Abstract
Objective:
To evaluate the frequency, determinants and sleep characteristics of lucid dreaming in narcolepsy
Settings:
University hospital sleep disorder unit
Design:
Case-control study
Participants:
Consecutive patients with narcolepsy and healthy controls
Methods:
Participants were interviewed regarding the frequency and determinants of lucid dreaming. Twelve narcolepsy patients and 5 controls who self-identified as frequent lucid dreamers underwent nighttime and daytime sleep monitoring after being given instructions regarding how to give an eye signal when lucid.
Results:
Compared to 53 healthy controls, the 53 narcolepsy patients reported more frequent dream recall, nightmares and recurrent dreams. Lucid dreaming was achieved by 77.4% of narcoleptic patients and 49.1% of controls (P < 0.05), with an average of 7.6 ± 11 vs. 0.3 ± 0.8 lucid dreams/month (P < 0.0001). The frequency of cataplexy, hallucinations, sleep paralysis, dyssomnia, HLA positivity, and the severity of sleepiness were similar in narcolepsy with and without lucid dreaming. Seven of 12 narcoleptic (and 0 non-narcoleptic) lucid dreamers achieved lucid REM sleep across a total of 33 naps, including 14 episodes with eye signal. The delta power in the electrode average, in delta, theta, and alpha powers in C4, and coherences between frontal electrodes were lower in lucid than non-lucid REM sleep in spectral EEG analysis. The duration of REM sleep was longer, the REM sleep onset latency tended to be shorter, and the percentage of atonia tended to be higher in lucid vs. non-lucid REM sleep; the arousal index and REM density and amplitude were unchanged.
Conclusion:
Narcoleptics have a high propensity for lucid dreaming without differing in REM sleep characteristics from people without narcolepsy. This suggests narcolepsy patients may provide useful information in future studies on the nature of lucid dreaming.
Citation:
Dodet P, Chavez M, Leu-Semenescu S, Golmard JL, Arnulf I. Lucid dreaming in narcolepsy. SLEEP 2015;38(3):487–497.
Keywords: dreaming, lucid dreaming, REM sleep, narcolepsy, spectral power, EEG coherence
INTRODUCTION
Lucid dreaming is the experience of being aware of dreaming while asleep and continuing to dream.1,2 As many as 51% adults in a German representative sample of 922 subjects reports that they had experienced a lucid dream at least once,3 while the monthly frequency of lucid dreams is higher in children than in teenagers and adults.4 Lucid dreams generally arise in REM sleep.5 Compared to non-lucid REM sleep, lucid REM sleep is associated with local frontal lobe EEG changes in the 40 Hz band, increased brain coherence,6 and increased activity on functional MRI in the bilateral precuneus, cuneus, parietal lobules, and prefrontal (and especially the dorsolateral prefrontal cortex) and occipito-temporal cortices, which may correspond to restored reflective consciousness.7 Models of lucid dreaming have recently been used to determine the cortical signature associated with dreamed motor activity (e.g., clenching the left and right hands).8 Although this model is fascinating when used to address several questions about dreamed versus imagined and physically performed activities, as well as mind-body interactions during sleep, most studies have been limited by the difficulty to achieve lucid dreaming in a sleep laboratory and within a functional brain imaging device. Consequently, their results are based on one to three healthy subjects and require several nights of monitoring before achieving lucidity.6–10
Patients with narcolepsy experience excessive daytime sleepiness with rapid entry into REM sleep as well as several symptoms associated with dissociated REM sleep, including cataplexy, hypnagogic hallucinations, sleep paralysis, and REM sleep behavior disorder.11 These patients also report frequent dreams and nightmares.12–14 While interviewing narcolepsy patients in our reference center for narcolepsy, we serendipitously noted that they reported frequently being aware of dreaming without any specific training. We decided to study the frequency and determinants of lucid dreaming in narcolepsy and to challenge patients' alleged ability to achieve lucid dreaming using sleep monitoring during nighttime and daytime sleep.
METHODS
Participants
From February to May 2013, we prospectively interviewed all patients with narcolepsy who presented to the outpatient clinic of the reference center for narcolepsy of a university hospital, regardless of whether they were new or follow-up patients. They had to meet the international criteria for narcolepsy,15 including: (i) excessive daytime sleepiness occurring daily for ≥ 3 months; (ii) A mean sleep latency < 8 min on the multiple sleep latency tests (5 tests performed at 08:00, 10:00, 12:00, 14:00, and 16:00 after attended nighttime polysomnography including EEG [Fp1/A2, C3/A2, C3/O1], left and right EOG, chin and left and right tibialis anterior EMGs, nasal pressure, abdominal and thoracic efforts, tracheal sound, EKG, pulse oximetry and position); (iii) ≥ 2 periods of REM sleep onset during these tests; and (iv) no other causes for these findings, including sleep apnea syndrome, acute and chronic sleep deprivation, circadian sleep disorders, shift work disorder, depression and recent withdrawal of an antidepressant or neuroleptic drug. The presence of cataplexy was identified by interview and, in rare cases, by direct observation of sudden loss of axial muscle tone with transient absence of deep tendon reflexes. The CSF hypocretin-1 levels were not measured, as diagnostic criteria were met without this test in all cases. The presence of the DQB1*0602 HLA genotype was assessed in all patients. Among patients with narcolepsy who reported lucid dreams, 12 volunteered to participate in the lucid dreaming experiment performed in the sleep laboratory. Healthy controls were recruited among friends, families, hospital employees, and students to match the narcolepsy patients in terms of age and gender. Controls were interviewed by a sleep physician who ensured the absence of any sleep disorders. In addition, 5 frequent lucid dreamers, without any disease, were recruited through word of mouth. The protocol was approved by the local ethics committee (Comité de Protection des Personnes - Ile de France 06), and participants gave written informed consent. Individuals who took part in the experimental portion of the sleep study received monetary compensation for their participation.
Questionnaire
The same sleep neurologist (PD) conducted in-person interviews with the patients and the controls regarding their demographic and clinical characteristics, including gender, age, body mass index, cataplexy, excessive daytime sleepiness (score on the Epworth Sleepiness Scale,16 sleep attacks, monthly number of naps), nighttime sleep characteristics (usual sleep time, number of awakenings, sleep talking, restless leg syndrome, observed sleep apnea, sleep paralysis, hypnagogic hallucinations, dyssomnia), automatic behaviors, and history of treatment. The Epworth Sleepiness Scale score, mean daytime sleep latency, number of sleep onset in REM periods, and HLA genotyping at the time of narcolepsy diagnosis were collected from patients' medical files. The systematic questionnaire about dreams included an evaluation of the monthly frequency of dreams, nightmares, prominent emotions in dreams (negative, positive, both), dreams of false awakening, recurrent dreams, enchained dreams (defined as the ability to resume the same dream after a long awakening exactly at the time in the scenario when the dream was interrupted by the awakening), and lucid dreaming (defined as the awareness of being in a dream). Examples of lucid dreams were provided. Subjects with lucid dreams were asked to evaluate their ability to change the dream scenario, identify the elements (themselves, other characters, the scene, change/cut the scenario) that they were able to modify in their dreams, determine the predominant emotion within the lucid dreams (positive, negative, euphoric), and assess the similarities between lucid dreams and awake life. Most questions were derived from the Lucidity and Consciousness in Dreams Scale (LuCiD).2 Subjects were also asked to evaluate the utility of their lucid dreams (e.g., to stop nightmares, to have fun, to train oneself) and assess whether being lucid tended to induce waking or if the lucid dreaming experience was associated with fatigue upon awakening. In the statistical analysis of these measures, dichotomous measures were compared using the χ2 test, and continuous measures were compared with Student t-test.
Sleep Monitoring of Lucid Dreaming
The 17 subjects who participated in the experimental portion of the sleep study were asked to keep a dream diary for the entire week prior to the time of the experiment, to train nightly to access lucidity while asleep and to note their lucid dreams in the diary. Control subjects spent a single night in the sleep laboratory, while narcolepsy patients spent one night and the following day to include 6 to 7 discrete daytime naps from 08:00 to 19:00, interspersed with 1 to 2 hours of full wakefulness as in a prolonged multiple sleep latency test. Patients were active in the sleep laboratory (speaking, eating, working on a computer, walking around) until they were suggested to nap or spontaneously asked the investigator to lie on bed and sleep, as this is frequent in narcolepsy patients. Sleep monitoring used the same sensors as described above, plus extra EEG leads placed mostly in the frontal area, which has been shown to present with higher power in the 40 Hz band during lucid dreaming (Fp1, Fp2, F7, F8, C3, C4, O1, O2, using the Jasper 10/20 system). All monopolar signals were simultaneously acquired as on the polysomnograph Brainet (Medatec Ltd France), which has a notch filter at 48.5 Hz. Infrared video and sound recordings were also collected. Subjects were instructed to signal lucidity by scanning the horizon from right to left two times. The signal was trained during wakefulness. Eye movement signals were calibrated while each patient was awake using 2 targets that were located 2 meters away. Subjects reported the content of their dreams and the presence/ absence of lucidity during the night (upon spontaneous awakening in a few cases), after the full night's sleep (in all cases) and after each nap (in all cases). They completed the scale of lucidity upon awakening from each nap.2
Analysis of Sleep, Muscle, and Eye Activities
Sleep stages, arousals and respiratory events were scored according to international criteria.17,18 The eye signal was recognized on the EOG during REM sleep by two independent scorers, and the corresponding samples were named “signaled lucid REM sleep episodes.” Doubtful EOG signals were discarded. In addition, some patients reported that they were lucid after some REM sleep episodes; however, they did not display the eye signal or believed that they had performed the signal, but it was not visible for scorers. These REM sleep episodes were considered as “non-signaled lucid REM sleep episodes.” The number (termed “REM density” as the number per min of REM sleep time) and amplitude of the eye movements (including the signal during wakefulness and lucid REM sleep and the spontaneous movements during non-lucid and lucid REM sleep, in microvolts), the percentage of chin muscle atonia measured according to international criteria,19 and the arousal index during lucid and non-lucid REM sleep were manually measured. Because patients did not contribute equally to the number of lucid and non-lucid REM sleep episodes, measurements were adjusted for each individual subject and analyzed using a mixed linear model for quantitative paired variables and Turkey-Kramer post hoc tests if significant. This statistical calculation was performed using the SAS version 9 statistical package (SAS Institute Inc, Cary, NC, USA). All tests were two-tailed, and a probability level (P value) < 0.05 was considered to be statistically significant.
Power and Coherence EEG Analysis
Samples of EEG recordings (recorded at a sampling frequency of 256 Hz) were selected during quiet wakefulness with the eyes closed, non-lucid REM sleep, and signaled lucid REM sleep (from 6 sec before and after the left-right-left-right eye signal if isolated, and between 2 left-right-left-right eye signals distant < 20 sec). These samples were exported to perform spectral analysis on each electrode channel using the fast Fourier transform. Spectral analyses were performed on non-overlapping quasi-stationary segments of 20 sec (normalized to a zero mean in the 0 to 128 Hz frequency band). For each EEG activity, the power spectrum was estimated using the Welch averaged periodogram method.20 The degree of statistical association between the activities was estimated by means of classical coherence. Coherence is a measure of linear correlation between two signals x(t) and y(t) across frequencies. It is estimated from the cross-spectral density between the 2 waveforms and normalized by the power spectral density of each waveform (see the full methodology details from our group21). Coherence can range between 0 (when both signals are independent) and 1 (in case of a perfect dependency between 2 signals). Calculations were performed using the squared magnitude coherence function in MATLAB (MathWorks, Natick, MA) and Welch method applied to the N = 20 disjoint 1-sec windows. To determine the probability that coherence is significantly higher than expected from random fluctuations, we used Fisher z transform of coherence: zcoh = tanh−1 (square root of C).20 According to the independence hypothesis, zcoh is normally distributed with an expected mean of 0 and a variance var{zcoh} = 1/(2N), where N is the number of disjoint non-overlapping data segments used in the estimated coherence calculation (N = 20).22 Because the number and duration of samples were different between subjects, the estimated values (power or coherence) obtained in the segments were contrasted between different within-subject pairs of conditions (quiet wakefulness with eyes closed versus non-lucid REM sleep, quiet wakefulness with eye closed versus signaled lucid REM sleep, and non-lucid REM sleep versus signaled lucid REM sleep). To estimate the probability that coherence in one condition differed significantly from coherence in the other conditions, values were compared using a one-tailed t-test (justified by the normality of values), whereas power values were compared via a nonparametric test (Mann-Whitney U test) because power values did not exhibit a Gaussian distribution.
RESULTS
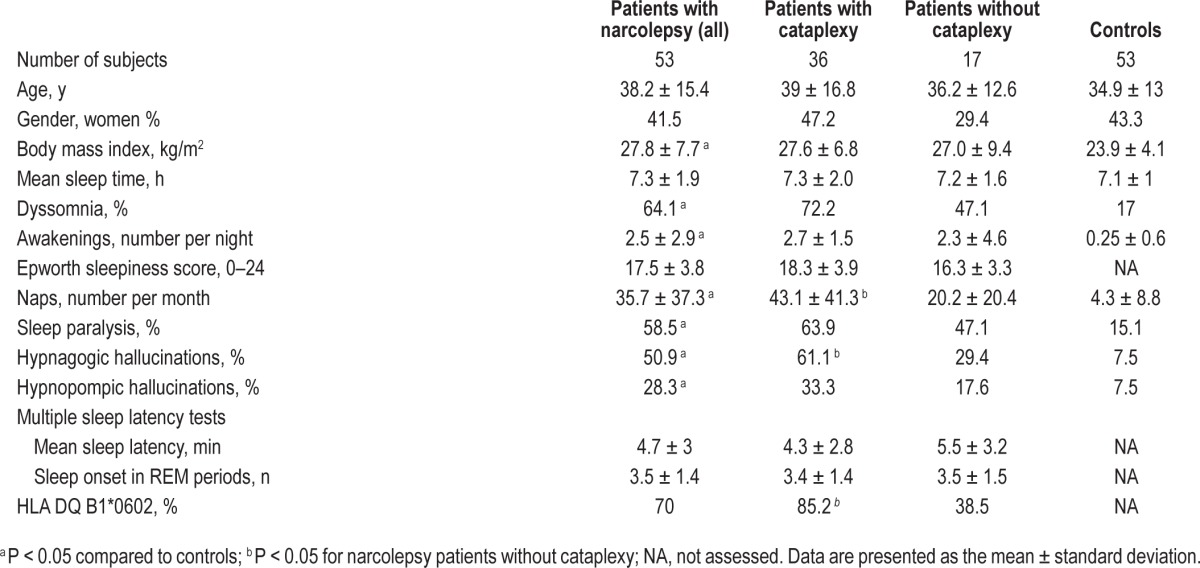
As expected by matching, groups did not differ by age or gender (Table 1). Patients with narcolepsy had higher body mass indices and a greater frequency of naps per week and awakenings per night. Narcolepsy patients also demonstrated dyssomnia, sleep paralysis, and hypnagogic and hypnopompic hallucinations more frequently than controls. Patients with cataplexy had more frequent naps, hypnagogic hallucinations, and HLA DQB1*0602 positivity than patients without cataplexy.
Table 1.
Demographic and clinical characteristics of patients with narcolepsy and controls.
Dreaming and Lucid Dreaming Characteristics in Interviews
Most dream characteristics differed between patients and controls; narcolepsy patients experienced more frequent dreams and nightmares and more recurrent and enchained dreams than controls (Table 2). Nightmares were more frequent in patients with cataplexy than in those without cataplexy. Dreams of false awakening were equally frequent in both groups. An example reported during a daytime nap in a patient with narcolepsy was:
“ A male nurse had been disagreeable with me. As revenge, I decided not to sleep as instructed, but instead stood up and typed on my computer. I realized after the nap that it was a dream.”
There were more lucid dreamers in the narcolepsy group (regardless of whether cataplexy was present) than in the control group, and narcoleptic lucid dreamers had significantly more lucid dreams per month. Narcoleptic patients with lucid dreams did not differ from patients without lucid dreams in terms of age, gender, presence of cataplexy, hypnagogic and hypnopompic hallucinations, sleep paralysis, sleepiness severity (Epworth sleepiness score, number of naps), dyssomnia, number of awakenings per night, number of dreams and nightmares per month, recurrent dreams, enchained dreams, dreams of false awakening, and HLA DQB1*0602 positivity. Narcoleptic patients with lucid dreams had higher body mass indices and longer sleep times than those without lucid dreams (Table 3). Lucid dreaming was clearly distinguished from sleep paralysis with and without sleep-related hallucinations. The 41 narcoleptic patients with lucid dreaming reported that lucid dreaming was equally frequent during nighttime sleep and daytime sleep (n = 19), more frequent during the night (n = 16), and more frequent during naps (n = 5); one did not know. Narcoleptic patients were more frequently able to achieve lucid dreaming during the daytime than controls (60% [24/40] versus 16% [4/25], P = 0.004). Lucid dreamers from all groups reported similar abilities to alter dream content, such as changing characters, oneself, and the dream scenario. Narcoleptic patients were able to change themselves more often than other characters in their lucid dreams (73.9% versus 43.4%, P = 0.036). These patients used lucid dreaming mostly to change nightmares and, more rarely, to have fun or train themselves. Patients with narcolepsy reported numerous examples of lucid nightmares:
“I was chased by an aggressor and had to kill myself by throwing myself onto an electrified fence to avoid the aggressor and wake up”;
“I had a recurrent nightmare of being flooded in a tsunami. Once I was in this dream again, I said to myself, ‘I am fed up with you, my tsunami dream.’ Suddenly the tsunami became a person and apologized for disturbing me. I never had this nightmare again”;
“I was chased by soldiers and chose to fly to escape them”;
“I built a panic room in myself where I mentally went during my nightmare to escape dangerous people”;
“I was seeing my family tortured to death and decided that it was not true, because we were rehearsing a play or a movie. I was behind the camera”; and
“I built myself a suit of armor made of lightning that protected me from my enemies.”
Patients with narcolepsy also reported pleasant lucid dreams:
“I was flying, I was piloting a spaceship over the places I chose, rode a giant horse, crossed the walls, traveled under the water while breathing easily”;
“I was in a castle (always the same castle), and decided to do exactly what I wanted. I went around discovering new parts of the castle, I tried to speak and shout loudly”; and
“I went out of my body and saw my physical body lying in the bed. Then I was able to fly, cross through ceilings, fly over the town and I chose to go to marvelous places with great people.”
Table 2.
Characteristics of dreams and nightmares in patients with narcolepsy and controls.
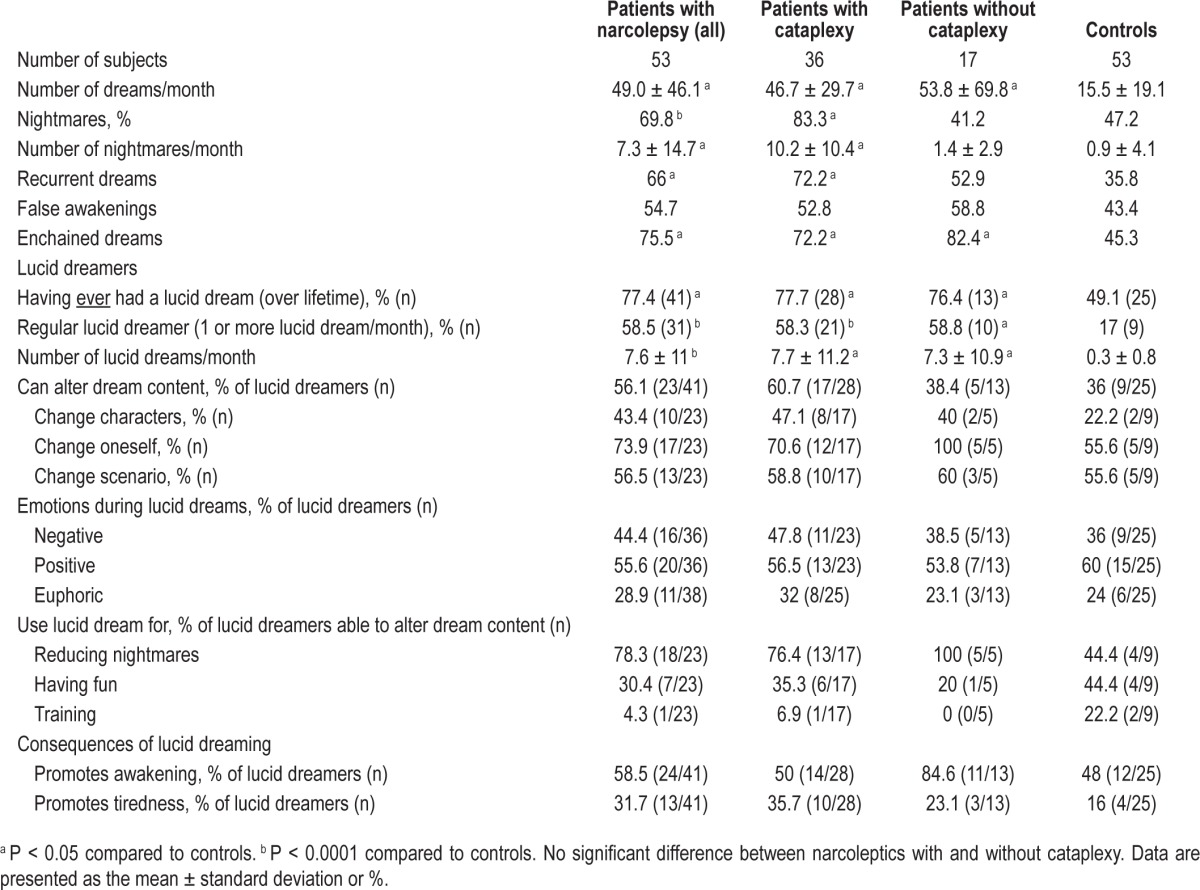
Table 3.
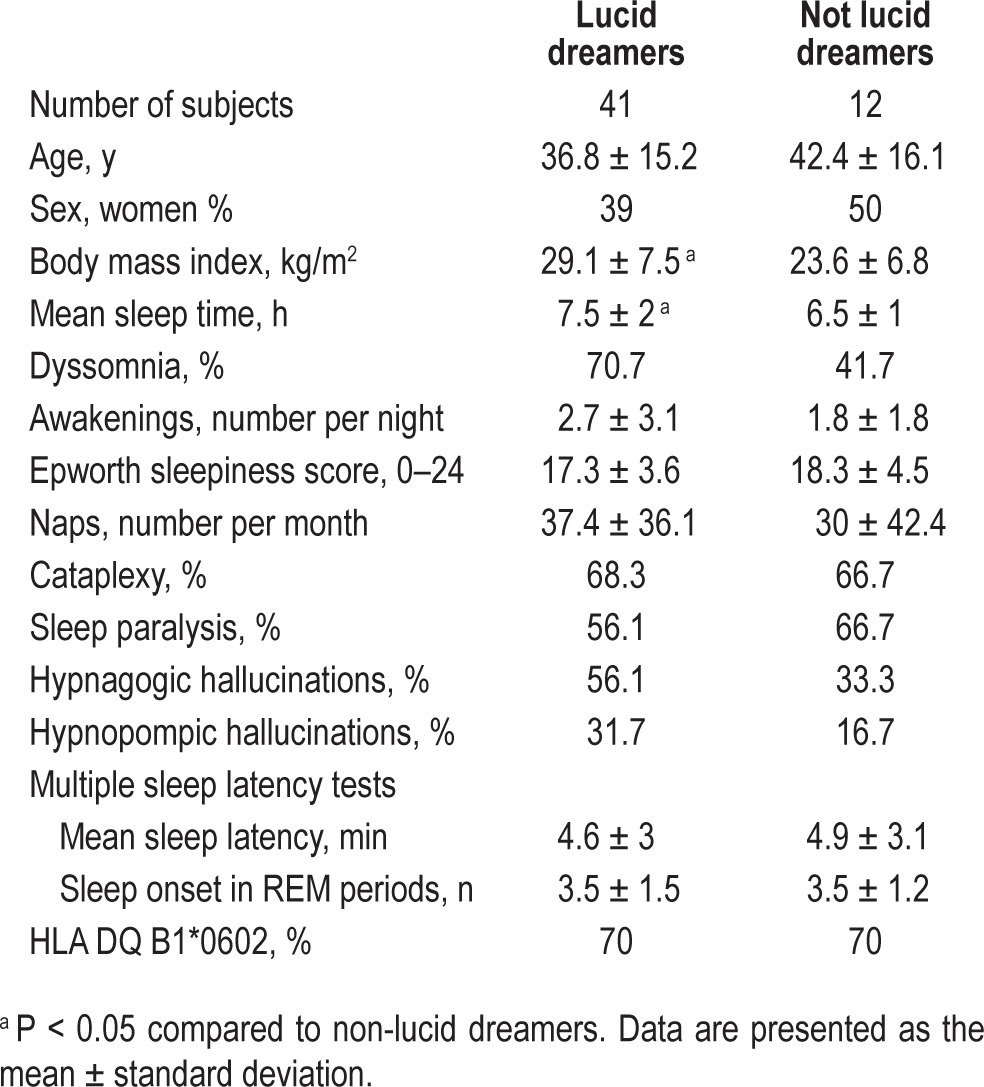
Demographic and clinical characteristics of narcoleptic patients with and without lucid dreaming.
Success in Achieving Lucid REM Sleep
Twelve narcoleptic patients (5 men, 7 women; 8 patients with cataplexy; aged 16 to 40 y) and 5 healthy controls (2 men, 3 women; aged 25 to 40 y; not included in the control group described above) were recruited as frequent lucid dreamers to participate in the experimental portion of the study. Healthy controls reported 12.9 ± 9.6 lucid dreams per month; narcoleptic patients reported 14.7 ± 12.6 lucid dreams per month (these values were not significantly different). Prior to nighttime sleep and naps, participants were instructed to signal lucid dreaming by scanning the horizon of their dream from right to left and then left to right 2 times. All subjects underwent a night polysomnography (including a total of 67 REM sleep episodes during the night), and the 12 narcoleptic patients underwent additional polysomnography during 6 to 7 daytime naps (including a total of 57 REM sleep episodes over 70 daytime naps). Three controls and all 12 narcoleptic patients reported that they achieved lucid dreaming at least once during the night; however, the eye signal could not be identified via behavioral observation during REM sleep in any of these participants. Two of the 5 controls made recurrent eye signals during clear wakefulness with diffuse alpha rhythms and reported dreamlike mental content that could have potentially corresponded to rich hypnagogic imagery. In contrast, during daytime sleep, narcoleptic patients reported 54 dreams during 70 naps, of which 41 were reported as lucid (corresponding to 33 naps) and 15 were reported as non-lucid (76% of all dream naps). Among these 41 lucid dreams, patients reported that they thought they had performed the eye signal in 38 instances. In sleep recordings, the eye signal was identified in 30 instances with certainty by 2 individual raters in 14 REM sleep episodes; the signal was possibly identified (with doubt) in 13 other instances. Overall, 7 narcoleptic patients and 0 non-narcoleptic controls performed the lucidity signal during REM sleep. These 7 lucid dreamers with narcolepsy had performed a total of 38 REM sleep episodes during the night, none of them containing a doubtless lucid eye signal.
Polygraphic Aspects of Lucid REM Sleep
Examples of lucid and non-lucid REM sleep epochs are shown in Figure 1. Most polysomnographic sleep characteristics were similar during non-lucid and lucid REM sleep, regardless of whether the lucid episodes contained the eye signal or if dreams were reported as lucid after waking from a nap during which the eye signal was not detected. REM sleep times were longer, REM sleep onset latency tended to be shorter, and the percentage of REM sleep with complete muscle atonia tended to be higher during signaled lucid REM sleep than during non-lucid REM sleep. The arousal index and REM density were not different between lucid and non-lucid REM sleep (Table 4). The amplitude of the eye movement signal was lower in lucid REM sleep than during wakefulness (Figure 2), representing that the horizontal gaze scanning spanned 104.8° during wakefulness and 26.5° during lucid REM sleep. Spontaneous rapid eye movements (REMs) had the same amplitude during lucid and non-lucid REM sleep, but the REMs amplitude was less than that of the lucidity eye signal during lucid REM sleep.
Figure 1.
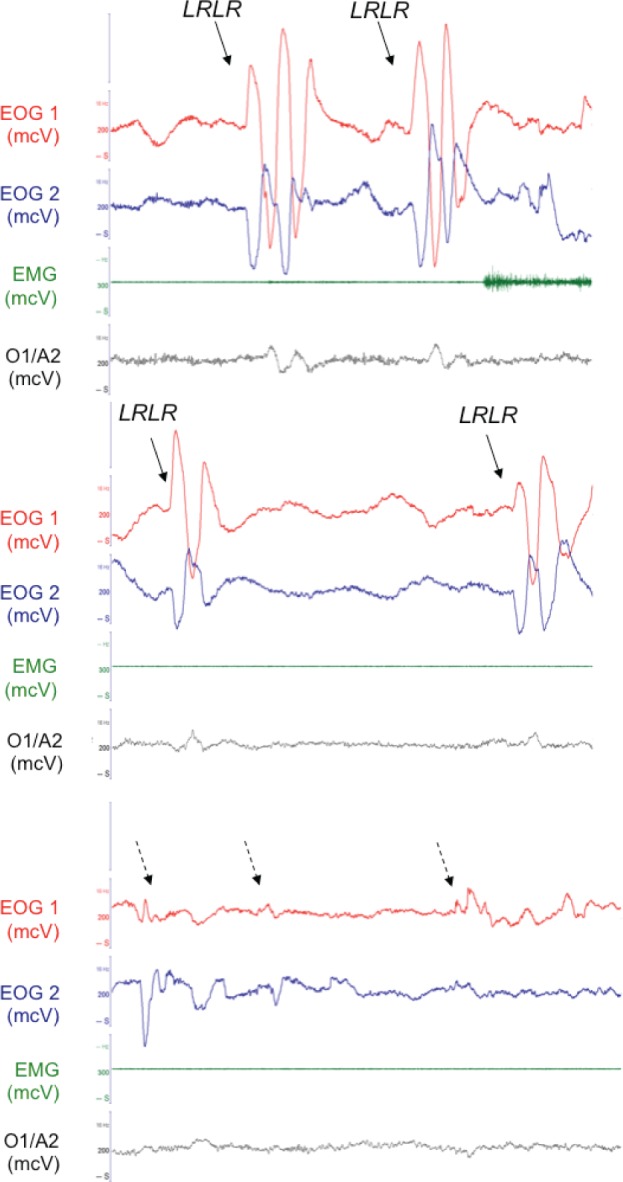
Polygraphic 30 sec epochs of wake (upper panel), lucid REM sleep (middle panel) and non-lucid REM sleep (lower panel) in the same subject. Plain arrows indicate the voluntary eye movements (signal was left right left right [LRLR] horizontal scanning of the horizon) and dashed arrows indicate spontaneous REMs. EOG, electro-oculograms in phase opposition; EMG, chin electromyogram; O1/A2, monopolar EEG with reference electrode on the right mastoid A2.
Table 4.
Sleep measures in daytime lucid and non-lucid REM sleep, and in nighttime REM sleep.
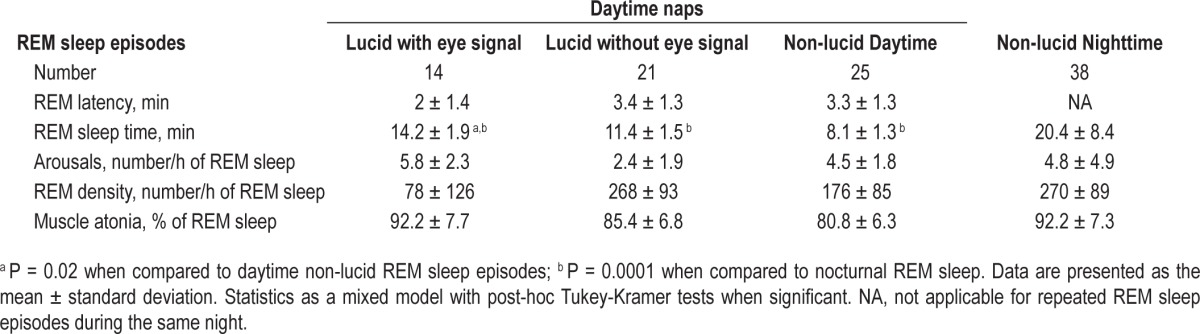
Figure 2.
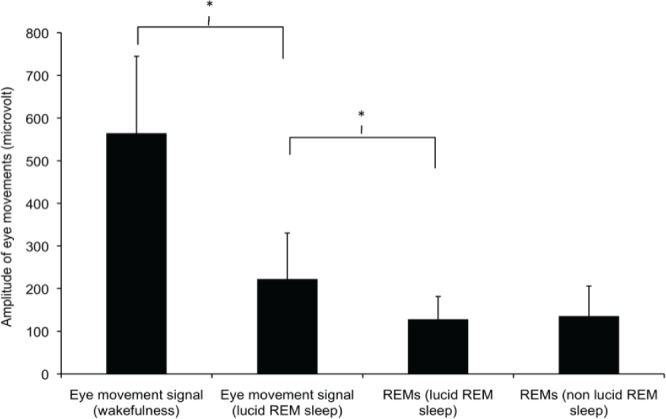
Amplitude of eye movements during wakefulness (instructed eye signal), lucid (instructed eye signal and spontaneous rapid eye movements, REMs) and during non-lucid REM sleep. Note that the amplitude of the eye signal was greater when performed during wakefulness than during lucid REM sleep. Spontaneous REMs had the same amplitude during lucid and non-lucid REM sleep, but the REMs amplitude was less than that of the eye signal during lucid REM sleep.
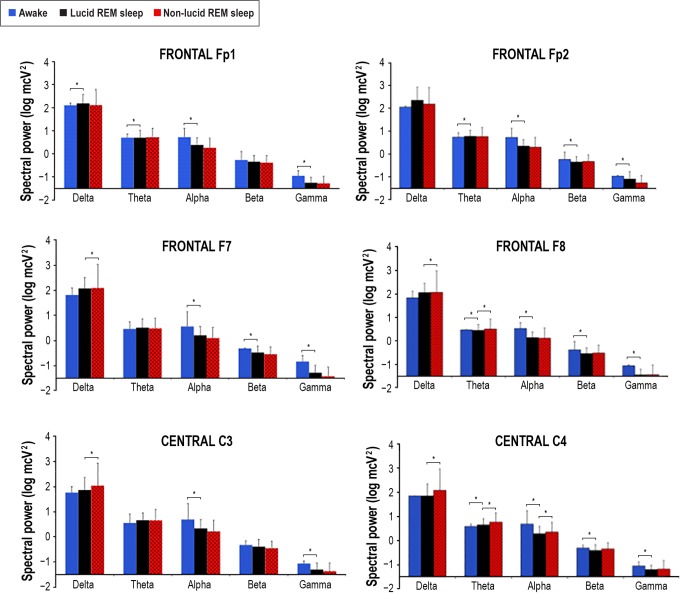
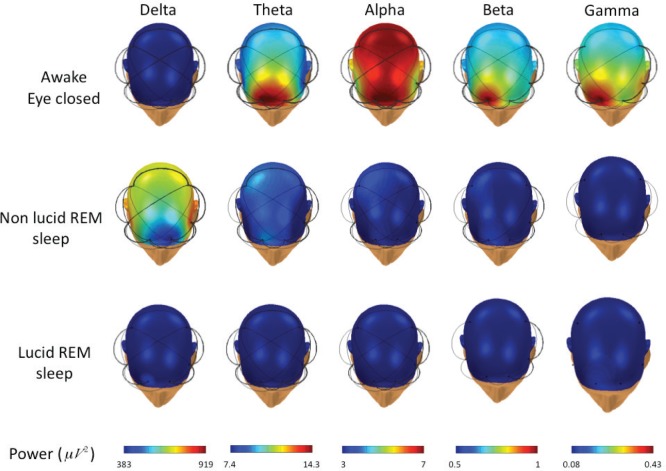
The spectral analysis was performed in all channels, except the occipital channels, which were frequently obscured by artifacts in the head-down lying position. There were no differences in the EEG power of the averaged EEG leads (Fp1, Fp2, F7, F8, C3, C4) between the 14 lucid and 21 non-lucid REM sleep episodes in the gamma, beta, theta, and alpha EEG bands. However, decreased power in the delta EEG band was observed during lucid dreaming compared with that during non-lucid REM sleep (Figure 3). The power analysis of each electrode placement site revealed that the power in the delta EEG band was reduced during lucid versus non-lucid REM sleep in F7, F8, C3 and C4; in F8 and C4 in the theta EEG band, and in C4 in the alpha band (Figure 4). No differences in spectral power were found in any band or electrode site when comparing lucid vs. non-lucid REM sleep. EEG power was lower during both lucid and non-lucid REM sleep than during waking in the alpha, beta and gamma bands. The typical peak in the alpha frequency band in wakefulness was not present in either lucid dreaming or non-lucid REM sleep. The coherences in waking with eyes closed were elevated in the alpha frequency band. The coherences between electrodes were lower in lucid than in non-lucid REM sleep in the gamma frequency bands, regardless of the combination of sites both in long and short distances (Figure 5). In the delta band, the coherence was lower in lucid vs. non-lucid REM sleep between Fp1 and Fp2, F8, C3 and C4; between Fp2 and F7, C3 and C4; between F7 and F8 and C4; between F8 and C3; and between C3 and C4. In the theta band, it was lower between Fp2 and F7, C3 and C4. In the beta band, it was lower between Fp2 and C3 and C4.
Figure 3.
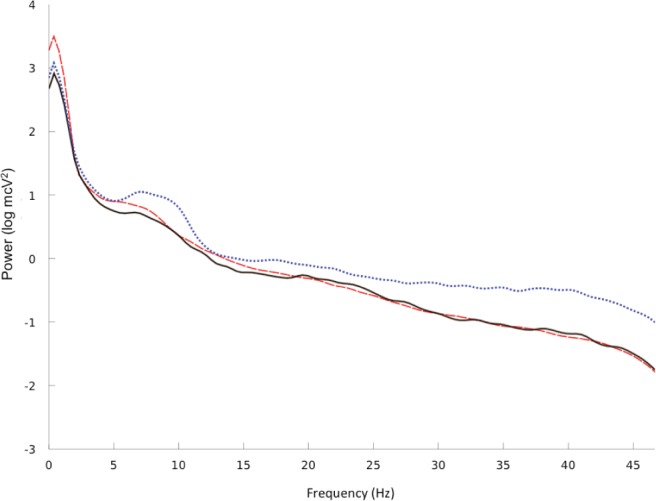
EEG spectral power (log transformed absolute power) during wakefulness (blue dotted line) and during non-lucid (red dashed line) and lucid (black continuous line) REM sleep in all electrodes and all lucid dreamers having displayed an eye signal.
Figure 4.
EEG spectral power, as a log transformed absolute power (log mcV2), during wakefulness (blue columns), non-lucid (red dashed columns) and lucid (black columns) REM sleep in the various electrode placement sites and EEG frequency bands (delta: 0.5–2.9 Hz; theta: 3–7.9 Hz; alpha: 8–12 Hz; beta: 12.1–30 Hz; gamma: 30–49.9 Hz). Significant differences between lucid REM sleep and other states are indicated with an asterisk.
Figure 5.
Topographical distribution (obtained by a spherical spline interpolation) of EEG spectral power during wakefulness (top row), non-lucid (middle row) and lucid (bottom row) REM sleep for different frequency bands. Significant couplings between the electrodes are indicated by the black links (the thickness is proportional to the coherence value). Colors from dark blue (lower EEG power) to dark red (higher EEG power) indicated for each EEG band in the Power line (bottom row).
DISCUSSION
We found that 78% of patients with narcolepsy in our sample were lucid dreamers, achieving on average 8 lucid dreams per month. The frequency of lucid dreaming was higher in narcoleptic lucid dreamers than in healthy lucid dreamers. In addition, only lucid dreamers with narcolepsy (i.e., no control subjects with frequent lucid dreams) were able to perform the instructed eye signal during REM sleep episodes. Narcolepsy patients also experienced lucid dreaming in approximately half of their naps. The arousal index, as well as the amplitude and REMs density, were similar between lucid and non-lucid REM sleep. EEG power was grossly similar in lucid than in non-lucid REM sleep, but lower in the delta band in the electrode grand average; and in the delta, theta and alpha frequency bands in the central right area. Coherence between electrodes was lower in lucid REM sleep than in non-lucid REM sleep, mostly in the delta and gamma frequency bands.
Patients with narcolepsy were lucid dreamers more frequently than the general population. They also experienced lucid dreams more frequently than controls with lucid dreams, to the point of having an average of two lucid dreams per week regularly, even without any training. This dramatic ability of being aware of dreaming in narcolepsy was incidentally noted in one patient.23 Some patients with narcolepsy used this ability to control their nightmares. As recurrent nightmares are a frequent complaint in patients with narcolepsy, this experience (and the numerous tricks reported by lucid patients to master their nightmares, such as recognizing their recurrent dreams, facing danger, flying away, building a safe place or armor, or killing themselves to wake up) could be shared with other non-lucid or partially lucid patients with narcolepsy, such that the latter may improve their ability to achieve lucid dreaming and change nightmare scenarios.
Why Are Narcoleptic Patients More Lucid during REM Sleep than Normal Subjects?
One may imagine that lucid REM sleep is a dissociated state with features of both waking (reflective consciousness, higher activity in the 40 Hz band in the frontal lobe)6 and REM sleep, which also shares similarities with numerous wake-REM dissociated states in narcolepsy. Such states include cataplexy, hypnagogic and hypnopompic hallucinations, sleep paralysis, and REM sleep behavior disorder. However, none of these symptoms (including cataplexy, which is the strongest marker of hypocretin-1 deficiency and is often associated with “genuine” narcolepsy)24 were associated with the ability to achieve lucid dreaming in narcolepsy. Of interest, patients with narcolepsy made a clear distinction between their experiences of lucid dreaming and sleep paralysis, indicating that even if lucid dreaming is a hybrid state between waking and REM sleep,6 it is different from being conscious in a paralyzed body as in sleep paralysis. Narcolepsy impacts the dreaming process, as highlighted by the high frequency of frightening dreams, including nightmares with recurrent thematic content (being chased, crawling in a tube, being attacked by monsters) and the high frequency of flying in narcoleptics dreams.13 Dream reports are longer and more story-like when collected after the first sleep onset in a REM period during the night than after the third REM sleep episode, suggesting that the dream process reaches its optimum earlier in narcolepsy than in the general population.25
The Important Role of SOREM Periods in Lucid Dreaming
The major difference in sleep structure between narcoleptics and controls is SOREM periods, i.e., the ability to enter directly from wakefulness to REM sleep, without any preceding EEG synchronization. Sleep onset in REM period may be a dissociated state of REM sleep per se, as illustrated for example by the absence of normal sympathetic activation during SOREM periods in patients with narcolepsy, compared to physiological REM sleep periods.26 This direct entry in REM sleep intensifies REM-dream emotion, especially anxiety/fear and joy/elation,12 and may also play a major role in promoting lucid dreaming. One may note indeed that the patients in our series were able to clearly display the eye signal only during daytime SOREM periods. In this direction, Fosse reported that reflective consciousness within dreams was judged as higher by 12 patients with narcolepsy than by controls.27 Reflective consciousness was greater during daytime SOREM, equally high during nighttime SOREM and late-night REM episodes, and lower during early-night REM episodes. These results suggest a synergistic effect of narcolepsy on reflective consciousness of circadian time (high consciousness during the end of the night and daytime REM episodes, possibly because the sleep process is low) and direct entry from wakefulness into REM sleep (SOREM). Some normal controls may use the dream re-entry method (a method of lucid dreaming induction in normal controls) to enter the dream state directly from a short period of waking after a dream, especially in the early morning hours.28 Sleep interruption may induce sleep paralysis when sleep resumes as well as SOREM periods29; this may promote lucid dreaming.
Lucid REM Sleep Is Not a Hybrid State between Waking and REM Sleep
The experimental portion of this study confirmed the high frequency at which narcoleptic patients achieve lucid REM sleep and revealed that non-narcoleptic healthy controls who reported frequent lucid dreaming were not able to achieve signaled lucid dreams during a single night. The EEG characteristics found during lucid REM sleep in narcolepsy were similar to those reported by Voss et al., including an EEG spectral power that is grossly similar between lucid and non-lucid REM sleep.6 However, EEG power was not increased in the frontal or fronto-polar electrodes in the gamma (40 Hz) band during lucid REM sleep in our subjects. EEG power was reduced in the delta band during lucid REM sleep and was variously reduced in other bands on individual sites compared to non-lucid REM sleep. Lower delta activity during lucid REM suggests that this state is more activated, at least within low frequencies, than non-lucid REM sleep. The differences between findings may be due to our use of a larger sample of lucid dreamers (7 as opposed to 3) and a greater number of lucid REM sleep segments (14 as opposed to 3). The increased frontal EEG power in the gamma band in healthy lucid dreamers was interpreted as a marker of metacognition during dreaming.6 As a consequence, some authors activated the prefrontal cortex in REM sleep with transcranial direct current stimulation, with the intention of increasing lucidity in dreams.8 As the results were somehow disappointing, it is possible that activation of a network of different brain areas is needed to achieve steady lucidity in dreams. Another possibility is that frontal activity in healthy lucid dreamers was not a marker of metacognition but a marker of the mental effort to achieve lucid REM sleep, while this state would be achieved without any mental effort in narcoleptic patients. In our study, the coherence between electrodes was often lower (and never higher) in lucid compared to non-lucid REM sleep. This result was also different from that reported by Voss et al. (who found greater coherence during lucid than non-lucid REM sleep)6 but is more statistically robust because it was obtained using a larger sample. Using our EEG montage, we mostly explored the coherence within the frontal lobe. The cognitive correlate of decreased coherence between electrodes within specific EEG bands is not completely understood; coherence is decreased not only prior to seizure but also within neuron pairs that are not recruited in a selective attention task.21 It has also been proposed that disrupted synchrony is related to the fragmented cognitive experience of patients with schizophrenia30; this is an experience that may share similarities with lucid dreaming. Intervening in one's dream proper may disturb its internal coherence. Overall, the power and coherence analysis in the present study revealed that lucid REM sleep does not share any EEG similarities with eyes-closed waking. This finding challenges the concept of lucid dreaming as a hybrid state between waking and REM sleep. As a result, lucid REM sleep appears to be a deep sleep state that is as far from waking as ordinary REM sleep. As our patients were recorded from only 6 EEG electrodes, we could not generate a more precise EEG spatial cartography of lucid REM sleep in narcolepsy.
Using the 14 signaled lucid REM sleep periods recorded in our study, we also found that several other markers of REM sleep, which have not been measured in previous studies, remain unchanged between lucid and non-lucid REM sleep. The arousal index was similar in lucid and non-lucid REM sleep, and the duration of REM sleep was longer during lucid dreaming. This finding indicated that attempts to achieve lucidity in narcolepsy do not promote more frequent awakenings from REM sleep (on the contrary, REM sleep time was of greater duration). Muscle atonia tended to be greater in lucid than in non-lucid REM sleep; this is consistent with increased H reflexes (which indicates a higher inhibition of the spinal lower motor neuron) during lucid REM sleep that were previously demonstrated in one subject.9 Increased atonia was unmasked in the present study in patients with narcolepsy because muscle tone is generally abnormally increased during usual REM sleep in narcolepsy.31 Another new finding is that the amplitude of the eye signal in REM sleep was lower than in waking. As subjects were instructed to scan the horizon in both lucid and non-lucid REM sleep, the horizon may be narrower in the dream state than in reality (approximately 26 degrees versus 105 degrees), which provides a novel model to measure the size of mental images. Alternatively, as the eyes and head work in concert to produce gaze, neck paralysis in REM sleep may mask the entire amplitude of the virtual gaze in lucid dreaming.32 The eye signal was greater than the usual REM activity in lucid REM sleep, providing a clue to recognize eye signals and more easily identify periods of lucidity. Identifying periods of lucidity may otherwise be difficult, unless a complex signal is used. Notably, only 14 REM sleep episodes contained without any doubt the eye signal, although participants reported that they achieved lucidity (and were certain they had performed the signal) after 33 REM sleep episodes. Even when subtracting the 9 episodes with a doubtful eye signal, 10 non-signaled lucid REM sleep episodes remain. This suggests that some subjects dreamed of having performed the eye signal without actually having done it physically.
Methodological Considerations
This study was realized under some constrained conditions. The dreaming interview (in the first part of the study) was based on retrospective reports rather than dream diaries. However, all participants were interviewed by the same interviewer. Additionally, the alleged ability to achieve lucidity in dreams was challenged by sleep monitoring in 17 participants. We discarded episodes with a doubtful eye signal to ensure the validity of the results. These neurophysiological markers were measured in 14 lucid REM sleep episodes obtained from 7 subjects, which provides a larger sample than in previous experiments investigating lucid dreaming. Whether our findings are restricted to lucid REM sleep in narcolepsy or if they may apply to lucid REM sleep in normal subjects cannot be directly tested because none of the healthy lucid dreamers were able to achieve signaled lucidity within a single evening.
CONCLUSION
In conclusion, we found a high propensity for lucid dreaming in patients with narcolepsy, which was confirmed via sleep monitoring. Lucid REM sleep did not differ from ordinary REM sleep for most EEG, EOG and EMG characteristics. Patients with narcolepsy may be excellent participants in future studies using our model of lucid dreaming, as they are (i) frequent lucid dreamers without any effort; (ii) able to rapidly enter REM sleep during several consecutive daytime naps, even when the environment is noisy; and (iii) able to achieve lucid REM sleep several times per day. Further, the participants can confirm more easily their lucidity upon awakening from a short nap with REM sleep than in the middle of the night. This is likely to facilitate future experiments regarding this fragile state performed inside noisy functional brain imaging devices. Whether the absence of frontal lobe activation, and decreased rather than increased EEG coherence in lucid REM sleep here were linked to the narcolepsy condition per se or to a more robust analysis performed on more episodes than previous studies should be tested in the future with more healthy lucid dreamers.
DISCLOSURE STATEMENT
This study was funded by the ADOREPS (Association pour le Développement et l'Organisation de la Recherche en Pneumologie et sur le Sommeil). Pauline Dodet received a grant from the Fondation pour la Recherche Medicale during her tenure in a master of neuroscience program. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Hervey de Saint Denys M. Les rêves et les moyens de les diriger: observations pratiques. Paris: Amyot; 1862. [Google Scholar]
- 2.Voss J, Schermelleh-Engel K, Windt J, Frenzel C, Hobson A. Measuring consciousness in dreams: the lucidity and consciousness in dreams scale. Conscious Cogn. 2013;22:8–21. doi: 10.1016/j.concog.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Schredl H, Erlacher D. Frequency of lucid dreaming in a representative German sample. Percept Mot Skills. 2011;112:104–8. doi: 10.2466/09.PMS.112.1.104-108. [DOI] [PubMed] [Google Scholar]
- 4.Voss U, Frenzel C, Koppehele-Gossel J, Hobson A. Lucid dreaming: an age-dependent brain dissociation. J Sleep Res. 2012;21:634–42. doi: 10.1111/j.1365-2869.2012.01022.x. [DOI] [PubMed] [Google Scholar]
- 5.LaBerge S, Nagel L, Dement W, Zarcone V. Lucid dreaming verified by volitional communication during REM sleep. Percept Mot Skills. 1981;52:727–32. doi: 10.2466/pms.1981.52.3.727. [DOI] [PubMed] [Google Scholar]
- 6.Voss U, Holzmann R, Tuin I, Hobson A. Lucid dreaming: a state of consciousness with features of both waking and non-lucid dreaming. Sleep. 2009;32:1191–200. doi: 10.1093/sleep/32.9.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dresler M, Wehrle R, Spoormaker VI, et al. Neural correlates of dream lucidity obtained from contrasting lucid versus non-lucid REM sleep: a combined EEG/fMRI case study. Sleep. 2012;35:1017–20. doi: 10.5665/sleep.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dresler M, Koch S, Wehrle R, et al. Dreamed movement elicits activation in the sensorimotor cortex. Curr Biol. 2011;21:1–5. doi: 10.1016/j.cub.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 9.Brylowski A, Levitan L, LaBerge S. H-reflex suppression and autonomic activation during lucid REM sleep: a case study. Sleep. 1989;12:374–8. doi: 10.1093/sleep/12.4.374. [DOI] [PubMed] [Google Scholar]
- 10.Stumbrys T, Erlacher D, Schredl M. Testing the involvement of the prefrontal cortex in lucid dreaming: a tDCS study. Conscious Cogn. 2013;22:1214–22. doi: 10.1016/j.concog.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Dauvilliers Y, Arnulf I, Mignot E. Narcolepsy with cataplexy. Lancet. 2007;369:499–511. doi: 10.1016/S0140-6736(07)60237-2. [DOI] [PubMed] [Google Scholar]
- 12.Fosse R, Stickgold R, Hobson JA. Emotional experience during rapid-eye-movement sleep in narcolepsy. Sleep. 2002;25:724–32. doi: 10.1093/sleep/25.7.724. [DOI] [PubMed] [Google Scholar]
- 13.Sturzenegger C, Bassetti C. The clinical spectrum of narcolepsy with cataplexy: a reappraisal. J Sleep Res. 2004;13:395–406. doi: 10.1111/j.1365-2869.2004.00422.x. [DOI] [PubMed] [Google Scholar]
- 14.Leu-Semenescu S, De Cock VC, Le Masson VD, et al. Hallucinations in narcolepsy with and without cataplexy: contrasts with Parkinson's disease. Sleep Med. 2011;12:497–504. doi: 10.1016/j.sleep.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 15.American Academy of Sleep Medicine. The international classification of sleep disorders. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 16.Johns MH. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 17.Rechstchaffen A, Kales A. Los Angeles: UCLA Brain Information Service/Brain Research Institute; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. [Google Scholar]
- 18.Iber C, Ancoli-Israel S, Chesson A, Quan S. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 19.Lapierre O, Montplaisir J. Polysomnographic features of REM sleep behavior disorder: development of a scoring method. Neurology. 1992;42:1371–4. doi: 10.1212/wnl.42.7.1371. [DOI] [PubMed] [Google Scholar]
- 20.Brillinger D. Time series: data analysis and theory. Philadelphia, PA: Society for Industrial and Applied Mathematics; 2001. [Google Scholar]
- 21.Varela F, Lachaux J, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large scale integration. Nat Rev Neurosci. 2001;2:229–39. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- 22.Enochson L, Goodman N. Gaussian approximations to the distribution of sample coherence. Wright-Patterson Air Force Base, Ohio: Air Force Flight Dynamics Laboratory Technical Report; 1965. [Google Scholar]
- 23.Tang H, Sharma N, Whyte KF. Lucid dreaming during multiple sleep latency test (MSLT) Sleep Med. 2006;7:462–3. doi: 10.1016/j.sleep.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- 25.Cipolli C, Bellucci C, Mattarozzi K, Mazzetti M, Tuozzi G, Plazzi G. Story-like organization of REM-dreams in patients with narcolepsy– cataplexy. Brain Res Bull. 2008;77:206–13. doi: 10.1016/j.brainresbull.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Donadio V, Liguori R, Vandi S, et al. Sympathetic and cardiovascular changes during sleep in narcolepsy with cataplexy patients. Sleep Med. 2014;15:315–21. doi: 10.1016/j.sleep.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Fosse M. REM mentation in narcoleptics and normals: an empirical test of two neurocognitive theories. Conscious Cogn. 2000;9:488–509. doi: 10.1006/ccog.2000.0466. [DOI] [PubMed] [Google Scholar]
- 28.Stumbrys T, Erlacher D, Schadlich M, Schredl M. Induction of lucid dreams: a systematic review of evidence. Conscious Cogn. 2012;21:1456–75. doi: 10.1016/j.concog.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Takeuchi T, Miyasita A, Sasaki Y, Inugami M, Fukuda K. Isolated sleep paralysis elicited by sleep interruption. Sleep. 1992:217–25. doi: 10.1093/sleep/15.3.217. [DOI] [PubMed] [Google Scholar]
- 30.Tononi G, Edelman G. Schizophrenia and the mechanisms of conscious integration. Brain Res Rev. 2000;31:391–400. doi: 10.1016/s0165-0173(99)00056-9. [DOI] [PubMed] [Google Scholar]
- 31.Dauvilliers Y, Rompre S, Gagnon JF, Vendette M, Petit D, Montplaisir J. REM sleep characteristics in narcolepsy and REM sleep behavior disorder. Sleep. 2007;30:844–9. doi: 10.1093/sleep/30.7.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leclair-Visonneau L, Oudiette D, Gaymard B, Leu-Semenescu S, Arnulf I. Do the eyes scan dream images during rapid eye movement sleep? Evidence from the rapid eye movement sleep behaviour disorder model. Brain. 2010;133:1737–46. doi: 10.1093/brain/awq110. [DOI] [PubMed] [Google Scholar]