Abstract
Background
Over 3000 persons undergo allogeneic hematopoietic stem-cell transplantation (allo-HSCT) in Germany every year. Advances in allo-HSCT have prolonged the survival of treated patients but have concomitantly increased the risk of long-term complications that impair their quality of life.
Methods
This literature review of the long-term sequelae of allo-HSCT is based on pertinent articles that were retrieved by a selective search of PubMed, and on current international guidelines. Case reports were excluded from consideration.
Results
Hardly any randomized clinical trials have been performed to investigate the long-term outcome of allo-HSCT, but international consensus-based guidelines have been published. 50% to 70% of patients treated with allo-HSCT develop chronic graft-versus-host disease (cGVHD) within ten years of treatment. Transplant recipients are at higher risk of infection, including the reactivation of dormant herpes viruses; therefore, vaccination is recommended, as described in the current guidelines. Gonadal dysfunction arises in up to 92% of men and up to 99% of women; its frequency depends on the timing of transplantation, on radiotherapy, and on other factors. The medications that transplant recipients need to take can impair liver function, and transfusion-associated hemosiderosis can do so as well. 40% to 50% of patients suffer from lipid metabolic disturbances that increase the risk of myocardial infarction, peripheral arterial occlusive disease, and stroke. Their life expectancy is shorter than that of the overall population.
Conclusion
Measures should be taken to prevent the potential long-term complications of allo-HSCT. All patients who have been treated with allo-HSCT should receive individualized, risk-adapted, and multidisciplinary follow-up care, so that any complications that arise can be correctly diagnosed and appropriately treated. Long-term follow-up care could be improved by prospective clinical trials investigating the long-term sequelae of allo-HSCT, as well as by consistent, uniform documentation of these sequelae in supra-regional data registries.
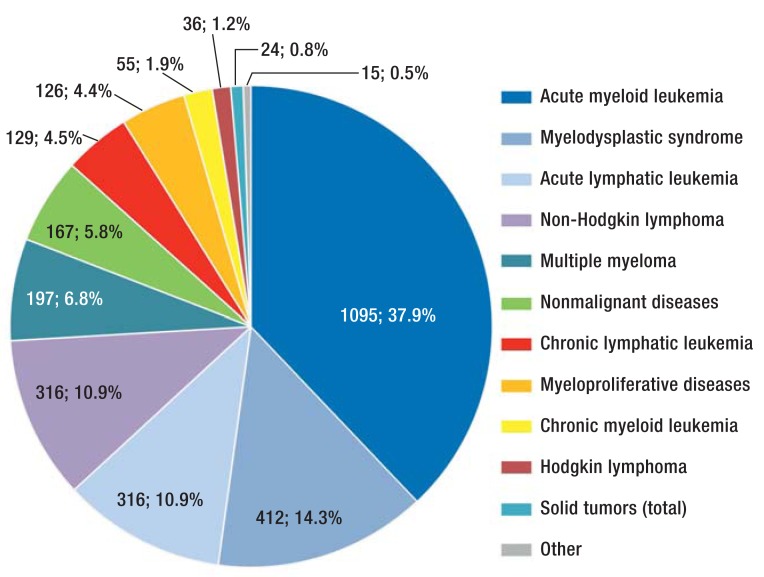
Transplantation numbers have been rising steadily in recent years, both in Germany and abroad. First-time allogeneic hematopoietic stem cell transplantations (alloHSCTs) in Germany increased from 1118 in 1998 to 2866 in 2012 (e1). As shown in Figure 1, the most common indication for an alloHSCT is acute leukemia (48.8%) (e1). Total mortality among patients who underwent HSCT in Seattle fell from 63% between 1993 and 1997 to 47% between 2003 and 2007 (hazard ratio [HR] = 0.59, p <0.001). At the same time, non-relapse mortality fell from 41% to 26% (HR = 0.48, p <0.001) (1). In a study by the Center for International Blood and Marrow Transplant Research (CIBMTR), the 10-year survival rate of patients who had survived at least two years after alloHSCT without relapse of their underlying disease was 85% (2). Their life expectancy was lower than that of the general population, however (2).
Figure 1.
Frequency of diseases for which allogeneic stem cell transplantation is performed in Germany. Figure based on data of the German Stem Cell Transplantation Register 2013 (e1)
In another study, 66% of transplant patients but only 39% of their siblings developed at least one chronic health condition; 18% and 8% respectively developed a serious or life-threatening disease (3). The prevalence of chronic health conditions after alloHSCT was 71% (3). Patients suffered cardiovascular diseases more frequently than their siblings (relative risk [RR] = 3.0), and the same was true of deterioration in hearing or vision (RR = 3.7), gastrointestinal complaints (RR = 6.0), and musculoskeletal diseases (RR = 7.1) (3).
Current analyses even report that more than 90% of long-term survivors have at least one chronic health condition (e2, e3). Long-term complications pose an even greater challenge in patients who receive a transplant before the age of 35 years (4).
There is a broad spectrum of long-term complications, ranging from organ-specific complications and infections to secondary malignancies (Table 1). As a result, physicians in all specialties, and primary care physicians, are confronted with unclear symptoms and complications in long-term survivors more frequently than in the past. In order to ensure swift patient care or prompt referral to a transplant center, we would like to make physicians who treat these patients in the first instance aware of the most common complications.
Table 1. Long-term effects of alloHSCT in adults, including pediatric issues*.
| Organ/tissue/organ system | Frequency (%) | Long-term sequelae | Risk factors (selected examples) |
|---|---|---|---|
| Immune system | Associated with cGVHD |
|
cGVHD, long-term immunosuppressive treatment HSCT for non-malignant diseases with subsequent mixed chimerism |
| Eyes | 20%, in cases with cGVHD up to 40% |
|
cGVHD, total body irradiation, busulfan treatment, long-term corticosteroid treatment |
| Ears |
|
Radiation of the skull, cyclophosphamide treatment | |
| Mouth | 26 to 50% with cGVHD |
|
cGVHD, total body irradiation or irradiation of the skull Transplantation at an early age |
| Skin and skin adnexa | Approx. 70% in cGVHD |
|
cGVHD, UV exposure, total body irradiation |
| Endocrine system | 9 to 35% 21% 92 to 99% 50%/40% |
|
Total body irradiation, long-term corticosteroid ‧treatment, transplantation at an early age cGVHD, corticosteroids, calcineurin and mTOR ‧inhibitors |
| Liver | 32 to 58% |
|
Cumulative number of transfusions, drug toxicity, cGVHD, viral infections |
| Cardiovascular system | 5 to 22% |
|
Total body or mediastinal irradiation, anthracycline treatment, pre-existing cardiac risk factors, metabolic syndrome, older age, hemosiderosis |
| Lungs | Approx. 5 to 15% |
|
Total body irradiation, bleomycin treatment, busulfan treatment, BCNU treatment cGVHD, nicotine abuse |
| Genitourinary tract | Approx. 30% 12% |
|
Pre-existing kidney disease, nephrotoxic drug treatment, infections, total body irradiation |
| Nervous system | Approx. 20 to 42% |
|
Complicated transplant course, calcineurin inhibitor treatment, cGVHD, infections, radiation of the skull, intrathecal chemotherapy, young age |
| Skeletal system | 4 to 50% |
|
cGVHD, long-term corticosteroid treatment, total ‧body irradiation, physical inactivity, hypogonadism |
| Psychosocial | 9 to 36% |
|
Pre-existing psychological disorders |
| Secondary malignancies | 8.8% |
|
cGVHD, age, total body irradiation |
This table shows the long-term sequelae of allogeneic stem cell transplantation (alloHSCT), together with frequencies for individual organ systems and risk factors.
*Specific pediatric issues are underlined in the table.
alloHSCT: allogeneic hematopoietic stem cell transplantation; BCNU: bis-chloroethyl nitrosourea; cGVHD: chronic graft-versus-host disease; CMV: cytomegalovirus; mTOR: mammalian target of rapamycin; PAOD: peripheral artery occlusive disease; UV: ultraviolet radiation; VZV: varicella zoster virus; CNS: central nervous system
On the basis of a selective search of the PubMed literature of the last 25 years, this article summarizes organ-specific long-term complications and lists the screening examinations that should be performed as part of long-term follow-up of patients after alloHSCT as a result. The search of the literature used the terms “late effect,” “long-term,” “stem cell transplantation,” and “bone marrow transplantation” and combined them with each other. While the list of screening examinations was compiled on the basis of international consensus recommendations (5, 6), only a small number of the recommendations for secondary prevention of long-term complications are evidence-based, as there is a lack of prospective studies. However, they are in line with our experience that complications after transplantation must be diagnosed early and treated promptly. Treating physicians sometimes consult specialists in this process. In addition to the screening examinations recommended in (Table 2, patients’ medical history should be taken and organ systems should be clinically examined.
Table 2. Screening during long-term follow-up after alloHSCT* (X: always indicated; O: indicated if there is a history of abnormal findings).
| Time since alloHSCT | 6 months | 1 year | 10 years | Annually | Comments |
|---|---|---|---|---|---|
Immune system
|
X X X |
X X X |
X X O |
O O |
|
Eyes
|
O |
X |
X |
O |
|
Mouth
|
O |
X |
X |
X |
|
| Skin | O | X | X | X |
|
Endocrine system
|
X X X X X |
X X X X X |
X X X |
X O O X O |
|
Liver
|
X O |
X X O |
X |
X O |
|
Cardiovascular system
|
X X X O |
X X X X X |
X X X O X |
X X X O O |
|
Lungs
|
X O X |
X O X |
X X X |
X O X |
|
Gastrointestinal tract
|
O |
X X |
O |
|
|
Genitourinary tract
|
X X O |
X X X |
X X X |
X X X |
|
Nervous system
|
X |
X |
X |
X |
|
Skeletal system
|
X |
O |
|
||
Psychosocial
|
X X X X |
X X X X |
X X X X |
X X X X |
|
*Recommended screening examinations at various times during follow-up after allogeneic stem cell transplantation, based on international consensus recommendations (5), 6))
alloHSCT: allogeneic hematopoietic stem cell transplantation; cGVHD: chronic graft-versus-host disease; DXA: dual-energy X-ray absorptiometry; HRCT: high-resolution computed tomography; Ig: immunoglobulin; MRI: magnetic resonance imaging; NT-proBNP: n-terminal pro-B natriuretic peptide
Pediatric issues
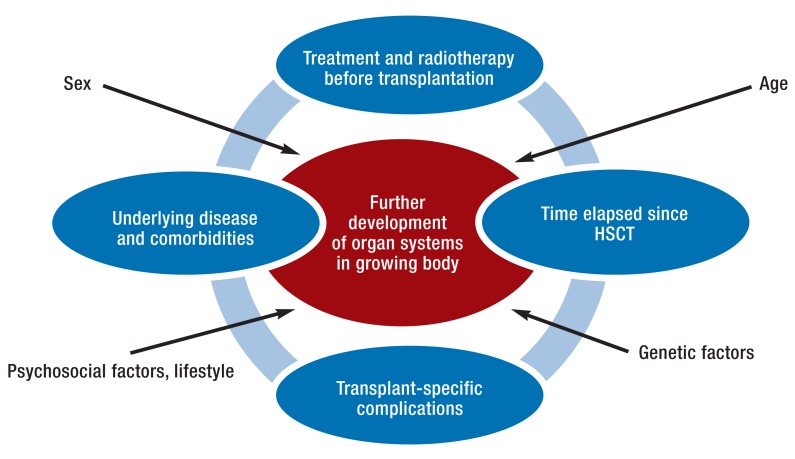
In the long-term follow-up of children and adolescents after alloHSCT it is particularly important to monitor the effects of the underlying disease, earlier chemotherapy and radiotherapy, and transplant-specific complications in the growing body (Figure 2). Organs that have not yet matured seem to be more vulnerable, which can affect future development potential (7). Young age (less than 10 years) at alloHSCT is the most common risk factor for most long-term sequelae, with the exception of hypogonadism.
Figure 2.
Aspects of long-term follow-up of children and adolescents following allogeneic stem cell transplantation (alloHSCT)
Providing adequate long-term follow-up care is often problematic, partly because long-term sequelae develop after some time has elapsed and partly because the transition from pediatric to adult medical care can be difficult. Several guidelines have been published to date for pediatric patients (5) (www.survivorshipguidelines.org,www.cclg.org.uk), but only a very small number of prospective studies. It is worth highlighting that a nonmalignant disease with specific comorbidities or risk factors is the indication for an alloHSCT in approximately one-third of all pediatric patients (7).
Chronic graft-versus-host disease
Both chronic graft-versus-host disease (cGVHD), which develops in up to 70% of all patients following alloHSCT, and its treatment can cause complications to be more frequent or more severe. They cause a particularly large increase in infections and also an increase in secondary malignancies. The working group for this review article recently published recommendations for the treatment of cGVHD in Deutsches Ärzteblatt International (8). Because cGVHD and its secondary complications are complex, patients require multidisciplinary treatment in specialized outpatient departments at transplant centers.
Immunodeficiency and infections
In addition to the immunosuppressive medication required to treat cGVHD, the immune system itself is a target of immune responses. This is sometimes associated with long-term T and B lymphocytopenia and functional asplenia. Transplant recipients must therefore be informed in detail of the increased risk of infection, including reactivation of dormant herpes viruses such as cytomegalovirus and zoster virus. Patients must also be made aware that prophylaxis of infection as well as prompt antibiotic treatment of early symptoms of infection and/or fever are required. The possibility of prescribing standby medication can be considered, although this must not replace an immediate visit to a doctor. It is recommended that vaccinations be administered in line with guidelines; this also includes repeat primary immunization (8). Immunoglobulin substitution should be considered if there is evidence of IgG deficiency (<400 mg/dL) or immunoglobulin subclass deficiency, and for recurring infections (8).
Endocrine dysfunction
Conditioning therapy administered for alloHSCT, including both total body irradiation and long-term corticosteroid therapy for cGVHD, can lead to various endocrine dysfunctions (Table 1). The examinations required for long-term follow-up are listed in Table 2.
Gonadal dysfunction develops in up to 92% of men and up to 99% of women after alloHSCT (6). Complete azoospermia developed in 65% of male patients after alloHSCT. Risk factors were total body irradiation (RR = 7.1), age over 25 years at transplantation (RR = 2.4), and cGVHD in patients with no history of total body irradiation (e4). Hormone replacement therapy can be considered, depending on the patient’s hormonal status and age (9, e5). This is particularly recommended for female patients who were previously premenopausal, in order to prevent complications of early menopause such as osteoporosis and arteriosclerosis. Spontaneous recovery of spermatogenesis or ovarian function has been described only occasionally (10, 11, e5, e6).
The possibility of secondary adrenal insufficiency, which is a particular risk during long-term steroid therapy (10), must be considered in emergency or stress situations.
Liver problems
The causes of liver dysfunction (Table 1) range from drug toxicity side effects via involvement in GVHD to transfusion-associated hemosiderosis or reactivation of earlier viral hepatitis (12). Hemosiderosis is associated with secondary damage to a very wide range of organ systems and increased mortality (13). It occurs in 32% to 58% of transplant recipients (14, 15). Iron overload of the liver can be diagnosed using laboratory testing, MRI, or in rarer cases liver biopsy (14– 16). It is treated using phlebotomy and/or iron chelators (17); phlebotomy is more cost-effective and has fewer side effects and should therefore be preferred if blood count is normal.
Hepatitis B virus (HBV) reactivation increases the risk of liver cirrhosis (e7). The risk of hepatic transplantation-associated complications is higher in carriers of chronic hepatitis C (e8): 24% after 20 years (e9).
Cardiovascular problems
The incidence of cardiovascular events such as coronary heart disease, peripheral arterial occlusion, and apoplexy 25 years after alloHSCT is 22% (18). The risk 10 years after alloHSCT depends on the number of cardiovascular risk factors present: it lies at 4.7% if there are no risk factors and 11.2% if there are more than two (19). Anthracycline therapy or chest radiation before alloHSCT and pre-existing cardiovascular risk factors increase the risk of cardiovascular diseases (19). Lipid metabolism disorders can be detected in 40% to 50% of patients after alloHSCT. They are associated with accelerated vascular sclerosis. Dyslipidemia after alloHSCT (e10) is caused by both cGVHD and cGVHD treatment in the form of corticosteroids, calcineurin inhibitors (CNIs) such as cyclosporine and tacrolimus, or mTOR inhibitors such as everolimus or sirolimus, and also by history of total body irradiation.
Prophylaxis, diagnosis, and treatment of cardiovascular complications should be performed in line with the recommendations of cardiology societies. Examinations recommended as part of long-term follow-up are listed in Table 2.
Lung problems
Delayed pulmonary effects of alloHSCT are very challenging, as they appear gradually and are difficult to treat. In addition to bronchiolitis obliterans syndrome (BOS) as part of cGVHD, recurring infections may lead to serious long-term complications (20, 21). Early diagnosis followed by swift start of therapy are essential to improving patients’ chances of survival (22). All patients should therefore undergo consistent screening, including lung function testing, from day 100 after alloHSCT onwards, from the age of six years. This should be performed every three months for the first two years and thereafter at least once a year and after lung infections (22). In addition to pharmacological treatment, pulmonary rehabilitation consisting of specific breathing and movement training, dietary counseling, and psychosocial support improves the physical activity of patients with BOS (e11).
Renal insufficiency
The incidence of chronic renal insufficiency after alloHSCT is approximately 30% (23). It is often caused by multiple factors, including intensity of conditioning therapy, use of nephrotoxic drugs, infections, and radiation of the renal area. Recommended screening examinations (24) are listed in Table 2.
Neurological issues
Neurological problems are observed in 31% to 42% of transplant recipients (25– 27). Damage to the peripheral nervous system, e.g. after a complicated transplant course or CNI therapy (25), manifests as polyneuropathy. Complications affecting the central nervous system are often caused by multiple factors (infections, hemorrhages, leukoencephalopathy, local relapse of underlying disease, central nervous system involvement in GVHD) (28) and are frequently fatal.
In the event of neurological symptoms, neurological assessment by a specialist should be performed, including imaging diagnostics and possibly lumbar puncture.
Musculoskeletal problems
Reduction in bone density develops early (29– 33). It is correlated with use of CNIs and corticosteroids and with loss of muscle mass (32); it affects 24% to 50% of patients (e12). Physiotherapy and stamina training (34) improve muscle strength, performance, and stamina. Calcium and vitamin D as prophylaxis for osteoporosis (35, 36), together with measurement of bone density using dual-energy X-ray absorptiometry (DXA), are recommended for all patients during the first year and in the event of abnormal findings or continued immunosuppressant therapy (35, e12). This must take account of the age-adapted Z-score. Bisphosphonates should be considered as prophylaxis for high-risk patients and as treatment for osteoporosis (35). Hormonal status and dietary counseling are part of multidisciplinary treatment.
The five-year incidence of avascular osteonecrosis, e.g. of the femoral head, is between 4% and 7% (e12, e13). Risk factors are GVHD and/or corticosteroid therapy, underlying disease, age, and male sex (e13, e14).
Psychosocial issues, quality of life
Transplant recipients frequently suffer from insomnia or fatigue (e15). Some also experience cognitive dysfunction or symptoms of an anxiety disorder. Listlessness is reported by 36% of patients after alloHSCT (e16), and depression is described in 9% to 20% of long-term survivors (e17). Suicide and accident rates are higher than in the general population (37). Sexual dysfunction such as erectile or ejaculatory disorders in men, vaginal dryness or dyspareunia in women, and reduced libido in both sexes contribute to a reduced quality of life and should therefore be the subject of targeted enquiry.
Screening for psychosocial burdens such as depression or an anxiety disorder should be provided to all transplant recipients (5). Psychomotor development must be monitored in children and adolescents. Sight and hearing tests and developmental diagnostic testing are recommended in the event of abnormalities or difficulties at school.
Secondary malignancies
The risk of secondary neoplasia increases steadily with time since transplant and is between 1.4 and 2.1 times higher than in the general population (38, 39). The 20-year cumulative incidence of secondary malignancy after alloHSCT is 8.8% (3). The risk is particularly high for secondary neoplasia of the oral cavity, skin, liver, CNS, hematopoietic system, and thyroid. Risk factors are cGVHD (3), age, and history of total body irradiation.
Because early diagnosis of secondary neoplasia contributes to improved overall survival, regular, lifelong follow-up is recommended (39). In addition to medical history and thorough physical examination, skin cancer screening should be performed in line with universally valid screening guidelines. The oral cavity should also be examined, particularly for patients with cGVHD.
It is recommended that women undergo mammograms every one to two years from the age of 40 years onwards (6). Younger female patients who have undergone radiation of the thorax of at least 20 Gy should be screened from the age of 25 years onwards or from eight years after their radiotherapy onwards, whichever comes first (e18). Due to the risk of cervical cancer sexually active female patients and women from the age of 21 years onwards should undergo PAP smear screening (6).
Discussion
Recent advances (19) achieved thanks to lower-intensity conditioning regimens and improvements in supportive therapy following alloHSCT are allowing more and more transplant recipients to survive for long periods. This means that long-term complications are becoming more and more significant. Long-term follow-up of alloHSCT patients is a challenge and requires close cooperation between primary care physicians, other specialists in private practice, transplant recipients, and transplantation centers. Various factors are hindering structured follow-up care. These include long distances between patients’ homes and transplant centers, lack of reimbursement of transport costs, staff shortages or turnover, and inadequate remuneration, in addition to a lack of knowledge and awareness of the long-term sequelae of alloHSCT (40). In adolescents, the transition from pediatric to adult care is often difficult but can be made easier with joint transitional doctors’ appointments.
Knowledge of the long-term sequelae of alloHSCT allows for tiered intervention. This involves counseling and educating patients, regular screening, and prompt treatment of long-term sequelae. A compromise should be sought between use of practical and financial resources on the one hand and the closest possible clinical monitoring on the other. Tailor-made, risk-adapted, comprehensive follow-up programs should be put together for all patients after alloHSCT and discussed perspicuously with patients. In addition, a special follow-up card for transplant recipients might improve and structure long-term follow-up. In view of the serious effects of GVHD on long-term outcomes and their complexity, multidisciplinary treatment in specialized outpatient departments at transplant centers is desirable.
In addition to prospective studies, long-term outcomes should be systematically documented using multicenter registry studies, in order to optimize the management of long-term alloHSCT survivors in the future too.
Key Messages.
Total survival following alloHSCT is steadily improving. Knowledge of its long-term sequelae is therefore becoming more important.
Long-term survivors after alloHSCT are at increased risk of comorbidities, which may have a negative impact on quality of life and contribute to late mortality following alloHSCT.
In addition to preventive measures such as vaccination and cancer screening, structured follow-up programs are required to prevent long-term sequelae or to diagnose and treat them promptly.
Joint transitional consultings ease the transition to adult care of patients who received HSCT as children.
Patients with cGVHD, in particular, require multidisciplinary treatment in specialized outpatient departments at transplant centers.
Acknowledgments
Translated from the original German by Caroline Devitt, M.A.
Prof. Wolff is backed by the German José Carreras Leukemia Foundation.
Footnotes
Conflict of interest statement
Dr. Hilgendorf has received lecture fees from MSD and Gilead.
Prof. Dr. Wolff has received research funding from Novartis.
The other authors declare that no conflict of interest exists.
References
- 1.Gooley TA, Chien JW, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wingard JR, Majhail NS, Brazauskas R, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2011;29:2230–2239. doi: 10.1200/JCO.2010.33.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun CL, Francisco L, Kawashima T, et al. Prevalence and predictors of chronic health conditions after hematopoietic cell transplantation: a report from the Bone Marrow Transplant Survivor Study. Blood. 2010;116:3129–3139. doi: 10.1182/blood-2009-06-229369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armenian SH, Sun CL, Francisco L, et al. Health behaviors and cancer screening practices in long-term survivors of hematopoietic cell transplantation (HCT): a report from the BMT Survivor Study. Bone Marrow Transplant. 2012;47:283–290. doi: 10.1038/bmt.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Majhail NS, Rizzo JD, Lee SJ, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Bone Marrow Transplant. 2012;47:337–341. doi: 10.1038/bmt.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rizzo JD, Wingard JR, Tichelli A, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation: joint recommendations of the European Group for Blood and Marrow Transplantation, the Center for International Blood and Marrow Transplant Research and the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2006;12:138–151. doi: 10.1016/j.bbmt.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Baker KS, Bhatia S, Bunin N, et al. NCI, NHLBI first international consensus conference on late effects after pediatric hematopoietic cell transplantation: state of the science, future directions. Biol Blood Marrow Transplant. 2011;17:1424–1427. doi: 10.1016/j.bbmt.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolff D, Bertz H, Greinix H, et al. The treatment of chronic graft-versus-host disease: consensus recommendations of experts from Germany, Austria and Switzerland. Dtsch Arztebl Int. 2011;108:732–740. doi: 10.3238/arztebl.2011.0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tauchmanova L, Selleri C, Rosa GD, et al. High prevalence of endocrine dysfunction in long-term survivors after allogeneic bone marrow transplantation for hematologic diseases. Cancer. 2002;95:1076–1084. doi: 10.1002/cncr.10773. [DOI] [PubMed] [Google Scholar]
- 10.Kauppila M, Koskinen P, Irjala K, et al. Long-term effects of allogeneic bone marrow transplantation (BMT) on pituitary, gonad, thyroid and adrenal function in adults. Bone Marrow Transplant. 1998;22:331–337. doi: 10.1038/sj.bmt.1701337. [DOI] [PubMed] [Google Scholar]
- 11.Gunasekaran U, Agarwal N, Jagasia MH, Jagasia SM. Endocrine complications in long-term survivors after allogeneic stem cell transplant. Semin Hematol. 2012;49:66–72. doi: 10.1053/j.seminhematol.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Kida A, McDonald GB. Gastrointestinal, hepatobiliary, pancreatic, and iron-related diseases in long-term survivors of allogeneic hematopoietic cell transplantation. Semin Hematol. 2012;49:43–58. doi: 10.1053/j.seminhematol.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Altes A, Remacha AF, Sureda A, et al. Iron overload might increase transplant-related mortality in haematopoietic stem cell transplantation. Bone Marrow Transplant. 2002;29:987–989. doi: 10.1038/sj.bmt.1703570. [DOI] [PubMed] [Google Scholar]
- 14.Majhail NS, DeFor T, Lazarus HM, Burns LJ. High prevalence of iron overload in adult allogeneic hematopoietic cell transplant survivors. Biol Blood Marrow Transplant. 2008;14:790–794. doi: 10.1016/j.bbmt.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Rose C, Ernst O, Hecquet B, et al. Quantification by magnetic resonance imaging and liver consequences of post-transfusional iron overload alone in long term survivors after allogeneic hematopoietic stem cell transplantation (HSCT) Haematologica. 2007;92:850–853. doi: 10.3324/haematol.11063. [DOI] [PubMed] [Google Scholar]
- 16.Au WY, Lam WM, Chu WC, et al. A magnetic resonance imaging study of iron overload in hemopoietic stem cell transplant recipients with increased ferritin levels. Transplant Proc. 2007;39:3369–3374. doi: 10.1016/j.transproceed.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 17.Majhail NS, Lazarus HM, Burns LJ. A prospective study of iron overload management in allogeneic hematopoietic cell transplantation survivors. Biol Blood Marrow Transplant. 2010;16:832–837. doi: 10.1016/j.bbmt.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Tichelli A, Bucher C, Rovo A, et al. Premature cardiovascular disease after allogeneic hematopoietic stem-cell transplantation. Blood. 2007;110:3463–3471. doi: 10.1182/blood-2006-10-054080. [DOI] [PubMed] [Google Scholar]
- 19.Armenian SH, Sun CL, Vase T, et al. Cardiovascular risk factors in hematopoietic cell transplantation survivors: role in development of subsequent cardiovascular disease. Blood. 2012;120:4505–4512. doi: 10.1182/blood-2012-06-437178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solh M, Arat M, Cao Q, et al. Late-onset noninfectious pulmonary complications in adult allogeneic hematopoietic cell transplant recipients. Transplantation. 2011;91:798–803. doi: 10.1097/TP.0b013e31820c85fa. [DOI] [PubMed] [Google Scholar]
- 21.Patriarca F, Skert C, Sperotto A, et al. Incidence, outcome and risk factors of late-onset noninfectious pulmonary complications after unrelated donor stem cell transplantation. Bone Marrow Transplant. 2004;33:751–758. doi: 10.1038/sj.bmt.1704426. [DOI] [PubMed] [Google Scholar]
- 22.Hildebrandt GC, Fazekas T, Lawitschka A, et al. Diagnosis and treatment of pulmonary chronic GVHD: report from the consensus conference on clinical practice in chronic GVHD. Bone Marrow Transplant. 2011;46:1283–1295. doi: 10.1038/bmt.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellis MJ, Parikh CR, Inrig JK, et al. Chronic kidney disease after hematopoietic cell transplantation: a systematic review. Am J Transplant. 2008;8:2378–2390. doi: 10.1111/j.1600-6143.2008.02408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abboud I, Peraldi MN, Hingorani S. Chronic kidney diseases in long-term survivors after allogeneic hematopoietic stem cell transplantation: monitoring and management guidelines. Semin Hematol. 2012;49:73–82. doi: 10.1053/j.seminhematol.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Gallardo D, Ferra C, Berlanga JJ, et al. Neurologic complications after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1996;18:1135–1139. [PubMed] [Google Scholar]
- 26.Teive HA, Funke V, Bitencourt MA, et al. Neurological complications of hematopoietic stem cell transplantation (HSCT): a retrospective study in a HSCT center in Brazil. Arq Neuropsiquiatr. 2008;66:685–690. doi: 10.1590/s0004-282x2008000500014. [DOI] [PubMed] [Google Scholar]
- 27.de Brabander C, Cornelissen J, Smitt PA, et al. Increased incidence of neurological complications in patients receiving an allogenic bone marrow transplantation from alternative donors. J Neurol Neurosurg Psychiatry. 2000;68:36–40. doi: 10.1136/jnnp.68.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solaro C, Murialdo A, Giunti D, et al. Central and peripheral nervous system complications following allogeneic bone marrow transplantation. Eur J Neurol. 2001;8:77–80. doi: 10.1046/j.1468-1331.2001.00160.x. [DOI] [PubMed] [Google Scholar]
- 29.Buchs N, Helg C, Collao C, et al. Allogeneic bone marrow transplantation is associated with a preferential femoral neck bone loss. Osteoporos Int. 2001;12:880–886. doi: 10.1007/s001980170041. [DOI] [PubMed] [Google Scholar]
- 30.Kauppila M, Irjala K, Koskinen P, et al. Bone mineral density after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1999;24:885–889. doi: 10.1038/sj.bmt.1701989. [DOI] [PubMed] [Google Scholar]
- 31.Petropoulou AD, Porcher R, Herr AL, et al. Prospective assessment of bone turnover and clinical bone diseases after allogeneic hematopoietic stem-cell transplantation. Transplantation. 2010;89:1354–1361. doi: 10.1097/TP.0b013e3181d84c8e. [DOI] [PubMed] [Google Scholar]
- 32.Schulte CM, Beelen DW. Bone loss following hematopoietic stem cell transplantation: a long-term follow-up. Blood. 2004;103:3635–3643. doi: 10.1182/blood-2003-09-3081. [DOI] [PubMed] [Google Scholar]
- 33.Kashyap A, Kandeel F, Yamauchi D, et al. Effects of allogeneic bone marrow transplantation on recipient bone mineral density: a prospective study. Biol Blood Marrow Transplant. 2000;6:344–351. doi: 10.1016/s1083-8791(00)70061-9. [DOI] [PubMed] [Google Scholar]
- 34.Wiskemann J, Dreger P, Schwerdtfeger R, et al. Effects of a partly self-administered exercise program before, during and after allogeneic stem cell transplantation. Blood. 2011;117:2604–2613. doi: 10.1182/blood-2010-09-306308. [DOI] [PubMed] [Google Scholar]
- 35.McClune BL, Polgreen LE, Burmeister LA, et al. Screening, prevention and management of osteoporosis and bone loss in adult and pediatric hematopoietic cell transplant recipients. Bone Marrow Transplant. 2011;46:1–9. doi: 10.1038/bmt.2010.198. [DOI] [PubMed] [Google Scholar]
- 36.Robien K, Strayer LG, Majhail N, et al. Vitamin D status among long-term survivors of hematopoietic cell transplantation. Bone Marrow Transplant. 2011;46:1472–1479. doi: 10.1038/bmt.2010.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tichelli A, Labopin M, Rovo A, et al. Increase of suicide and accidental death after hematopoietic stem cell transplantation: a cohort study on behalf of the Late Effects Working Party of the European Group for Blood and Marrow Transplantation (EBMT) Cancer. 2013;119:2012–2021. doi: 10.1002/cncr.27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Majhail NS, Brazauskas R, Rizzo JD, et al. Secondary solid cancers after allogeneic hematopoietic cell transplantation using busulfan-cyclophosphamide conditioning. Blood. 2011;117:316–322. doi: 10.1182/blood-2010-07-294629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rizzo JD, Curtis RE, Socie G, et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. 2009;113:1175–1183. doi: 10.1182/blood-2008-05-158782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Majhail NS, Rizzo JD. Surviving the cure: long term follow up of hematopoietic cell transplant recipients. Bone Marrow Transplant. 2013;48:1145–1151. doi: 10.1038/bmt.2012.258. [DOI] [PubMed] [Google Scholar]
- e1.Deutsches Register für Stammzelltransplantationen (DRST) DRST. Jahresbericht. 2013. www.drst.de/download/jb2013.pdf (last accessed on 13 November 2014)
- e2.Gifford G, Sim J, Horne A, et al. Health status, late effects and long-term survivorship of allogeneic bone marrow transplantation: a retrospective study. Intern Med J. 2014;44:139–147. doi: 10.1111/imj.12336. [DOI] [PubMed] [Google Scholar]
- e3.Jesudas R, Malesky A, Chu R, et al. Reviewing the follow-up care of pediatric patients status post-hematopoietic stem cell transplantation for the primary care pediatricician. Clin Pediatr (Phila) 2013;52:487–495. doi: 10.1177/0009922813483361. [DOI] [PubMed] [Google Scholar]
- e4.Rovò A, Aljurf M, Chiodi S, et al. Ongoing graft-versus-host disease is a risk factor for azoospermia after allogeneic hematopoietic stem cell transplantation: a survey of the Late Effects Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2013;98:339–345. doi: 10.3324/haematol.2012.071944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e5.Schubert MA, Sullivan KM, Schubert MM, et al. Gynecological abnormalities following allogeneic bone marrow transplantation. Bone Marrow Transplant. 1990;5:425–430. [PubMed] [Google Scholar]
- e6.Loren AW, Chow E, Jacobsohn DA, et al. Pregnancy after hematopoietic cell transplantation: a report from the late effects working committee of the Center for International Blood and Marrow Transplant Research (CIBMTR) Biol Blood Marrow Transplant. 2011;17:157–166. doi: 10.1016/j.bbmt.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e7.Iloeje UH, Yang HI, Su J, et al. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678–686. doi: 10.1053/j.gastro.2005.11.016. [DOI] [PubMed] [Google Scholar]
- e8.Nakasone H, Kurosawa S, Yakushijin K, et al. Impact of hepatitis C virus infection on clinical outcome in recipients after allogeneic hematopoietic cell transplantation. Am J Hematol. 2013;88:477–484. doi: 10.1002/ajh.23436. [DOI] [PubMed] [Google Scholar]
- e9.Peffault de Latour R, Lévy V, Asselah T, et al. Long-term outcome of hepatitis C infection after bone marrow transplantation. Blood. 2004;103:1618–1624. doi: 10.1182/blood-2003-06-2145. [DOI] [PubMed] [Google Scholar]
- e10.Kagoya Y, Seo S, Nannya Y, Kurokawa M. Hyperlipidemia after allogeneic stem cell transplantation: prevalence, risk factors, and impact on prognosis. Clin Transplant. 2012;26:E168–E175. doi: 10.1111/j.1399-0012.2012.01628.x. [DOI] [PubMed] [Google Scholar]
- e11.Tran J, Norder EE, Diaz PT, et al. Pulmonary rehabilitation for bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18:1250–1254. doi: 10.1016/j.bbmt.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e12.Hautmann AH, Elad S, Lawitschka A, et al. Metabolic bone diseases in patients after allogeneic hematopoietic stem cell transplantation: report from the Consensus Conference on Clinical Practice in chronic graft-versus-host-disease. Transplant Intern. 2011;24:867–879. doi: 10.1111/j.1432-2277.2011.01264.x. [DOI] [PubMed] [Google Scholar]
- e13.Schulte CM, Beelen DW. Avascular osteonecrosis after allogeneic hematopoietic stem-cell transplantation: diagnosis and gender matter. Transplantation. 2004;78:1055–1063. doi: 10.1097/01.tp.0000138026.40907.38. [DOI] [PubMed] [Google Scholar]
- e14.Socie G, Cahn JY, Carmelo J, et al. Avascular necrosis of bone after allogeneic bone marrow transplantation: analysis of risk factors for 4388 patients by the Societe Francaise de Greffe de Moelle (SFGM) Br J Haematol. 1997;97:865–870. doi: 10.1046/j.1365-2141.1997.1262940.x. [DOI] [PubMed] [Google Scholar]
- e15.Grulke N, Albani C, Bailer H. Quality of life in patients before and after hematopoietic stem cell transplantation measured with the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Core Questionnaire QLQ-C30. Bone Marrow Transplant. 2012;47:473–482. doi: 10.1038/bmt.2011.107. [DOI] [PubMed] [Google Scholar]
- e16.Wu LM, Austin J, Valdimarsdottir H, et al. Cross-sectional study of patient-reported neurobehavioral problems following hematopoietic stem cell transplant and health-related quality of life. Psychooncology. 2014 May 21; doi: 10.1002/pon.3554. doi: 10.1002/pon.3554. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- e17.Syrjala KL, Martin PJ, Lee SJ. Delivering care to long-term adult survivors of hematopietic stem cell transplantation. J Clin Oncol. 2012;30:3746–3751. doi: 10.1200/JCO.2012.42.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e18.Majhail NS. Secondary cancers following allogeneic haematopoietic cell transplantation in adults. Br J Hematol. 2011;154:301–310. doi: 10.1111/j.1365-2141.2011.08756.x. [DOI] [PubMed] [Google Scholar]
- e19.Negrin RS. Introduction to the review series on “Advances in hematopoietic cell transplantation”. Blood. 2014;124 doi: 10.1182/blood-2014-05-566679. [DOI] [PubMed] [Google Scholar]