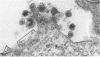
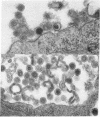
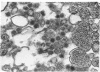
Abstract
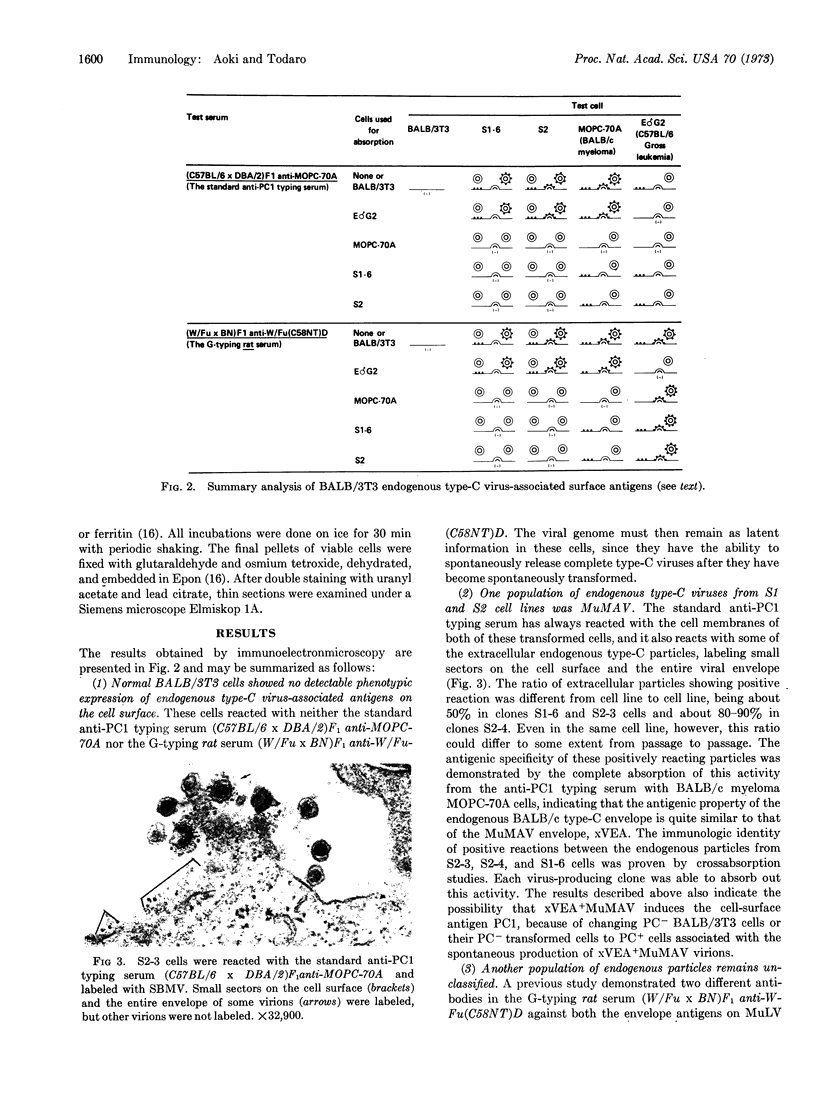
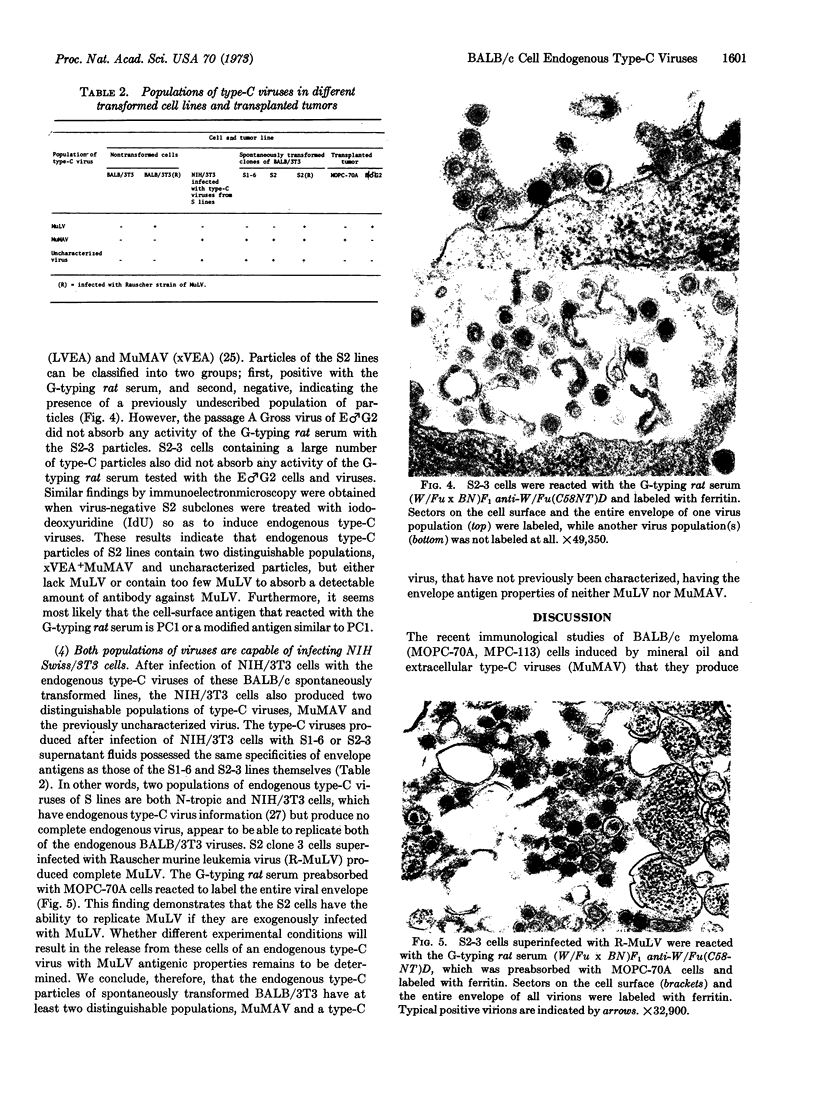
During long-term tissue culture of spontaneously transformed clones from BALB/c 3T3 mouse-embryo cells, some clones spontaneously begin to produce high titers of endogenous murine type-C viruses. The antigenic properties of these viruses have been analyzed by indirect immunoelectronmicroscopy and can be classified into two distinguishable populations: (a) BALB/c murine myeloma-associated extracellular viruses that carry a specific envelope antigen, xVEA, different from the typical murine leukemia viral envelope antigens; and (b) previously uncharacterized type-C viruses that have neither xVEA nor the murine leukemia viral envelope antigens. The former produces PC1 antigen and the latter might induce a new cell-surface antigen. Neither of these two populations of BALB/3T3 endogenous type-C viruses was able to infect BALB/c cells but both could infect NIH Swiss cells. A single BALB/3T3 clone, then, can release infectious endogenous type-C viruses with at least two different antigenic properties. We conclude that BALB/c somatic cells contain preexisting genetic information for production of at least closely related but, nevertheless, distinct type-C viruses.
Keywords: immunoelectronmicroscopy, cell-surface antigens
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Hartley J. W., Todaro G. J. Mouse leukemia virus: "spontaneous" release by mouse embryo cells after long-term in vitro cultivation. Proc Natl Acad Sci U S A. 1969 Sep;64(1):87–94. doi: 10.1073/pnas.64.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaronson S. A., Todaro G. J. Basis for the acquisition of malignant potential by mouse cells cultivated in vitro. Science. 1968 Nov 29;162(3857):1024–1026. doi: 10.1126/science.162.3857.1024. [DOI] [PubMed] [Google Scholar]
- Aaronson S. A., Todaro G. J. Development of 3T3-like lines from Balb-c mouse embryo cultures: transformation susceptibility to SV40. J Cell Physiol. 1968 Oct;72(2):141–148. doi: 10.1002/jcp.1040720208. [DOI] [PubMed] [Google Scholar]
- Aaronson S. A., Todaro G. J., Scolnick E. M. Induction of murine C-type viruses from clonal lines of virus-free BALB-3T3 cells. Science. 1971 Oct 8;174(4005):157–159. doi: 10.1126/science.174.4005.157. [DOI] [PubMed] [Google Scholar]
- Aoki T. An analysis of antigens on the surface of murine leukemia viruses and cells. Bibl Haematol. 1973;39:307–315. doi: 10.1159/000427857. [DOI] [PubMed] [Google Scholar]
- Aoki T., Herberman R. B., Johnson P. A., Liu M., Sturm M. M. Wild-type gross leukemia virus: classification of soluble antigens (GSA). J Virol. 1972 Dec;10(6):1208–1219. doi: 10.1128/jvi.10.6.1208-1219.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki T., Takahashi T. Viral and cellular surface antigens of murine leukemias and myelomas. Serological analysis by immunoelectron microscopy. J Exp Med. 1972 Mar 1;135(3):443–457. doi: 10.1084/jem.135.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARSKI G., CASSINGENA R. Malignant transformation in vitro of cells from C57BL mouse normal pulmonary tissue. J Natl Cancer Inst. 1963 May;30:865–883. [PubMed] [Google Scholar]
- Geering G., Old L. J., Boyse E. A. Antigens of leukemias induced by naturally occurring murine leukemia virus: their relation to the antigens of gross virus and other murine leukemia viruses. J Exp Med. 1966 Oct 1;124(4):753–772. doi: 10.1084/jem.124.4.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilden R. V., Oroszlan S. Group-specific antigens of RNA tumor viruses as markers for subinfectious expression of the RNA virus genome. Proc Natl Acad Sci U S A. 1972 Apr;69(4):1021–1025. doi: 10.1073/pnas.69.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W. T., Andresen W. F., Sanford K. K., Evans V. J., Hartley J. W. Virus particles and murine leukemia virus complement-fixing antigen in neoplastic and nonneoplastic cell lines. Science. 1967 Apr 7;156(3771):85–88. doi: 10.1126/science.156.3771.85. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Aoki T. Immune and natural antibodies to syngeneic murine plasma cell tumors. J Exp Med. 1972 Jul 1;136(1):94–111. doi: 10.1084/jem.136.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindig D. A., Kirsten W. H. Virus-like particles in established murine cell lines: electron-microscopic observations. Science. 1967 Mar 24;155(3769):1543–1545. doi: 10.1126/science.155.3769.1543. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Rowe W. P., Teich N., Hartley J. W. Murine leukemia virus: high-frequency activation in vitro by 5-iododeoxyuridine and 5-bromodeoxyuridine. Science. 1971 Oct 8;174(4005):155–156. doi: 10.1126/science.174.4005.155. [DOI] [PubMed] [Google Scholar]
- Old L. J., Boyse E. A., Stockert E. The G (Gross) leukemia antigen. Cancer Res. 1965 Jul;25(6):813–819. [PubMed] [Google Scholar]
- Parks W. P., Livingston D. M., Todaro G. J., Benveniste R. E., Scolnick E. M. Radioimmunoassay of mammalian type C viral proteins. 3. Detection of viral antigen in normal murine cells and tissues. J Exp Med. 1973 Mar 1;137(3):622–635. doi: 10.1084/jem.137.3.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks W. P., Scolnick E. M., Ross J., Todaro G. J., Aaronson S. A. Immunological relationships of reverse transcriptases from ribonucleic acid tumor viruses. J Virol. 1972 Jan;9(1):110–115. doi: 10.1128/jvi.9.1.110-115.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Hartley J. W., Lander M. R., Pugh W. E., Teich N. Noninfectious AKR mouse embryo cell lines in which each cell has the capacity to be activated to produce infectious murine leukemia virus. Virology. 1971 Dec;46(3):866–876. doi: 10.1016/0042-6822(71)90087-0. [DOI] [PubMed] [Google Scholar]
- SANFORD K. K., MERWIN R. M., HOBBS G. L., FIORAMONTI M. C., EARLE W. R. Studies on the difference in sarcoma-producing capacity of two lines of mouse cells derived in vitro from one cell. J Natl Cancer Inst. 1958 Jan;20(1):121–145. [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Old L. J., Boyse E. A. Surface alloantigens of plasma cells. J Exp Med. 1970 Jun 1;131(6):1325–1341. doi: 10.1084/jem.131.6.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J. Spontaneous release of type C viruses from clonal lines of spontaneously transformed Blab-3T3 cells. Nat New Biol. 1972 Nov 29;240(100):157–160. doi: 10.1038/newbio240157a0. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Friis R. R., Katz E., Vogt P. K. Induction of avian tumor viruses in normal cells by physical and chemical carcinogens. Virology. 1971 Dec;46(3):920–938. doi: 10.1016/0042-6822(71)90091-2. [DOI] [PubMed] [Google Scholar]