three communities (Figure 2b). The fumonisin values ranged from 0.74–11.0 ppm with a mean of 5.4 ppm in Ejura town, 1.9–6.8 ppm with a mean of 4.2 ppm in Hiawoawu and Dromankuma had a range of 1.5–11.0 ppm with a mean of 4.3 ppm.
Figure 2.
Mean of aflatoxin concentrations in Weanimix. (A) Aflatoxin (AFB1) concentration. (B) Fumonisin (FB1) concentrations.
Discussion
Weaning is a transition period of a child from breast milk to other sources of food which often results in a marked decrease in nutrient intake in developing countries.16 One possible variable contributing to poor child health is the increase in exposure to mycotoxin contaminated foods following weaning. The present studies have shown that, homemade weanimix meant to improve child nutrition is significantly contaminated with the mycotoxins, aflatoxin and fumonisin.
Aflatoxin exposure early in life has been associated with impaired growth, particularly stunting.17 This early exposure is a potential risk for synergistic interactions with other toxins as subjects grow in later years.18,9 Other studies elsewhere in Africa have shown high levels of aflatoxin contamination in staple foods. In a study 55% of maize products in Kenya had aflatoxin levels greater than the action limit of 20ppb, 35% of the products have levels exceeding 100µg/kg and 7% above 1000µg/kg as reported.20 A survey in 38 cities and towns in Benin, Togo and Ghana, showed AFB1 contamination of maize grains from market stores in the range of 24–117.5µg/kg, 0.7–108.8µg/kg, and 0.4–490.7µg/kg respectively.21 In the present study aflatoxin levels in homemade weanimix ranged from 7.9 –500ppb (ug/kg). The data obtained showed that two out of the 36 samples (from different communities) have levels of 460 and 500ppb (ug/kg).
Fumonisins have been found in maize samples worldwide, with levels of FB1 reaching > 10 ppm in the United States and > 100ppm in parts of Africa.22 Studies in Cote d'Ivoire23 and Ghana24 have shown cooccurrence of aflatoxins and fumonisins in maize-based foodstuffs. The present study shows that, 83.3% of homemade weanimix contaminated with aflatoxin was above the action limit of 20ppb and 58.3% of the weanimix contaminated with fumonisin was above the action limit of 4ppm. This indicates that, the consumption of contaminated groundnuts and maize in these communities is likely to be very high.
In a study involving anaemia among pregnant women in Ghana, strong association was shown between aflatoxin and women with malaria and even stronger when those with iron deficiency anaemia were excluded, there appeared to be a risk of low birth weights when high levels of blood aflatoxin biomarker (>11.34pg AFB1/mg albumin) were observed in pregnant women.25
In another study involving 507 Ghanaian participants, AFB1-lysine adduct levels were statistically higher in subjects who had low levels of both vitamins A and E compared to subjects who had high vitamins A and E.26 A cohort study involving 472 Gambian children of ages 6–9 years were recruited for analysis of possible correlation of aflatoxin exposure and immune status. Immune parameters included secretory IgA (sIgA) in saliva and cell-mediated immunity (CMI). It was found out that, saliva IgA (sIgA) was markedly lower in children with detectable aflatoxin-lysine compared with those with non-detectable levels of 50.4µg/mg protein.7
The co-occurance of (70.8%) of aflatoxins and fumonisins contaminations seen in this study with the 53% as reported by27 indicates about 0.74-fold increase which may be due to poor storage conditions of the maize, groundnuts and beans used to prepare weanimix. The toxicosis that AFB1 and FB1 cause in humans and animals as well as the possible carryover of aflatoxins into consumable animal products, such as milk and infant feed is of widespread concern for child health in Sub-Saharan African countries.28
The results of the present study suggest that very young children from the Ejura-Sekyedumase district of the Ashanti Region of Ghana are likely to be exposed to high levels of aflatoxin from consumption of Aflatoxin contaminated homemade weanimix. The possible consequences on child immunity and growth in the district warrant further studies.
Conclusion
From this study homemade weanimix, an important food for feeding very young children was found to be highly contaminated with aflatoxin and fumonisin. This could negatively impact their health with respect to immunity and growth faltering, particularly stunting. The observations emphasize the need for aflatoxin exposure intervention strategies in high-risk countries, possibly targeted at the weaning period. Therefore there is a critical need to educate mothers on the dangers of mycotoxins exposure and to develop an economically feasible strategy to eliminate exposure of children fed homemade weanimix to aflatoxin and fumonisin.
Acknowledgement
The study was supported by a grant from The Peanut Collaborative Research Program, TAM 149 USAID. The Authors are also grateful to Mr. Ebenezer Ofori-Atta of Clinical Pathology department, Noguchi Memorial Institute for Medical Research for his technical support.
References
- 1.International Agency for Research on Cancer Monographs on the Evaluation of Carcinogenic Risks to Humans, author. IARC Scientific Publications no.56. Lyon: IARC; 2002. Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Armonatic Amines and Mycotoxins. [Google Scholar]
- 2.Stockmann-Juvalla H, Savolainen K. “A review of the toxic effects and mechanisms of action of fumonisin B1”. Human & Experimental Toxicology. 2008;27(11):799–809. doi: 10.1177/0960327108099525. [DOI] [PubMed] [Google Scholar]
- 3.Martins ML, Martins HM, Bernardo F. Aflatoxins in spices marketed in Portugal. Food Addit Contam. 2001;18(4):315–319. doi: 10.1080/02652030120041. [DOI] [PubMed] [Google Scholar]
- 4.Center for Disease Control and Prevention, author. Outbreak of aflatoxin poisoning Eastern and Central Provinces, Kenya. 2004. Morb Mortal. 2004;53:790–793. [PubMed] [Google Scholar]
- 5.Ozturk M. p53 mutation in hepatocellular carcinoma after aflatoxin exposure. Lancet. 1991;338:1356–1359. doi: 10.1016/0140-6736(91)92236-u. [DOI] [PubMed] [Google Scholar]
- 6.Ali MV, Mohiuddin SM, Reddy MV. Effect of dietary aflatoxin on cell mediated immunity and serum proteins in broiler chicken. Indian Vet. 1994;71:760–762. J. [Google Scholar]
- 7.Turner PC, Moore SE, Hall AJ, Prentice AM, Wild CP. Modification of immune function through exposure to dietary aflatoxin in Gambian children. Environ Health Persp. 2003;(111):217–220. doi: 10.1289/ehp.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blom HJ, Shaw GM, Heijer M, Finnell RH. Neural tube defects and folate: case far from closed. Nat Rev Neurosci. 2006;7(9):724–731. doi: 10.1038/nrn1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stockmann-Juvalla H, Savolainen K. “A review of the toxic effects and mechanisms of action of fumonisin B1”. Human & Experimental Toxicology. 2008;27(11):799–809. doi: 10.1177/0960327108099525. [DOI] [PubMed] [Google Scholar]
- 10.Gong YY, Egal S, Hounsa A, Turner PC, Hall AJ, Cardwell KF, Wild CP. Determinants of aflatoxin exposure in young children from Benin and Togo, West Africa: the critical role of weaning. Int J Epidemiol. 2003;32:556–562. doi: 10.1093/ije/dyg109. [DOI] [PubMed] [Google Scholar]
- 11.Ankrah NA, Ekuban FA, Asamoah EB. Depressant effect of very low levels of aflatoxin B1 on mouse glyoxylase-I activity and methylglyoxal disposal. Biochem Pharmacol. 1990;39(7):1261–1263. doi: 10.1016/0006-2952(90)90272-m. [DOI] [PubMed] [Google Scholar]
- 12.Awuah RT, Kpodo KA. High incidence of Aspergillus flavus and aflatoxins in stored groundnut in Ghana and the use of a microbial assay to assess the inhibitory effects of plant extracts on aflatoxin synthesis. Mycopathologia. 1996;134(2):109–114. doi: 10.1007/BF00436873. [DOI] [PubMed] [Google Scholar]
- 13.Jolly P, Jiang Y, Ellis W, Awuah R, Nnedu O, Phillips T, Wang Js, Afriyie-Gyawu E, Tang L, Person S, Williams J, Jolly C. Determinants of aflatoxin levels in Ghanaians: sociodemographic factors, knowledge of aflatoxin and food handling and consumption practices. Int J Hyg Environ Health. 2006;209(4):345–358. doi: 10.1016/j.ijheh.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Robinson A, Johnson NM, Strey A, Taylor JF, et al. Calcium montmorillonite clay reduces urinary biomarkers of fumonisin B1 exposure in rats and humans. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2012;29(5):809–818. doi: 10.1080/19440049.2011.651628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang P, Afriyie-Gyawu E, Tang Y, Johnson NM, Xu L, Tang L, Huebner HJ, Ankrah NA, Ofori-Adjei D, Ellis W, Jolly PE, Williams JH, Wang JS, Phillips TD. NovaSil clay intervention in Ghanaians at high risk for aflatoxicosis: II. Reduction in biomarkers of aflatoxin exposure in blood and urine. Food Additive and Contaminants. Part A Chem Anal Control Expo Risk Assess. 2008;25(5):622–634. doi: 10.1080/02652030701598694. [DOI] [PubMed] [Google Scholar]
- 16.Susheela TP, Narasinga-Rao BS. Energy density of diet in relation to energy intake of pre-school children from urban and rural communities of different economic status. Human Nutr Clin Nutr. 1983;37:133–137. [PubMed] [Google Scholar]
- 17.Gong YY, Cardwell K, Hounsa A, Turner PC, Hall AJ, Wild CP. Dietary aflatoxin exposure and impaired growth in young children from Benin and Togo: cross sectional study. Br Med J. 2002;325:20–21. doi: 10.1136/bmj.325.7354.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asare GA, Bronz M, Naidoo V, Kew MC. Interactions between aflatoxin B1 and dietary iron overload in hepatic mutagenesis. Toxicoology. 2007;234(3):157–166. doi: 10.1016/j.tox.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Kew MC. Synergistic Interaction between Aflatoxin B1 and Hepatitis B Virus in Hepatocarcinogenesis. Liver Int. 2003;23(6):405–409. doi: 10.1111/j.1478-3231.2003.00869.x. [DOI] [PubMed] [Google Scholar]
- 20.Lewis L, Onsongo M, Schurz-Rogers H, Luber G, et al. Aflatoxin Contamination of Commercial Maize Products during an Outbreak of Acute Aflatoxicosis in Eastern and Central Kenya. Environ Health Persp. 2005;113(12):1763–1767. doi: 10.1289/ehp.7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.James B, Adda C, Cardwell K, et al. Public information campaign on aflatoxin contamination of maize grains in market stores in Benin, Ghana and Togo. Food Addit Contam. 2007;24(11):1283–1291. doi: 10.1080/02652030701416558. [DOI] [PubMed] [Google Scholar]
- 22.International Agency for Research on Cancer Monographs on the Evaluation of Carcinogenic Risks to Humans, author. IARC Scientific Publications no. 56. Lyon: IARC; 2002. Some Naturally Occurring Substances: Food Items and Constitutents, Heterocyclic Armonatic Amines and Mycotoxins. [Google Scholar]
- 23.Sangare-Tigori B, Moukha S, Kouadio HJ, Betbeder AM, Dano DS, Creppy EE. Co-occurrence of aflatoxin B1, fumonisin B1, ochratoxin A and zear-alenone in cereals and peanuts from Côte d'Ivoire. Food Addit Contam. 2006;23(10):1000–1007. doi: 10.1080/02652030500415686. [DOI] [PubMed] [Google Scholar]
- 24.Kpodo K, Thrane U, Hald B. Fusaria and fumonisins in maize from Ghana and their co-occurrence with aflatoxins. Int J Food Microbio. 2000;61(2–3):147–157. doi: 10.1016/s0168-1605(00)00370-6. 1. [DOI] [PubMed] [Google Scholar]
- 25.Shuaib FM, Jolly PE, Ehiri JE, et al. Association between birth outcomes and aflatoxin B1 biomarker blood levels in pregnant women in Ku-masi, Ghana. Trop Med Int Health. 2010;15(2):160–167. doi: 10.1111/j.1365-3156.2009.02435.x. [DOI] [PubMed] [Google Scholar]
- 26.Tang L, Xu L, Afriyie-Gyawu E, et al. Aflatoxin-albumin adducts and correlation with decreased serum levels of vitamins A and E in an adult Ghanaian population. Food Addit Contam. Part A Chem Anal Control Expo Risk Assess. 2009;26(1):108–118. doi: 10.1080/02652030802308472. [DOI] [PubMed] [Google Scholar]
- 27.Kpodo K, Thrane U, Hald B. Fusaria and fumonisins in maize from Ghana and their cooccurrence with aflatoxins. Int J Food Microbio. 2000;61(2–3):147–157. doi: 10.1016/s0168-1605(00)00370-6. 1. [DOI] [PubMed] [Google Scholar]
- 28.Williams JH, Phillips TD, Jolly PE, Stiles JK, Jolly CM, Aggarwal D. Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences and intervenetions. Am J Clin Nutr. 2004;80:1106–1122. doi: 10.1093/ajcn/80.5.1106. [DOI] [PubMed] [Google Scholar]