Summary
Background
Creatinine (Cr) has been implicated as an independent predictor of hypertension and exercise has been reported as adjunct therapy for hypertension. The purpose of the present study was to investigate the effect of continuous training programme on blood pressure and serum creatinine concentration in black African subjects with hypertension.
Methods
Three hundred and fifty seven male patients with mild to moderate (systolic blood pressure [SBP] between 140–180 & diastolic blood pressure [DBP] between 90–109 mmHg) essential hypertension were age matched and randomly grouped into continuous & control groups. The continuous group involved in an 8 weeks continuous training (60–79% HR reserve) of between 45minutes to 60 minutes, 3 times per week, while the control group remain sedentary. SBP, DBP, VO2max, serum Cr, body mass index (BMI), waist hip ratio (WHR) and percent (%) body fat. Analysis of covariance (ANCOVA) and Pearson correlation tests were used in data analysis.
Results
Findings of the study revealed significant decreased effects of continuous training programme on SBP, DBP, Cr, BMI, WHR, % body fat and significant increase in VO2max at p< 0.05. Serum Cr is significantly and negatively correlated with SBP (−.335), DBP (.194), BMI (.268), WHR (−.258) and % body fat (−.190) at p<0.05.
Conclusion
The present study demonstrated a rationale bases for the adjunct therapeutic role of moderate intensity continuous exercise training as a multi-therapy in the down regulation of blood pressure, serum Cr, body size and body fat in hypertension.
Keywords: Hypertension, Blood pressure, Creatinine, body size, body fat, Africa
Introduction
Hypertension is particularly prevalent among African subjects, with 59% being affected. 1,2 Because of the high and increasing prevalence of hypertension and its concomitant risks of cardiovascular events (such as stroke, kidney disease, decreased disability adjusted and mortality), hypertension has been claimed to be a major global health problem and public-health challenge; demanding a vast proportion of health care resources directly and indirectly.3,4
Hypertension can lead to kidney disease or exist as a co-morbid condition of kidney disease and can contribute to kidney disease progression. The relationship between blood pressure and incidence of renal disease has been shown to be positive and continuous throughout the entire spectrum of blood pressure categories. However, serum creatinine concentration is widely interpreted as a measure of the glomerular filtration rate (GFR) and is used as an index of renal function in clinical practice.
Hypertension can cause damage to the kidney's filtration ability; hypertension-associated renal dysfunction is manifested primarily by increases in serum creatinine level. However, genetics, ethnicity and interracial differences in the development of hypertension, kidney disease and serum creatinine concentration have been reported and blacks are more prevalent to the development of hypertension, kidney disease and elevated serum creatinine level than whites.5–9
It has also been postulated that the genetic factor increasing the propensity of black people of sub-Saharan African descent to develop high blood pressure is the relatively high activity of creatine kinase, predominantly in vascular and cardiac muscle tissue. Such greater activity of creatine kinase has been reported in skeletal muscle of black untrained subjects and has also been reported to be almost twice the activity found in white subjects. Therefore, greater cellular activity of creatine kinase might explain the greater hypertension risk and the clinical characteristics of hypertensive disease observed in the black population. 10
To the best of our knowledge, there are few large randomized controlled trials investigating the association between exercise training and serum creatinine concentration in hypertension, and of those few studies, none has investigated these effects on pure black African population. However, heredity11 and genetical12–14 factors have been implicated in the causative of hypertension and creatinine production. There is also the possibility of the effects of genotypes in responses to exercise and physical activity in hypertension.14 These interpersonal and interracial differences clearly indicate the needs for study on pure older black African population. Therefore, the purpose of the present study was to investigate the effect of continuous training programme on blood pressure and serum creatinine concentration in black African subjects with hypertension.
Materials and Methods
Study design
In the present study, age-matched randomized independent pretest--posttest--control group design was used to determine the influence of the continuous training programme on blood pressure and serum creatinine concentration.
Subjects
The population for the study was male essential hypertensive subjects attending the hypertensive clinic of Murtala Muhammed Specialist Hospital Kano Nigeria. Subjects were fully informed about the experimental procedures, risk, and protocol, after which they gave their informed consent.
Inclusion criteria
Only those who volunteered to participate in the study were recruited. Subjects between the age range of 50 and 70 years with chronic mild to moderate and stable (> 1 year duration) hypertension (systolic blood pressure [SBP] between 140–179 & diastolic blood pressure [DBP] between 90–109 mmHg) were selected. Only those who had stopped taking antihypertensive drugs or on a single antihypertensive medication were recruited. 15 They were sedentary and have no history of psychiatry or psychological disorders or abnormalities.
Exclusion criteria
Underweight and obese (BMI < 18.5 & > 30 kg/m2 respectively), smokers, alcoholic, diabetic, other cardiac, renal, respiratory disease patients were excluded. Those involved in vigorous physical activities and above averagely physically fit (VO2max >27 and >33 ml/kg.min for over 60 and 50 years old respectively) were also excluded.
Randomization of subjects
Subjects' age were arranged in ascending order (50 to 70 years) and then assigned to continuous and control groups in an alternating pattern (age matched).
Intervention
Outcome measures: The study outcome measures included the SBP, DBP, and VO2max and serum creatinine concentration.
Procedures
Pretest procedure:
All subjects on antihypertensive drugs were asked to stop all forms of medication and in replacement, were given placebo tablets (consisted of mainly lactose and inert substance) in a double blind method. 16 Also subjects including those not on any antihypertensive medications were placed on placebo tablets for one week (7 days); this is known as “Wash out period”. The purpose of the wash out period was to get rid of the effects of previously taken antihypertensive medications. During the wash out period all subjects were instructed to report to the hypertensive clinic for daily blood pressure monitoring and general observation. All pretest procedures were conducted at the last day of the wash out period. Subjects resting (pre training) heart rate (HR), SBP, and DBP were monitored from the right arm as described by Musa et al. 17 using an automated digital electronic BP monitor (Omron digital BP monitor, Medel 11 EM 403c; Tokyo Japan).
Anthropometric measurement: Subjects' physical characteristics (weight [kg] & height [m]) and body composition (body mass index [BMI] (kg/m2)) assessment was done in accordance with standardized anthropometric protocol. 18Blood Sample Collection (Venipuncture Method): Pre-treatment venous blood samples were obtained after about 12 hour overnight fast (fasting blood sample). Five ml syringe was used for blood sample collection, using the procedure described by Bachorik. 19 Serum creatinine concentrations: serum creatinine concentration was determined using Colorimetric method with deproteinization with the Randox kit and manuals by Randox Laboratory, Antrim, United Kingdom.
Pretest stress test:
The Young Men Christian Association (YMCA) submaximal cycle ergometry test protocol was used to assess subject's aerobic power (VO2max) as described by ACSM.20
The stress test (pre & post training) was conducted under the supervision of experts in basic life support care and the emergency unit of the hospital was made ready to accommodate any incident that might occur during the stress test.
Test (training) procedure
Training programme: Following stress test and prior to the exercise training, all subjects in both control and continuous groups were re-assessed by the physician and were prescribed with antihypertensive drug; methyldopa as necessary. Methyldopa was preferred because it does not alter normal haemodynamic responses to exercise 21 and it is a well-tolerated antihypertensive drug in Africa 22 and mostly prescribed in the northern part (Kano) of Nigeria where the study was conducted and useful in the treatment of mild to moderately severe hypertension. 23 Subjects maintain these prescriptions with regular medical consultation and observation through-out the period of exercise training.
Intervention
The exercise group (group 1): Subjects in the exercise group exercised on a bicycle ergometer at a low intensity of between 60–79% of their HR reserve as recommended by ACSM.24 The starting workload was 100 kgm (17 watts) at a pedal speed of 50rpm; the workload was later increased to obtain a HR reserve of 60%. This was increased in the first two weeks to and levelled up at 79% HR reserve throughout the remaining part of the training period. The exercise session was increased from 45 minutes in the first two weeks of training to and leveled up at 60 minutes throughout the remaining part of the training. Exercise session of three times per week was maintained throughout the 8 weeks training period for continuous group.
The control group (group 2): Subjects in the control group were advised not to undertake or involve in any organized sports or structured exercise programme during the 8weeks period of study.
Posttest procedure
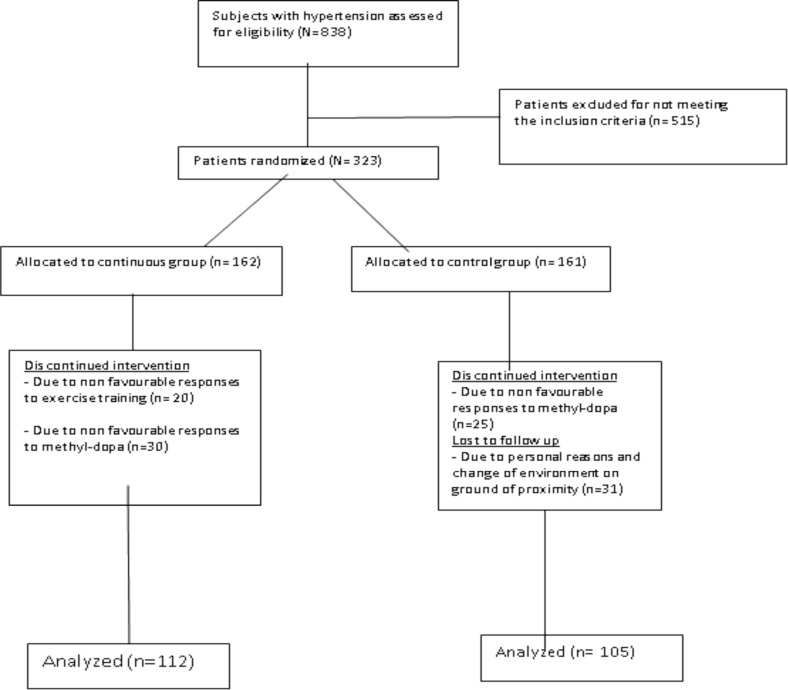
Posttest wash out Period: At the end of the 8 weeks training period, all subjects were asked to stop methyldopa and subjects were prescribed placebo tablets for one week as in pretest procedure. Posttest SBP, DBP, VO2max and serum creatinine concentration and post-test stress test were conducted as earlier described in the pretest procedures using standardized protocols, techniques and methods. All posttest procedures were conducted at the last day of the posttest wash out period. A total of 323 chronic and stable, essential mild to moderate male hypertensive patients satisfied the necessary study criteria. Subjects were aged matched and randomly grouped into exercise (162) and control (161) groups. Two hundred and seventeen subjects (112 from exercise, and 105 from control group) completed the eight weeks training program. One hundred and six subjects (50 from exercise and 56 from control group) had dropped out because of non-compliance, unfavorable responses to methyldopa and exercise training or had incomplete data; therefore, the data of 217 subjects (exercise [112] and control [105]) were used in the statistical analysis (Figure 1).
Figure 1.
Study design flow chat
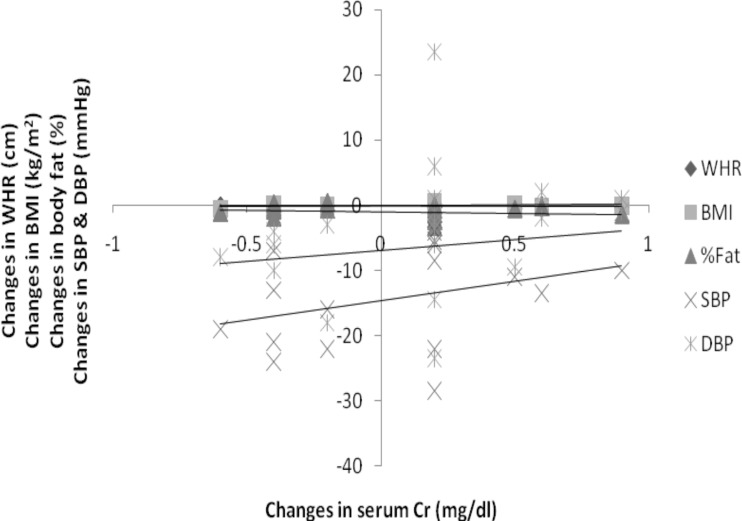
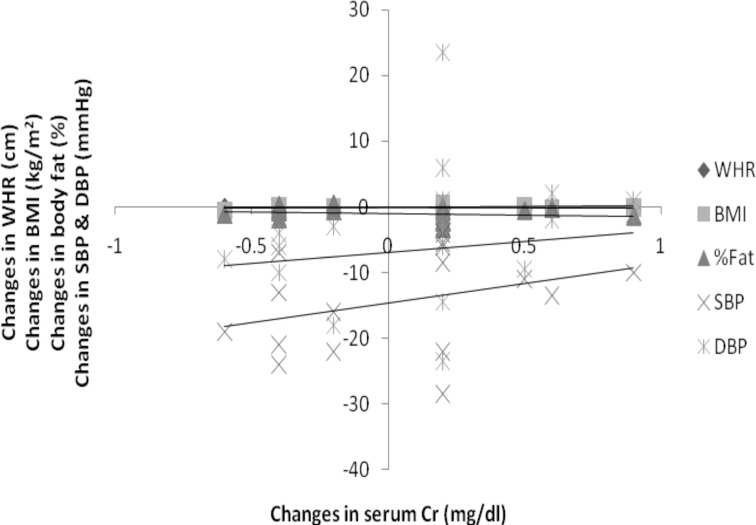
WHR= −.258*, BMI=.268*, % Fat= −.190*, SBP=.335**, DBP= .194*
Statistical analysis
Following data collection, the measured and derived variables were statistically analyzed. The descriptive statistics (Means and standard deviations) of the subjects' physical characteristics, estimated VO2 max, CRP, psychosocial status, and cardiovascular parameters were determined. Analysis of covariance (AN-COVA) was used to assess treatment outcomes; in the ANCOVA, the post-test values were the outcome variables and pretest values as covariates. Pearson product moment correlation test were computed for the variables of interest. In the correlation test, changed score (the difference between subjects post-training and pre-training measurements) were used as dependent measures. All statistical analyses were performed on a Toshiba compatible microcomputer using the statistical package for the social science (SPSS), (Version 16.0 Chicago IL, USA). The probability level for all the above tests was set at 0.05 to indicate significance.
Results
The subjects' age ranged between 50 and 70years. Mean age, height and weight: of subjects in exercise group were (58.63±7.22years, 1.73±6.97m and 67.49±10.16kg) respectively while for the Control group Mean age, height and weight were (58.27 ±6.24years, 1.68±5.31m, & 68.47±17.07 kg) respectively. There was no significant difference in age (t= 215, p= .697), SBP (t=.540, p=.597), DBP (t=.530, p=.597) and VO2max (t=-.406, p= .685) between groups. The physical characteristics of the subjects are comparable (Table 1).
Table1.
Groups mean± SD base line physical characteristics and Independent t-test (N=217)
| Variables | Continuous group pretest (n=112) X±SD |
Control group pretest (n=105) X±SD |
t-values | p-values |
| Age(years) | 58.63±7.22 | 58.27±6.24 | 0.390 | .697 |
| SBP(mmHg) | 170.45±15.57 | 160.87±13.23 | 0.540 | .597 |
| DBP(mmHg) | 97.56±7.53 | 97.17±1.43 | 0.530 | .597 |
| VO2max(ml/kg/min) | 20.69±12.49 | 21.23±5.76 | 0.406 | .685 |
| Weight (kg) | 67.48±10.16 | 68.47±17.07 | -0.514 | .608 |
*Significant, p< 0.05
Subjects' pre and post treatment mean BP ± SD mmHg; serum creatinine and VO2max (ml.kg−1.min−1) for the exercise and control groups are depicted in Table 2. Groups changed score values and ANCOVA test results (Table 3) indicated a significant effect in the exercise groups over control in SBP, DBP, serum creatinine, WHR, BMI, %body fat and VO2 max at p<0.05.
Table 2.
Groups mean(X) and standard deviation (SD) pre and posttest values (N=217)
| Variables | Continuous pretest X±SD |
Continuous posttest X±SD |
Control pretest X±SD |
Control posttest X±SD |
| SBP(mmHg) | 170.45±15.57 | 157.82±23.91 | 160.87±13.23 | 163.47±14.88 |
| DBP(mmHg) | 97.56±7.53 | 94.83±7.21 | 97.17±1.43 | 96.10±2.61 |
| VO2max (ml/kg/min) | 20.69±12.49 | 28.68±13.60 | 21.23±5.76 | 22.82±7.44 |
| Cr (mg/dl) | 0.81±0.17 | 0.85±0.39 | 0.81±0.26 | 0.99±0.45 |
| BMI (kg/m2) | 22.48±2.89 | 22.41±2.95 | 24.16±4.91 | 24.30±5.01 |
| WHR (cm) | 0.98±0.16 | 0.88±0.08 | 0.92±0.14 | 0.93±0.07 |
| Body fat (%) | 11.90±5.30 | 10.89±5.80 | 22.27±9.82 | 22.25±9.82 |
Table 3.
Groups changed scores mean(X) ± standard deviation (SD) and ANCOVA test (F) values (N = 217)
| Variables | Changed score values X±SD |
F-values | p-values | |
| Continuous group n=112 |
Control group n=105 |
|||
| SBP(mmHg) | −13.94± 6.95 | 2.61± 7.85 | 514.611 | .000* |
| DBP(mmHg) | −7.41± 6.26 | −1.07±1.76 | 488.715 | .000* |
| VO2max(ml/kg/min) | 7.99± 6.62 | 1.59± 3.52 | 135.488 | .000* |
| Cr (mg/dl) | 0.08±0.41 | 0.17±0.35 | 16.192 | 0.000* |
| BMI (kg/m2) | −0.07±0.32 | 0.14±0.40 | 4746.008 | .000* |
| WHR (cm) | −0.08±0.13 | 0.02±0.13 | 49.589 | .000* |
| Body fat (%) | −1.01±1.02 | −0.02±0.07 | 8609.141 | .000* |
Significant, p< 0.05
There was a significant positive correlation between changes in serum Cr and BMI (r= 0.268), SBP (0.355), DBP (0.194) and serum Cr negatively correlated with WHR (−0.258) and %body fat (−0.190) at p<0.01 (Figure 2).
Figure 2.
Correlation between training changes in serum creatinine and other variables (WHR, BMI, %fat, SBP & DBP) of interest (N=112)
Discussion
Findings of the present study indicated significant reduction in SBP, DBP and significant increase in VO2max as a result of continuous exercise training; several previous studies25,27 have reported similar findings. The present study also demonstrated a significant reduction in creatinine production in the exercise group over the control group. A contradictory finding was reported by Lo et al 28 who examined the effects of a 12-week exercise program on blood biochemistry in 13 patients undergoing continuous ambulatory peritoneal dialysis (CAPD; Thirteen subjects (men 6 & 7 women) with mean age of 46.5 ± 12.8 years.
The patients underwent exercise training on treadmill, bike, and arm ergometers thrice weekly; they reported no significant changes in serum creatinine. Similar non-significant effect of aerobic training on creatine kinase (CK) was reported by Rahnma et al.29 In their study, twenty male students were randomly assigned into either a control (n=10) or aerobic training (10) groups. They reported no significant effect of aerobic training on serum CK levels at rest and after exercise to exhaustion. The study of Johnson et al 30 investigated the effects of a combined functional and aerobic exercise program on aerobic capacity and serum creatine kinase level on 7 participants with sporadic IBM before and after a 12-week exercise program. They reported no significant change in the serum creatine kinase level after the exercise period. Peng et al31 investigated if swimming exercise training was supposed to be beneficial to its recovery. In their study, Doxorubicin-induced CKD (DRCKD) rat model was performed. Swimming training was programmed three days per week, 30 or 60 min per day for a total period of 11 weeks. They reported slight elevation of serum creatinine, the levels raised to 1.0–1.1 mg/dL at week 11. Swimming exercise did not show any effect on its restoration when referring to the normal serum creatinine range for SD rats 0.2–0.8 mg/dL.
The mechanism for reduction in serum creatinine following aerobic training could be related to the theoretical and empirical reports that creatinine concentration is positively associated with BMI, body fat and fat distribution.32 However, it has been reported that cratine (creatinine, the end product of creatinine metabolism) may potentially reduce the amount of stress placed on the cardiovascular system during aerobic activities thus improving the cardiovascular endurance by slowing the burning of oxygen in the muscles when active. 33, 34 The causes of elevated serum creatinine levels are tissue damage as a result of vigorous exercise and chronic kidney disease (CKD) and sedentary (physical inactivity) lifestyle.35 However, low to moderate aerobic training appeared to reduce and prevent tissue damage by the reduction in BMI, body fat and fat distribution as reported in the present study.
Though, all studies including the present study demonstrated that aerobic exercise slow down the rate of creatinine production compared to control but dispersed at the level of significance. However, disparity in findings could be related to methodology differences, types of subjects recruited for the studies and differences in exercise parameters in terms of exercise mode, duration and intensity.
Conclusion
The results of this study suggest that regular, moderate intensity aerobic training is an effective adjunct means of blood pressure reduction and a non-pharmacologic intervention to protect and attenuate tissue damage through creatinine production.
Clinical Implication
The possibility that reduction in creatinine level as a result of moderate intensity reflected the protection of such training programme on subclinical muscle damage cannot be excluded.
This also reflects the positive association between blood pressure and serum creatinine in the present study. The correlation between blood pressure and serum creatinine level provides evidence that the level of creatine kinase (CK) activity may modulate the function and dysfunction of cardiovascular tissue; particularly in resistant arteries, a small increase in CK activity may markedly increase contractility, with a potentially large impact on blood pressure levels. It has also been reported that high activity of creatine kinase, the central regulatory enzyme of energy metabolism, facilitates the development of high blood pressure.36
Clinical application
The study demonstrated a rationale for the adjunct therapeutic role of moderate intensity continuous training in the down regulation of blood pressure and serum creatinine. Therefore, specialists and other professionals in hypertension management should feel confident in the use of this mode of training in the non-pharmacological adjunct multi-therapy of hypertension.
References
- 1.Derman EW, Whitesman S, Dreyer M, Patel DN, Nossel C, Schwellnus MP. Healthy lifestyle interventions in general practice Part 7: Lifestyle and hypertension. South Africa Fam Pract. 2009;51:382–386. [Google Scholar]
- 2.Sliwa K, Wilkinson D, Hansen C, Ntyintyane L, Tibazarwa K, Becker A. Spectrum of heart disease and risk factors in a black urban population in South Africa (the Heart of Soweto Study): a cohort study. Lancet. 2008;371:915–922. doi: 10.1016/S0140-6736(08)60417-1. [DOI] [PubMed] [Google Scholar]
- 3.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 4.Williams B, Poulter NR, Brown MJ, Davis M, McInnes GT, Potter JF. British Hypertension Society. Guidelines for management of hypertension: report of the fourth working party of the British Hypertension Society, 2004-BHS IV. J Hum Hypertens. 2004;18:139–185. doi: 10.1038/sj.jhh.1001683. [DOI] [PubMed] [Google Scholar]
- 5.Coresh J, Wei GL, McQuillan G, Brancati FL, Levey AS, Jones C, Klag MJ. Prevalence of High Blood Pressure and Elevated Serum Creatinine Level in the United States: Findings From the Third National Health and Nutrition Examination Survey (1988–1994) Arch Intern Med. 2001;161(9):1207–1216. doi: 10.1001/archinte.161.9.1207. [DOI] [PubMed] [Google Scholar]
- 6.Klag MJ, Whelton PK, Randall BL. Blood pressure and end-stage renal disease in men. N Engl J Med. 1996:33413–33418. doi: 10.1056/NEJM199601043340103. [DOI] [PubMed] [Google Scholar]
- 7.Perry HM, Jr, Miller JP, Fornoff JR. Early predictors of 15-year end-stage renal disease in hypertensive patients. Hypertension. 1995:25587–25594. doi: 10.1161/01.hyp.25.4.587. [DOI] [PubMed] [Google Scholar]
- 8.Flack JM, Neaton JD, Daniels B, Esunge P. Ethnicity and renal disease: lessons from the Multiple Risk Factor Intervention Trial and the Treatment of Mild Hypertension Study. Am J Kidney Dis. 1993:2131–2140. doi: 10.1016/s0272-6386(12)80859-6. [DOI] [PubMed] [Google Scholar]
- 9.Perrone RD, Madias NE, Levey AS. Serum Creatinine as an Index of Renal Function: New Insights into Old Concepts. Clinical Chemistry. 1992;38(10):1933–1953. [PubMed] [Google Scholar]
- 10.Brewster LM, Clark JF, VanMontfrans GA. Is greater tissue activity of creatine kinase the genetic factor increasing hypertension risk in black people of sub-Saharan African descent? J Hypertension. 2000;18(11):1537–1544. doi: 10.1097/00004872-200018110-00002. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization Expert Committee on hypertension control, author. Hypertension control. Geneva: 1996. pp. 24–31. [PubMed] [Google Scholar]
- 12.Bouchard C, An P, Rice T, Skinner JS, Wilmore JH, Gagnon J, Perusse L, Leon AS, Rao DC. Familial aggregation for VO2max response to exercise training: results from the HERITAGE Family Study. Journal of Applied Physiology. 1999;87:1003–1008. doi: 10.1152/jappl.1999.87.3.1003. [DOI] [PubMed] [Google Scholar]
- 13.McArdle WD, Katch FI, Katch VL. Exercise physiology: Energy, nutrition, and human performance. Baltimore, Maryland: Williams & Wilkins; 1996. [Google Scholar]
- 14.Terry C, Loukaci V, Green FR. Cooperative influence of genetic polymorphisms on interleukin 6 transcription regulation. J Biol Chem. 2000;275:18138–18144. doi: 10.1074/jbc.M000379200. [DOI] [PubMed] [Google Scholar]
- 15.American College of Sport Medicine, author. Guide lines for exercise testing and Prescription. 4th Edition. Philadelphia: Lea & Febiger; 1991. [Google Scholar]
- 16.Townsend RR, Mcfadden TC, Ford V, Cadee JA. A randomized double blind, placebo-controlled trial of casein protein hydrolysnte (C12 peptide) in human essential hypertension. American Journal of Hypertension. 2004;17:1056–1058. doi: 10.1016/j.amjhyper.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 17.Musa DI, Ibrahim DM, Toriola AL. Cardiorespiratory fitness and risk factors of CHD in pre-adolescent Nigerian girls. J Human Movement Studies. 2002;42:455–455. [Google Scholar]
- 18.International Society for the Advancement of Kinanthropometry (ISAK), author International standards for anthropometric assessment. Patche Fstroom, South Africa: ISAK; 2001. [Google Scholar]
- 19.Bachorik PS. Collection of blood sample for lipoprotein analysis. Clin Chem. 1982;28:1375–1378. [PubMed] [Google Scholar]
- 20.American College of Sports Medicine, author. ASCM's guidelines for exercise testing and prescription. 5th Edition. Baltimore: Williams & Wilkins; 1995. [Google Scholar]
- 21.Katzung BG. Basic and clinical pharmacology. 7th ed. New York: Lange Medical Books/Craw Hill; 1998. [Google Scholar]
- 22.Mancia G, Ferari L, Gregorini L, Leonett L, Terzoli L, Biachini C. Effects of treatment with methyldopia on basal haemodynamic and on rural control. In: Robertson JS, Pickering GW, Goldwell ADS, editors. The therapeutics of hypertension. London: Royal Society of Medicine and Academic Press Inc. Ltd; 1980. pp. 70–78. [Google Scholar]
- 23.Salako LA. Treatment of hypertension: cardiovascular disease in Africa. Ibadan: Ciba Geigy Ltd; 1976. [Google Scholar]
- 24.American College of Sport Medicine, author. Physical activity,physical fitness and hypertension. Med Sci Sports Exer. 1993;25:i–x. [PubMed] [Google Scholar]
- 25.Georgiades A, Sherwood A, Gullette ECD, Babyak MA, Hinderliter A, Waugh R. Effects of exercise and weight loss on mental stress- induced cardiovascular responses in individuals with high blood pressure. Hypertension. 2000;36:171–176. doi: 10.1161/01.hyp.36.2.171. [DOI] [PubMed] [Google Scholar]
- 26.Pierce TW, Madden DJ, Siegel WC, Blumenthal JA. Effects of aerobic exercise on cognitive and psychosocial functioning in patients with mild hypertension. Health Psychology. 1993;12:286–291. doi: 10.1037//0278-6133.12.4.286. [DOI] [PubMed] [Google Scholar]
- 27.Kohut ML, McCann DA, Russell DW, Konoplan DN, Cunnick JE, Frank WD. Aerobic exercise, but not flexibility/resistance, reduces serum IL-18, CRP and IL-6, independent of beta-blockers, BMI and psychosocial factor in older adults. Brain Behav Immun. 2006;20:201–209. doi: 10.1016/j.bbi.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Lo CY, Lo L, Lo WK, Chan ML, So E, Tang S, Yuen MC, Cheng IK, Chan TM. Benefits of exercise training in patients on continuous ambulatory peritoneal dialysis. Am J Kidney Dis. 1998;32(6):1011–1018. doi: 10.1016/s0272-6386(98)70076-9. [DOI] [PubMed] [Google Scholar]
- 29.Rahnman N, Gaeni AA, Hamedinia MR. Oxidative stress responses in physical education students during 8 weeks aerobic training. J Sports Med Phys Fitness. 2007;47(1):119–123. [PubMed] [Google Scholar]
- 30.Johnson LG, Collier KE, Edwards DJ, Philippe DL, Eastwood PR, Walters SE, Thickbroom GW, Mastaglia FL. Improvement in aerobic capacity after an exercise program in sporadic inclusion body myositis. J Clin Neuromcul Dis. 2009;10(4):178–184. doi: 10.1097/CND.0b013e3181a23c86. [DOI] [PubMed] [Google Scholar]
- 31.Peng CC, Chen KC, Hsieh CL, Peng RY. Swimming Exercise Prevents Fibrogenesis in Chronic Kidney Disease by Inhibiting the Myofibroblast Transdifferentiation. PLoS ONE. 2012;7(6):e37388. doi: 10.1371/journal.pone.0037388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerchman F, Tong J, Utzschneider KM, Zraika S, Udayasankar J, McNeely MJ, Carr DB, Leonetti DL, Young BA, de Boer IH, Boyko EJ, Fujimoto WY, Kahn SE. Body Mass Index Is Associated with Increased Creatinine Clearance by a Mechanism Independent of Body Fat Distribution. J Clin Endocrinol Metab. 2009;94:3781–3788. doi: 10.1210/jc.2008-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harriman D. Creatine and cardio performance. http://www.Livestrong.com/article/462138-creatine-and-cardio-performance/&q.201.1. [Google Scholar]
- 34.Longo N, Ardon O, Vanzo R, Schwatz E, Pasquali M. 20 Disorders of creatine transport and metabolism. Am J Med Genet. 2011;99:1–7. doi: 10.1002/ajmg.c.30292. [DOI] [PubMed] [Google Scholar]
- 35.Nicholson GA, McLeod JG, Morgan G, Meerkin M, Cowan J, Bretag A, Graham D, Hill G, Robertson E, Sheffield L. Variable distributions of serum creatine kinase reference values: relationship to exercise activity. J Neurol Sci. 1985;71:233–245. doi: 10.1016/0022-510x(85)90062-0. [DOI] [PubMed] [Google Scholar]
- 36.Brewster LM, Clark JF, van Montfrans GA. Is greater tissue activity of creatine kinase the genetic factor increasing hypertension risk in black people of sub-Saharan African descent? J Hypertens. 2000;18:1537–1544. doi: 10.1097/00004872-200018110-00002. [DOI] [PubMed] [Google Scholar]